Abstract
Age-related macular degeneration (AMD) is a major cause of central vision loss in persons over 55 years of age in developed countries. AMD is a complex disease in which genetic, environmental and inflammatory factors influence its onset and progression. Elevation in serum anti-retinal autoantibodies, plasma and local activation of complement proteins of the alternative pathway, and increase in secretion of proinflammatory cytokines have been seen over the course of disease. Genetic studies of AMD patients confirmed that genetic variants affecting the alternative complement pathway have a major influence on AMD risk. Because the heterogeneity of this disease there is no sufficient strategy to identify the disease onset and progression sole based eye examination, thus identification of reliable serological biomarkers for diagnosis, prognosis and response to treatment by sampling patient's blood. This review provides an outline of the current knowledge on possible serological (autoantibodies, complement factors, cytokines, chemokines) and related genetic biomarkers relevant to the pathology of AMD, and discusses their application for prediction of disease activity and prognosis in AMD.
Keywords: autoimmunity, age-related macular degeneration, autoantibodies, complement factors, Factor H, cytokines, IL-17, chemokines, retina, aging
1. Incidence and Etiology
In modern medicine biomarkers are important tools helping in diagnosis, drug discovery, and clinical care of any disease, in particular, complex degenerative diseases such as age-related macular degeneration (AMD) [1]. AMD is a major cause of legal blindness in persons over 55 years of age in developed countries [2]. It is estimated that 6 to 10 million Americans are blind from AMD and new cases are diagnosed in the U.S. each year [3]. It has been reported that annual incidence of advanced stage AMD (neovascular AMD and geographic atrophy) in Caucasian Americans was 293, 000 new cases per year (3.5 per 1000 persons) and was 38% higher in women compared to men [4].
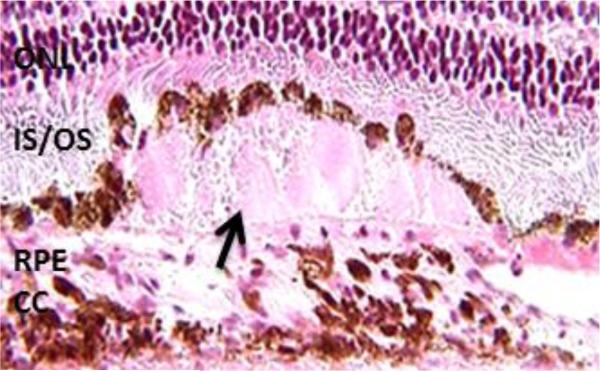
Macular degeneration is characterized by the disruption of the macula, the central part of retina responsible for high acuity vision [5]. AMD is a complex disease in which genetic as well as environmental risk factors, such as cigarette smoking, diet, and lifetime light exposure influence its progression [6]. The thickening of the Bruch's membrane and accumulation of debris (drusen) on the retinal pigment epithelium/Bruch's membrane are the ocular signs of AMD (Figure 1) [7-9]. It starts with small numbers of drusen and then larger numbers, in effect leading to end-stage AMD and loss of central vision [10, 11]. Although the initial drusen deposits are not associated with macular blindness, those individuals with drusen are considered at risk for developing more advanced forms of AMD and loss of vision [12]. End-stage AMD occurs in one of two forms: geographic atrophy or choroidal neovascularization. Approximately 90% of patients with AMD have the non-neovascular “dry” form characterized by atrophy of the retinal pigment epithelium (RPE), and loss of photoreceptor cells in the macula [2, 12].
Fig. 1.
Retina with subretinal debris (drusen) present beneath multiple layers of retinal pigment epithelium (RPE) cells (arrow). ONL- outer nuclear layer, IS/OS – inner segments/outer segments of photoreceptor cells; CC – choriocapillaris
Choroidal neovascularization, the “wet form” of AMD is characterized by the development of new blood vessels, originating in the choroid, that break through Bruch's membrane and the RPE and invade the subretinal space or sub-RPE space [12]. These new blood vessels leak blood into the retina, causing distortion of vision and in consequence loss of central vision. Also, these blood vessels can hemorrhage in the compartment between the foveal photoreceptors and RPE, leading to immediate blindness [13]. The neovascular form affects about 10% persons with the disease [12]. AMD stages were recently defined by the Age-Related Eye Disease Study (AREDS) classification scheme, which was based on results, obtained from examining retinal and fundus color photographs and is shown in Table 1 [11].
Table 1.
