Abstract
Objective
To measure the effects of a five-minute delay (DCC) versus immediate cord clamping (ICC) on residual placental blood volume (RPBV) at birth, and hemoglobin and serum bilirubin at 24 to 48 hours of age.
Study Design
In this prospective randomized controlled trial, seventy-three women with term (37 to 41 weeks) singleton fetuses were randomized to DCC (≥5 minutes; n=37) or ICC (<20 seconds; n=36).
Results
Maternal and infant demographics were not different between groups. Mean cord clamping time was 303 ± 121 (DCC) versus 23 ± 59 (ICC) seconds (p<0.001) with 10 protocol violations. Cord milking was the proxy for DCC (n = 11) when the provider could not wait. Infants randomized to DCC compared to ICC had significantly less RPBV (20.0 vs 30.8 mL/kg, p<0.001), higher hemoglobin levels (19.4 vs 17.8 g/dL, p=0.002) at 24 to 48 hours, with no difference in bilirubin levels.
Conclusions
Term infants had early hematological advantage of DCC without increases in hyperbilirubinemia or symptomatic polycythemia.
Keywords: delayed cord clamping, umbilical cord milking, hemoglobin, bilirubin, residual placental blood volume, placental transfusion
Introduction
At each birth, obstetrical providers decide when to clamp and cut the umbilical cord. Immediate cord clamping (ICC) has become the routine practice in the United States without scientific evaluation of its potential impact on an infant’s health and development. Clear evidence to guide a provider’s decision for term infants is not sufficient at this time.1 Fear of hyperbilirubinemia and polycythemia are concerns that have hindered the adoption of delayed cord clamping (DCC) as routine care.
The amount of blood an infant obtains with placental transfusion is considerable. When cord clamping is delayed at birth or the umbilical cord is milked, infants obtain a placental transfusion resulting in approximately a 20 to 30% increase in blood volume and a 50% increase in red cell volume.2, 3 Although changes in practice are beginning to favor DCC, evidence of long-term outcomes is limited.
Older physiologic studies on placental transfusion or DCC show advantages for term infants that include increased red cell volume and higher hematocrit levels 4, 5, 6; improved cutaneous perfusion and higher skin temperature 7, 8; increased renal blood flow with higher urine output and less sodium loss 9; and an increased red blood cell flow to the brain and gut.10 None of these studies followed the infants beyond hospitalization. None reported complications or adverse events.
In the latest Cochrane review on term infants, McDonald et al (2013) found no significant differences between infants with ICC or DCC for most neonatal morbidities such as Apgar score less than seven at five minutes, admission to the neonatal intensive care unit, or clinical jaundice.11 However, in the DCC group, mean birthweight (mean increase of 101 grams) and hemoglobin concentrations in infants at 24 to 48 hours were significantly higher. While there was no difference in the diagnosis of clinical jaundice, 2% fewer infants in the ICC group required phototherapy compared to the DCC group. Improvement in iron stores appeared to persist into infancy. Infants receiving ICC were twice as likely to be iron deficient at three to six months compared with infants receiving DCC (RR 2.65, 95% CI 1.04 to 6.73).
We have conducted two pilot randomized controlled trials involving full-term infants. The first (n = 24) demonstrated that milking the umbilical cord (UCM) five times after cesarean section, in contrast to ICC, resulted in significantly higher hemoglobin and hematocrit levels at 24–48 hours and less residual placental blood volume (RPBV) with no reports of adverse neonatal events.12 The second pilot study compared RPBV for infants (n=36) placed on the maternal abdomen and assigned to one of four groups: ICC, a delay of two minutes, a delay of five minutes, or UCM. Infants placed on the maternal abdomen immediately after birth who were assigned to a five-minute delay in cord clamping time received a significantly larger placental transfusion than those with a two-minute delay.13 The results of these two pilot feasibility studies formed the basis for our current study design.
Our current randomized trial tests the effects of DCC for five minutes with term infants placed skin-to-skin on the maternal abdomen with the aim of assessing brain myelin deposition and developmental outcomes at four months, twelve months and two years of age (NCT01620008). This paper reports on the birth data, clinical safety issues, and the two-day hospital outcomes for the cohort of infants and mothers. We predicted that DCC would result in less RPBV, higher hematocrit and hemoglobin at 48 hours of age, and no differences in hyperbilirubinemia or polycythemia.
