Abstract
This study aimed to investigate the effects of vitamin and antioxidant supplements in the prevention of bladder cancer using a meta-analysis of randomized controlled trials (RCTs). Fourteen RCTs were included in the final analysis. In a fixed-effect meta-analysis, vitamin and antioxidant supplements showed no preventive effect for bladder cancer (relative risk [RR] = 1.04; 95% confidence interval [CI] 0.92–1.17; I2 = 39.7%). Also, there was no preventive effect of these supplements in the subgroup meta-analyses by various factors such as type of supplements, type of cancer prevention, methodological quality, providers of supplements, type of control group, and number of participants. Among the subgroup analyses by type of supplements, beta-carotene supplementation alone marginally increased the risk of bladder cancer (RR = 1.44; 95% CI 1.00–2.09; I2 = 0.0%; n = 3). The current meta-analysis found that vitamin and antioxidant supplements have no preventive effect against bladder cancer.
Keywords: Antioxidant, Bladder Cancer, Meta-Analysis, Randomized Controlled Trials, Vitamin
Graphical Abstract
INTRODUCTION
There were an estimated 429,000 new cases and 165,000 deaths of bladder cancer in 2012 worldwide, ranking the ninth among the most common cancers (1). Bladder cancer is more common in developed countries than underdeveloped ones and develops in men more than in women (2).
There are several risk factors for the development of bladder cancer, such as cigarette smoking, chronic bladder Schistosoma infection, and occupational exposures (3,4). Previous observational studies suggested that the intake of fruits and vegetables was associated with a reduced risk of bladder cancer (5,6). A recent meta-analysis also reported that there was an inverse association between the consumption of fruits and vegetables and the bladder cancer risk (7). Vegetables and fruits are rich in various vitamins or antioxidants, which may have anticarcinogenic activities by inhibition of oxidative stress and inflammation (8).
Recently, several randomized controlled trials (RCTs) (9,10,11,12,13,14,15,16,17,18,19,20,21,22) reported the association between the use of vitamin or antioxidant supplements and the risk of bladder cancer. However, those findings remain inconsistent, and some studies even reported that vitamin and antioxidant supplements increased the risk of bladder cancer (15).
This study aimed to investigate the preventive effect of vitamin and antioxidant supplements on bladder cancer by using a meta-analysis of RCTs according to various factors such as type of cancer prevention, type of vitamin or antioxidant supplements, dose of supplements, methodological quality of studies, providers of supplements, and type of control group.
MATERIALS AND METHODS
Literature search
We searched PubMed, EMBASE, and the Cochrane Library in April 2015 first, by using common keywords related to the use of vitamin or antioxidant supplements and the risk of bladder cancer in RCTs. Additionally, in July 2016, we searched additional RCTs. The keywords were as follows: ‘vitamin,’ ‘antioxidant,’ ‘vitamin A,’ ‘retinol,’ ‘retinal,’ ‘retinoic acid,’ ‘retinoid,’ ‘tretinoin,’ ‘fenretinide,’ ‘etretinate,’ ‘acitretin,’ ‘beta-carotene,’ ‘vitamin B,’ ‘vitamin B1,’ ‘vitamin B2,’ ‘vitamin B3,’ ‘vitamin B5,’ ‘vitamin B6,’ ‘vitamin B7,’ ‘vitamin B9,’ ‘vitamin B12,’ ‘thiamine,’ ‘riboflavin,’ ‘niacin,’ ‘nicotinic acid,’ ‘pantothenic acid,’ ‘pyridoxine,’ ‘biotin,’ ‘folic acid,’ ‘cobalamin,’ ‘vitamin C,’ ‘ascorbic acid,’ ‘vitamin D,’ ‘vitamin E,’ ‘alpha-tocopherol,’ ‘selenium,’ ‘lipoic acid,’ ‘glutathione,’ ‘catechin,’ ‘isoflavone,’ ‘lycopene,’ ‘resveratrol,’ or ‘coenzyme q10,’ and ‘bladder cancer.’ Also, the bibliographies of relevant articles were reviewed to locate studies that were not included from the database search. There was no restriction to publication languages.
Selection criteria
We included RCTs that evaluated the efficacy of vitamin or antioxidant supplements for the prevention of bladder cancer. The main outcome measure was bladder cancer incidence. We excluded studies related to etretinate which was removed from the market in Canada and USA due to the high risk of birth defects. If the data from the same study were duplicated in more than 1 article, we included the first published or largest study.
Selection of relevant studies
Based on the pre-determined selection criteria, 2 evaluators (Park SJ and Lee YJ) independently screened all studies searched from the databases and bibliographies by reviewing those titles and abstracts. When there was a disagreement between evaluators concerning the inclusion of studies, we discussed and reached a consensus. We thoroughly reviewed the full texts that were selected from the first screening and included the studies meeting the selection criteria in the final analysis.
Assessment of methodological quality
We assessed the methodological quality of the trials based on the Jadad scale (23). This scale includes points for randomization (mentioned as randomized, 1 point; if the randomization is appropriate, e.g., table of random numbers or computer-generated randomization, additional 1 point), double-blind (mentioned as double-blind, 1 point; if the blinding is appropriate, e.g., masking such as identical placebo, additional 1 point), and follow-up (the numbers and reasons for withdrawal in each group are described; 1 point) reported in individual RCTs. We classified all studies into 2 groups, trials with a score of 2 or less as low quality and a score of 3 to 5 as high quality.
