Abstract
Data on the frequency of nosocomial infections during extracorporeal membrane oxygenation (ECMO) in adult populations remain scarce. We investigated the risk factors for nosocomial infections in adult patients undergoing venoarterial ECMO (VA-ECMO) support. From January 2011 to December 2015, a total of 259 patients underwent ECMO. Of these, patients aged 17 years or less and patients undergoing ECMO for less than 48 hours were excluded. Of these, 61 patients diagnosed with cardiogenic shock were evaluated. Mean patient age was 60.6 ± 14.3 years and 21 (34.4%) patients were female. The mean preoperative Sequential Organ Failure Assessment (SOFA) score was 8.6 ± 2.2. The mean duration of ECMO support was 6.8 ± 7.4 days. The rates of successful ECMO weaning and survival to discharge were 44.3% and 31.1%, respectively. There were 18 nosocomial infections in 14 (23.0%) patients. These included respiratory tract infections in 9 cases and bloodstream infections in a further 9. In multivariate analysis, independent predictors of infection during ECMO were the preoperative creatinine level (hazard ratio [HR], 2.176; 95% confidence interval [CI], 1.065–4.447; P = 0.033) and the duration of ECMO support (HR, 1.400; 95% CI, 1.081–1.815; P = 0.011). A higher preoperative creatinine level and an extended duration of ECMO support are risk factors for infection. Therefore, to avoid the development of nosocomial infections, strategies to shorten the length of ECMO support should be applied whenever possible.
Keywords: Extracorporeal Membrane Oxygenation Support, Infection
Graphical Abstract
INTRODUCTION
In the time since the first successful application of extracorporeal membrane oxygenation (ECMO) support in 1972 (1), ECMO has become one of the most important therapeutic modalities for severe cardiopulmonary failure (2). The use of ECMO to treat adults has increased greatly since the H1N1 influenza pandemic of 2009 and publication of the findings of a multicenter randomized trial of treatments for severe adult respiratory failure (the CESAR trial) (3,4).
Although the experience of clinicians with ECMO therapy in adult populations is growing, ECMO is still associated with high-level mortality and many potential complications. Of these, nosocomial infection (NI) is associated with high-level mortality (5,6). Adult patients undergoing ECMO are at higher risk for nosocomial infection than neonate and pediatric patients (6.1% of neonates and pediatric patients vs. 20.5% of adults develop culture-proven infections during ECMO) (5).
However, data on NIs acquired during ECMO in the adult populations remain scarce. Although a few studies have explored this topic (7,8), the works have been limited in terms of patient numbers considerable heterogeneity in patient baseline characteristics including the ECMO mode employed (venovenous vs. venoarterial), the indications for ECMO, and the ECMO cannulation site (percutaneous vs. intrathoracic). Such heterogeneity may affect the incidence of NI development during ECMO. Thus, further work with a homogeneous patient group is required to precisely identify risk factors for NI in patients on ECMO support.
Therefore, we investigated the risk factors for NIs in patients with cardiogenic shock who underwent percutaneous venoarterial ECMO (VA-ECMO) support.
MATERIALS AND METHODS
Patients
From January 2011 to December 2015, 259 consecutive patients underwent ECMO at Chonnam National University Hospital. Patients aged 17 years or less were excluded. Patients who underwent ECMO for < 48 hours were not included in this study since they were not exposed to ECMO long enough to identify ECMO-related infection events. A total of 95 adult patients underwent VA-ECMO for cardiogenic shock. Of these, 34 adult patients underwent ECMO support for < 48 hours. Among the 34 patients, 2 patients survived and 32 patients were non-survivors. The cause of death in the 32 non-survivor patients included hypoxic brain death in 8 patients and multi-organ failure in 24. However, there was no sepsis-related death in these patients. Finally, cardiogenic shock patients (n = 61) undergoing ECMO for ≥ 48 hours were evaluated. Weaning success rate, survival to discharge, and infection incidence on ECMO were assessed through hospital records. Baseline characteristics and operative information including age, gender, underlying conditions, pre-ECMO laboratory findings, Sequential Organ Failure Assessment (SOFA) score, chest X-ray state (1 point per quadrant infiltrated) before ECMO, transfusion history before ECMO, hospital and intensive care unit (ICU) stay before ECMO, type and mode of ECMO, type of anticoagulation, and duration of ECMO support were collected and evaluated. Each patient's survival at the time of ECMO de-cannulation and at the time of discharge was also evaluated in terms of weaning success rate and survival to discharge.