Materials and Methods
Enrollment for this prospective randomized controlled trial was conducted from July 2013 to November 2015 at Women and Infants Hospital (WIH) in Providence, Rhode Island after approval by the WIH and the University of Rhode Island Institutional Review Boards. Follow-up for this study is out to 24 months of age and will be finished in November 2017. An independent data safety and monitoring committee reviewed the data after 42 infants were randomly assigned. No concerns were identified.
Pregnant women were recruited from the WIH obstetric clinic, private obstetric offices, WIH childbirth and breastfeeding classes, at community affairs and brochures placed in obstetric and pediatric offices. Women were eligible if they were expecting a healthy singleton pregnancy in the vertex position at term (37–416/7 weeks), had no evidence of medical or obstetrical complications (i.e. hypertension, pre-eclampsia, diabetes, smoking, substance abuse, suspected intrauterine growth restriction), were planning on breastfeeding, spoke English, and were at least 18 years of age. Infants with evidence of intrauterine growth restriction and serious congenital anomalies were excluded. All participants were screened and informed about the study by registered nurses (RNs) who completed the written informed consent with each mother. Doulas were assigned as research assistants (RAs) to each woman to support communication both pre-labor and postpartum and to attend the births, assign randomization, collect the RPBV, conduct two-day hospital visits, and assist with follow-up.
A statistician not involved in the trial established the randomization plan before the study began. Blocked, stratified randomization of subjects by gender (or unknown gender) was used to assign the groups with a pre-specified equal probability. Sequenced and sealed envelopes identifying the stratification and group assignment on cards were prepared and kept in a secured area on the Labor and Delivery Unit. The study RAs randomized mothers based on the next study card designation when women went into active labor.
At the time of birth, infants were randomized to either ICC (the control group) or DCC (the intervention group). The provider placed the infant skin-to-skin on the maternal abdomen immediately at birth where they were dried and stayed for at least 30 minutes. Those randomized to ICC received the hospital’s usual practice of cord clamping (≤20 seconds). For infants receiving DCC, the RAs used a stopwatch to notify the provider to clamp at five minutes. If unable to carry out the DCC protocol, or if delivery was by cesarean section, the cord was milked five times before being clamped (n=11).
The RPBV was collected via a blood collection bag and weighed (1 gm = 1mL). The provider inserted the blood bag needle into the umbilical vein when collection was in-utero. Ex-utero collection was done by suspending the placenta and draining into blood bag via needle inserted in the vein. The cord was drained until there was no more blood returned usually about one to two minutes. Blood was collected with the placenta in-utero at vaginal deliveries (n = 54) and ex-utero after cesarean section (n = 19) by necessity. Cord blood samples were collected for hemoglobin, hematocrit, ferritin, total serum bilirubin (TSB), and C-reactive protein (CRP) either by the obstetrical provider or the RAs. At 24–48 hours, when the state recommended newborn metabolic screening sample was drawn, laboratory personnel drew a capillary sample from the infant’s heel for hemoglobin, hematocrit, and TSB. All subsequent nursery care was routine.
Outcome variables for the neonatal data were RPBV at birth, hematocrit, hemoglobin, and TSB levels at 24 to 48 hours, hyperbilirubinemia, symptomatic polycythemia, anemia at 24 to 48 hours, and admission to the NICU. RPBV was defined as the amount of blood (mL/kg of birth weight) obtained from the umbilical cord by drainage into a blood bag (Blood-Pack Unit, Fenwal Inc., Lake Zurich, IL, USA) after birth with the placenta either in-utero (vaginal delivery) or ex-utero (cesarean section). Hematocrit and hemoglobin were peripheral capillary samples and analyzed in the WIH laboratory (Coulter LH750 Hematology Analyzer, Beckman-Coulter, Miami, FL, USA). Risk of hyperbilirubinemia was assessed by plotting the TSB levels by age (in hours) [birth and 24–48 hour samples] on the Bhutani Nomogram.14 This allowed us to quantify hyperbilirubinemia risk and compare TSB levels between groups. We analyzed the peak level each infant reached at any time during hospitalization (range 31–56 hours of age). Symptomatic polycythemia was defined as hematocrit over 65% with evidence of newborn distress as assessed by the pediatric providers.15, 16 Anemia was defined as capillary hematocrit below 47%.17
The intervention could not be masked because of the obvious nature of cord clamping. Staff who attended each birth were asked not to reveal the infant’s randomization group in the medical record. Laboratory and nursery personnel were masked to the randomization group and results of the 24–48 hour blood work. Research staff collecting on-going clinical data and follow-up data remained blinded to the infants’ randomization.
