Abstract
Biliary atresia (BA) is the major cause of cholestasis and the leading indication for liver transplantation (LT). However, the incidence of BA in Korea has not been reported. The aim of this study was to investigate the incidence and clinical outcomes of BA in Korea. We used the Korean universal health insurance database and extracted data regarding BA patients younger than 18 years of age admitted between 2011 and 2015. The incidence of BA was calculated by dividing the number of BA patients by the number of live births. Two hundred forty infants were newly diagnosed with BA. A total of 963 BA patients younger than 18 years of age were followed up for 5 years. The overall incidence of BA was 1.06 cases per 10,000 live births. The incidence of BA was 1.4 times higher for female patients than for male patients. Additionally, significant seasonal variation was observed; in particular, the incidence of BA was 2 times higher from June through August than from December through February. Congenital anomalies were found in 38 of 240 patients (15.8%). Congenital heart diseases were major associated congenital anomalies (6.3%). Several complications developed during the study period, including cholangitis (24.0%), varix (6.2%), and gastrointestinal bleeding (4.4%). Three hundred and one of the 963 BA patients under 18 years of age (31.3%) received LT for BA. The incidence of BA is higher in Korea than that in Western countries. We also report significant gender-associated differences and seasonal variation with respect to the incidence of BA.
Keywords: Biliary Atresia, Incidence, Liver Transplantation
Graphical Abstract
INTRODUCTION
Biliary atresia (BA) is the major cause of neonatal cholestatic jaundice, and untreated BA can cause chronic liver disease, hepatic failure, and death in children (1,2,3,4,5,6,7,8,9,10,11). BA is well established as the leading indication for liver transplantation (LT) in children in Korea. Furthermore, BA is the most common cause of LT in infants under 12 months (12).
The incidence of BA is slightly higher in Eastern countries (1.04 to 1.79/10,000 live births) than in Western countries (0.42 to 0.71/10,000) (1,2,3,5,6,7,8,9,13,14,15,16,17,18,19,20,21). This difference in incidence rates may be due to a combination of factors, including genetics, environmental factors, and infectious pathogens (5,7,20). The incidence of BA in Korea has not been reported. The aim of this study was to investigate the incidence and clinical outcomes of BA in Korea.
MATERIALS AND METHODS
Data source
We used the Health Insurance Review and Assessment Service (HIRA) database based on the Korean universal health insurance system. We retrieved information about patient's age, sex, diagnosis and treatments, and diagnosis, which was presented by the Korean Classification of Disease, 7th Revision (KCD-7) code.
Patient selection and definition of cases
We extracted the following information from the database: KCD-7, Q442, or Q443 BA status in patients under 18 years of age from January 2011 to December 2015. We defined 2 BA groups: the “newly diagnosed BA group,” which included patients with BA who received the Kasai operation (Q735–Q737) or LT (Q814) before their first birthday, and the “total BA group,” which included all BA patients between 0 and 18 years of age during the 5-year study period.
The newly diagnosed BA group was used to calculate the incidence of BA and the percentage of congenital anomalies because definite and suspected BA cases could not be discriminated using the KCD-7. The total BA group was used to calculate the percentages of complications and LT.
The incidence of BA was calculated by dividing newly diagnosed BA patients by the population of live births. We defined the date of diagnosis as the first day that a patient visited a medical facility for BA. Month of diagnosis was grouped into 4 seasons: winter (December–February), spring (March–May), summer (June–August), and autumn (September–November). Incidence is presented as the number of BA patients per 10,000 live births with a 95% confidence interval (CI).
We investigated the percentage of congenital anomalies such as spleen anomalies, situs inversus, and heart disease in the newly diagnosed BA group. If different congenital anomalies were present in a single patient, each anomaly was counted separately. Percentages of complications and LT were calculated by dividing the number of applicable cases by the size of the total BA group. We also evaluated patients subjected to endoscopic variceal bleeding control. For the total BA group, we determined the number of patients who underwent LT during the 5-year study period and the number of patients who had already undergone LT prior to 2011.
