Abstract
Korean red ginseng (KRG) and ginsenosides exhibit diverse biological effects, including anti-inflammatory and anti-allergic. We aimed to investigate the therapeutic effect of KRG in a murine model of atopic dermatitis (AD) is mediated whether by diminishing the pruritus or by suppressing the inflammation. Thirty NC/Nga mice were randomly divided to 5 groups. AD-like skin lesions were induced by percutaneous challenge with 2,4,6-trinitro-1-chrolobenzene (TNCB) on the ears and backs of NC/Nga mice. KRG extract, evening primrose oil, cyclosporine, and phosphate-buffered saline were administered orally by a gastric tube. Each study group was also divided into scratching-permitted and scratching-restricted subgroups to evaluate the impact of scratching behavior on AD. The effects of KRG and the other agents were assessed by measuring the clinical severity score, ear thickness, extent of transepidermal water loss (TEWL), number of scratching movements, total systemic immunoglobulin E (IgE) and interleukin (IL)-31 levels, histologic changes of cutaneous lesions, and mRNA expression levels of tumor necrosis factor (TNF)-α, interferon (IFN)-γ, thymic stromal lymphopoietin (TSLP), and IL-31. KRG exerts therapeutic effects against AD by inhibiting the T helper 2 (Th2) mediated inflammation as well as by diminishing the itching sensation. Moreover, restricting scratching behavior suppresses the vicious cycle of itching and scratching, thus reducing clinical and systemic inflammation in our murine model of AD.
Keywords: Dermatitis, Atopic, Panax ginseng, Pruritus
Graphical Abstract
INTRODUCTION
Itching is the most serious clinical symptom and a major diagnostic criterion of atopic dermatitis (AD) (1). Itching provokes the scratch reflex; this reflex is harmful because the itching-scratching cycle aggravates the existing skin lesions (2). Scratching behavior not only intensifies pruritus, but also further compromises the skin barrier and induces inflammation (3). Controlling inflammation and the sensation of abnormal itching are important for successfully managing AD (4). Several studies have shown that red ginseng has anti-inflammatory and anti-allergic properties (5,6,7,8,9). However, few studies have determined whether red ginseng has an anti-pruritic effect.
We previously examined the effects of systemic administration of Korean red ginseng (KRG) on a 2,4,6-trinitro-1-chrolobenzene (TNCB)-induced model of AD in NC/Nga mice and found that KRG prevented the development of acute AD-like lesions and the late onset of chronic AD-like lesions in NC/Nga mice model (7,8). KRG extract significantly reduced the total clinical severity score and the level of systemic immunoglobulin E (IgE) in a mouse model of AD. In the chronic stage, oral administration of KRG not only reduced the T helper 1 (Th1)-mediated response, demonstrated by the reductions in tumor necrosis factor (TNF)-α and interferon (IFN)-γ levels, but also inhibited regulatory T (Treg) cells and Langerhans cells (LCs) (7). Oral administration of KRG was found to partially suppress thymic stromal lymphopoietin (TSLP), dendritic cells (DCs), and the T helper 2 (Th2) response of early AD-like skin lesions in NC/Nga mice (8).
The authors attempted to identify whether the therapeutic effects of KRG on AD is the anti-inflammatory effect on the primary inflammatory process or is mediated by reducing itching to decrease the secondary inflammation by itch-scratch cycle in this experiment. In the present study, we developed a novel method of protecting the skin from scratching.
MATERIALS AND METHODS
Animals
Thirty SPF NC/Nga female mice (5 weeks old) were purchased from Charles River Laboratories (Kanagawa, Japan) and housed at 22°C with a 12 hours light-dark cycle. Food and water were freely available.
Induction of AD-like skin lesions
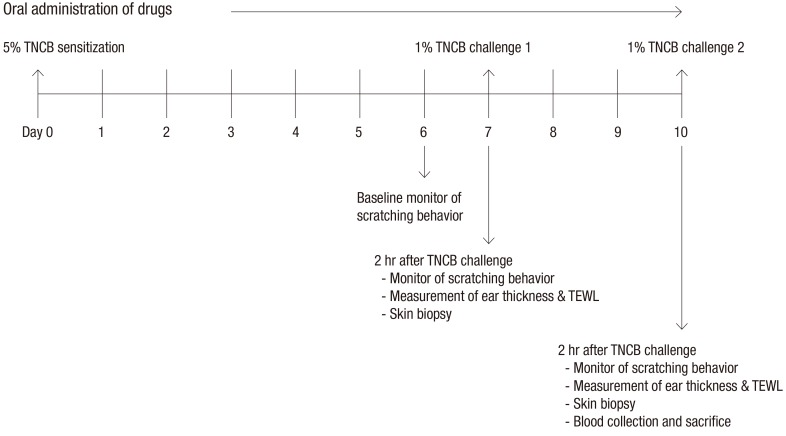
TNCB was purchased from Sigma (St. Louis, MO, USA) and used to induce AD-like skin lesions as previously described (7,8). Briefly, the abdomen of each mouse was sensitized epicutaneously with 150 µL of 5% TNCB dissolved in a 4:1 mixture of ethanol and acetone. On day 7 postsensitization, the dorsal skin and ears of the mice were challenged with 190 µL of 1% TNCB dissolved in a 4:1 mixture of acetone and olive oil. Three days after the first challenge, a 1% TNCB solution was repeatedly applied to the dorsal skin and ears. The study design is summarized in Fig. 1.