Main and subgroup analyses
We investigated the association between the use of vitamin or antioxidant supplements and the risk of bladder cancer. Additionally, we performed subgroup meta-analyses according to type and dose of vitamin or antioxidant supplements; type of cancer prevention (primary prevention in subjects without a history of bladder cancer or secondary prevention in bladder cancer survivors); methodological quality of study (high vs. low); duration of treatment (< 5 years vs. ≥ 5 years); providers of supplements (pharmaceutical industry vs. non-pharmaceutical industry); type of control (placebo vs. no treatment); number of participants (< 10,000 vs. ≥ 10,000).
Statistical analysis
We used both the fixed-effect model and random-effects models in order to estimate the pooled relative risk (RR) with its 95% confidence interval (CI). Higgins I2 was calculated to assess heterogeneity across studies. Higgins I2 means the percentage of total variation across trials (24). I2 was calculated as follows:
| I2 = 100% × (Q – df)/Q, |
where Q is Cochran's heterogeneity statistic, and df indicates the degrees of freedom. Negative values of I2 are put equal to 0. Therefore, I2 ranges between 0% (no observed heterogeneity) and 100% (maximal heterogeneity). An I2 value greater than 50% was considered as having substantial heterogeneity. When substantial heterogeneity was not found, we reported the pooled RR with its 95% CI based on the fixed-effect model; when substantial heterogeneity was found, we reported that based on the random-effects model.
Publication bias was evaluated by using Begg's funnel plot and Egger's test. When Begg's funnel plot shows asymmetry or P value from Egger's test is less than 0.05, it is considered that publication bias exists. Stata SE version 12.1 software package (StataCorp., College Station, TX, USA) was used for all statistical analyses.
RESULTS
Identification of studies
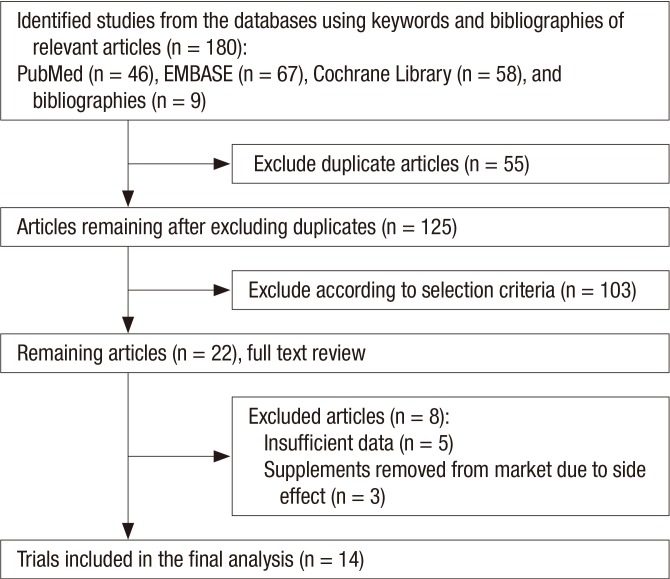
Fig. 1 shows a flow diagram for identifying relevant trials. By the searches of 3 databases and relevant bibliographies, a total of 180 articles were identified. During the first screening, we excluded 55 duplicate articles and 103 articles not meeting the selection criteria. After reviewing the full text of the remaining 22 articles, we excluded 8 articles because of insufficient data (n = 5) and the use of etretinate supplements removed from market due to the high risk of birth defects (n = 3). A total of 14 RCTs (9,10,11,12,13,14,15,16,17,18,19,20,21,22) were included in the final analysis.
Fig. 1.
Flow diagram for identification of relevant clinical trials.
Characteristics of selected studies
A total of 14 RCTs included 147,383 participants, which involved 89,972 in the supplement group and 57,411 in the control group. Table 1 shows the main characteristics of the selected RCTs. The studies were published between 1977 and 2012, spanning 35 years. They were conducted in the following countries: USA (n = 8), Italy (n = 2), Canada (n = 1), Finland (n = 1), Iran (n = 1), and US/Canada/Puerto Rico (n = 1). The supplementation and follow-up periods ranged between 1 and 13 years. Among the 14 trials, 8 trials were primary prevention trials (male smokers [11], people with high risk of developing lung cancer [12], general populations [13,21], male physicians [15], patients with nonmelanoma skin cancer [16], and patients with stage I or II squamous head and neck cancer [17,18]), and the other 6 trials were secondary prevention trials (bladder cancer survivors). The sample size of the trials ranged from 46 to 39,876. Eleven of these studies used a placebo group as a control (2 studies used recommended daily allowance [RDA] multivitamins as placebo [10,20]), and 3 studies used a control group without a placebo (14,17,22). In all trials, vitamins, antioxidants, and placebos were administered orally either singly or in combination. The types of vitamin and antioxidant supplements were as follows: vitamin A, vitamin B6, vitamin C, vitamin D, vitamin E, beta-carotene, folic acid, and selenium. The dosage regimens in individual trials were as follows: vitamin A (200 mg or 25,000, 36,000, or 40,000 IU daily), vitamin B6 (25 or 100 mg daily), vitamin C (2,000 mg daily), vitamin D (1,600 IU daily), vitamin E (50 mg or 400 IU daily), beta-carotene (20 or 30 mg daily; 50 mg alternate day; 75 mg daily for 3-month cycles), folic acid (1.6 mg daily), and selenium (200 μg daily).