ECMO procedures
Cannulation of ECMO was performed in a sterile fashion with chlorhexidine painting. In ECMO-eligible patients, an emergency bypass system (n = 38, 62.3%; Terumo Inc., Tokyo, Japan) or a permanent life support system (n = 23, 37.7%; Maquet Inc., Hirrlingen, Germany) was applied. The oxygenator of the permanent life support system is a polymethylpentene (PMP) type. The emergency bypass system is a non-PMP type oxygenator. Recently, the Maquet system has been more commonly used than the Terumo system, which is known to be the more durable and thrombo-resistant device (9). Heparin or nafamostat mesilate was used as an anticoagulant. Cannulation mode, size, and approach site were determined by the surgeon considering the patient's body weight, height, and vessel size. A peripheral approach using femoral artery and vein was the predominant approach in the study. The management protocol for ECMO follows the Extracorporeal Life Support Organization (ELSO) guidelines (10).
Definition of nosocomial infection during ECMO support
The definition of nosocomial infections during ECMO support was based on the Centers for Disease Control and Prevention definitions for nosocomial infections (11). Nosocomial infection during ECMO support was defined as a case with confirmed organisms from one or more blood, respiratory, or urinary cultures during the period 48 hours after the initiation of ECMO to 24 hours after ECMO weaning. Microbiological isolations were correlated with clinical symptoms and typical inflammatory characteristics in blood samples and radiographic findings.
Antibiotics strategy
Prophylactic antibiotics with broad spectrum drug was prescribed to patients at the initiation of ECMO support. In patients who had already been taking antibiotics, antibiotic agents were maintained. If infection developed during ECMO support, antibiotics were changed according to the culture results. With regard to dosing of antibiotics, conventional dose was started and adjusted according to the drug concentration monitoring. Every antibiotic therapy was discussed with the department of infection and adjusted according to the patient's clinical situations.
Statistical analysis
Categorical variables are presented as frequencies and percentages and continuous variables are expressed as means ± standard deviations. Student's t-tests and χ2 tests were used to compare factors related to nosocomial infection. We performed the logistic regression using nosocomial infection as the dependent variable. P values < 0.05 were considered statistically significant. SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Ethics statement
This study was approved by Institutional Review Board of Chonnam National University Hospital, which waived the requirement for informed patient consent based on the retrospective nature of the work (IRB No: CNUH-2016-228).
RESULTS
Baseline characteristics
The 61 patients with cardiogenic shock underwent ECMO for a total of 416.0 days. The mean age of patients at the time of ECMO support was 60.6 ± 14.3 years, and 34.4% (n = 21) were female. A total of 42.6% (n = 26) of patients had hypertension and 41.0% (n = 25) diabetes mellitus. The infiltration status of pre-ECMO chest X-rays was 1.6 ± 1.4. Eighteen patients (29.5%) had a transfusion history before ECMO. Pre-ECMO hospital and ICU stays before ECMO were 4.4 ± 6.5 and 1.8 ± 2.5 days, respectively (Table 1). Venoarterial type was the predominant ECMO type in 95.1% (n = 58) of the patients. Venoarterial-venous type of ECMO was applied in 3 patients (4.9%). According to the ECMO oxygenator type, the non-PMP oxygenator was used in 38 patients (62.3%) and the PMP type in 23 patients (37.7%). Anticoagulation was performed using heparin in 88.6% (n = 54) of patients. The duration of ECMO support was 6.8 ± 7.4 days (Table 2).
Table 1. Baseline characteristics before ECMO support.