Statistical analysis
We analyzed the data using SAS Enterprise version 6.1 (SAS Institute, Cary, NC, USA). The 95% CI of BA incidence was calculated using the Poisson distribution. The seasonal, annual, and gender variation in the occurrence of BA was measured using the Poisson regression test. We used linear regression to evaluate the linear trend in LT over time.
Ethics statement
This study was exempted from Institutional Review Board (IRB) review because it contained open data and does not include any personal identifiable information (IRB No. E-1604-002-750).
RESULTS
Incidence
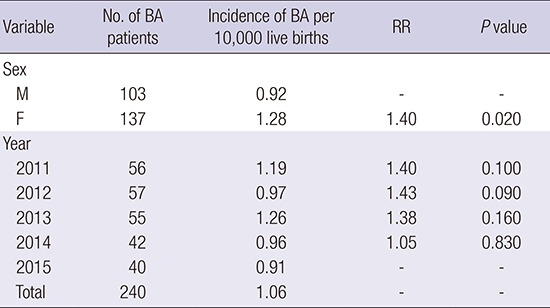
A total of 240 BA patients (137 females, 103 males) were born during the 5-year study period (56 in 2011, 57 in 2012, 55 in 2013, 42 in 2014 and 40 in 2015).
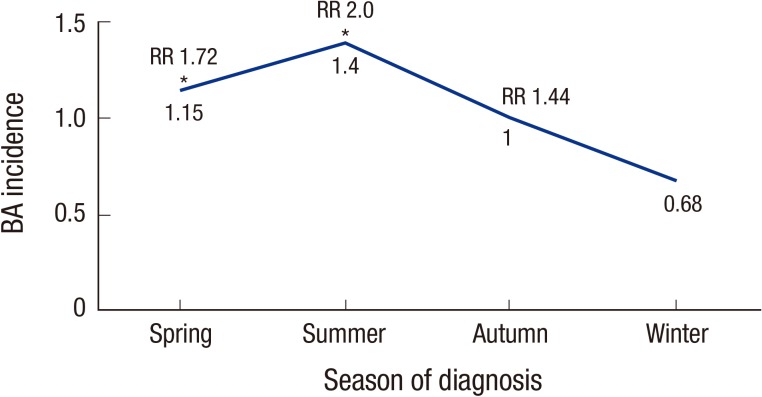
The incidence of BA was 1.06/10,000 (95% CI, 0.93–1.19), (females 1.28, males 0.92). The incidence of BA was 1.4 times higher for female than male patients, and this difference was statistically significant (P = 0.200) (Table 1). When the months were grouped into 1 of 4 seasons, the rate from June through August (summer) was 2 times higher than that from December through February (winter) (P < 0.010) (Fig. 1). There was no statistically significant difference in annual incidence.
Table 1. Incidence and RR of BA by sex and year in Korea, 2011–2015.
| Variable | No. of BA patients | Incidence of BA per 10,000 live births | RR | P value |
|---|---|---|---|---|
| Sex | ||||
| M | 103 | 0.92 | - | - |
| F | 137 | 1.28 | 1.40 | 0.020 |
| Year | ||||
| 2011 | 56 | 1.19 | 1.40 | 0.100 |
| 2012 | 57 | 0.97 | 1.43 | 0.090 |
| 2013 | 55 | 1.26 | 1.38 | 0.160 |
| 2014 | 42 | 0.96 | 1.05 | 0.830 |
| 2015 | 40 | 0.91 | - | - |
| Total | 240 | 1.06 | - | - |
BA = biliary atresia, RR = relative risk.
Fig. 1.
Seasonal variation in the incidence of BA.
BA = biliary atresia, RR = relative risk.
*P < 0.05.