Table 1. Characteristics of trials included in the final meta-analysis (n = 14).
| No. | Source (project name) | Journal | Country | Design (type of prevention) | Participants (average age, yr; Women, %) | Duration of supplemen-tation, yr (follow-up period, yr) | Intervention vs. control | No. of bladder cancer patients/No. of participants | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||||
| 1 | 1977 Byar et al. | Urology | USA | OLRCT (Secondary) | 118 patients with stage Ⅰ bladder cancer (NA) | 2 (2.0) | 25 mg of pyridoxine (vitamin B6) vs. placebo per day | 15/32 | 29/48 |
| 2 | 1994 Lamm et al. | J Urol | USA | RDBPCT (Secondary) | 65 bladder cancer (67; 17) | 3.8 (3.8) | RDA plus | 14/35 | 24/30 |
| 40,000 IU of vitamin A + 100 mg of vitamin B6 + 2,000 mg of vitamin C + 400 IU of vitamin E + 90 mg of zinc vs. RDA multivitamin per day | |||||||||
| 3 | 1994 ATBC | N Engl J Med | Finland | RDBPCT (Primary) | 29,133 male smokers 50 to 69 years of age (57; 0) | 1.5 (1.5) | 50 mg of vitamin E + 20 mg of beta carotene vs. placebo per day | Vit E 79/14,560 | Vit E 76/14,573 |
| Beta carotene 81/14,564 | Beta carotene 74/14,569 | ||||||||
| 4 | 1996 CARET | J Natl Cancer Inst | USA | RDBPCT (Primary) | 18,314 men and women at high risk of developing lung cancer (58; 44) | 4 (4.0) | 30 mg of beta-carotene + 25,000 IU of vitamin A vs. placebo per day | 42/9,420 | 36/8,894 |
| 5 | 1999 WHS | J Natl Cancer Inst | USA | RDBPCT (Primary) | 39,876 healthy women (55; 100) | 2.1 (4.1) | 50 mg of beta-carotene vs. placebo on alternate day | 5/19,939 | 6/19,937 |
| 6 | 2000 Decensi et al. | Cancer Epidemiol Biomarkers Prev | Italy | RDBPCT (Secondary) | 99 subjects with resected superficial bladder cancer (63; 19) | 2 (3.0) | 200 mg of fenretinide (vitamin A) per day vs. no treatment | 22/49 | 29/50 |
| 7 | 2000 PHS | Cancer Causes Control | USA | RDBPCT (Primary) | 22,071 US male physicians (40–84; 0) | 13(13.0) | 50 mg of beta-carotene vs. placebo on alternate day | 62/11,036 | 41/11,035 |
| 8 | 2002 NPC | Cancer Epidemiol Biomarkers Prev | USA | RDBPCT (Primary) | 1,250 nonmelanoma skin cancer patients (63; 25) | 13 (13.0) | 200 µg of selenium vs. placebo per day | 10/621 | 8/629 |
| 9 | 2003 IHNCSG | Oncol Rep | Italy | OLRCT (Primary) | 214 patients with a radically treated stage Ⅰ–Ⅱ squamous head and neck tumor (61; 18) | 3 (5.0) | 75 mg of beta-carotene daily for 3-month cycles within 1-month intercycle intervals vs. no treatment | 1/104 | 0/110 |
| 10 | 2005 Bairati et al. | J Clin Oncol | Canada | RDBPCT (Primary) | 540 patients with stage Ⅰ or Ⅱ head and neck cancer (63; 21) | 3 (4.3) | 400 IU of α-tocopherol (vitamin E) + 30 mg of beta-carotene vs. placebo per day | 2/273 | 0/267 |
| 11 | 2008 Sabichi et al. | Clin Cancer Res | USA | RDBPCT (Secondary) | 137 patients with non-muscle-invasive bladder TCC (67; 18) | 1 (1.3) | 200 mg of fenretinide (vitamin A) vs. placebo per day | 22/70 | 22/67 |
| 12 | 2010 Nepple et al. | J Urol | USA | RDBPCT (Secondary) | 670 patients with nonmuscle invasive bladder cancer (68; 24) | 3.1 (4.0) | 36,000 IU of vitamin A + 25 mg of vitamin B6 + 2,000 mg of vitamin C + 1,600 IU of vitamin D + 400 IU of vitamin E + 1.6 mg of folate + 30.4 mg of zinc vs. RDA vitamin per day | 118/334 | 113/336 |
| 13 | 2012 SELECT | J Urol | USA, Canada, Puerto Rico | RDBPCT (Primary) | 34,888 men (62; 0) | 6 (7.1) | 200 µg of selenium + 400 IU of vitamin E vs. placebo per day | 171/26,192 | 53/8,696 |
| 14 | 2012 Mazdak et al. | Int J Prev Med | Iran | OLRCT (Secondary) | 46 patients with a single, low-grade, superficial bladder cancer (60; 11) | 2 (2.0) | 400 IU of vitamin E per day vs. no treatment | 4/21 | 9/25 |
NA = not applicable, ATBC = the Alpha-tocopherol Beta-carotene Cancer Prevention Study, CARET = the Beta-Carotene and Retinol Efficacy Trial, IHNCSG = the Italian Head and Neck Chemoprevention Study Group, NPC = Nutritional Prevention of Cancer, PHS = the Physicians' Health Study, RDA = recommended daily allowance, SELECT = Selenium and Vitamin E Cancer Prevention Trials, WHS = the Women's Health Study.