| Characteristics | Value* |
|---|---|
| No. of patients | 61 |
| Age, yr | 60.6 ± 14.3 |
| Female gender | 21 (34.4) |
| Body weight, kg | 62.1 ± 11.8 |
| Underlying condition | |
| Hypertension | 26 (42.6) |
| Diabetes mellitus | 25 (41.0) |
| Coronary artery disease | 10 (16.4) |
| Chronic renal disease | 5 (8.2) |
| Acute kidney injury | 3 (4.9) |
| Dialysis | 3 (4.9) |
| COPD | 1 (1.6) |
| Cerebrovascular accident | 2 (3.3) |
| Laboratory findings | |
| White blood cell (103/mm3) | 12.8 ± 5.9 |
| Hemoglobin, g/dL | 11.9 ± 2.8 |
| Platelets (103/mm3) | 204.7 ± 77.8 |
| CRP, mg/dL | 4.6 ± 6.2 |
| Lactate, mmol/L | 8.9 ± 4.7 |
| Total bilirubin, mg/dL | 1.1 ± 0.9 |
| Creatinine, mg/dL | 1.5 ± 1.2 |
| SOFA score | 8.6 ± 2.2 |
| Infiltration status on CXR† | 1.6 ± 1.4 |
| Transfusion history | 18 (29.5) |
| Pre-ECMO ICU stay, day | 1.8 ± 2.5 |
| Pre-ECMO hospital stay, day | 4.4 ± 6.5 |
ECMO = extracorporeal membrane oxygenation, COPD = chronic obstructive lung disease, CXR = chest X-ray, CRP = C-reactive protein, ICU = intensive care unit, SOFA = Sequential Organ Failure Assessment.
*Data are presented as numbers of cases (%) for categorical variables or as means ± standard deviations for continuous variables; †One point for each quadrant infiltrated (0–4).
Table 2. ECMO data of patients.
| Parameters of ECMO | Value* |
|---|---|
| No. of patients | 61 |
| ECMO support | |
| Venoarterial mode | 58 (95.1) |
| Venoarterial-venous mode | 3 (4.9) |
| Type of ECMO oxygenator | |
| Non-PMP membrane | 38 (62.3) |
| PMP membrane | 23 (37.7) |
| Anticoagulation drug used | |
| Heparin | 54 (88.6) |
| Nafamostat mesilate | 7 (11.4) |
| Duration of ECMO support, day | 6.8 ± 7.4 |
ECMO = extracorporeal membrane oxygenation, PMP = polymethylpentene.
*Data are presented as numbers of cases (%) for categorical variables or as means ± standard deviations for continuous variables.
Early outcomes and infection incidence
The rates of weaning success and survival to discharge were 44.3% (27/61) and 31.1% (19/61), respectively. There were 18 nosocomial infection events of 14 (23.0%) patients. The incidence of ECMO-related nosocomial infection was 43.3 cases per 1,000 ECMO-days. These included bloodstream infection (BSI) in 9 cases, and respiratory tract infection (RTI) in 9 cases (Table 3). However, there was no urinary tract infection (UTI) and surgical site wound infection.
Table 3. Microorganisms causing infections during ECMO support.
| Microorganism species | RTI (n = 9) | BSI (n = 9) |
|---|---|---|
| Gram-negative pathogens | ||
| A. baumannii | 8 | 2 |
| P. aeruginosa | 0 | 2 |
| Enterobacter cloacae | 0 | 0 |
| Serratia marcescens | 0 | 1 |
| Klebsiella pneumonia | 0 | 1 |
| Gram-positive pathogens | ||
| S. aureus | 0 | 1 |
| Staphylococcus epidermidis | 0 | 1 |
| Corynebacterium striatum | 0 | 1 |
| Fungi | ||
| Candida albicans | 1 | 0 |
| Candida tropicalis | 0 | 0 |
ECMO = extracorporeal membrane oxygenation, RTI = respiratory tract infection, BSI = bloodstream infection.
Causative microorganisms of nosocomial infection
The offending organisms associated with nosocomial infection are shown in Table 3. Gram-negative pathogens were predominant in both RTI and BSI. Acinetobacter baumannii (n = 8, 88.9%) was the most common respiratory tract pathogens. In terms of BSI, A. baumannii (n = 2), and Pseudomonas aeruginosa (n = 2) were the most common pathogens.
The characteristics and ECMO outcomes of patients with and without nosocomial infections
The baseline characteristic data for 47 patients without nosocomial infection and 14 patients with nosocomial infection are shown in Tables 4 and 5. There were no significant differences between the 2 groups in terms of age, sex, body weight, underlying condition, laboratory findings, infiltration state in chest X-ray, ICU and hospital stay before ECMO, ECMO oxygenator type and anticoagulation drug type. However, patients with nosocomial infection underwent ECMO for a longer period than patients without nosocomial infection (14.5 ± 12.4 days vs. 4.5 ± 2.4 days, P = 0.010). The rates of weaning success and survival to discharge were higher in patients without nosocomial infection than in patients with nosocomial infection, (48.9% vs. 28.6% and 36.2% vs. 14.3%, respectively); however, the differences were not significant (P = 0.228 and P = 0.190, respectively).