Congenital anomalies
Congenital anomalies were found in 38/240 of patients in the newly diagnosed BA group (15.8%); specifically, this group included 2 patients with spleen anomalies (0.8%) and 1 patient with situs inversus (0.4%). Congenital heart diseases comprised major associated congenital anomalies (15 patients, 6.3%), which ranged from relatively minor disease (atrial septal defect, patent ductus arteriosus) to life threatening severe disease (hypoplastic left heart syndrome, pulmonary atresia). Two patients had more than 2 congenital heart diseases. Ten patients (4.2%) had biliary anomalies (choledochal cyst or duplication of the biliary duct), and 8 patients (3.3%) were diagnosed with intestinal anomalies such as small bowel atresia, malrotation, tracheoesophageal fistula, and Meckel's diverticulum. One patient with congenital cystic lung and another with congenital vesicoureteral reflux were reported (Table 2).
Table 2. Frequency of congenital anomalies in BA patients.
| Congenital anomalies | No. (%) |
|---|---|
| Congenital heart disease | 15 (6.3) |
| Atrial septal defect | 10 (4.2) |
| Patent ductus arteriosus | 1 (0.4) |
| Pulmonary stenosis | 4 (1.3) |
| Pulmonary valve atresia | 1 (0.4) |
| Hypoplastic left heart syndrome | 1 (0.4) |
| Biliary anomalies | 10 (4.2) |
| Choledochal cyst | 3 (1.3 ) |
| Biliary duct duplication | 5 (2.1) |
| Other congenital malformations of the gallbladder | 2 (0.8) |
| Gastrointestinal | 8 (3.3) |
| Esophageal atresia, tracheoesophageal fistula | 2 (0.8) |
| Small bowel stenosis, small bowl atresia, malrotation, meckel diverticulum | 6 (2.5) |
BA = biliary atresia.
Complications
The total BA group included 963 BA patients. Two hundred thirty-one patients (24%) experienced at least 1 episode of cholangitis, and their median age was 1 year (Table 2). There were 60 patients (6.2%) who had esophageal and gastric varices, with a median age of 7 years. Gastrointestinal bleeding was reported in 42 patients (4.4%), and there were 44 patients (4.6%) who received endoscopic bleeding control (EBC). Three patients received repetitive EBC, with 1 patient who received EBC 6 times during a 5-year period.
Two patients developed hepatocellular carcinoma (HCC) before they turned 18 years (at age 3 and 7 years) and 2 other patients showed secondary pulmonary hypertension (at age 4 and 13 years).
LT
One hundred twenty-four patients with BA underwent LT (including re-transplantation) during the 5-year study period, and the median age at LT was 1 year. Sixty-five of these 124 patients (52.4%) received deceased donor LT (DDLT); split LT was the preferred approach (34.0%), and 7 of these 65 patients received re-DDLT (split DDLT in 4 cases and total DDLT in 3 cases). The remaining 59 of the aforementioned 124 patients (47.6%) received living donor LT (LDLT); typically, the left lateral segment was used (37.3%), and 2 of these 59 patients received re-LDLT using the left lateral segment (Table 3). The cases of deceased DDLT (split DDLT) increased (P = 0.100), while LDLT decreased over time (P = 0.720), but there was no statistically significant difference. Fifty-three of 115 patients (46.0%) received LT prior to 1 year of age. Eleven patients (8.7%) received re-LT, and 3 of these 11 patients were less than 1 year of age.