Methodological quality
Table 2 shows the methodological quality of trials assessed by using the Jadad scale. Twelve studies were considered as having a high quality, receiving a total of 3 points or more, whereas the remaining 2 studies receiving 2 points were considered as having a low quality.
Table 2. Methodological quality of trials based on the Jadad scale (n = 14).
| No. | Source (project name) | Randomization | Description of randomization methods | Double-blind | Using identical placebo | Follow-up reporting | Total score |
|---|---|---|---|---|---|---|---|
| 1 | 1977 Byar and Blackard | 1 | 0 | 0 | 0 | 1 | 2 |
| 2 | 1994 Lamm et al. | 1 | 1 | 1 | 1 | 1 | 5 |
| 3 | 1994 ATBC | 1 | 1 | 1 | 1 | 1 | 5 |
| 4 | 1996 CARET | 1 | 0 | 1 | 1 | 1 | 4 |
| 5 | 1999 WHS | 1 | 1 | 1 | 1 | 1 | 5 |
| 6 | 2000 Decensi et al. | 1 | 1 | 1 | 0 | 1 | 4 |
| 7 | 2000 PHS | 1 | 0 | 1 | 1 | 1 | 4 |
| 8 | 2002 NPC | 1 | 1 | 1 | 1 | 1 | 5 |
| 9 | 2003 IHNCSG | 1 | 1 | 0 | 0 | 1 | 3 |
| 10 | 2005 Bairati et al. | 1 | 1 | 1 | 1 | 1 | 5 |
| 11 | 2008 Sabichi et al. | 1 | 1 | 1 | 1 | 1 | 5 |
| 12 | 2010 Nepple et al. | 1 | 1 | 1 | 1 | 1 | 5 |
| 13 | 2012 SELECT | 1 | 1 | 1 | 1 | 1 | 5 |
| 14 | 2012 Mazdak and Zia | 1 | 0 | 0 | 0 | 1 | 2 |
ATBC = the Alpha-tocopherol Beta-carotene Cancer Prevention Study, CARET = the Beta-Carotene and Retinol Efficacy Trial, WHS = the Women's Health Study, PHS = the Physicians' Health Study, NPC = Nutritional Prevention of Cancer, IHNCSG = the Italian Head and Neck Chemoprevention Study Group, SELECT = Selenium and Vitamin E Cancer Prevention Trials.
Main analysis
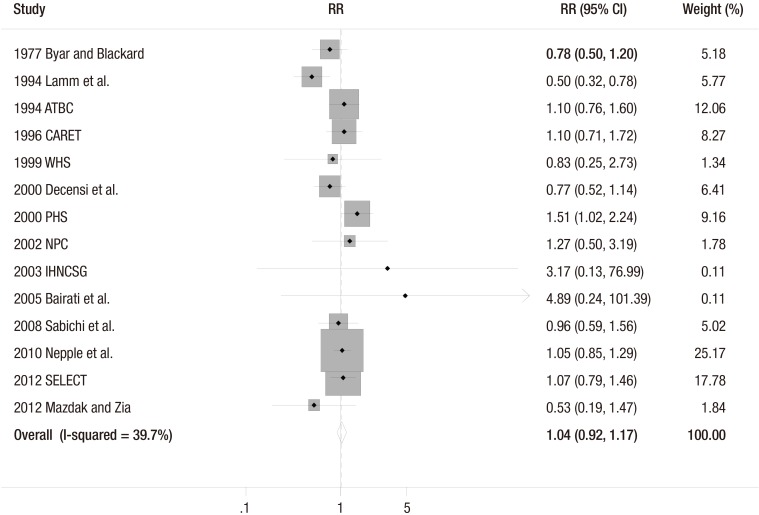
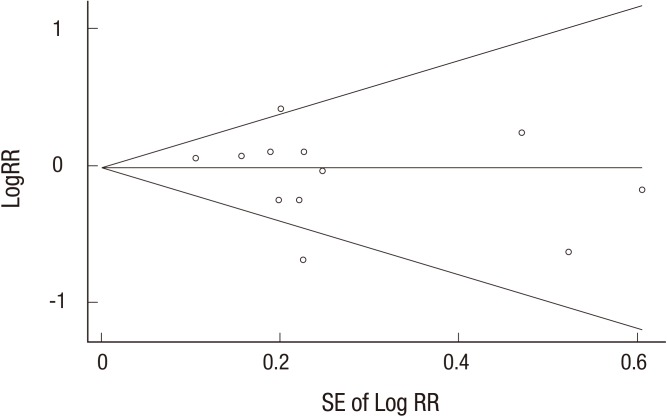
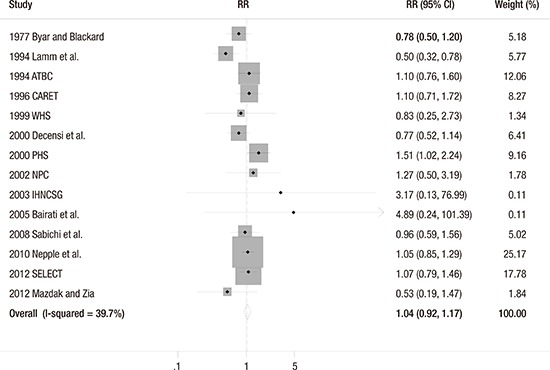
The fixed-effect meta-analysis of all 14 trials showed that vitamin or antioxidant supplementation was not associated with the risk of bladder cancer (RR = 1.04; 95% CI 0.92–1.17; I2 = 39.7%) (Fig. 2). Publication bias was not observed (Begg's funnel plot, symmetrical; Egger's test, P for bias = 0.378) (Fig. 3).