Table 4. The characteristics of patients with and without nosocomial infections during ECMO.
| Risk factors | Without infection*(n = 47) | With infection*(n = 14) | P value |
|---|---|---|---|
| Age, yr | 61.3 ± 14.9 | 58.5 ± 12.5 | 0.529 |
| Female gender | 14 (29.8) | 7 (50.0) | 0.206 |
| Body weight, kg | 62.9 ± 12.1 | 59.6 ± 10.5 | 0.369 |
| Underlying condition | |||
| Hypertension | 19 (40.4) | 7 (50.0) | 0.553 |
| Diabetes mellitus | 18 (38.3) | 7 (50.0) | 0.540 |
| Coronary artery disease | 7 (14.9) | 3 (21.4) | 0.683 |
| Chronic renal disease | 3 (6.4) | 2 (14.3) | 0.322 |
| Acute kidney injury | 3 (6.4) | 0 | > 0.999 |
| Dialysis | 2 (4.3) | 1 (7.1) | 0.549 |
| COPD | 0 | 1 (7.1) | 0.230 |
| Cerebrovascular accident | 2 (4.3) | 0 | > 0.999 |
| Laboratory findings | |||
| White blood cell (103/mm3) | 12.5 ± 6.1 | 13.7 ± 5.2 | 0.490 |
| Hemoglobin, g/dL | 12.1 ± 2.8 | 11.2 ± 3.0 | 0.310 |
| Platelets (103/mm3) | 204.8 ± 82.2 | 204.4 ± 63.5 | 0.989 |
| CRP, mg/dL | 4.8 ± 6.6 | 3.7 ± 5.1 | 0.563 |
| Lactate, mmol/L | 8.7 ± 4.4 | 9.7 ± 5.7 | 0.499 |
| Total bilirubin, mg/dL | 1.1 ± 0.9 | 1.1 ± 1.1 | 0.819 |
| Creatinine, mg/dL | 1.4 ± 0.8 | 2.1 ± 2.1 | 0.227 |
| SOFA score | 8.5 ± 2.4 | 9.0 ± 1.7 | 0.473 |
| Infiltration status on CXR | 1.6 ± 1.4 | 1.6 ± 1.6 | 0.874 |
| Peak body temperature, ℃ | 37.3 ± 1.3 | 37.2 ± 1.0 | 0.667 |
| Transfusion history | 17 (36.2) | 1 (7.1) | 0.047 |
| Pre-ECMO ICU stay, day | 1.7 ± 2.0 | 2.1 ± 3.7 | 0.599 |
| Pre-ECMO hospital stay, day | 4.5 ± 7.0 | 3.9 ± 4.3 | 0.747 |
ECMO = extracorporeal membrane oxygenation, COPD = chronic obstructive lung disease, CRP = C-reactive protein, CXR = chest X-ray, ICU = intensive care unit, SOFA = Sequential Organ Failure Assessment.
*Data are presented as numbers of cases (%) for categorical variables or as mean ± standard deviation for continuous variables.
Table 5. Outcomes of ECMO support in patients with and without nosocomial infections.
| Outcomes | Without infection* (n = 47) | With infection* (n = 14) | P value |
|---|---|---|---|
| ECMO oxygenator type | 0.756 | ||
| Non-PMP membrane | 30 (63.8) | 8 (57.1) | |
| PMP membrane | 17 (36.2) | 6 (42.9) | |
| Anticoagulation drug given | 0.710 | ||
| Heparin | 42 (89.3) | 12 (85.7) | |
| Nafamostat mesilate | 5 (10.7) | 2 (14.3) | |
| ECMO support duration, day | 4.5 ± 2.4 | 14.5 ± 12.4 | 0.010 |
| ECMO weaning | 0.228 | ||
| Success | 23 (48.9) | 4 (28.6) | |
| Failure | 24 (51.1) | 10 (71.4) | |
| Survival to discharge | 0.190 | ||
| Yes | 17 (36.2) | 2 (14.3) | |
| No | 30 (63.8) | 12 (85.7) |
ECMO = extracorporeal membrane oxygenation, PMP = polymethylpentene.
*Data are presented as numbers of cases (%) for categorical variables or as means ± standard deviations for continuous variables.