AMD Clinical Classification
| Classification of AMD | Definition1 |
|---|---|
| No apparent aging changes | No drusen and No AMD pigmentary abnormalities |
| Normal aging changes | Only drupelets (small drusen ≤63μm) and no AMD pigmentary abnormalities* |
| Early AMD | Medium drusen >63 μm and ≤125μm and no AMD pigmentary abnormalities* |
| Intermediate AMD | Large drusen > 125 μm and/or Any AMD pigmentary A abnormalities* |
| Late AMD | Neovascular AMD and/or Any geographic atrophy |
AMD - age-related macular degeneration
lesions assessed within 2 disc diameters of fovea in either eye
AMD pigmentary abnormalities = any definite hyper- or hypopigmentary abnormalities associated with medium or large drusen but not associated with known disease entities.
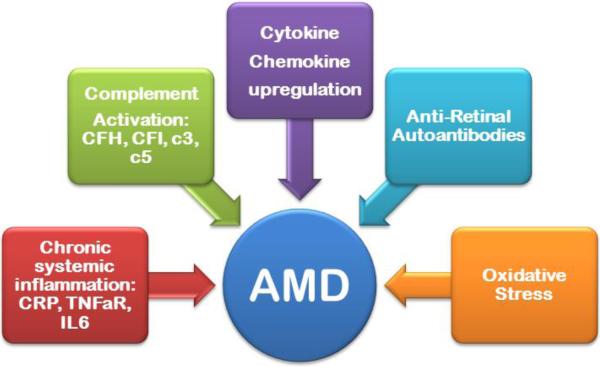
Pathology of AMD involves the disruption of many physiological pathways, but chronic inflammation is thought to play a major role in AMD progression (Figure 2) [14]. Low-grade inflammation is mediated by many factors and stimulated by a complement system of an alternative pathway, which starts within Bruch's membrane, and then leads to early and advanced, exudative AMD forms [15-17]. It is important to point out that the low-grade inflammatory process exists also in the aging retina under physiologic conditions, however, persistent inflammation may trigger early and then advanced forms of the disease [18, 19]. Yet, most individuals do not progress to end stage AMD. Even if inflammatory activities are a cause of early AMD, it is not known whether therapeutic interventions that reduce systemic inflammation will reduce the incidence of early AMD [20]. As a result, there is no adequate strategy to identify the disease stage based only on the risk factors.
Fig. 2.
A diagram showing potential factors influencing early AMD.
In recent years, biomarkers have become major indicators of personalized medicine, in particular, molecular biomarkers that provide minimally invasive objective procedures that help in diagnosis, prognosis and response to treatment [21, 22]. The aim of this review is to provide an overview of the current knowledge on possible serological and genetic biomarkers in relation to different stages of AMD, and discuss their application for prediction of disease activity and prognosis in AMD.
2. AMD and serological biomarkers
Serological biomarkers such as autoantibodies (AAbs), complement proteins and cytokines/chemokines can be measured in the blood, using minimally invasive methods. In the study of AMD, there are reports of the relationship between serum C-reactive protein (CRP), complement proteins, tumor necrosis factor-α receptor 2, interleukin-6, and soluble vascular cell adhesion molecule-1 independent of age, smoking status, and other factors [23]. Also, serum anti-retinal AAbs were detected at a much higher incidence in individuals with early AMD than in persons without AMD, suggesting their diagnostic value [24-26]. Below, we discuss the association of inflammatory mediators, autoantibodies, and complement proteins in early and advance AMD. Investigating markers associated with disease, one should establish whether a given biomarker shows an increased or decreased presence in disease as compared to healthy individuals.
2.1. Inflammatory mediators in AMD: Cytokines and chemokines
Inflammation has been proposed as a central mechanism in the pathogenesis of AMD [7, 27]. The presence of hematopoietic cells in the macular choroid and the analysis of drusen from human retinal lesions clearly proved the presence of inflammatory mediators and innate immune cells, such as macrophages and microglia that strongly supported their role in disease [20, 28]. At all stages of AMD, macrophages are one of the main inflammatory cell types that are correlated with choroidal neovascularization (CNV), drusen, geographic atrophy, and ruptures of the Bruch's membrane [29, 30]. Moreover, T cells and M1 macrophages can be activated by oxidative damage in AMD pathogenesis [31]. The RPE cells are also an important source of cytokines in the posterior segment of the eye [32, 33]. When activated, they can produce several cytokines, chemokines (MCP-1), and vascular endothelial growth factor (VEGF) [34].