Fig. 1.
Summary of the study design.
TNCB = 2,4,6-trinitro-1-chrolobenzene, TEWL = transepidermal water loss.
Animal groups and drugs
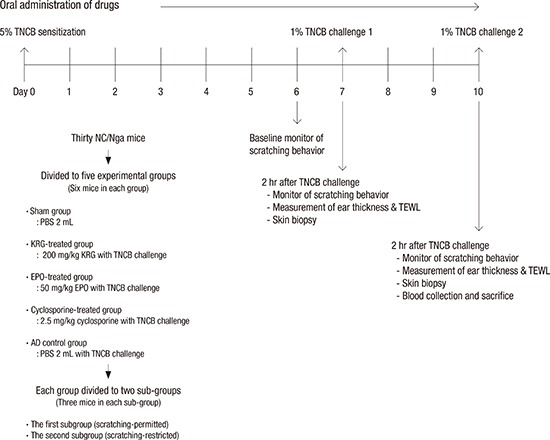
Thirty mice were divided randomly into 5 groups: AD control, KRG-treated (KRG), cyclosporine-treated (cyclosporine), evening primrose oil-treated (EPO), and sham. Each experimental group consisted of 6 mice. The AD control and sham group received 0.2 mL phosphate-buffered saline (PBS). The KRG, cyclosporine, and EPO groups were orally administered 200 mg/kg KRG, 2.5 mg/kg cyclosporine, and 50 mg/kg EPO for 10 days, respectively and they did not received PBS. The AD control, KRG, cyclosporine, and EPO groups was applied TNCB on the back and ears. The sham group received only PBS (0.2 mL) by a gastric tube without TNCB challenge.
Each study group was divided into 2 subgroups (Fig. 2A). Mice in the first subgroup (scratching-permitted) were allowed to freely scratch their TNCB-induced AD-like dorsal lesions. In contrast, mice in the second subgroup (scratching-restricted) were prevented from scratching their skin via a custom-designed plastic chamber affixed to their backs before starting the experiment (Fig. 2B). Each subgroup consisted of 3 mice. The scratching-restricted subgroups are designated with the “@” symbol after the drug names in the figures.
Fig. 2.
Description of mouse experiment and gross view of mice. (A) Animal groups. (B) A custom-designed plastic chamber affixed to scratching-restricted mice backs before starting the experiment.
TNCB = 2,4,6-trinitro-1-chrolobenzene, PBS = phosphate-buffered saline, KRG = Korean red ginseng, EPO = evening primrose oil, AD = atopic dermatitis.
The KRG extract was manufactured from the roots of a 6-year-old fresh ginseng plant (Panax ginseng Meyer). The ginseng was harvested in the Republic of Korea by the Korea Ginseng Corporation (Seoul, Korea). The resultant KRG extract was analyzed via high-performance liquid chromatography. The KRG extract contained major ginsenosides, including −Rg1: 1.79 mg/g, −Rb1: 7.44 mg/g, −Rg1+Rb1: 9.23 mg/g, −Rg3S: 1.39 mg/g, −Re:1.86 mg/g, −Rc: 3.04 mg/g, −Rb2: 2.59 mg/g, −Rd: 0.91 mg/g, −Rf: 1.24 mg/g, −Rh1: 1.01 mg/g, and −Rg2S: 12.04 mg/g, in addition to other minor ginsenosides. Cyclosporine (Cipol-N®) was purchased from Chong Kun Dang (Seoul, Korea); EPO (Evoprim®) was obtained from Dalim Biotech (Seoul, Korea).
Measurement of spontaneous scratching behavior
AD-like skin lesions and scratching behavior were induced by repeated application of TNCB solution onto the ears and backs of NC/Nga mice as previously described (7,8). Baseline spontaneous scratching movements were obtained by recording the scratching movements on the abdomen for 2 hours after TNCB sensitization (day 6). On days 7 and 10, scratching behavior was monitored by video for 2 hours after 1% TNCB challenge on the ears and back. The number of scratching movements after challenge was compared with baseline measurements.
Measurement of total clinical severity score and ear thickness
The severity of AD was evaluated by scoring clinical signs and symptoms as previously described (7,8). The total clinical severity score was defined as the sum of the scores for erythema/hemorrhage, edema, excoriation/erosion, and scaling/dryness on the following scale: 0 (none), 1 (mild), 2 (moderate), and 3 (severe). Assessment was performed by an investigator who was blinded to group assignment. Ear thickness was measured using a dial caliper (Kori Seiki MFG, Tokyo, Japan) on day 0 to obtain a baseline measurement and then again on days 7 and 10 after TNCB challenge on the ears and back.
Measurement of transepidermal water loss (TEWL)
TEWL was assessed on the dorsal skin of the NC/Nga mice using a VapoMeter instrument (Delfin Technologies, Kuopio, Finland). TEWL was measured at baseline (day 0) and on days 7 and 10.