Table 3. Cases of LT in BA patients during 2011–2015.
| LT types | 2011 | 2012 | 2013 | 2014 | 2015 | No. (%) |
|---|---|---|---|---|---|---|
| DDLT | 10 | 6 | 16 | 14 | 19 | 65 (52.4) |
| Total liver | 4 | 1 | 3 | 5 | 4 | 17 (13.7) |
| Split liver | 6 | 5 | 13 | 9 | 15 | 48 (38.7) |
| LDLT | 9 | 13 | 13 | 14 | 10 | 59 (47.6) |
| Lt lat. | 8 | 11 | 12 | 10 | 8 | 49 (39.5) |
| Lt lobe | 0 | 2 | 0 | 3 | 2 | 7 (5.6) |
| Rt lobe | 1 | 0 | 1 | 1 | 0 | 3 (2.4) |
| Total | 19 | 19 | 29 | 28 | 29 | 124 (100.0) |
LT = liver transplantation, BA = biliary atresia, DDLT = deceased donor liver transplantation, LDLT = living donor liver transplantation; Lt = left; Rt = right, lat. = lateral segment.
Three hundred one of the 963 patients in the total BA group (31.3%) received LT, including 124 patients who first received LT during the 5-year study period and 177 patients who underwent LT prior to 2011.
DISCUSSION
This is the first report of BA incidence in Korea using the national health system database and the entire population of children born in the country. We identified the differences in BA incidence by gender and season, and we also reported the associated congenital anomalies and clinical outcomes of BA.
The incidence of BA in Korea (1.06/10,000) is similar to that in other East Asian countries, including Japan (1.0–1.1) (15,17) and Taiwan (1.5–1.8) (7,9), but higher than that in European countries such as France (0.5) (2), the UK (0.6) (3,19), Scotland (0.7) (16), and Switzerland (0.6) (18) as well as the USA cities of Atlanta (0.7) (1) and New York (0.9) (5). Because our incidence calculations only included patients who underwent the Kasai operation or LT before their first birthday, we may not have accounted for certain patients who died before receiving either of these operations or ceased receiving treatment.
The etiology of BA is unknown. However, environmental factors, including infections, toxins, and ischemia, are purported to cause this disease. Several studies describe seasonal and geographic variation in the incidence of BA (1,2,5,7,9,16,17,18,19,21,22). In Atlanta, significant seasonal clustering of BA was reported, with rates 3 times higher from December through March than rates from April through July (1,7,19). We also reported the seasonal variation of BA, and these results are consistent with environmental factors such as viral infection. This epidemiologic observation suggests that further investigation into the timing of possible viral infection around the perinatal period and subsequent identification of BA is necessary.
However, other studies insist that genetic factors are important to the pathogenesis of BA, showing the ethnic and gender variations in the incidence of BA (1,6,7,13,17,19,23). In our study, the incidence of BA was 1.4 times higher in females than in males; this finding is consistent with the results of many previous studies (1,6,7,13,17,19,23).
In this study, 15.8% of 240 BA patients had associated congenital anomalies; similar findings have been obtained in other studies (with corresponding percentages of 21.5%, 20.8%, 19.6%, 14.0%, and 12.6% for the UK, Switzerland, Japan, USA, and Canada, respectively) (1,3,6,15,18,20,24). Congenital heart disease (6.3%) was the major associated anomaly in our study, much like in other countries such as Canada (7.5%) and the USA (8.7%), followed by biliary anomalies (4.2%) and gastrointestinal anomalies (3.3%) (20,24).
In our total BA group, 2 HCC patients (0.2%, 3 and 7 years of age) were identified. At King's College Hospital, 3 of 387 BA patients (0.8%) developed histologically proven HCC, which was detected at a median age of 2.1 years (range 1.8–4.9 years); in Belgium, an infant who was affected by BA complicated by HCC when he was 6 months old was treated via LT, suggesting that HCC can develop at an early age in children with BA (25,26,27). Pulmonary hypertension was reported in 5 of the 617 BA patients who underwent LDLT (0.5%); this condition is more common among patients who undergo LDLT than among BA patients with naïve livers because the former patients are LT candidates with end-stage liver disease (28,29). We also found 2 secondary pulmonary hypertension patients (of 4 and 13 years of age) in the total BA group.
