Abstract
The cause of death in patients with tuberculosis (TB) may differ according to the phase of anti-tuberculosis treatment. However, there are limited data regarding this issue in Korea. We compared the cause of death of TB patients who died during the early intensive and late continuation phase of treatment. Twenty (56%) of the 36 early deaths were due to TB-related causes, whereas 34 (89%) of the 38 late deaths were due to TB-unrelated causes. This finding suggests that TB-related early deaths mainly attributable to delayed diagnosis should be improved to further reduce the overall TB deaths.
Keywords: Tuberculosis, Early Deaths, Late Deaths, Drug Susceptibility Test
Graphical Abstract
Tuberculosis (TB) remains a significant cause of mortality worldwide. TB deaths are one of the crucial indicators in TB program monitoring (1), especially in areas with intermediate or high TB prevalence. In Korea, TB is a major health problem, with a mortality rate of 3.8/100,000 persons reported in 2014 (1). Data on TB deaths provide us with a better understanding of the cause of death and help guide interventions aimed at reducing mortality. The majority of identified studies have addressed TB deaths occurring at any time during anti-tuberculosis treatment (ATT) (2). Few studies have focused on early deaths, defined as death occurring during the initial 2-month intensive phase of ATT. The cause of death in TB patients may differ according to the phase of ATT. However, there are limited data regarding this issue in Korea. This study compared the clinical characteristics, results of drug susceptibility testing (DST), and cause of death between patients with TB who died during the intensive and continuation phases of ATT.
Patients who were diagnosed with Mycobacterium tuberculosis culture-positive TB at Kyungpook National University Hospital in Korea between January 2013 and December 2015 and who died during ATT were included in the study. Those who had human immunodeficiency virus infection were excluded. The World Health Organization's definition of TB death (3) was used in this study, defined as patients with TB who died from any cause during treatment. These patients were divided into 2 groups according to whether death occurred within the initial 2-month intensive phase or during the continuation phase of ATT, the early and late death groups, respectively. The clinical characteristics, DST results, and cause of death were retrospectively reviewed and compared between the early and late death groups. Comorbid conditions were defined as previously described (4). DST was routinely performed even in cases with culture-positive results identified after early death. The specific causes of death were identified, and some of them were defined as follows: TB-related respiratory failure as a condition requiring mechanical ventilator support due to TB progression; TB-related septic shock as hypotension persisting despite adequate fluid resuscitation, which was induced by TB without other etiology of infection; TB-unrelated septic shock as that due to other new infection in the absence of TB progression.
A standard regimen consisting of isoniazid, rifampicin, ethambutol, and pyrazinamide was administered during the intensive phase, as recommended by the National Tuberculosis Program (5), unless drug modification was necessary due to drug adverse reactions or drug resistance detected by rapid molecular diagnostic methods. The subsequent continuation regimen was modified according to the DST results when they become available; ethambutol and pyrazinamide were discontinued in patients confirmed to have no resistance to any first-line drug. Outpatient therapy was self-administered with the support of trained nurses in a Public-Private Mix project in both groups (6).
During the study period, 760 patients were diagnosed with culture-positive TB and 74 (10%) died during ATT. Thirty-six (5%) patients died during the intensive phase of ATT; their median time from ATT initiation to death was 21 (interquartile range [IQR] 7–38) days. Another 38 (5%) patients died during the continuation phase of ATT, before completion of treatment; their median time from ATT initiation to death was 131 (IQR 99–226) days. The clinical characteristics, DST results, and cause of death between the 2 groups are given in Table 1. The median age in the early and late groups was 78 and 76 years, respectively, and approximately 80% of patients in both groups were > 70 years old. Men accounted for 26 (72%) and 25 (66%) cases in the early and late groups, respectively. The frequency of a body mass index < 18.5 kg/m2 and history of previous TB was not significantly different between the 2 groups. In both groups, approximately 70% of patients had a chronic illness and 80% were anemic. There were more patients with malignancy in the late group (P = 0.005). More than 80% of cases in both groups had pulmonary TB alone. The frequency of severe pulmonary TB according to the classification of National Tuberculosis Association (7) was higher in the early death group with a marginal trend (P = 0.065). The proportion of patients with smear positive sputum was similar in both groups. The time from presentation to hospital to initiation of ATT was significantly shorter in the early group than in the late group (P < 0.001).