Fig. 2.
Efficacy of vitamin and antioxidant supplements in the prevention of bladder cancer by a fixed-effect model meta-analysis of randomized controlled trials.
RR = relative risk, CI = confidence interval, RCT = randomized controlled trial.
Fig. 3.
Funnel plots and egger's test for identifying publication bias (P = 0.393) in a meta-analysis of trials (n = 13).
RR = relative risk, SE = standard error.
Subgroup meta-analyses
Table 3 presents the findings from the subgroup meta-analyses according to various factors. Regarding types of supplements, any type of vitamin and antioxidant supplements had no beneficial effect on the risk of bladder cancer: vitamin A (RR = 0.86; 95% CI 0.65–1.13; I2 = 61.7%; n = 5), vitamin B6 (RR = 0.77; 95% CI 0.49–1.20; I2 = 78.8%; n = 3), vitamin C (RR = 0.74; 95% CI 0.36–1.54; I2 = 88.8%; n = 2), vitamin D (RR = 1.05; 95% CI 0.85–1.29; n = 1), vitamin E (RR = 0.91; 95% CI 0.69–1.19; I2 = 60.9%; n = 6), beta-carotene (RR = 1.19; 95% CI 0.96–1.46; I2 = 0.0%; n = 6), folate (RR = 1.05; 95% CI 0.85–1.29; n = 1), and selenium (RR = 1.09; 95% CI 0.81–1.46; I2 = 0.0%; n = 2).
Table 3. Efficacy of vitamin and antioxidant supplements in the prevention of bladder cancer in subgroup meta-analyses.
| Factors | No. of trials | RR (95% CI) | Heterogeneity, I2, % | Model |
|---|---|---|---|---|
| All | 14 | 1.04 (0.92–1.17) | 39.7 | Fixed-effects |
| Type of supplements | ||||
| Vitamin A | 5 | 0.86 (0.65–1.13) | 61.7 | Random-effects |
| Vitamin A (25,000–40,000 IU) | 3 | 0.85 (0.54–1.34) | 79.0 | Random-effects |
| Fenretinide (200 mg/day) | 2 | 0.85 (0.63–1.16) | 0.0 | Fixed-effects |
| Vitamin B6 | 3 | 0.77 (0.49–1.20) | 78.8 | Random-effects |
| Low dose (25 mg/day) | 2 | 1.00 (0.83–1.21) | 34.8 | Fixed-effects |
| High dose (100 mg/day) | 1 | 0.50 (0.32–0.78) | NA | NA |
| Vitamin C | 2 | 0.74 (0.36–1.54) | 88.8 | Random-effects |
| Vitamin D | 1 | 1.05 (0.85–1.29) | NA | NA |
| Vitamin E | 6 | 0.91 (0.69–1.19) | 60.9 | Random-effects |
| Low dose (50 mg/day) | 1 | 1.09 (0.80–1.50) | NA | NA |
| High dose (400 IU/day) | 5 | 0.84 (0.58–1.22) | 67.0 | Random-effects |
| Beta-carotene | 6 | 1.19 (0.96–1.46) | 0.0 | Fixed-effects |
| Low dose (20–30 mg/day) | 5 | 1.18 (0.96–1.45) | 0.0 | Fixed-effects |
| High dose (75 mg/day) | 1 | 3.17 (0.13–76.99) | NA | NA |
| Beta-carotene alone | 3 | 1.44(1.00–2.09) | 0.0 | Fixed-effects |
| Folic acid | 1 | 1.05 (0.85–1.29) | NA | NA |
| Selenium | 2 | 1.09 (0.81–1.46) | 0.0 | Fixed-effects |
| Type of cancer prevention | ||||
| Primary | 8 | 1.18 (0.99–1.41) | 0.0 | Fixed-effects |
| Secondary | 6 | 0.79 (0.62–1.02) | 54.1 | Random-effects |
| Methodological quality | ||||
| High quality | 12 | 1.06 (0.94–1.20) | 41.1 | Fixed-effects |
| Low quality | 2 | 0.71 (0.47–1.07) | 0.0 | Fixed-effects |
| Duration of treatment | ||||
| < 5 | 11 | 0.96 (0.84–1.10) | 33.5 | Fixed-effects |
| ≥ 5 | 3 | 1.22 (0.97–1.55) | 0.0 | Fixed-effects |
| Provider of supplements | ||||
| Pharmaceutical | 10 | 1.09 (0.95–1.24) | 44.9 | Fixed-effects |
| Non-pharmaceutical | 4 | 0.75 (0.57–0.99) | 0.0 | Fixed-effects |
| High quality | 2 | 0.78 (0.53–1.15) | 0.0 | Fixed-effects |
| Low quality | 2 | 0.71 (0.47–1.07) | 0.0 | Fixed-effects |
| Type of control | ||||
| No treatment | 3 | 0.75 (0.52–1.09) | 0.0 | Fixed-effects |
| Placebo | 11 | 1.06 (0.94–1.20) | 43.6 | Fixed-effects |
| No. of participants in each trial | ||||
| < 10,000 | 9 | 0.92 (0.79–1.07) | 40.7 | Fixed-effects |
| ≥ 10,000 | 5 | 1.16 (0.97–1.39) | 0.0 | Fixed-effects |
NA = not applicable, RR = relative risk.