LT, including re-LT, was performed in 124 BA patients during the 5-year study period, and the median age at LT was 1 year. Other studies have found median ages for LT for BA of 1.1 years in Taiwan, 11–14 months in Canada, and 12 months in the UK and Switzerland, which are similar to the age calculated in our study (3,7,18,20).
In a study of patients from Seoul National University Hospital in Korea, a total of 152 children received LT from 1992 to 2010; 92 of these patients (60.5%) had BA, and 33 (35.9%) patients received LT for BA before 1 year of age (12). Our study produced similar results: 53 of 115 patients (46%) received LT before 1 year of age. In Asian countries, LDLT has been the main form of LT because of a shortage of deceased donors attributable to cultural and ethical concerns (12). However, the number of pediatric DDLT increased from 2006 to 2010 due to increased total deceased donation rates and the pediatric split graft policy (30). In our report, the number of cases involving DDLT (split DDLT) increased over the study period, whereas the number of cases involving LDLT decreased during this time. Three hundred one of the 963 patients (31.3%) received LT; this rate is slightly lower than that observed in the USA (40.3%) but higher than the corresponding rates in Taiwan (26%) and Croatia (20.7%) (7,8,24).
Our study has some limitations. The HIRA database only contains 5 years (2011–2015) of data; thus, this investigation utilized relatively short-term data. The database did not provide information regarding mortality; therefore, we could not examine survival rates.
In conclusion, this is the first report describing the incidence and clinical outcomes of BA in Korea using the national health insurance system database. In Korea, BA incidence is relatively higher than those in other Western countries but is similar to that in other East Asian countries and shows statistically significant female dominancy and seasonal clustering.
Footnotes
Funding: This research was supported by the Department of Pediatrics, Seoul National University College of Medicine (No. 2016-001).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Ko JS. Resources: Lee KJ, Kim JW. Formal analysis: Lee KJ, Kim JW. Writing - original draft: Lee KJ. Writing - review & editing: Moon JS, Ko JS.
References
- 1.Yoon PW, Bresee JS, Olney RS, James LM, Khoury MJ. Epidemiology of biliary atresia: a population-based study. Pediatrics. 1997;99:376–382. doi: 10.1542/peds.99.3.376. [DOI] [PubMed] [Google Scholar]
- 2.Chardot C, Carton M, Spire-Bendelac N, Le Pommelet C, Golmard JL, Auvert B. Epidemiology of biliary atresia in France: a national study 1986-96. J Hepatol. 1999;31:1006–1013. doi: 10.1016/s0168-8278(99)80312-2. [DOI] [PubMed] [Google Scholar]
- 3.McKiernan PJ, Baker AJ, Kelly DA. The frequency and outcome of biliary atresia in the UK and Ireland. Lancet. 2000;355:25–29. doi: 10.1016/S0140-6736(99)03492-3. [DOI] [PubMed] [Google Scholar]
- 4.Perlmutter DH, Shepherd RW. Extrahepatic biliary atresia: a disease or a phenotype? Hepatology. 2002;35:1297–1304. doi: 10.1053/jhep.2002.34170. [DOI] [PubMed] [Google Scholar]
- 5.Caton AR, Druschel CM, McNutt LA. The epidemiology of extrahepatic biliary atresia in New York State, 1983-98. Paediatr Perinat Epidemiol. 2004;18:97–105. doi: 10.1111/j.1365-3016.2003.00536.x. [DOI] [PubMed] [Google Scholar]