Table 1. Clinical characteristics, causes of death, and DST results of patients with early and late death during ATT.
| Variables | Early death (n = 36) | Late death (n = 38) | P value |
|---|---|---|---|
| Age, yr | 78 (71–85) | 76 (71–83) | 0.327 |
| Age > 70 yr, No. (%) | 28 (78) | 30 (79) | 0.903 |
| Male, No. (%) | 26 (72) | 25 (66) | 0.550 |
| Low BMI (< 18.5 kg/m2), No. (%) | 12 (33) | 9 (24) | 0.357 |
| Prior TB history, No. (%) | 3 (8) | 5 (13) | 0.712 |
| Comorbidities, No. (%) | |||
| None | 12 (33) | 10 (26) | 0.509 |
| Diabetes mellitus | 10 (28) | 8 (21) | 0.500 |
| Chronic heart disease | 5 (14) | 6 (16) | 0.818 |
| Chronic lung disease | 5 (14) | 1 (3) | 0.103 |
| Chronic liver disease | 5 (11) | 1 (3) | 0.103 |
| Chronic kidney disease | 2 (6) | 5 (13) | 0.431 |
| Neurologic disease | 3 (8) | 1 (3) | 0.351 |
| Malignancy | 4 (11) | 15 (40) | 0.005 |
| Anemia (male < 13 g/dL, female < 12 g/dL), No. (%) | 28 (78) | 29 (76) | 0.881 |
| Site of TB, No. (%) | 0.602 | ||
| Pulmonary alone | 29 (81) | 31 (82) | |
| Pulmonary + extrapulmonary | 6 (17) | 4 (11) | |
| Extrapulmonary alone | 1 (3) | 3 (8) | |
| Severe degree of pul-TB on chest X-ray, No. (%) | 19 (53) | 12 (32) | 0.065 |
| Smear-positive sputum, No. (%) | 19/34 (56) | 20/32 (63) | 0.585 |
| Time from presentation to hospital to ATT, day | 1 (1–3) | 5 (1–14) | < 0.001 |
| Time from ATT to death, day | 21 (7–38) | 131 (99–226) | < 0.001 |
| DST to first-line drugs, No. (%) | 0.707 | ||
| All sensitive | 30 (83) | 32/37 (86) | - |
| Any resistance | 6 (17) | 5/37 (14) | 1.000 |
| Isoniazid resistance | 4 (11) | 5/37 (14) | 0.240 |
| Multi-drugs resistance | 2 (6) | 0/37 (0) | - |
| Cause of death | < 0.001 | ||
| TB-related | 20 (56) | 4 (11) | |
| TB-unrelated | 16 (44) | 34 (89) |
BMI = body mass index, TB = tuberculosis, pul-TB = pulmonary tuberculosis, ATT = anti-tuberculosis treatment, DST = drug susceptibility test, IQR = interquartile range.
Data are presented as median (IQR) or number (%). Differences were analyzed by Mann-Whitney test and χ2 or Fisher's exact tests.
DST results were available for all but one case in the late group. In the early group, 30 (83%) patients had M. tuberculosis sensitive to all anti-TB drugs tested, 6 (17%) showed resistance to at least one first-line anti-TB drug; 4 (11%) had resistance to isoniazid, and 2 (6%) had multidrug resistant M. tuberculosis, defined as resistance to at least isoniazid and rifampicin. One patient with multidrug resistance received a second-line anti-TB drugs regimen based on GeneXpert MTB/RIF test (Cepheid, Sunnyvale, CA, USA) results that identified rifampicin resistance. The other died of respiratory failure on the day of admission. The overall DST results in the late group were comparable to those of the early group.