Tissue samples and histopathologic examination
On days 7 and 10, 4 mm punch biopsies were performed on the backs of mice and tissue samples were also excised from each ear. The samples were fixed in 4% paraformaldehyde (PFA) for hematoxylin and eosin (H & E) and toluidine blue staining. Sections were evaluated at × 400 magnification in 3 consecutive microscopic fields for quantitative analysis.
Reverse transcription-polymerase chain reaction (RT-PCR)
The expression levels of various mRNA transcripts were determined by RT-PCR using the following primers: TNF-α (forward: 5'-CAGGCGGTGCCTATGTCTC-3', reverse: 5'-CGATCACCCCGAAGTTCAG-TAG-3'), TSLP (forward: 5'-ACTGCAACTTCACGTCAATTACG-3', reverse: 5'-TTGCTCGAACTTAGCCCCTTT-3'), Interleukin (IL)-31 (forward: 5'-ATACAGCTGCCGTGTTTCAG-3', reverse: 5'-AGCCATCTTATCACCCAAGAA-3'), and IFN-γ (forward: 5'-TACACACTGCATCTTGGCTTTG-3', reverse: 5'-CTTCCACATCTATGCCACTTGAG-3'). RT-PCR analysis was performed on an Applied Biosystems 7900HT Fast RT-PCR System (Thermo Fisher Scientific Inc., Waltham, MA, USA). Reactions were set up in a total volume of 20 µL using 0.5 µL of cDNA and 10 µL of FastStart Universal SYBR Green Master Mix (Roche, Mannheim, Germany). For amplification, the samples were heated to 50°C for 2 minutes, to 95°C for 10 minutes, and then subjected to 40 of the following thermocycles: 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds.
Enzyme-linked immunosorbent assay (ELISA)
Blood samples were collected from the retro-orbital plexus using heparinized glass capillary tubes at the end of the experiment. Serum was obtained by centrifugation and stored at −20°C until use. Serum concentrations of total IgE and IL-31 were determined using a mouse IgE ELISA kit (Bethyl, Montgomery, TX, USA) and a mouse IL-31 ELISA kit (R & D Systems, Minneapolis, MN, USA), respectively, according to the manufacturers' instructions.
Statistical analysis
The significance of differences among experimental groups was examined using Student's t-test and analysis of variance (ANOVA). Treatment group comparisons were evaluated using ANOVA. For subgroup comparison in each group and comparison of the KRG group and other groups we used the Student's t-test. All calculations were performed in SPSS v19.0 (SPSS Inc., Chicago, IL, USA) and all values are expressed as mean ± standard error of the mean (SEM). P values less than 0.05 were considered statistically significant.
Ethics statement
The animal care, handling and experimental procedures were carried out in accordance with a protocol approved by the Animal Care and Use Committee of Catholic University in Korea (CIMH-2013-010).
RESULTS
KRG, EPO, and cyclosporine effectively suppressed AD-like symptoms are evoked by TNCB challenge
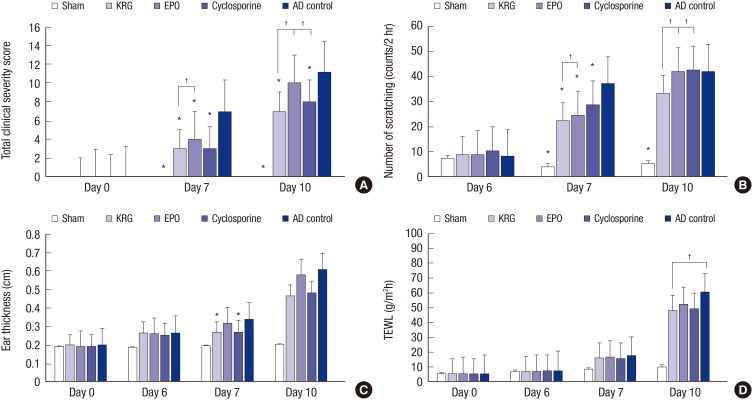
AD-like symptoms such as erythema, edema, excoriation, dryness, and scaling appeared after sensitization and percutaneous challenge with TNCB on the backs and ears of NC/Nga mice. Total clinical severity scores of the KRG, EPO, cyclosporine, and AD control groups were significantly increased compared with those of the sham group on days 7 and 10 (P < 0.001). Moreover, total clinical severity scores were significantly elevated in the AD control group compared with the sham, KRG, EPO, and cyclosporine groups on day 7 and compared with the sham, KRG, and cyclosporine groups on day 10 (P < 0.001; Fig. 3A). Systemic administration of KRG, effectively suppressed clinical symptoms compared with the EPO group on day 7 (3.00 ± 0.26 vs. 4.00 ± 0.37, P = 0.049) and compared with the EPO and cyclosporine groups on day 10 (7.00 ± 0.37 vs. 10.00 ± 0.37, P < 0.001 and 7.00 ± 0.37 vs. 8.00 ± 0.26, P = 0.049, respectively). Clinical severity scores were also significantly higher in the scratching-permitted subgroups on day 10 in both the KRG and EPO groups (7.67 ± 0.33 vs. 6.33 ± 0.33, P = 0.047 and 10.67 ± 0.33 vs. 9.33 ± 0.33, P = 0.047, respectively). In general, clinical severity scores tended to be higher in the scratching-permitted subgroups than in the scratching-restricted subgroups, although these differences were not always statistically significant.