Multivariate analysis of risk factors for nosocomial infection
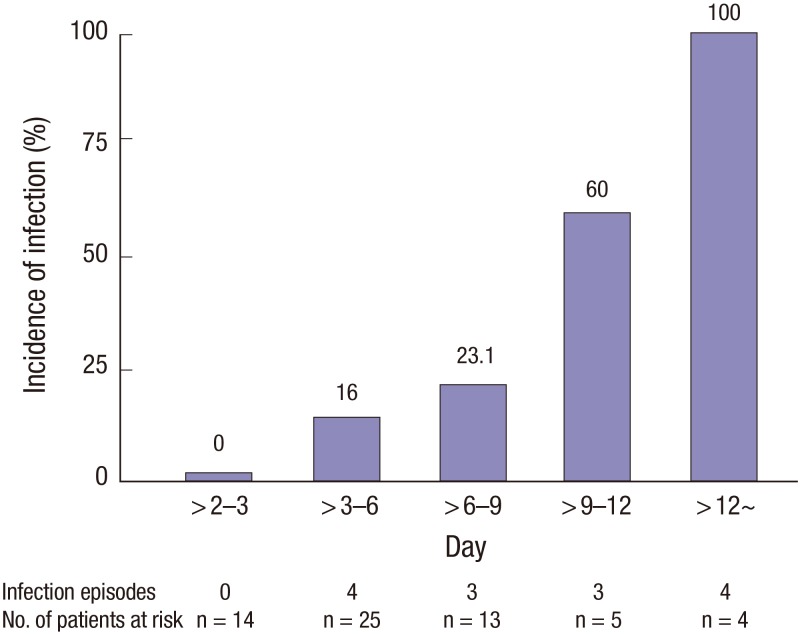
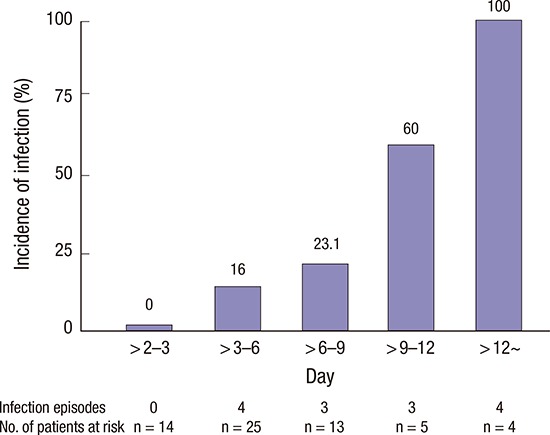
In the logistic regression analysis, pre-specified covariates were included in this analysis. Independent predictors of infection during ECMO were a higher preoperative creatinine level (adjusted odds ratio [OR], 2.176; 95% confidence interval [CI], 1.065–4.447; P = 0.033) and the duration of ECMO support (adjusted OR, 1.400; 95% CI, 1.081–1.815; P = 0.011) (Table 6). Fig. 1 shows the incidence of infection according to the duration of ECMO.
Table 6. Multivariate analysis of risk factors for nosocomial infection.
| Variables | Adjusted OR (95% CI) | P value |
|---|---|---|
| Pre-ECMO creatinine level, mg/dL | 2.176 (1.065–4.447) | 0.033 |
| Duration of ECMO support, day | 1.400 (1.081–1.815) | 0.011 |
OR = odds ratio, CI = confidence interval, ECMO = extracorporeal membrane oxygenation.
*Variables with a probability valve < 0.20 in univariate analyses were candidates for multivariate analysis. The candidate variables were gender, pre-ECMO creatinine level, transfusion history, and duration of ECMO support.
Fig. 1.
The incidence of infection according to the duration of ECMO.
ECMO = extracorporeal membrane oxygenation.
DISCUSSION
We found that the incidence of nosocomial infection in an adult population undergoing ECMO was 43.3 cases per 1,000 ECMO-days. In multivariate analyses, both a higher preoperative creatinine level and the duration of ECMO support were independently associated with nosocomial infection. ECMO patients are at an increased risk for infections as a consequence of being in an ICU. Mechanical ventilators, central venous catheters, indwelling urinary catheters, surgical incisions, and health care providers are all sources of infection for ECMO patients. In general, the rate of nosocomial infection in the ICU has ranged from 13.9% to more than 44.0% (12,13).
In terms of the site of nosocomial infection, Vincent et al. (12) analyzed data from 1,417 ICUs in Europe to determine the incidence of pathogens and infections; pneumonia and other lower RTIs (64.7%) were the most common diseases, followed by UTI (17.6%) and BSI (12.0%). However, among infections during ECMO support, BSI was the most common, occurring in 32.6% to 89.4%, and BSIs were predominant in most studies, followed by RTIs, UTIs, and surgical site infections (7,14,15). In our study, the infection site differed from that of the general ECMO cohort. We found that the incidence of RTI was similar to that of BSI.