Overall, there was no significant effect of vitamin and antioxidant supplements in the subgroup meta-analyses by various factors such as dose of supplements, type of cancer prevention, methodological quality, duration of treatment, provider of supplements, type of control, and number of participants. However, the risk of bladder cancer was marginally increased in trials with the use of beta-carotene alone (RR = 1.44; 95% CI 1.00–2.09; I2 = 0.0%; n = 3). There was a preventive effect in trials not supplied with supplements by pharmaceutical industry (RR = 0.75; 95% CI 0.57–0.99; I2 = 0.0%; n = 4). However, those beneficial effects disappeared in the subgroup meta-analysis of high quality RCTs among those trials (RR = 0.78; 95% CI 0.53–1.15; I2 = 0.0%; n = 2).
DISCUSSION
The present meta-analysis of RCTs found that overall, there was no preventive effect of vitamin or antioxidant supplements on bladder cancer. Furthermore, no association was found in the subgroup meta-analyses by type and dose of supplements, type of cancer prevention, methodological quality, duration of treatment, providers of supplements, type of control, and number of participants in individual trials.
The findings of our meta-analysis are inconsistent with those from the previous in vitro laboratory or in vivo animal studies regarding the association between vitamins or antioxidants and the development of bladder cancer (25,26,27). This advocates that the findings of the preclinical experimental studies may not represent the biological processes in humans (28).
Such a discrepancy is also found between the meta-analyses of observational epidemiologic studies and those of RCTs. The meta-analyses of observational studies reported the use of vitamin A, vitamin C, vitamin D, vitamin E, folate, and selenium was associated with a lower risk of bladder cancer (29,30,31,32). Also, ours are inconsistent with another meta-analysis reporting that the intake of vegetables and fruits rich in vitamin and antioxidant may significantly prevent the development of bladder cancer (7). There are several possible explanations for this discrepancy. First, there might be differences in absorptions and functions between natural and synthetic vitamins or antioxidants. For example, synthetic beta-carotene is made of only all-trans beta-carotene, whereas natural beta-carotene includes both all-trans beta-carotene and 9-cis beta-carotene. Consequently, there is a difference in absorption between natural and synthetic forms of beta-carotene (33). Second, there are some important biases in case-control and cohort studies. In general, case-control studies are prone to recall bias due to the use of retrospective assessment. Patients with bladder cancer might recall wrongly their diet, and healthy controls might report a healthy diet (34). Also, cases or controls are not representative of their population, thereby selection bias could affect the results. Although cohort studies are less prone to biases than case-control studies, they are unable to confirm the causality. Furthermore, vitamin or antioxidant supplements in the RCTs are not equivalent to the consumption of fruits and vegetables in observation epidemiological studies, which also contain other micronutrients and antioxidants. Preventive effects of vitamins or antioxidants might be attributable to a combination of those with various nutrients.
Interestingly, we found that the use of beta-carotene supplements alone was marginally significantly associated with an increased risk of bladder cancer. This finding is similar to those from the previous meta-analyses (35,36). Regarding this finding, there are plausible biological mechanisms. Beta-carotene can serve as an antioxidant or a prooxidant according to intrinsic properties or biological environments in which it acts (37). Beta-carotene may increase the prooxidant character under chronic oxidative stress such as smoking (38). The carotenoid could exhibit an increase in DNA oxidative damage and modify cell proliferation and apoptosis in cells exposed to toxin; it might eventually lead to cancer (39). Although we are unable to confirm this effect due to a paucity of data, our analysis supports previous findings regarding the association between the use of beta-carotene supplements and mortality (40).
Also, we found that there was a preventive effect in trials with supplements not provided by the pharmaceutical industry. However, beneficial effects were not observed when we performed the subgroup meta-analysis of high quality RCTs within its category.
Our study has several limitations. First, the included trials in our study involved only synthetic vitamin and antioxidant supplements. Thus, our findings could not be applicable to natural vitamins or antioxidants occurring in fruits and vegetables. Second, we were unable to investigate whether vitamin and antioxidant supplements are beneficial in the prevention of bladder cancer among people who are deficient in vitamins or antioxidants. Further RCTs are required to evaluate this association. Last, we assessed the methodological quality of individual trials by only using the data shown in each article. Therefore, we might not have assessed the actual performance or biases in individual trials.