Patients with AMD have increased plasma levels of inflammatory cytokines such as IL-1α, IL-1β, IL-4, IL-5, IL-13, and IL-17 [35]. The strongest links with disease are related to the pro-inflammatory cytokines IL-1, IL-6, and TNF-α, which are released from the choroid of patients with AMD and can immune-mediated retinal damage [17].
Interleukin 17 (IL-17) a proinflammatory cytokine secreted by the T helper 17 (Th17) lymphocytes, seems to play a particularly important role in all forms of AMD [36, 37]. Also, IL-17C and its receptor IL-17RC have been found in diseased eyes and blood, suggesting their pathogenic participation. [38] [17, 36, 39]. IL-17A can induce the destruction of photoreceptor cells and the RPE layer, which is evident in the dry form of AMD [36]. IL-17 can stimulate retinal angiogenesis and choroidal neovascularization (CNV) during the exudative form of AMD either directly, by enhancing the growth of endothelial cells in the presence of angiogenic factors, and/or indirectly, by inducing the production of VEGF by other cells types [36, 40]. In addition, complement C5a can induce a cytokine expression, including IL-22 and IL-17 by human CD4+ T lymphocytes, which were found to be significantly elevated in AMD patients compared to patients without AMD (p<0.0001 and p=0.0005, respectively) [41]. Based on these findings, potential therapeutic strategy for AMD could focus on targeting IL-17, IL-17RC, and cells producing IL-17 to stop retinal degeneration [36]. Caution should be taken of adverse effects of individual therapeutic agents because genetic background and clinical manifestation of each patient disease may influence the benefits of such a therapy. Nevertheless, IL-17 plays an important pro-inflammatory role and its elevated serum presence could be considered as a biomarker of advanced AMD along with other factors.
In addition to cytokines, recent clinical studies showed AMD association with increased intraocular levels of certain chemokines, including CCL2 (MCP-1) [42, 43]. Chemokines are chemotactic cytokines that drive the migration of leukocytes throughout the body under physiological and pathological conditions [44]. In AMD, chemokines such as CCL2 and CX3CL1 (C-X3-C Motif Chemokine Ligand 1) that control recruitment and migration of specific monocytes, participate in subretinal accumulation of microglia and macrophage, and in the development of retinal degeneration as well as choroidal neovascularization [29, 45]. Also, the dysfunction of chemokine CX3CL1 and its receptor CX3CR1 leads to the influx of microglia and macrophages into subretinal space with damaging effects on the RPE and photoreceptors [45]. Elevated levels of inflammation-related chemokines, including CXCL10, CCL14, CXCL16, CXCL7, and CCL22, in the aqueous humor of AMD patients may further suggest their pathogenic role for inflammation [46]. The mice deficient in CCL2 or CCR2 and later double-knockout mice (Ccl2−/−/Cx3cr1−/−) have developed AMD-like retinal lesions and they have been used to study the role chemokines in pathogenicity of AMD [47-49]. Taken together, CCL2 and CX3CL1 appear to be essential in the accumulation of subretinal microglia and macrophage, participation in pathophysiology of retinal degeneration, and choroidal neovascularization [45]. Analyzing their specific role in the early stages of AMD and their contribution to late stage AMD might help in the development of more specific therapeutic approaches. It is also important to note that most of these markers are universal for inflammation instead of being specific to pathophysiology of AMD [50]. Nevertheless, both CCL2 and CX3CL1 could be considered as important biomarkers for AMD.
2.2. Serum Autoantibodies in AMD
Adaptive immunity may also be involved in AMD pathogenesis because serum autoantibodies (AAbs) that bind retinal proteins on the western blot and on retinal tissue section have been detected in many AMD patients in much higher frequencies than age matched controls [17, 25, 51-53]. Although we do not know when such autoantibodies were generated to be detected in the blood, it is likely they were made years before the destruction of retinal cells that led to manifestation of visual symptoms [54]. It has been shown that AAbs can develop on average 3–15 years prior to the first clinical signs [55]. Many AAbs have been associated with different stages of AMD, and those AAbs can bind to nuclei, nucleoli, and nuclear membranes in the outer and inner nuclear layers of the retina [53]. However, the role of AAbs in the induction or acceleration of retinal deterioration is not well defined.