Power analyses for RPBV and two day hematocrit and hemoglobin require less than 15 infants in each group. Consequently we based our sample size on the study’s four-month outcome variable, ferritin level (μg/L), for the continuing longitudinal study. Data from prior studies suggest that without adjustment sample sizes of 30 subjects per group would have more than 80% power at alpha of 0.05 to detect a 20% difference in ferritin levels between the two treatment arms.18, 19, 20
Data analyses included two-sided Pearson’s chi-square tests, t-tests, and Wilcoxon rank-sum tests for non-normally distributed variables. Odds ratios and 95% confidence intervals were examined as appropriate. Primary analyses were conducted using intention-to-treat, and sensitivity analyses were conducted using actual treatment in order to assess the robustness of the findings and to examine results of the biologic variables.
Results
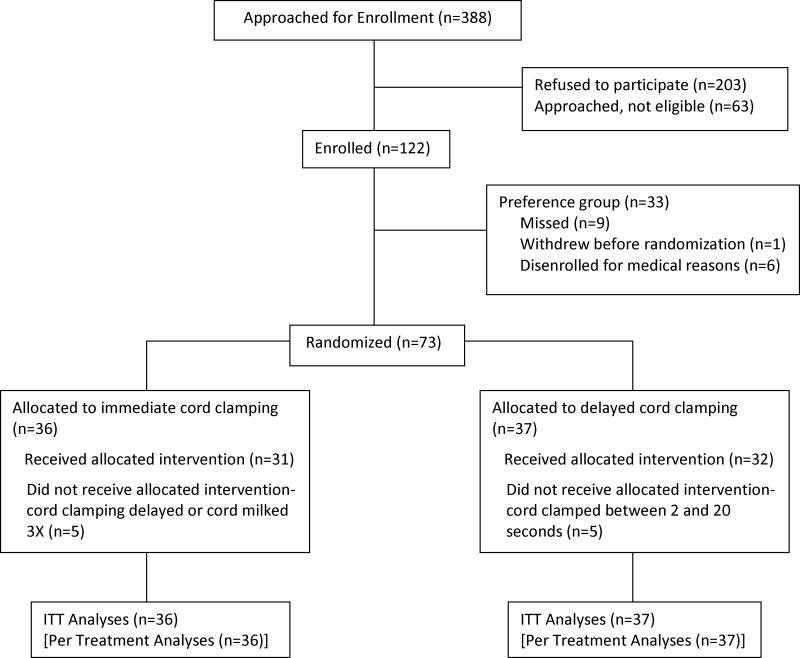
Seventy-three pregnant women and their fetuses between 37 and 416/7 weeks gestation were randomized at birth from July 2013 through November 2015 (Figure 1). Thirty six infants were randomized to the ICC group and 37 to the DCC group.
Figure 1.
Flow Chart
Table 1 shows that there were no significant differences between the DCC and ICC groups with respect to maternal demographics or clinical variables. Table 2 shows no significant differences between the two groups on the neonatal demographics and clinical variables except for cord clamping time and RPBV. Per protocol, cord-clamping time was significantly longer in the DCC group. Cord clamping time including and excluding those infants who received UCM is noted to assess compliance with the protocol. As expected, RPBV was less in those infants who received DCC or UCM (mean difference of 10.8 mL/kg). Infants delivered by cesarean section had less RPBV than infants born vaginally but this did not reach a level of significance. There were 10 protocol violations. Reasons included 1) the clinical situation of meconium or shoulder dystocia (n=4), 2) the provider preference for the DCC or UCM group (n=2), 3) a misunderstanding between RAs and provider (n=2), and 4) parental request at time of delivery (n=2).
Table 1. Maternal Demographics and Clinical Variables at Birth (ITT).
| Maternal Characteristics | DCC (n=37) | ICC (n=36) | P value |
|---|---|---|---|
|
| |||
| Age (years) | 28.3 ± 5.5 | 27.2 ± 5 | 0.35 |
|
| |||
| Primipara | 15 (41) | 14 (39) | 0.89 |
|
| |||
| Maternal education (years) | 14 ± 2.7 | 14 ± 2.6 | 0.96 |
|
| |||
| Insurance, public | 23 (62) | 21 (58) | 0.74 |
|
| |||
| Hemoglobin at admission (g/dL) | 11.6 ± 1 | 12.0 ± 1 | 0.14 |
|
| |||
| Lead level at admission (mcg/dL) | 1.0 ± 0.4 | 1.1 ± 0.4 | 0.76 |
|
| |||
| Ferritin at admission (ng/mL) | 23.4 ± 22 | 16.9 ± 14 | 0.07 |
|
| |||
| Mode of delivery | |||
| Spontaneous vaginal | 27 (73) | 25 (69) | 0.28 |
| Instrumented vaginal | 2 (5) | 0 (0) | |
| Cesarean section | 8 (22) | 11 (31) | |
N (%) or mean ± SD
Table 2. Infant Demographics and Clinical Variables at Birth (ITT).