Fig. 3.
Comparison of therapeutic effects of KRG, EPO, and cyclosporine. (A) Total clinical severity score. (B) Scratching counts. (C) The ear thickness. (D) TEWL of back. All group values are expressed as mean ± SEM.
KRG = Korean red ginseng; EPO = evening primrose oil, TEWL = transepidermal water loss, SEM = standard error of the mean, ANOVA = analysis of variance, AD = atopic dermatitis.
*P < 0.05 compared with the AD control group (n = 6; ANOVA). †P < 0.05 compared with the KRG group (n = 6, t-test).
KRG treatment reduces scratching behaviors in TNCB-induced NC/Nga mice
We monitored the scratching behavior of each group to determine the effect of each agent on pruritus. To clinically evaluate the extent of pruritus, we monitored scratching movements at baseline before TNCB challenge (day 6) and for 2 hours after TNCB challenge (days 7 and 10). This approach was based on a previous study by Inagaki et al. (10) showing that most scratching movements occurred in the first hour after initial 2,4-dinitrofluorobenzene (DNFB) challenge and in the first 2 hours after the ninth challenge.
The number of scratching movements increased over time after the first TNCB challenge. The sham (4.00 ± 0.45), KRG (22.17 ± 1.17), EPO (24.33 ± 1.63), and cyclosporine (28.67 ± 0.84) groups exhibited significantly less scratching movements compared with the AD control group (37.00 ± 3.11) on day 7 after the first percutaneous challenge of TNCB (sham, KRG, and EPO P < 0.001, and cyclosporine P = 0.019; Fig. 3B). On day 7, the KRG group had significantly lower scratching counts compared to the EPO group (P = 0.001). On day 10, KRG mice exhibited fewer scratching movements (33.17 ± 1.35) compared to EPO (41.67 ± 3.10) and cyclosporine (42.50 ± 2.29) mice (P = 0.041 and P = 0.006, respectively). These results suggest that systemic administration of KRG has anti-pruritic effects in our mouse model of AD. Significant differences were also observed between the 2 AD subgroups on day 7 (scratching permitted subgroup 43.33 ± 2.60 vs. scratching-restricted subgroup 30.67 ± 1.20, P = 0.012) and in the 2 EPO subgroups group on day 10 (scratching permitted subgroup 48.33 ± 2.08 vs. scratching-restricted subgroup 35.33 ± 1.86, P = 0.010). The number of scratching movements was also lower in other scratching-restricted subgroups, although these differences were not significant.
Treatment with KRG and cyclosporine significantly reduces ear thickness
The mean ear thickness increased over time from day 0 to day 10 after initial TNCB application. Ear thickness of the KRG (0.27 ± 0.01 cm) and cyclosporine (0.27 ± 0.02 cm) groups was significantly decreased on day 7 compared with the AD control group (0.34 ± 0.03 mm; P = 0.017 and P = 0.020, respectively; Fig. 3C). Thus, both KRG and cyclosporine exert anti-inflammatory effects when administered systemically. Significant differences were also observed between the 2 AD subgroups on day 7 (scratching-permitted subgroup 0.32 ± 0.01 mm vs. scratching-restricted subgroup 0.36 ± 0.01 mm, P = 0.031). No significant differences were observed between the scratching-permitted and scratching-restricted subgroups within the sham, KRG, EPO, and cyclosporine groups on day 7 and within all groups on day 10, presumably because the ears were not protected from scratching.
KRG treatment prevented TEWL increase
We measured TEWL on the backs of NC/Nga mice on day 0 (baseline), day 6 (before the first TEWL challenge), day 7 (2 hours after the first TEWL challenge), and day 10 (2 hours after the second TEWL challenge) using a VapoMeter instrument (Delfin Technologies). TEWL increased over time from day 0 to day 10 after initial TNCB application. The KRG group (48.02 ± 1.26 g/m2h) exhibited significantly reduced TEWL compared with the AD control group (60.17 ± 5.29 g/m2h) on day 10 (P = 0.049; Fig. 3D). EPO and cyclosporine treatments did not have any significant effect on TEWL increase. No significant differences were observed in TEWL between the scratching-permitted and scratching-restricted subgroups.
Histopathologic analysis of KRG treatment on TNCB-induced AD-like skin lesions
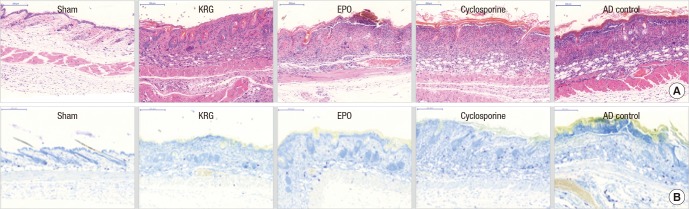
After repeated application of TNCB, excessive epidermal hyperplasia, hyperkeratosis, epidermal parakeratosis, marked leukocyte infiltration in the dermis, and dermal edema were observed on the backs and ears of mice in the AD control groups. Oral administration of KRG, EPO, and cyclosporine effectively reduced these epidermal changes and dermal infiltrations (Fig. 4).