In conclusion, we found that vitamin and antioxidant supplements have no overall preventive effect against bladder cancer in the meta-analysis of RCTs. Instead, subgroup meta-analyses showed that beta-carotene supplementation marginally increased the risk of bladder cancer. Even though further large, high-quality trials are required to confirm these associations, the effects (either beneficial or harmful) of vitamin or antioxidant supplements on bladder cancer should not be overemphasized.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Park SJ, Myung SK. Data curation: Park SJ, Lee YJ. Investigation: Park SJ, Myung SK. Writing - original draft: Park SJ, Myung SK. Writing - review & editing: Park SJ, Myung SK, Lee Y, Lee YJ.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer Incidence and Mortality Worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Letašiová S, Medve’ová A, Šovčíková A, Dušinská M, Volkovová K, Mosoiu C, Bartonová A. Bladder cancer, a review of the environmental risk factors. Environ Health. 2012;11(Suppl 1):S11. doi: 10.1186/1476-069X-11-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Park SY, Ollberding NJ, Woolcott CG, Wilkens LR, Henderson BE, Kolonel LN. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the Multiethnic Cohort Study. J Nutr. 2013;143:1283–1292. doi: 10.3945/jn.113.174920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakauchi F, Mori M, Washio M, Watanabe Y, Ozasa K, Hayashi K, Miki T, Nakao M, Mikami K, Ito Y, et al. Dietary habits and risk of urothelial cancer incidence in the JACC Study. J Epidemiol. 2005;15(Suppl 2):S190–5. doi: 10.2188/jea.15.S190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Wang XC, Hu GH, Guo ZF, Lai P, Xu L, Huang TB, Xu YF. Fruit and vegetable consumption and risk of bladder cancer: an updated meta-analysis of observational studies. Eur J Cancer Prev. 2015;24:508–516. doi: 10.1097/CEJ.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 8.Root MM, McGinn MC, Nieman DC, Henson DA, Heinz SA, Shanely RA, Knab AM, Jin F. Combined fruit and vegetable intake is correlated with improved inflammatory and oxidant status from a cross-sectional study in a community setting. Nutrients. 2012;4:29–41. doi: 10.3390/nu4010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byar D, Blackard C. Comparisons of placebo, pyridoxine, and topical thiotepa in preventing recurrence of stage I bladder cancer. Urology. 1977;10:556–561. doi: 10.1016/0090-4295(77)90101-7. [DOI] [PubMed] [Google Scholar]
- 10.Lamm DL, Riggs DR, Shriver JS, vanGilder PF, Rach JF, DeHaven JI. Megadose vitamins in bladder cancer: a double-blind clinical trial. J Urol. 1994;151:21–26. doi: 10.1016/s0022-5347(17)34863-2. [DOI] [PubMed] [Google Scholar]
- 11.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 12.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr, Valanis B, Williams JH, Jr, et al. Risk factors for lung cancer and for intervention effects in CARET, the beta-carotene and retinol efficacy trial. J Natl Cancer Inst. 1996;88:1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 13.Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women’s Health Study. J Natl Cancer Inst. 1999;91:2102–2106. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- 14.Decensi A, Torrisi R, Bruno S, Costantini M, Curotto A, Nicolò G, Malcangi B, Baglietto L, Bruttini GP, Gatteschi B, et al. Randomized trial of fenretinide in superficial bladder cancer using dna flow cytometry as an intermediate end point. Cancer Epidemiol Biomarkers Prev. 2000;9:1071–1078. [PubMed] [Google Scholar]
- 15.Cook NR, Le IM, Manson JE, Buring JE, Hennekens CH. Effects of beta-carotene supplementation on cancer incidence by baseline characteristics in the Physicians’ Health Study (United States) Cancer Causes Control. 2000;11:617–626. doi: 10.1023/a:1008995430664. [DOI] [PubMed] [Google Scholar]
- 16.Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Jr, Slate EH, Fischbach LA, Marshall JR, Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 17.Toma S, Bonelli L, Sartoris A, Mira E, Antonelli A, Beatrice F, Giordano C, Benazzo M, Caroggio A, Cavalot AL, et al. Beta-carotene supplementation in patients radically treated for stage I–II head and neck cancer: results of a randomized trial. Oncol Rep. 2003;10:1895–1901. [PubMed] [Google Scholar]
- 18.Bairati I, Meyer F, Gélinas M, Fortin A, Nabid A, Brochet F, Mercier JP, Têtu B, Harel F, Abdous B, et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Clin Oncol. 2005;23:5805–5813. doi: 10.1200/JCO.2005.05.514. [DOI] [PubMed] [Google Scholar]
- 19.Sabichi AL, Lerner SP, Atkinson EN, Grossman HB, Caraway NP, Dinney CP, Penson DF, Matin S, Kamat A, Pisters LL, et al. Phase III preventiontrial of fenretinide in patients with resected non-muscle-invasive bladder cancer. Clin Cancer Res. 2008;14:224–229. doi: 10.1158/1078-0432.CCR-07-0733. [DOI] [PubMed] [Google Scholar]