In recent years, the growing number of serum AAbs with various specificities to retinal proteins and in much higher than the age-matched controls, has been reported in association with AMD, but which autoantibody could be used a marker of disease development was not clear. The identified antigens were the components of drusen, RPE or Bruch's membrane. For example, AAbs to elastin, heparan sulfate, fibronectin, histone H2B, collagen III, and collagen IV were significantly elevated in sera of AMD patients compared to normal controls [56]. Another study identified more autoantigens, including αβ-crystallin, α-actinin, amyloid, C1q protein, chondroitin, collagen I, III, and IV, elastin, histone H2A and H2B, laminin, vimentin, vitronectin, aldolase C, and pyruvate kinase M2 [16]. AAbs against cyclic nucleotide phosphodiesterase, phosphatidylserine (PS), and proliferating cell nuclear antigen [56], glial fibrillary acidic protein (GFAP), and α-enolase were also documented. [26, 53, 57] Anti-PS IgGs were significantly increased in patients who had advanced AMD with choroidal neovascularization, suggesting that such AAbs might contribute to the angiogenesis associated with choroidal neovascularization [56]. They showed in an in vitro study that purified AAbs induced more tube formation on choroidal–retinal endothelial cells compared to AAbs obtained from healthy donors [56].
Autoantibodies related to both neovascular and geographic atrophy AMD were found to be against retinol-binding protein 3 (RBP3, 120-kDa) [58], retinol-binding protein 1 (RLBP1, 36-kDa) [58], aldolase C (39-kDa) [58], and glial fibrillary acidic protein (GFAP, 52-kDa) [57]. Anti-GFAP AAbs occurred in 44% of the NV AMD patients’ population studied. Anti-aldolase C (40-kDa) and RAB3 were targeted in wet AMD whereas pyruvate kinase M2 (60-62-kDa) was targeted in both wet and dry AMD [58]. In recent study another five candidate antigens were found in sera collected from participants in the Age-Related Maculopathy Ancillary (ARMA) Study relation to the early stages of AMD that included heat shock protein members such as HSPA8, HSPA9, HSPB4 (also known as α crystalline), annexin A5, and S100 calcium-binding protein A9 (S100-A9, calgranulin B) [59]. These antigenic proteins could be implicated in autophagy, immunomodulation, and protection from oxidative stress as well as apoptosis, so they could be linked to AMD pathogenesis. However, AAbs specific for αB-crystalline, which is the basic structural component of multiple HSPs, are not only specific to AMD but they are also found in sera of patients with uveitis [60], glaucoma [61], and multiple sclerosis [62]. Overall, the presence of serum AAbs confirms their autoimmune involvement in disease and the possibility that autoimmunity disrupts the maintenance of self-tolerance within the retina of AMD patients leading to disease [17].
One of the antigens that could be potentially used as a biomarker of AMD is α-enolase that belongs to a family of glycolytic enzymes, but also has other cellular functions unrelated its activity in glycolysis [63]. Our study using sera from well-defined participants in the Age-related Eye Disease Study (AREDS) showed that AAbs against α-enolase were detected in 40% patients with large drusen and 46% with geographic atrophy (GA), but only 29% of neovascular (NV) patients and controls [26]. Joachim et al found much larger frequency of anti-enolase AAbs (67%) in the NV AMD patients [57]. In spite of this, a differential expression of α-enolase in tissues and presence of anti-enolase AAbs have also been associated with several other pathologies, such as cancer, Alzheimer's disease, autoimmune diseases, and rheumatoid arthritis, among others [63]. Important to note, that AAbs against α-enolase have been strongly associated with retinal degeneration, including cancer-associated retinopathy (CAR) and autoimmune retinopathy [64, 65]. In vitro and in vivo investigations showed that anti-enolase AAbs from patients with CAR were able to induce apoptotic cell death of retinal cells, and provided a potential mechanism for antibody-mediated retinal degeneration in humans [66]. Thus, chronic access of AAbs to the retina results in the inhibition of enolase catalytic function, depletion of ATP, and elevation in intracellular Ca2+ leading to deregulation of glycolysis in retinal cells and their destruction [67].