Fig. 4.
Histopathologic evaluation after repeated application of TNCB on the back of NC/Nga mice. (A) H & E staining, × 200. (B) Toluidine blue staining, × 200 for mast cell infiltration.
TNCB = 2,4,6-trinitro-1-chrolobenzene, KRG = Korean red ginseng, EPO = evening primrose oil, H & E = hematoxylin and eosin, AD = atopic dermatitis.
The KRG (19.67 ± 1.71) and cyclosporine (18.00 ± 2.27) groups exhibited significantly reduced mast cell infiltration on the back compared with the AD control group (28.00 ± 2.50; P = 0.043 and P = 0.011; Table 1). EPO mice (20.67 ± 0.80) exhibited less mast cell infiltration on the back compared with the AD control group, but this was not significant (P = 0.092). Cyclosporine was more effective for reducing mast cell infiltration than either KRG or EPO, but this difference was not significant (P = 0.973 and P = 0.868, respectively). No significant differences were observed between scratching-permitted and scratching-restricted subgroups.
Table 1. Numbers of mast cells and mRNA expression of TNF-α, TSLP, IL-31 and IFN-γ in TNCB-induced skin lesions, and serum levels of IgE and IL-31 by ELISA.
| Subject group | No. of mast cells (at × 400 magnification) | mRNA expression change | ELISA (serum) | ||||
|---|---|---|---|---|---|---|---|
| TNF-α | TSLP | IL-31 | IFN-γ | IgE, ng/mL | IL-31, pg/mL | ||
| Sham group | 17.6 ± 2.25* | 1.30 ± 0.40 | 1.49 ± 0.31 | 0.48 ± 0.12 | 2.59 ± 0.77*† | 3.82 ± 1.38*† | 5,41.58 ± 39.07*† |
| KRG group | 19.67 ± 1.71* | 7.47 ± 3.35 | 19.30 ± 7.09 | 5.82 ± 2.64 | 31.37 ± 6.50 | 102.90 ± 8.79* | 1,524.18 ± 75.49* |
| EPO group | 20.67 ± 0.80 | 16.88 ± 7.98 | 8.64 ± 4.00 | 6.58 ± 3.61 | 30.64 ± 3.49 | 109.51 ± 3.97* | 1,602.99 ± 113.67* |
| Cyclosporine group | 18.00 ± 2.27* | 11.50 ± 5.45 | 21.97 ± 15.75 | 5.89 ± 2.85 | 26.91 ± 10.69 | 132.23 ± 9.08*† | 2,041.25 ± 147.39† |
| AD control group | 28.00 ± 2.50 | 22.84 ± 3.66† | 35.89 ± 4.98 | 7.31 ± 2.66 | 49.82 ± 10.17 | 168.30 ± 9.98† | 2,241.29 ± 80.41† |
Quantification of serum IgE and IL-31 levels by ELISA after collection of blood samples from the retro-orbital plexus; All group values are expressed as means ± SEMs.
ELISA = enzyme-linked immunosorbent assay, TNF = tumor necrosis factor, TSLP = thymic stromal lymphopoietin, IL = interleukin, IFN = interferon, IgE = immunoglobulin E, KRG = Korean red ginseng, EPO = evening primrose oil, AD = atopic dermatitis, SEM = standard error of the mean, ANOVA = analysis of variance.
*ANOVA, P < 0.05 compared with the AD control group. †t-test, P < 0.05 compared with the KRG group.
Effects of KRG on TNCB-induced upregulation of TNF-α, IFN-γ, TSLP, and IL-31 mRNA expression in AD-like skin lesions
TNF-α is a proinflammatory cytokine that initiates inflammation in the acute phase of AD by recruiting inflammatory cells to the lesion (11). mRNA expression of TNF-α was reduced by KRG (7.47 ± 3.35), EPO (16.88 ± 7.98), and cyclosporine (11.50 ± 5.45) treatment compared to the AD control group (22.84 ± 3.66). KRG treatment significantly suppressed mRNA expression of TNF-α compared to AD control group (P = 0.011; Table 1). TNF-α mRNA expression was lower in the scratching-restricted subgroups, although these differences were not significant.
TSLP is produced by keratinocytes and activates DCs to stimulate naïve T cells to produce Th2 cytokines (12). TSLP mRNA expression was reduced by EPO treatment (8.64 ± 4.00) compared with the AD control group (35.89 ± 4.98) to a greater extent than it was by KRG (19.30 ± 7.09) and cyclosporine (21.97 ± 15.75) treatments, but these differences were not significant (P = 0.182; Table 1). TSLP mRNA expression tended to be lower in the scratching-restricted subgroups, although this difference was only significant in the AD control group (46.25 ± 3.44 vs. 25.53 ± 2.20, P = 0.007).
IL-31 is upregulated in both lesional and nonlesional skin in AD and also induces a severe itching sensation and dermatitis in transgenic mice (13). The extent of scratching correlates with the expression of IL-31 mRNA (14). IL-31 mRNA levels of the KRG (5.82 ± 2.64), EPO (6.58 ± 3.61), and cyclosporine (5.89 ± 2.85) treatment groups were not significantly lower than that of the AD control group (7.31 ± 2.66; Table 1). IL-31 mRNA levels of the cyclosporine subgroups varied significantly, however (11.35 ± 3.30 vs. 0.42 ± 0.18, P = 0.030).