We found that the hazard ratio for nosocomial infection in patients with higher levels of preoperative creatinine was 2.176 (P = 0.033). Although the precise mechanism by which increased preoperative creatinine increases the risk of nosocomial infection remains unknown, some data suggest that increased serum levels of creatinine correlate with nosocomial infection. An increase in creatinine levels reflects the presence of kidney disease; an injured kidney may compromise the immune response via systemic release from leukocytes in the kidneys and renal tubular cells (16,17,18,19,20). Such changes in the host immune response may be associated with nosocomial infection.
We also found that the duration of ECMO was a risk factor for infection. Many previous studies showed that the duration of ECMO support was the most important risk factor of acquired infection during ECMO and was associated with a significantly increased rate of death (7,14,15). Burket et al. (21) reported that the rate of BSI increased with the duration of ECMO support. Among patients who underwent ECMO for 3–10 days, the rate of BSI was 9.5 cases per 1,000 ECMO-days, and among those who underwent ECMO for 11–20 days and 21–30 days, the rates of BSI increased to 27.2 cases per 1,000 ECMO-days and 64.5 cases per 1,000 ECMO-days, respectively. We found that a longer duration of ECMO was associated with nosocomial infection. The receiver operation characteristic (ROC) curve showed that a cut-off point of 3.7 days was significant. The incidence of nosocomial infection was higher in patients who underwent ECMO for > 3.7 days than for shorter times. However, nosocomial infection did not affect survival to discharge. This may be because of our small patient numbers. Further studies on larger cohorts are required.
In terms of the causative microorganisms, large-scale international data show that Staphylococcus aureus and other Staphylococcus species (38%) are the most common pathogens causing BSIs, followed by Escherichia coli (24%), in critically ill patients without ECMO support (12,22). In addition, Candida species are often isolated in the ICU and can cause pneumonia and BSIs with a mortality rate of up to 47% (12,22). On the other hand, microbes are commonly associated with medical device-related infections in patients with ECMO support, such as coagulase-negative Staphylococcus, Pseudomonas, and Candida species (7). However, the balance is shifting from multi-resistant staphylococci to highly resistant classes of A. baumannii and P. aeruginosa (23). In our study, A. baumannii was the most common pathogen in both blood and the respiratory tract, which would be a common pathogen in the ICU environment. We encountered no case of fungal infection (for example, Candida) in the present study. This may have been due to the short duration of ECMO compared with those in other ECMO cohort studies.
This study had the limitations inherent in retrospective work using observational data from a single center. Although our patient numbers were too low to allow us to draw strong conclusions, we sought to identify precise risk factors in a homogeneous group.
A higher preoperative creatinine level and an extended duration of ECMO support were risk factors for nosocomial infection. Therefore, to avoid the development of nosocomial infections, strategies shortening the length of ECMO support should be applied whenever possible.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Kim GS, Lee KS, Jeong IS, Ahn BH. Data curation: Kim GS, Kang SK, Kim S. Investigation: Kim GS, Lee KS, Park CK, Kang SK, Kim DW. Supervision: Kim GS, Lee KS, Park CK, Kang SK, Kim DW, Oh SG, Oh BS, Jung Y, Kim S, Yun JS, Song SY, Na KJ, Jeong IS, Ahn BH. Writing - original draft: Kim GS, Lee KS, Oh SG, Oh BS, Kim S, Yun JS, Song SY, Na KJ, Jeong IS, Ahn BH. Writing - review & editing: Kim GS, Lee KS, Oh SG, Oh BS, Kim S, Yun JS, Song SY, Na KJ, Jeong IS, Ahn BH.