| Infant Characteristics | DCC (n=37) | ICC (n=36) | P value |
|---|---|---|---|
| Gestational Age, weeks | 39.5 ± 1 (37–41) | 39.4 ± 1 (37–41) | 0.54 |
| Male: Female | 19:18 | 18:18 | 0.91 |
| Cord Clamping Time (sec) (includes UCM) Range | 192 ± 171 (5–647) | 23.1 ± 59 (3–360) | 0.001 |
| Cord Clamping Time (sec) (without UCM) Range | 303 ± 121 (55–647) | 10 ± 6 (3–25) | 0.001 |
| Apgar Scores 1 minute, median (range) 5 minutes |
8 (2–9) 9 (8–10) |
8 (2–9) 9 (5–9) |
0.65 0.24 |
| Apgar score ≤7 at 5 min | 0 (0) | 1 (3) | 0.31 |
| Temperature <36.6°C in first 15 minutes | 1 (3) | 0 (0) | 0.32 |
| Birth Weight (g) Range | 3584 ± 497 (2455–4410) | 3433 ± 454 (2730–4630) | 0.18 |
| RPBV (mL/kg) Range | 20.0 ± 8.5 (0–36.8) | 30.8 ± 9.6 (14.1–65.2) | 0.001 |
| Cord blood Hemoglobin (g/dL) Range | 14.8 ± 2 (12.0–19.9) | 15.2 ± 2 (12–18.9) | 0.29 |
| Cord blood Hematocrit (%)Range | 44.2 ± 6.3 (24.5–59.2) | 45.9 ± 4.7 (36–53) | 0.24 |
| Cord blood Ferritin (ng/mL) Range | 154.3 ± 115 (16.9–570.4) | 134.6 ± 81 (18.1–365.1) | 0.42 |
| Protocol Deviations | 5 (14) | 5 (14) | 0.96 |
| Breastfeeding at time of discharge | 18 (50) | 19 (56) | 0.62 |
N (%) or mean ± SD (range)
Table 3 shows blood values at approximately 24 to 48 hours of age as well as the incidence of jaundice and polycythemia comparing groups by intention to treat and actual treatment. As expected, there were significant differences in hemoglobin and hematocrit levels without differences in peak TSB levels or symptomatic polycythemia between groups. None of the six infants in the DCC group with hematocrit levels > 65% exhibited symptoms or received any treatment for polycythemia. Two infants were admitted to the NICU briefly (DCC = 1; ICC = 1) for observation for respiratory distress (DCC) and on day two for hypoglycemia (ICC). Two infants (ICC) were readmitted for hyperbilirubinemia: one on day three for a bilirubin level of 17.3 mg/dL and the other on day 12 for “likely” breast milk jaundice. Five infants (14%) in the ICC group had a two-day hematocrit level < 47% but none of the infants were diagnosed with anemia by pediatric providers.
Table 3. Measures of 24 to 48 hour blood values by Intention-to-Treat and Sensitivity Analyses (Actual treatment).
| Two Day Values | Intention-to-treat | Actual Treatment | ||||
|---|---|---|---|---|---|---|
| DCC (n=37) | ICC (n=36) | P value | DCC (n=37) | ICC (n=36) | P value | |
| Hemoglobin (g/dL), (capillary) | 19.4 ± 2 (16.3–25.0) | 17.8 ± 2 (14.5–22.5) | 0.002 | 19.5 ± 2.1 (16.3–25) | 17.7 ± 1.8 (14.5–22.2) | 0.002 |
| Hematocrit (%), (capillary) | 58 ± 6.2 (50.3–73.7) | 53 ± 5.4 (42.4–63.8) | 0.001 | 58.4 ± 6.2 (50.9–73.7) | 52.5 ± 5.2 (42.4–64.4) | 0.001 |
| Peak total serum bilirubin (TSB) level, (mg/dL)* | 9 ± 3 (1.8–15.3) | 8.5 ± 3 (1.7–13.5) | 0.50 | 8.5 ± 3 (1.8–14.9) | 9.0 ± 3.2 (1.7–15.3) | 0.45 |
| Bilirubin Nomogram High risk zone (≥95%) (bilitool.org) | 2 (5) | 2 (6) | 0.95 | 1 (3) | 3 (9) | 0.28 |
| Phototherapy (initial hospital stay) | 4 (11) | 0 (0) | 0.04 | 2 (5) | 2 (6) | 0.98 |
| Symptomatic Polycythemia | 0 | 0 | -- | 0 | 0 | -- |
| Capillary Hematocrit > 65% | 6 (16) | 0 (0) | 0.01 | 6 (16) | 0 (0) | 0.01 |
| Capillary Hematocrit >70% | 1 (3) | 0 (0) | 0.33 | 1 (3) | 0 (0) | 0.32 |
| Capillary Hematocrit < 47% | 0 (0) | 5 (14) | 0.02 | 0 (0) | 5 (14) | 0.02 |
N (%) or mean ± SD (range);
TSB collection time ranged from 31 to 56 hours of age
While analysis by intention-to-treat showed significantly more infants in the DCC group needing phototherapy, analysis by actual treatment revealed that there were two infants in each group who received phototherapy during hospital stay. Treatment was provided at the discretion of the managing pediatrician.