IFN-γ is a characteristic Th1-type cytokine that is reduced in acute AD and promotes chronic inflammation (11). mRNA levels of IFN-γ were similar between the KRG (31.37 ± 6.50), EPO (30.64 ± 3.49), and cyclosporine (26.91 ± 10.69) groups; moreover, the AD control group exhibited a statistically similar IFN-γ mRNA level (49.82 ± 10.17; Table 1). The IFN-γ mRNA levels of the cyclosporine subgroups varied significantly (47.22 ± 12.33 vs. 6.59 ± 2.57, P = 0.032).
KRG and EPO prevents the elevation of systemic IgE levels in mice with TNCB-induced AD
Decreased systemic IgE elevation was observed in the KRG (102.90 ± 8.79), EPO (109.51 ± 3.97), and cyclosporine (132.23 ± 9.08) treatment groups compared with the AD control group (168.30 ± 9.98; P < 0.001, P < 0.001, and P = 0.002, respectively; Table 1). The KRG treatment more effectively suppressed systemic IgE elevation than cyclosporine treatment (P = 0.043). No significant differences were observed between any of the scratching-permitted and -restricted subgroups.
KRG effectively prevents the elevation of systemic IL-31 levels in mice with TNCB-induced AD
IL-31 is mainly produced by Th2 cells, and some evidence indicates that IL-31 production correlates with the severity of pruritus and inflammation in the context of AD (13,15). We evaluated serum IL-31 levels using ELISA and IL-31 mRNA expression levels in cutaneous tissue via RT-PCR. The increase in systemic IL-31 was significantly reduced by oral administration of KRG (1,524.181 ± 75.490) and EPO (1,602.99 ± 113.67) compared with the AD control group (2,241.29 ± 80.41; P < 0.001 and P = 0.001, respectively), although cyclosporine (2,041.25 ± 147.39) did not have a significant effect (Table 1). KRG and EPO controlled serum IL-31 elevation more effectively than cyclosporine (t-test, P = 0.010 and P = 0.035, respectively), The overall systemic IL-31 levels in the scratching-restricted subgroups were lower than those in the scratching-permitted subgroups, but this difference was not significant.
DISCUSSION
A range of studies have shown the diverse biological effects of Panax ginseng (5,6,7,8,9,16). We previously examined the anti-inflammatory effects of systemic administration of KRG on a TNCB-induced model of AD in NC/Nga mice and found that KRG prevented the development of acute AD-like lesions and late-onset chronic AD-like lesions (7,8). As an extension of this previous research, we evaluated the anti-pruritic effect as well as the anti-inflammatory effect of systemic KRG in a murine model of AD.
Clinical severity score, number of scratching movements, ear thickness, and TEWL were measured to evaluate the macroscopic effects of KRG, EPO, and cyclosporine. Systemic administration of KRG, EPO, and cyclosporine significantly reduced TNCB-induced clinical signs and symptoms. KRG was more effective than EPO on day 7 and more effective than EPO and cyclosporine on day 10. Compared to the AD control group, KRG and cyclosporine mitigated ear swelling and ear thickness up to day 7. These results indicate the clinical anti-inflammatory effects of systemic KRG.
The KRG group exhibited significantly less TEWL elevation compared with the AD control group on day 10. This was consistent with our previous study (8). Cho et al. (8) showed that 6 hours after TNCB application, KRG- and cyclosporine-treated groups had lower TEWL values compared to the AD control group. In this study, we observed a correlation between scratching movements and TEWL changes. KRG, EPO, and cyclosporine treatment all reduced the number of scratching movements, with KRG being the most at day 10. Thus, we presumed that TEWL changes correlated with protection against mechanical stimuli (scratching) and anti-the inflammatory effects of systemic KRG.
Samukawa et al. (16) demonstrated that topical application of tacrolimus and red ginseng extract (RGE) significantly inhibited scratching behavior in a DNFB induced AD mouse model, whereas dexamethasone did not. Topical RGE also significantly reduced DNFB-induced nerve growth factor expression and nerve fiber extensio. In addition, topical application of RGE suppressed histamine-induced scratching behavior (16). In the present study as well as our previous studies, KRG was administered via a systemic method, not topically. Our previous demonstrated that oral administration of KRG suppresses the expression of protein gene product 9.5 and substance P in nerve fibers (7). In this study, the systemic KRG, EPO, and cyclosporine groups all exhibited reduced scratching behavior compared with the AD control group after initial TNCB challenge. KRG suppressed scratching behaviors more effectively than EPO on day 7 and more effectively than EPO and cyclosporine on day 10. This finding suggests that systemic administration of KRG has an anti-pruritic effect in this mouse model of AD. In the present study, we divided each study group into 2 subgroups to evaluate the clinical and systemic effects of the itching-scratching cycle in our AD mouse model. Clinical severity scores were higher in the scratching-permitted subgroups on day 10, especially in the KRG- and EPO-treated groups. These results imply that the itching-scratching cycle exacerbates skin lesions in AD and restriction of scratching behavior might be an effective approach for preventing this vicious cycle in patients with AD.