- 6.Wada H, Muraji T, Yokoi A, Okamoto T, Sato S, Takamizawa S, Tsugawa J, Nishijima E. Insignificant seasonal and geographical variation in incidence of biliary atresia in Japan: a regional survey of over 20 years. J Pediatr Surg. 2007;42:2090–2092. doi: 10.1016/j.jpedsurg.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Tiao MM, Tsai SS, Kuo HW, Chen CL, Yang CY. Epidemiological features of biliary atresia in Taiwan, a national study 1996-2003. J Gastroenterol Hepatol. 2008;23:62–66. doi: 10.1111/j.1440-1746.2007.05114.x. [DOI] [PubMed] [Google Scholar]
- 8.Grizelj R, Vuković J, Novak M, Batinica S. Biliary atresia: the Croatian experience 1992-2006. Eur J Pediatr. 2010;169:1529–1534. doi: 10.1007/s00431-010-1266-8. [DOI] [PubMed] [Google Scholar]
- 9.Lin YC, Chang MH, Liao SF, Wu JF, Ni YH, Tiao MM, Lai MW, Lee HC, Lin CC, Wu TC, et al. Decreasing rate of biliary atresia in Taiwan: a survey, 2004-2009. Pediatrics. 2011;128:e530–6. doi: 10.1542/peds.2011-0742. [DOI] [PubMed] [Google Scholar]
- 10.Utterson EC, Shepherd RW, Sokol RJ, Bucuvalas J, Magee JC, McDiarmid SV, Anand R, SPLIT Research Group Biliary atresia: clinical profiles, risk factors, and outcomes of 755 patients listed for liver transplantation. J Pediatr. 2005;147:180–185. doi: 10.1016/j.jpeds.2005.04.073. [DOI] [PubMed] [Google Scholar]
- 11.McDiarmid SV, Anand R, Lindblad AS, SPLIT Research Group Studies of Pediatric Liver Transplantation: 2002 update. An overview of demographics, indications, timing, and immunosuppressive practices in pediatric liver transplantation in the United States and Canada. Pediatr Transplant. 2004;8:284–294. doi: 10.1111/j.1399-3046.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 12.Byun J, Yi NJ, Lee JM, Suh SW, Yoo T, Choi Y, Ko JS, Seo JK, Kim H, Lee HW, et al. Long term outcomes of pediatric liver transplantation according to age. J Korean Med Sci. 2014;29:320–327. doi: 10.3346/jkms.2014.29.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, Sokol RJ. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology. 1996;23:1682–1692. doi: 10.1002/hep.510230652. [DOI] [PubMed] [Google Scholar]
- 14.Davenport M. Biliary atresia: outcome and management. Indian J Pediatr. 2006;73:825–828. doi: 10.1007/BF02790394. [DOI] [PubMed] [Google Scholar]
- 15.Nio M, Ohi R, Miyano T, Saeki M, Shiraki K, Tanaka K. Japanese Biliary Atresia Registry. Five- and 10-year survival rates after surgery for biliary atresia: a report from the Japanese Biliary Atresia Registry. J Pediatr Surg. 2003;38:997–1000. doi: 10.1016/s0022-3468(03)00178-7. [DOI] [PubMed] [Google Scholar]
- 16.Houwen RH, Kerremans II, van Steensel-Moll HA, van Romunde LK, Bijleveld CM, Schweizer P. Time-space distribution of extrahepatic biliary atresia in The Netherlands and West Germany. Z Kinderchir. 1988;43:68–71. doi: 10.1055/s-2008-1043419. [DOI] [PubMed] [Google Scholar]
- 17.Nakamizo M, Toyabe S, Kubota M, Komata O, Suzuki H, Akazawa K. Seasonality in the incidence of biliary atresia in Japan. Acta Paediatr. 2006;95:509–510. doi: 10.1080/08035250500411957. [DOI] [PubMed] [Google Scholar]
- 18.Wildhaber BE, Majno P, Mayr J, Zachariou Z, Hohlfeld J, Schwoebel M, Kistler W, Meuli M, Le Coultre C, Mentha G, et al. Biliary atresia: Swiss national study, 1994-2004. J Pediatr Gastroenterol Nutr. 2008;46:299–307. doi: 10.1097/MPG.0b013e3181633562. [DOI] [PubMed] [Google Scholar]