All study recruitment was completed during the prenatal period (late second and early third trimester). We did not have access to the data of women who refused enrollment or who were not enrolled.
Discussion
This is the first study of DCC to be conducted in the United States on healthy term infants and the first in North America since 1980.21 It is also the most recent study to use a five-minute delay with infants placed skin-to-skin on the maternal abdomen. In 2012, the ACOG statement on DCC offered a consensus that evidence was lacking to recommend DCC or UCM for term infants. This current study is designed to examine short-term and long-term outcomes over a two-year span. In this report, we have shown that term infants with DCC of five minutes (or UCM x 5) have lower amounts of RPBV associated with higher hematocrit and hemoglobin at 24 to 48 hours and no increase in jaundice, symptomatic polycythemia, or other adverse effects.
Our use of a five-minute delay along with placement on the maternal abdomen is unique among current cord clamping studies. Our pilot data showed that placing the term infant on the maternal abdomen during a delay in cord clamping slows the transfer of placental blood necessitating a five-minute delay to obtain a full placental transfusion.13 Others have shown no difference in weight from immediately after birth to after clamping at 2 minutes with the infants placed either on the maternal abdomen or at the introitus.22 However, the overall weight gain by the infants in Vain’s study was 52 to 59 grams – slightly more than half of the weight gain predicted by the meta-analysis.11 This reinforces our position that a two minute delay is not long enough for a full placental transfusion when the infant is placed on the maternal abdomen. We attempted to obtain the largest placental transfusion possible to maximize blood volume for comparisons of long-term infant outcomes with ICC versus DCC.
There is currently no ethical way to measure blood volume of healthy term infants thus necessitating the use of measures of inference such as two-day hematocrit and hemoglobin and RPBV. Measurement of the RPBV can be used to approximate the amount of blood transfused to the infant during the first few minutes after birth. While documentation of RPBV is not essential in studies of DCC, we included this measure to suggest mechanisms of action if our longitudinal study data should show DCC to be an advantage. Our findings are consistent with a previous study that measured RPBV by drainage following vaginal birth 4, 23 and with a study showing more transfer of placental blood to the infant with DCC via weighing infants with attached cord over the first five minutes after birth.3 Recent studies of cord blood harvesting report higher total volume of blood and stem cell yields with in-utero rather than ex-utero collection.24, 25 We used ex-utero collection at cesarean section to get better drainage and allow the surgeon to close the uterus and a few times at vaginal birth when the placenta delivered quickly.
There is a widespread belief that placental transfusion (DCC/UCM) increases an infant's risk for jaundice or polycythemia. This has hindered the adoption of placental transfusion techniques in clinical practice.26 The most recent Cochrane review on term infants reports no increase in clinical jaundice although they report a 2% increase of infants needing phototherapy.11 Bilirubin is a known antioxidant. Zahir and colleagues (2015) suggest that bilirubin levels that are elevated but still within a normal range (associated with normal physiologic jaundice) may provide a unique protective antioxidant effect, especially for the developing brain.27 None of the published RCTs on term infants have demonstrated an increase in jaundice 11, 20, 28, 29 and experts refute that DCC causes hyperbilirubinemia (A. McDonagh, personal communication, 2014). We used the Bhutani Nomogram for designation of hyperbilirubinemia risk to define hyperbilirubinemia in our subjects.14 This allowed a quantification of levels of risk and a comparison of the two groups of infants.14 We included results by sensitivity analyses to show the actual effect of DCC on hyperbilirubinemia rather than the effect of being assigned to receive DCC. Intention-to-treat analyses show the compliance with the protocol. Analyses by actual treatment are needed to assess and clarify differences in biologic variables affected by the protocol. Experts suggest hemolytic disorders (i.e. ABO incompatibility) and genetic predisposition (i.e. G6PD) are most frequently the cause for severe hyperbilirubinemia.30, 31 Even in infants with red cell alloimmunization who required in utero transfusion, Garabedian et al (2016) found that DCC at birth resulted in improved hemoglobin levels at birth, less need for postnatal exchange transfusions, and no increase in severe hyperbilirubinemia.32 Thus, evidence that the practice of DCC is associated with severe hyperbilirubinemia is lacking.