In the present study, histopathological analysis also revealed that KRG administration markedly reduced the extent of epidermal hyperplasia, hyperkeratosis, parakeratosis, and dermal infiltration of leukocytes. In addition, KRG and cyclosporine treatment reduced mast cell infiltration in the back and cyclosporine treatment was most effective. This finding is consistent with a previous study reporting that cyclosporine inhibits mast cell degranulation and the release of inflammatory mediators (17). In AD, mast cells are sensitized by IgE and modulate the differentiation of naïve T cells and induce Th2 polarization (11). Elevated levels of IgE autoantibodies also correlated with early onset of disease, intense pruritus, and bacterial infection relapse (18,19). TNCB -induced elevation of serum IgE was also significantly lowered by KRG, EPO and cyclosporine treatment. KRG was more effective than cyclosporine in suppressing total IgE elevation. These results were suggesting that systemic administration of KRG has anti-allergic effects and oral KRG might reduce the severity of AD lesions and prevent progression to respiratory atopy.
TNF-α is one of proinflammatory cytokines and responses to environmental damage and inflammation (20). Systemic administration of KRG significantly suppressed TNF-α mRNA expression compared to the AD control group. Kim et al. (6) showed that topical KRG as well as 0.05% betamethasone significantly suppressed TNCB-induced TNF-α mRNA expression in NC/Nga mice. Other previous studies showed that systemic KRG treatment also effectively suppressed TNF-α mRNA expression in atopic mouse model (7,8). Thus, systemic administration of KRG was effective to reduce production of TNF-α from keratinocyte. EPO inhibited TSLP mRNA expression most effectively, although this was not significant. This result is similar with our previous study, which showed that oral administration of EPO effectively reduced total IgE level and mRNA expression of TSLP (8). EPO is a natural source of gamma-linolenic acid (GLA), and GLA is converted to dihomo-GLA (DGLA) in humans. Dietary DGLA supplementation induced prostaglandin D1 (PGD1) production by mast cells in a mouse study (21). PGD1 suppressed IgE mediated degranulation and TSLP gene expression in keratinocytes and topical application of PGD1 also reduced scratching behavior in an AD mouse model (21). These results confirm those of our previous study and this study, in that oral administration of EPO was effective for reducing clinical symptoms, scratching movements, serum IgE, and IL-31 levels in a mouse model of AD.
KRG and EPO significantly reduced the serum level of IL-31 compared to the AD control group. IL-31 secretion was similar to the observed scratching patterns (14), suggested that KRG and EPO exerted more potent anti-pruritic effects, as demonstrated by their dampening of the scratching behavior and systemic IL-31 levels compared with cyclosporine. Oral administration of cyclosporine also had an anti-pruritic effect on day 7, but it did not significantly alter the serum IL-31 level. We hypothesize that the anti-pruritic effect of cyclosporine is mainly due to its suppression of histamine release by mast cells. Since the mechanism of action of KRG is different from immunosuppressants such as cyclosporine, concurrent use of KRG and cyclosporine could be synergistic. Systemic IL-31 levels in the scratching-restricted subgroups were lower than those in the scratching-permitted subgroups. This finding suggests that scratching behavior and serum IL-31 levels may have a cyclic feed-forward relationship.
There are several limitations to our study. First, this is a pilot study evaluating the anti-pruritic and anti-allergic effects of systemic KRG for AD. Thus, future animal studies with larger sample sizes and human research will be needed to confirm these findings. Second, we focused on mouse scratching behavior than previous our study, and used a novel chamber for scratching restriction. There have been several observational studies about scratching behavior in mice, but there have been few experiments using scratching restriction. In addition, we used indirect values such as scratching movement counts and serum IL-31 levels for objective evaluation of pruritus, which have been used in prior studies (13,14,15,22). To evaluate the role of mechanical injury due to scratching in AD and its relation to anti-pruritic effects in humans, further studies are needed.
In summary, we confirmed the anti-pruritic and anti-allergic effects of KRG in a NC/Nga mice model. KRG decreased clinical signs and symptoms and the number of scratching movements; the mechanism of these effects may be a reduction in mast cell infiltration and/or suppression of systemic IL-31 and IgE. Second, KRG protected skin barrier function as indicated by TEWL elevation. Third, KRG exert anti-inflammatory effects by reducing the mRNA levels of TNF-α and by histopathologic findings such as a markedly diminished extent of epidermal hyperplasia, hyperkeratosis, parakeratosis, and dermal infiltration of leukocytes and mast cells. Thus, proactive systemic administration of KRG may effectively reduce early allergic sensitization and suppress the Th2 response. Restriction of scratching in the early stages could also help prevent the itching-scratching cycle, thus reducing clinical and systemic inflammation.
ACKNOWLEDGMENT
The authors thank Si-Kwan Kim for excellent assistance in the preparation of this study.
Footnotes
Funding: This work was supported by the 2013 grant from the Korean Society of Ginseng funded by Korea Ginseng Corporation.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Lee HJ, Cho SH. Data curation: Lee HJ. Formal analysis: Lee HJ. Investigation: Lee HJ, Cho SH. Writing - original draft: Lee HJ, Cho SH. Writing - review & editing: Lee HJ, Cho SH.