References
- 1.Hill JD, O’Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, Gerbode F. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286:629–634. doi: 10.1056/NEJM197203232861204. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett RH. Extracorporeal life support: history and new directions. ASAIO J. 2005;51:487–489. doi: 10.1097/01.mat.0000179141.08834.cb. [DOI] [PubMed] [Google Scholar]
- 3.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR, Registry EL. Extracorporeal life support organization registry report 2012. ASAIO J. 2013;59:202–210. doi: 10.1097/MAT.0b013e3182904a52. [DOI] [PubMed] [Google Scholar]
- 4.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 5.Aubron C, Cheng AC, Pilcher D, Leong T, Magrin G, Cooper DJ, Scheinkestel C, Pellegrino V. Infections acquired by adults who receive extracorporeal membrane oxygenation: risk factors and outcome. Infect Control Hosp Epidemiol. 2013;34:24–30. doi: 10.1086/668439. [DOI] [PubMed] [Google Scholar]
- 6.Haneke F, Schildhauer TA, Schlebes AD, Strauch JT, Swol J. Infections and extracorporeal membrane oxygenation: incidence, therapy, and outcome. ASAIO J. 2016;62:80–86. doi: 10.1097/MAT.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 7.Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P, Extracorporeal Life Support Organization Task Force on Infections, Extracorporeal Membrane Oxygenation Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011;12:277–281. doi: 10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Bréchot N, Hariri S, Guiguet M, Luyt CE, Makri R, Leprince P, Trouillet JL, Pavie A, Chastre J, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis. 2012;55:1633–1641. doi: 10.1093/cid/cis783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Undar A, Wang S, Palanzo DA. Impact of polymethylpentene oxygenators on outcomes of all extracorporeal life support patients in the United States. Artif Organs. 2013;37:1080–1081. doi: 10.1111/aor.12242. [DOI] [PubMed] [Google Scholar]
- 10.Annich G, Lynch W, MacLaren G, Wilson J, Bartlett R. ECMO: Extracorporeal Cardiopulmonary Support in Critical Care. 4th ed. Ann Arbor, MI: Extracorporeal Life Support Organization; 2012. [Google Scholar]
- 11.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 12.Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, Wolff M, Spencer RC, Hemmer M. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274:639–644. [PubMed] [Google Scholar]
- 13.Maillet JM, Guérot E, Novara A, Le Guen J, Lahjibi-Paulet H, Kac G, Diehl JL, Fagon JY. Comparison of intensive-care-unit-acquired infections and their outcomes among patients over and under 80 years of age. J Hosp Infect. 2014;87:152–158. doi: 10.1016/j.jhin.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Schutze GE, Heulitt MJ. Infections during extracorporeal life support. J Pediatr Surg. 1995;30:809–812. doi: 10.1016/0022-3468(95)90753-x. [DOI] [PubMed] [Google Scholar]
- 15.Brown KL, Ridout DA, Shaw M, Dodkins I, Smith LC, O’Callaghan MA, Goldman AP, Macqueen S, Hartley JC. Healthcare-associated infection in pediatric patients on extracorporeal life support: the role of multidisciplinary surveillance. Pediatr Crit Care Med. 2006;7:546–550. doi: 10.1097/01.PCC.0000243748.74264.CE. [DOI] [PubMed] [Google Scholar]
- 16.Bihorac A, Efron PA, Ang D, Maier RV, Moldawer LL. Acute kidney injury is associated with nosocomial infections and surgical site infections after trauma. Surg Infect (Larchmt) 2011;12:S017. [Google Scholar]
- 17.Bihorac A, Baslanti TO, Cuenca AG, Hobson CE, Ang D, Efron PA, Maier RV, Moore FA, Moldawer LL. Acute kidney injury is associated with early cytokine changes after trauma. J Trauma Acute Care Surg. 2013;74:1005–1013. doi: 10.1097/TA.0b013e31828586ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoste EA, De Corte W. Clinical consequences of acute kidney injury. Contrib Nephrol. 2011;174:56–64. doi: 10.1159/000329236. [DOI] [PubMed] [Google Scholar]
- 19.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19:547–558. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DW, Faubel S, Edelstein CL. Cytokines in acute kidney injury (AKI) Clin Nephrol. 2011;76:165–173. doi: 10.5414/cn106921. [DOI] [PubMed] [Google Scholar]
- 21.Burket JS, Bartlett RH, Vander Hyde K, Chenoweth CE. Nosocomial infections in adult patients undergoing extracorporeal membrane oxygenation. Clin Infect Dis. 1999;28:828–833. doi: 10.1086/515200. [DOI] [PubMed] [Google Scholar]
- 22.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyle JS, Buising KL, Thursky KA, Worth LJ, Richards MJ. Epidemiology of infections acquired in intensive care units. Semin Respir Crit Care Med. 2011;32:115–138. doi: 10.1055/s-0031-1275525. [DOI] [PubMed] [Google Scholar]