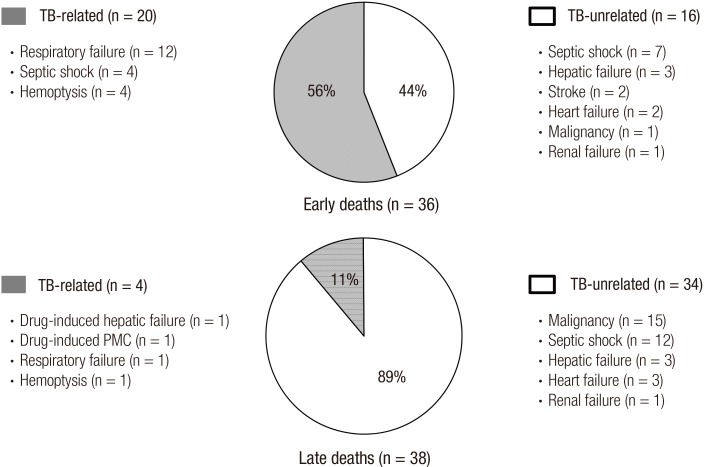
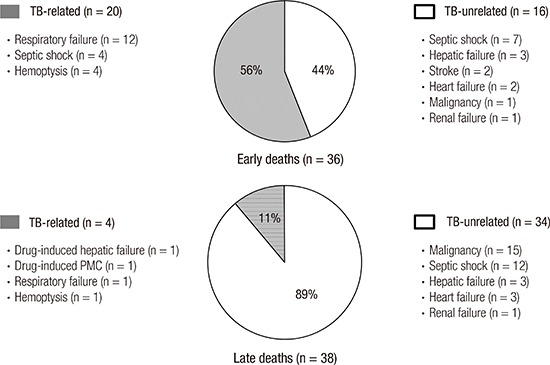
In terms of the cause of death, there were 20 (56%) TB-related and 16 (44%) TB-unrelated deaths in the early group. In contrast, most patients in the late group died due to TB-unrelated causes (n = 34, 89%); thus, the cause of death between the 2 groups differed significantly (P < 0.001). In the early group, respiratory failure (n = 12) was the most common cause of TB-related death, followed by TB-related septic shock (n = 4), and massive hemoptysis (n = 4) (Fig. 1). Regarding TB-unrelated deaths of the early group, TB-unrelated septic shock (n = 7) was the most common cause, followed by hepatic failure (n = 3), and stroke (n = 2). In the late group, underlying malignancy (n = 15) was the most common cause of TB-unrelated death, followed by TB-unrelated septic shock (n = 12). As the cause of TB-related deaths of the late group, there were anti-TB drug-induced hepatic failure (n = 1), anti-TB drug-induced pseudomembranous colitis (n = 1), respiratory failure (n = 1), and hemoptysis (n = 1).
Fig. 1.
Specific causes of TB-related and TB-unrelated deaths in patients with early and late deaths during anti-TB treatment.
TB = tuberculosis, PMC = pseudomembranous colitis.
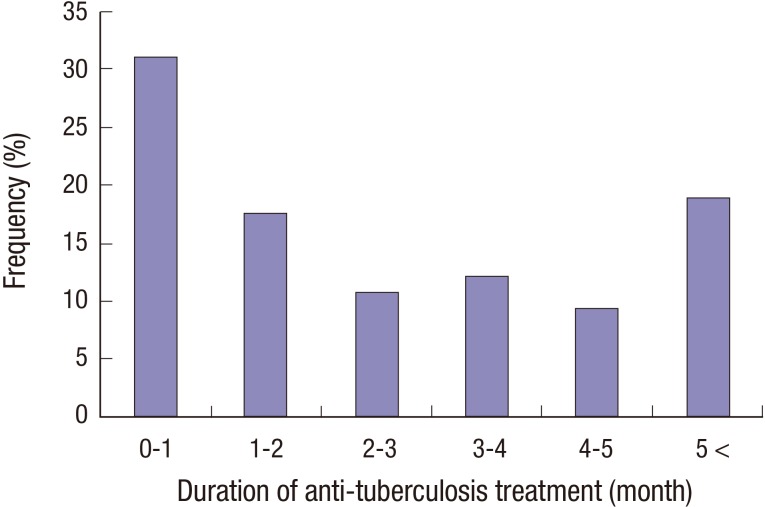
Fig. 2 shows the monthly distribution of the 74 TB deaths that occurred in 760 TB patients. The TB mortality was the highest within the first month after ATT. In addition, 15 (75%) of 20 patients with early TB-related death died within the first month.