References
- 1.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1980;92:44–47. [Google Scholar]
- 2.Yamaguchi T, Maekawa T, Nishikawa Y, Nojima H, Kaneko M, Kawakita T, Miyamoto T, Kuraishi Y. Characterization of itch-associated responses of NC mice with mite-induced chronic dermatitis. J Dermatol Sci. 2001;25:20–28. doi: 10.1016/s0923-1811(00)00099-2. [DOI] [PubMed] [Google Scholar]
- 3.Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, Chamlin SL, Cooper KD, Feldman SR, Hanifin JM, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327–349. doi: 10.1016/j.jaad.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong J, Buddenkotte J, Berger TG, Steinhoff M. Management of itch in atopic dermatitis. Semin Cutan Med Surg. 2011;30:71–86. doi: 10.1016/j.sder.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumiyoshi M, Sakanaka M, Kimura Y. Effects of red ginseng extract on allergic reactions to food in Balb/c mice. J Ethnopharmacol. 2010;132:206–212. doi: 10.1016/j.jep.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Kim HS, Kim DH, Kim BK, Yoon SK, Kim MH, Lee JY, Kim HO, Park YM. Effects of topically applied Korean red ginseng and its genuine constituents on atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol. 2011;11:280–285. doi: 10.1016/j.intimp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Cho SH. Korean red ginseng extract ameliorates skin lesions in NC/Nga mice: an atopic dermatitis model. J Ethnopharmacol. 2011;133:810–817. doi: 10.1016/j.jep.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Cho E, Cho SH. Effects of Korean red ginseng extract on the prevention of atopic dermatitis and its mechanism on early lesions in a murine model. J Ethnopharmacol. 2013;145:294–302. doi: 10.1016/j.jep.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Jung JH, Kang IG, Kim DY, Hwang YJ, Kim ST. The effect of Korean red ginseng on allergic inflammation in a murine model of allergic rhinitis. J Ginseng Res. 2013;37:167–175. doi: 10.5142/jgr.2013.37.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inagaki N, Shiraishi N, Igeta K, Itoh T, Chikumoto T, Nagao M, Kim JF, Nagai H. Inhibition of scratching behavior associated with allergic dermatitis in mice by tacrolimus, but not by dexamethasone. Eur J Pharmacol. 2006;546:189–196. doi: 10.1016/j.ejphar.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Mu Z, Zhao Y, Liu X, Chang C, Zhang J. Molecular biology of atopic dermatitis. Clin Rev Allergy Immunol. 2014;47:193–218. doi: 10.1007/s12016-014-8415-1. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nobbe S, Dziunycz P, Mühleisen B, Bilsborough J, Dillon SR, French LE, Hofbauer GF. IL-31 expression by inflammatory cells is preferentially elevated in atopic dermatitis. Acta Derm Venereol. 2012;92:24–28. doi: 10.2340/00015555-1191. [DOI] [PubMed] [Google Scholar]
- 14.Takaoka A, Arai I, Sugimoto M, Honma Y, Futaki N, Nakamura A, Nakaike S. Involvement of IL-31 on scratching behavior in NC/Nga mice with atopic-like dermatitis. Exp Dermatol. 2006;15:161–167. doi: 10.1111/j.1600-0625.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 15.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 16.Samukawa K, Izumi Y, Shiota M, Nakao T, Osada-Oka M, Miura K, Iwao H. Red ginseng inhibits scratching behavior associated with atopic dermatitis in experimental animal models. J Pharmacol Sci. 2012;118:391–400. doi: 10.1254/jphs.11182fp. [DOI] [PubMed] [Google Scholar]
- 17.Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012;106:9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto Y, Takaoka A, Sugimoto M, Honma Y, Sakurai T, Futaki N, Arai I. Itch-associated scratching contributes to the development of dermatitis and hyperimmunoglobulinaemia E in NC/Nga mice. Exp Dermatol. 2011;20:820–825. doi: 10.1111/j.1600-0625.2011.01337.x. [DOI] [PubMed] [Google Scholar]
- 19.Schmid-Grendelmeier P, Flückiger S, Disch R, Trautmann A, Wüthrich B, Blaser K, Scheynius A, Crameri R. IgE-mediated and T cell-mediated autoimmunity against manganese superoxide dismutase in atopic dermatitis. J Allergy Clin Immunol. 2005;115:1068–1075. doi: 10.1016/j.jaci.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 20.Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem. 2004;279:32633–32642. doi: 10.1074/jbc.M400642200. [DOI] [PubMed] [Google Scholar]
- 21.Amagai Y, Oida K, Matsuda A, Jung K, Kakutani S, Tanaka T, Matsuda K, Jang H, Ahn G, Xia Y, et al. Dihomo-gamma-linolenic acid prevents the development of atopic dermatitis through prostaglandin D1 production in NC/Tnd mice. J Dermatol Sci. 2015;79:30–37. doi: 10.1016/j.jdermsci.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, Alenius H, Dieu-Nosjean MC, Meller S, Rieker J, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]