- 20.Nepple KG, Lightfoot AJ, Rosevear HM, O’Donnell MA, Lamm DL, Bladder Cancer Genitourinary Oncology Study Group Bacillus Calmette-Guérin with or without interferon α-2b and megadose versus recommended daily allowance vitamins during induction and maintenance intravesical treatment of nonmuscle invasive bladder cancer. J Urol. 2010;184:1915–1919. doi: 10.1016/j.juro.2010.06.147. [DOI] [PubMed] [Google Scholar]
- 21.Lotan Y, Goodman PJ, Youssef RF, Svatek RS, Shariat SF, Tangen CM, Thompson IM, Jr, Klein EA. Evaluation of vitamin E and selenium supplementation for the prevention of bladder cancer in SWOG coordinated SELECT. J Urol. 2012;187:2005–2010. doi: 10.1016/j.juro.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazdak H, Zia H. Vitamin E reduces superficial bladder cancer recurrence: a randomized controlled trial. Int J Prev Med. 2012;3:110–115. [PMC free article] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Becci PJ, Thompson HJ, Grubbs CJ, Squire RA, Brown CC, Sporn MB, Moon RC. Inhibitory effect of 13-cis-retinoic acid on urinary bladder carcinogenesis induced in C57BL/6 mice by N-butyl-N-(4-hydroxybutyl)-nitrosamine. Cancer Res. 1978;38:4463–4466. [PubMed] [Google Scholar]
- 26.Wu HC, Lu HF, Hung CF, Chung JG. Inhibition by vitamin C of DNA adduct formation and arylamine N-acetyltransferase activity in human bladder tumor cells. Urol Res. 2000;28:235–240. doi: 10.1007/s002400000130. [DOI] [PubMed] [Google Scholar]
- 27.Ye C, Zhao W, Li M, Zhuang J, Yan X, Lu Q, Chang C, Huang X, Zhou J, Xie B, et al. δ-tocotrienol induces human bladder cancer cell growth arrest, apoptosis and chemosensitization through inhibition of STAT3 pathway. PLoS One. 2015;10:e0122712. doi: 10.1371/journal.pone.0122712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farbstein D, Kozak-Blickstein A, Levy AP. Antioxidant vitamins and their use in preventing cardiovascular disease. Molecules. 2010;15:8098–8110. doi: 10.3390/molecules15118098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang JE, Wang RJ, Zhong H, Yu B, Chen Y. Vitamin A and risk of bladder cancer: a meta-analysis of epidemiological studies. World J Surg Oncol. 2014;12:130. doi: 10.1186/1477-7819-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen F, Li Q, Yu Y, Yang W, Shi F, Qu Y. Association of vitamin C, vitamin D, vitamin E and risk of bladder cancer: a dose-response meta-analysis. Sci Rep. 2015;5:9599. doi: 10.1038/srep09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amaral AF, Cantor KP, Silverman DT, Malats N. Selenium and bladder cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:2407–2415. doi: 10.1158/1055-9965.EPI-10-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He H, Shui B. Folate intake and risk of bladder cancer: a meta-analysis of epidemiological studies. Int J Food Sci Nutr. 2014;65:286–292. doi: 10.3109/09637486.2013.866641. [DOI] [PubMed] [Google Scholar]
- 33.Patrick L. Beta-carotene: the controversy continues. Altern Med Rev. 2000;5:530–545. [PubMed] [Google Scholar]
- 34.Key TJ, Allen NE, Spencer EA, Travis RC. The effect of diet on risk of cancer. Lancet. 2002;360:861–868. doi: 10.1016/S0140-6736(02)09958-0. [DOI] [PubMed] [Google Scholar]
- 35.Jeon YJ, Myung SK, Lee EH, Kim Y, Chang YJ, Ju W, Cho HJ, Seo HG, Huh BY. Effects of beta-carotene supplements on cancer prevention: meta-analysis of randomized controlled trials. Nutr Cancer. 2011;63:1196–1207. doi: 10.1080/01635581.2011.607541. [DOI] [PubMed] [Google Scholar]
- 36.Bardia A, Tleyjeh IM, Cerhan JR, Sood AK, Limburg PJ, Erwin PJ, Montori VM. Efficacy of antioxidant supplementation in reducing primary cancer incidence and mortality: systematic review and meta-analysis. Mayo Clin Proc. 2008;83:23–34. doi: 10.4065/83.1.23. [DOI] [PubMed] [Google Scholar]
- 37.Cui Y, Lu Z, Bai L, Shi Z, Zhao WE, Zhao B. Beta-carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor gamma expression and reactive oxygen species production in MCF-7 cancer cells. Eur J Cancer. 2007;43:2590–2601. doi: 10.1016/j.ejca.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, Hercberg S. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer. 2010;127:172–184. doi: 10.1002/ijc.25008. [DOI] [PubMed] [Google Scholar]
- 39.Palozza P, Serini S, Trombino S, Lauriola L, Ranelletti FO, Calviello G. Dual role of beta-carotene in combination with cigarette smoke aqueous extract on the formation of mutagenic lipid peroxidation products in lung membranes: dependence on pO2. Carcinogenesis. 2006;27:2383–2391. doi: 10.1093/carcin/bgl074. [DOI] [PubMed] [Google Scholar]
- 40.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012:CD007176. doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]