Fig. 2.
The monthly distribution of 74 deaths that occurred in 760 patients with tuberculosis.
The present study shows that TB deaths occurred in patients with advanced age and comorbid illness in both groups. The clinical characteristics and DST results were not significantly different between the early and late death groups. However, more than half of the early deaths were due to TB-specific causes, whereas the majority of late deaths were due to TB-unrelated causes.
Advanced age and underlying comorbid illnesses are well known risk factors for TB deaths (2); these are unavoidable factors. However, despite available effective therapy, many TB-related deaths occurred during the intensive phase, particularly within the first month. Our results are consistent with previous studies performed in African countries (8,9). These early deaths imply delayed diagnosis and treatment of TB. In fact, delayed treatment has been linked to higher mortality rates in patients with TB (9,10,11,12,13). TB diagnosis after presentation to hospital was not delayed in our patients with early TB death. This suggests that, in the early death group, elderly patients likely delayed seeking medical care and were thus sicker at presentation. Although the burden of TB has improved over the last decade in Korea (1), the National Tuberculosis Program should address this problem to further reduce the TB mortality rate.
While rapid diagnosis and treatment of patients with infectious TB remain the cornerstones of TB control, targeted screening and treatment of high-risk subjects with latent TB infection (LTBI) is becoming increasingly important (14). As part of an ongoing effort, DST results in index cases are crucial for successful treatment of high-risk close contacts with LTBI (5), especially in areas with a high prevalence of drug resistance. The DST results in patients with early TB death have not been fully investigated in Korea (4,15,16); studies have focused mainly on risk factors for TB mortality. In the present study, the rate of isoniazid resistance was high; all 6 patients with early death and any drug resistance had resistance to isoniazid, which is the most commonly used drug for the treatment of subjects with LTBI. Furthermore, the GeneXpert test does not detect isoniazid resistance. Currently, while the national government supports all the costs of TB management, DST should be considered, even in TB cases with positive culture identified after early death.
This study was limited by the followings: its retrospective design, small sample size, and that it was performed in a single center. Thus, prospective studies with larger sample sizes are warranted.
In conclusion, our study provides the difference of cause of death between early and late TB deaths. These results suggest that TB-related early deaths mainly attributable to delayed diagnosis should be improved to further reduce the overall TB mortality rate.
Ethics statement
Permission was obtained from the Institutional Review Board (IRB) of Kyungpook National University Hospital to review and publish patient records retrospectively (IRB No. 2016-08-029). The need for informed consent was waived due to the retrospective nature of the study.
Footnotes
Funding: This work was supported by a grant of National Research Foundation of Korea, funded by the Korea government (2015R1C1A2A01052502).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Lee J, Kim CH. Data curation: Lee J, Nam HW, Choi SH, Yoo SS, Lee SY, Cha SI, Kim CH, Park JY. Investigation: Lee J, Kim CH. Writing - original draft: Lee J, Kim CH. Writing - review & editing: Lee J, Nam HW, Choi SH, Yoo SS, Lee SY, Cha SI, Park JY, Kim CH.
References
- 1.World Health Organization. Global tuberculosis report 2015 [Internet] [accessed on 9 February 2017]. Available at http://www.who.int/tb/data.