- 19.Livesey E, Cortina Borja M, Sharif K, Alizai N, McClean P, Kelly D, Hadzic N, Davenport M. Epidemiology of biliary atresia in England and Wales (1999-2006) Arch Dis Child Fetal Neonatal Ed. 2009;94:F451–5. doi: 10.1136/adc.2009.159780. [DOI] [PubMed] [Google Scholar]
- 20.Guttman OR, Roberts EA, Schreiber RA, Barker CC, Ng VL, Canadian Pediatric Hepatology Research Group Biliary atresia with associated structural malformations in Canadian infants. Liver Int. 2011;31:1485–1493. doi: 10.1111/j.1478-3231.2011.02578.x. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez-Rivera C, Jolin-Dahel KS, Fortinsky KJ, Gozdyra P, Benchimol EI. International incidence and outcomes of biliary atresia. J Pediatr Gastroenterol Nutr. 2013;56:344–354. doi: 10.1097/MPG.0b013e318282a913. [DOI] [PubMed] [Google Scholar]
- 22.Tayler R, Barclay AR, Rogers P, McIntyre K, Russell RK, Devadason D, Bisset WM, Ling SC, McGrogan P. Scottish outcomes for extra hepatic biliary atresia post-rationalisation of services. Arch Dis Child. 2013;98:381–383. doi: 10.1136/archdischild-2011-301608. [DOI] [PubMed] [Google Scholar]
- 23.Smith BM, Laberge JM, Schreiber R, Weber AM, Blanchard H. Familial biliary atresia in three siblings including twins. J Pediatr Surg. 1991;26:1331–1333. doi: 10.1016/0022-3468(91)90613-x. [DOI] [PubMed] [Google Scholar]
- 24.Shneider BL, Brown MB, Haber B, Whitington PF, Schwarz K, Squires R, Bezerra J, Shepherd R, Rosenthal P, Hoofnagle JH, et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148:467–474. doi: 10.1016/j.jpeds.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 25.Hol L, van den Bos IC, Hussain SM, Zondervan PE, de Man RA. Hepatocellular carcinoma complicating biliary atresia after Kasai portoenterostomy. Eur J Gastroenterol Hepatol. 2008;20:227–231. doi: 10.1097/MEG.0b013e3282cfb716. [DOI] [PubMed] [Google Scholar]
- 26.Hadžić N, Quaglia A, Portmann B, Paramalingam S, Heaton ND, Rela M, Mieli-Vergani G, Davenport M. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr. 2011;159:617–622.e1. doi: 10.1016/j.jpeds.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Brunati A, Feruzi Z, Sokal E, Smets F, Fervaille C, Gosseye S, Clapuyt P, de Ville de Goyet J, Reding R. Early occurrence of hepatocellular carcinoma in biliary atresia treated by liver transplantation. Pediatr Transplant. 2007;11:117–119. doi: 10.1111/j.1399-3046.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- 28.Shirouzu Y, Kasahara M, Takada Y, Taira K, Sakamoto S, Uryuhara K, Ogawa K, Doi H, Egawa H, Tanaka K. Development of pulmonary hypertension in 5 patients after pediatric living-donor liver transplantation: de novo or secondary? Liver Transpl. 2006;12:870–875. doi: 10.1002/lt.20758. [DOI] [PubMed] [Google Scholar]
- 29.Soh H, Hasegawa T, Sasaki T, Azuma T, Okada A, Mushiake S, Kogaki S, Matsushita T, Harada T. Pulmonary hypertension associated with postoperative biliary atresia: report of two cases. J Pediatr Surg. 1999;34:1779–1781. doi: 10.1016/s0022-3468(99)90311-1. [DOI] [PubMed] [Google Scholar]
- 30.Kim JM, Kim KM, Yi NJ, Choe YH, Kim MS, Suh KS, Kim SI, Lee SK, Lee SG. Pediatric liver transplantation outcomes in Korea. J Korean Med Sci. 2013;28:42–47. doi: 10.3346/jkms.2013.28.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]