We defined polycythemia as a hematocrit >65% using capillary samples. We used capillary samples to avoid collecting venous blood on two-day old healthy term infants. Only one study infant had a venous blood draw for a capillary hematocrit value of 61.5%. The infant did not have polycythemia. Oh and Lind (1966) reported venous samples to be 6 to 9 percentage points lower than capillary samples in infants with DCC.5 Thus our results likely err on the side of caution. No infant with a capillary hematocrit > 65% exhibited symptoms. The one infant who had a capillary hematocrit level >70% had a gentle easy birth, breastfed very well and remained asymptomatic. Hemoglobin and hematocrit are not routinely collected on all normal newborns at WIH therefore we felt it was ethical to mask nursery personnel to our laboratory findings.33 Blood hyperviscosity secondary to polycythemia (hematocrit > 65%) is frequently cited as a reason for providers to clamp and cut the cord immediately as a way to prevent over transfusion of red blood cells at the time of birth. Christensen and colleagues (2014) demonstrated that placental transfusion will not lead to neonatal hyperviscosity.34
Lack of equipoise in the community was an important factor that made enrollment difficult. There is a large amount of available information on the Internet extolling the benefits of DCC. Many women we approached did not want to be randomized. We revised our study plans to include a parallel "preference" group. The intention was to balance women who preferred DCC and women who preferred ICC for cord blood banking/harvesting. Early in the study, the local cord blood collection bank closed for financial reasons. As a result only one woman requested ICC so the balanced “preference” plan was unsuccessful. Although there were no differences in biologic variables between the randomized group (n = 73) and the preference group (n =33), the women in our preference group had significantly higher socio-economic status than our randomized group. Although data from our preference group are not analyzed in this paper, we plan to evaluate whether inclusion of those infants is appropriate when longitudinal data are examined over the two-year follow-up period. The follow-up assessments are ongoing until November 2017.
Term infants who were randomized to DCC left less residual blood volume behind in the placenta. The DCC infants had higher neonatal hemoglobin and hematocrit levels at 24 to 48 hours of age with no differences on peak TSB levels at 24 to 48 hours. There were no indicators of differences between groups in incidence of hyperbilirubinemia or symptomatic polycythemia. The study results support the early hematological advantage of DCC while demonstrating no association with an increase in hyperbilirubinemia or symptomatic polycythemia out to 48 hours of age.
Acknowledgments
This project was supported by the Bill and Melinda Gates Foundation, Grand Challenges Exploration, Phase I Grant, OPP1061070 and the NIH National Institute of Child Health & Human Development Grant 1R01HD076589. The authors would like to thank William Oh, MD and Betty Vohr, MD, for advising on the design of the study and Richard Tucker, BA for statistical consultation. We wish to thank the parents and infants who participated in this study and the research assistants and staff at Women and Infants Hospital. Without their trust and cooperation, this study would not have been possible.
Funding Source: Bill & Melinda Gates Foundation, Grand Challenges Exploration, Phase I Grant, OPP1061070; and NIH National Institute of Child Health & Human Development Grant 1R01HD076589
Abbreviations
- DCC
Delayed cord clamping
- ICC
Immediate cord clamping
- RAs
Research assistants
- RPBV
Residual placental blood volume
- TSB
Total serum bilirubin
- UCM
Umbilical cord milking
Footnotes
Financial Disclosure: None of the authors has any financial relationships relevant to this article to disclose. No one received any form of payment to produce the manuscript.
Conflict of Interest: The authors have no conflict of interest to disclose.