- 2.Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis. 2011;15:871–885. doi: 10.5588/ijtld.10.0352. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Global Tuberculosis Programme: Framework for Effective Tuberculosis Control. Geneva: World Health Organization; 1994. [Google Scholar]
- 4.Kwon YS, Kim YH, Song JU, Jeon K, Song J, Ryu YJ, Choi JC, Kim HC, Koh WJ. Risk factors for death during pulmonary tuberculosis treatment in Korea: a multicenter retrospective cohort study. J Korean Med Sci. 2014;29:1226–1231. doi: 10.3346/jkms.2014.29.9.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joint Committee for the Development of Koran Guidelines for Tuberculosis; Korean Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed [Internet] [accessed on 9 February 2017]. Available at http://www.lungkorea.org/thesis/file/korean_guidelines_for_tuberculosis_2014.pdf.
- 6.Kim HJ, Bai GH, Kang MK, Kim SJ, Lee JK, Cho SI, Lew WJ. A public-private collaboration model for treatment intervention to improve outcomes in patients with tuberculosis in the private sector. Tuberc Respir Dis (Seoul) 2009;66:349–357. [Google Scholar]
- 7.National Tuberculosis Association (US) Diagnostic Standards and Classification of Tuberculosis. 11th ed. New York, NY: National Tuberculosis Association; 1961. [Google Scholar]
- 8.Garin B, Glaziou P, Kassa-Kelembho E, Yassibanda S, Mbelesso P, Morvan J. High mortality rates among patients with tuberculosis in Bangui, Central African Republic. Lancet. 1997;350:1298. doi: 10.1016/S0140-6736(05)62475-0. [DOI] [PubMed] [Google Scholar]
- 9.Harries AD, Hargreaves NJ, Gausi F, Kwanjana JH, Salaniponi FM. High early death rate in tuberculosis patients in Malawi. Int J Tuberc Lung Dis. 2001;5:1000–1005. [PubMed] [Google Scholar]
- 10.Hansel NN, Merriman B, Haponik EF, Diette GB. Hospitalizations for tuberculosis in the United States in 2000: predictors of in-hospital mortality. Chest. 2004;126:1079–1086. doi: 10.1378/chest.126.4.1079. [DOI] [PubMed] [Google Scholar]
- 11.Asch S, Leake B, Anderson R, Gelberg L. Why do symptomatic patients delay obtaining care for tuberculosis? Am J Respir Crit Care Med. 1998;157:1244–1248. doi: 10.1164/ajrccm.157.4.9709071. [DOI] [PubMed] [Google Scholar]
- 12.Greenaway C, Menzies D, Fanning A, Grewal R, Yuan L, FitzGerald JM, Canadian Collaborative Group in Nosocomial Transmission of Tuberculosis Delay in diagnosis among hospitalized patients with active tuberculosis--predictors and outcomes. Am J Respir Crit Care Med. 2002;165:927–933. doi: 10.1164/ajrccm.165.7.2107040. [DOI] [PubMed] [Google Scholar]
- 13.Pablos-Méndez A, Sterling TR, Frieden TR. The relationship between delayed or incomplete treatment and all-cause mortality in patients with tuberculosis. JAMA. 1996;276:1223–1228. doi: 10.1001/jama.1996.03540150025026. [DOI] [PubMed] [Google Scholar]
- 14.Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161:S221–47. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 15.Kim CW, Kim SH, Lee SN, Lee SJ, Lee MK, Lee JH, Shinet KC, Yong SJ, Lee WY. Risk factors related with mortality in patient with pulmonary tuberculosis. Tuberc Respir Dis (Seoul) 2012;73:38–47. doi: 10.4046/trd.2012.73.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HJ, Lee CH, Shin S, Lee JH, Kim YW, Chung HS, Han SK, Shim YS, Kim DK. The impact of nutritional deficit on mortality of in-patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2010;14:79–85. [PubMed] [Google Scholar]