Clinical Trial Registration: ClinicalTrials.gov NCT01620008
References
- 1.ACOG. Committee Opinion No. 543: Timing of umbilical cord clamping after birth. Obstet Gynecol. 2012;120(6):1522–1526. doi: 10.1097/01.AOG.0000423817.47165.48. [DOI] [PubMed] [Google Scholar]
- 2.Yao AC, Lind J. Effect of gravity on placental transfusion. Lancet. 1969;2(7619):505–508. doi: 10.1016/s0140-6736(69)90213-x. [DOI] [PubMed] [Google Scholar]
- 3.Farrar D, Airey R, Law GR, Tuffnell D, Cattle B, Duley L. Measuring placental transfusion for term births: weighing babies with cord intact. BJOG. 2011;118(1):70–75. doi: 10.1111/j.1471-0528.2010.02781.x. [DOI] [PubMed] [Google Scholar]
- 4.Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet. 1969;2(7626):871–873. doi: 10.1016/s0140-6736(69)92328-9. [DOI] [PubMed] [Google Scholar]
- 5.Oh W, Lind J. Venous and capillary hematocrit in newborn infants and placental transfusion. Acta Paediatr Scand. 1966;55(1):38–48. doi: 10.1111/j.1651-2227.1966.tb15207.x. [DOI] [PubMed] [Google Scholar]
- 6.Cernadas J, Carroli G, Pellegrini L, Otano L, Ferreira M, Ricci C. The effect of timing of cord clamping on neonatal venous hematocrit values and clinical outcome at term: A randomized controlled trial. Obstetrical & Gynecolgical Survey. 2006;61(9):564–565. doi: 10.1542/peds.2005-1156. [DOI] [PubMed] [Google Scholar]
- 7.Oh W, Lind J. Body temperature of the newborn infant in relation to placental transfusion. Acta Paediatr Scand. 1967;172S:137–145. doi: 10.1111/j.1651-2227.1967.tb15289.x. [DOI] [PubMed] [Google Scholar]
- 8.Pietra GG, D'Amodio MD, Leventhal MM, Oh W, Braudo JL. Electron microscopy of cutaneous capillaries of newborn infants: effects of placental transfusion. Pediatr. 1968;42(4):678–683. [PubMed] [Google Scholar]
- 9.Oh W, Oh MA, Lind J. Renal function and blood volume in newborn infant related to placental transfusion. Acta Paediatr Scand. 1966;55:197–210. [Google Scholar]
- 10.Nelle M, Zilow EP, Bastert G, Linderkamp O. Effect of Leboyer childbirth on cardiac output, cerebral and gastrointestinal blood flow velocities in full-term neonates. Am J Perinatol. 1995;12(3):212–216. doi: 10.1055/s-2007-994455. [DOI] [PubMed] [Google Scholar]
- 11.McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013;7:Cd004074. doi: 10.1002/14651858.CD004074.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson-Owens DA, Mercer JS, Oh W. Umbilical cord milking in term infants delivered by cesarean section: a randomized controlled trial. J Perinatol. 2012;32(8):580–584. doi: 10.1038/jp.2011.159. [DOI] [PubMed] [Google Scholar]
- 13.Mercer JS, Erickson-Owens DA. Rethinking placental transfusion and cord clamping issues. JPNN. 2012;26(3):202–217. doi: 10.1097/JPN.0b013e31825d2d9a. [DOI] [PubMed] [Google Scholar]
- 14.AAP. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar S, Rosenkrantz TS. Neonatal polycythemia and hyperviscosity. Seminars in fetal & neonatal medicine. 2008;13(4):248–255. doi: 10.1016/j.siny.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Mimouni FB, Merlob P, Dollberg S, Mandel D. Neonatal polycythaemia: critical review and a consensus statement of the Israeli Neonatology Association. Acta Paediatr. 2011;100(10):1290–1296. doi: 10.1111/j.1651-2227.2011.02305.x. [DOI] [PubMed] [Google Scholar]
- 17.Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Seminars in fetal & neonatal medicine. 2007;12(1):54–63. doi: 10.1016/j.siny.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaparro CM, Neufeld LM, Tena Alavez G, Eguia-Liz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomised controlled trial. Lancet. 2006;367(9527):1997–2004. doi: 10.1016/S0140-6736(06)68889-2. [DOI] [PubMed] [Google Scholar]
- 19.Gupta R, Ramji S. Effect of delayed cord clamping on iron stores in infants born to anemic mothers: a randomized controlled trial. Indian Pediatr. 2002;39(2):130–135. [PubMed] [Google Scholar]
- 20.Andersson O, Hellstrom-Westas L, Andersson D, Domellof M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomized controlled trial. BMJ. 2011;343:d7157. doi: 10.1136/bmj.d7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson N, Enkin MW, Saigal S, Bennett KJ, Milner R, Sackett DL. A randomized trial of the Leboyer approach to childbirth. New Engl J Med. 1980;302:655–660. doi: 10.1056/NEJM198003203021203. [DOI] [PubMed] [Google Scholar]
- 22.Vain NE, Satragno DS, Gorenstein AN, Gordillo JE, Berazategui JP, Alda MG, et al. Effect of gravity on volume of placental transfusion: a multicentre, randomised, non-inferiority trial. Lancet. 2014;384(9939):235–240. doi: 10.1016/S0140-6736(14)60197-5. [DOI] [PubMed] [Google Scholar]
- 23.Nelle M, Zilow EP, Kraus M, Bastert G, Linderkamp O. The effect of Leboyer delivery on blood viscosity and other hemorheologic parameters in term neonates. Am J Obstet Gynecol. 1993;169(1):189–193. doi: 10.1016/0002-9378(93)90161-b. [DOI] [PubMed] [Google Scholar]
- 24.Bassiouny MR, El-Chennawi F, Mansour AK, Yahia S, Darwish A. Optimal method for collection of umbilical cord blood: an Egyptian trial for a public cord blood bank. Transfusion. 2015;55(6):1263–1268. doi: 10.1111/trf.12978. [DOI] [PubMed] [Google Scholar]
- 25.Wu S, Xie G, Wu J, Chen J, Lu Y, Li Y, et al. Influence of maternal, infant, and collection characteristics on high-quality cord blood units in Guangzhou Cord Blood Bank. Transfusion. 2015;55(9):2158–2167. doi: 10.1111/trf.13126. [DOI] [PubMed] [Google Scholar]
- 26.Jelin AC, Kuppermann M, Erickson K, Clyman R, Schulkin J. Obstetricians' attitudes and beliefs regarding umbilical cord clamping. J Matern Fetal Neonatal Med. 2014;27(14):1457–1461. doi: 10.3109/14767058.2013.864275. [DOI] [PubMed] [Google Scholar]
- 27.Zahir F, Rabbani G, Khan RH, Rizvi SJ, Jamal MS, Abuzenadah AM. The pharmacological features of bilirubin: the question of the century. Cell Mol Biol Lett. 2015;20(3):418–447. doi: 10.1515/cmble-2015-0012. [DOI] [PubMed] [Google Scholar]
- 28.Upadhyay A, Gothwal S, Parihar R, Garg A, Gupta A, Chawla D, et al. Effect of umbilical cord milking in term and near term infants: randomized control trial. Am J Obstet Gynecol. 2013;208(2):120, e121–126. doi: 10.1016/j.ajog.2012.10.884. [DOI] [PubMed] [Google Scholar]
- 29.Begum F, Zaman T, Khan RM. Effect of early and delayed umbilical cord clamping of term infants on mothers and neonates. Intl Gyn Obstret. 2012;119S3 (S295 Abstract 0097) [Google Scholar]
- 30.Christensen RD, Yaish HM. Hemolytic disorders causing severe neonatal hyperbilirubinemia. Clinics in perinatology. 2015;42(3):515–527. doi: 10.1016/j.clp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Bhutani VK, Srinivas S, Castillo Cuadrado ME, Aby JL, Wong RJ, Stevenson DK. Identification of neonatal haemolysis: an approach to predischarge management of neonatal hyperbilirubinemia. Acta Paediatr. 2016;105(5):e189–194. doi: 10.1111/apa.13341. [DOI] [PubMed] [Google Scholar]
- 32.Garabedian C, Rakza T, Drumez E, Poleszczuk M, Ghesquiere L, Wibaut B, et al. Benefits of delayed cord clamping in red blood Cell alloimmunization. Pediatrics. 2016;137(3):1–6. doi: 10.1542/peds.2015-3236. [DOI] [PubMed] [Google Scholar]
- 33.Strauss RG, Mock DM, Johnson KJ, Cress GA, Burmeister LF, Zimmerman MB, et al. A randomized clinical trial comparing immediate versus delayed clamping of the umbilical cord in preterm infants: short-term clinical and laboratory endpoints. Transfusion. 2008;48(4):658–665. doi: 10.1111/j.1537-2995.2007.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen RD, Baer VL, Gerday E, Sheffield MJ, Richards DS, Shepherd JG, et al. Whole-blood viscosity in the neonate: effects of gestational age, hematocrit, mean corpuscular volume and umbilical cord milking. J Perinatol. 2014;34(1):16–21. doi: 10.1038/jp.2013.112. [DOI] [PubMed] [Google Scholar]