In addition, AAbs against carboxyethylpyrrole (CEP), a lipid peroxidation product that accumulates in the retinas of dry AMD patients and is an oxidized component of drusen were found [31, 52]. CEP is present in the photoreceptor rod outer segments and RPE in mouse and human retinas [68, 69]. The study showed that the mean level of anti-CEP AAbs in AMD human plasma was significantly higher than in age-matched controls, and had higher immunoreactivity with retinal tissues (92%) than non-AMD controls [52]. These results suggest that both, CEP immunoreactivities and autoantibody titers may have diagnostic value in predicting AMD susceptibility. However, such protein modifications are not strictly unique to the retina and found in other tissue [70]. It can be speculated that the antigen does not need be derived from the retinal tissue to mount an immune response; anti-CEP response can be initiated by similar proteins but induce an antibody-mediated immune damage in the retina.
Anti-enolase AAbs alone may not be a sufficient disease marker. Based on the current data from different laboratories not one antibody but autoantibody panels (“autoantibody signatures”) might show a better correlation with intermediate and late stages of AMD with higher sensitivity and specificity. In fact, anti-40-kDa and 42-kDa AAbs were associated with intermediate AMD, while anti-30-kDa AAbs were primarily present in GA AMD. Anti-32-kDa, 35-kDa, and 60-kDa AAbs were more frequent in NV AMD [26]. Causal association of anti-retinal AAbs with AMD pathogenesis or progression will require a larger group of subjects at early and late stages of disease in individual persons and in groups. This might reveal distinct AAb signatures for early AMD, geographic GA and neovascular AMD [26]. The application of AAbs as predictors of pre-AMD disease in not feasible at this time, as the access to preclinical of sera is difficult. Serum samples collected from the same person, before and after AMD development as well as all stages in between, are not generally available, making it almost impossible to determine the exact moment when the AAbs appear and have an impact on disease progression.
2.3. Complement Proteins in AMD
AMD is a disease that manifests in at the aging macula. There is an increased complement protein deposition in the Bruch's membrane and in drusen [7]. Such local manifestation of systemic pathophysiology occurs as effect of low grade chronic inflammation, which damages the blood-retina-barrier, resulting in the breach of retinal-immune privilege and the development of retinal lesions [71]. The RPE and the choroidal vasculature are a source of the complement in drusen, which are composed of immunoglobulin (IgG), components of the complement cascade, and complement regulatory proteins (such as the membrane co-factor protein) [15, 72]. In fact, many potential activators of the complement cascade were found in drusen below the basal surface of the RPE, including IgG, nuclear fragments, phospholipids, cholesterol, and micro-fibrillary [3, 73, 74].
There is convincing evidence that complement is involved in the AMD disease process, including the complement-related proteins C3a, C5a, C5, C5b-9, complement factor H (CFH), CD35, and CD46 [15, 16, 75] [73]. The alternative pathway and has been implicated in AMD because of alternations in regulatory proteins and deficiencies in complement proteins [73,76, 77]. Complement disorders are autosomal recessive and genetic defects such as single nucleotide polymorphism (SNP) can result in formation of defective protein or gene deletion. Mutations in CFH, CFB, and C3 genes cause defects in the encoded proteins [78, 79]. CFH regulates the activation of C3b in the alternative complement pathway, both in serum and at host cell surfaces. In geographic and neovascular AMD, the Y402H gene polymorphism removes the protective function of CFH, leading to chronic inflammation mediated by C-reactive protein (CRP) [16]. CRP, an acute-phase protein and active regulator of the innate immune system, has been identified in drusen and other subretinal pigmented epithelium deposits [8]. Molins et al showed evidence that a monomeric form of CRP (mCRP) upregulates proinflammatory cytokines IL-8 and CCL2 levels in RPE cells and CFH binds mCRP to diminish its proinflammatory activity [76]. The increased levels of CRP in eyes with early AMD may be an indication of local inflammation and cellular injury in the RPE–choroid layer [75]. Elevated levels of C5a have been found in the serum of AMD patients. [41]. Also, deposition of C5b-9 in the retina is important because C5a can bind to its receptor, C5aR promoting an additional activation. Both complement components C3a and C5a induced VEGF in RPE cells as shown in experiments of laser-induced CNV, an accelerated model of neovascular AMD driven by VEGF and recruitment of leukocytes into the choroid [80].
Complement activation can be measured in plasma of affected patients by assessing their plasma levels and biosynthetic rates of the corresponding precursor proteins such as C3 and CFB, CFD and CFH [81]. Irrespective of known genotype, C3a, Bb, and C5a were strongly associated with an increased risk of advanced AMD [81]. C5a and SC5b-9 were markers of terminal pathway of complement activation, which were generated downstream of C3 and CFB [82]. Complement substrate and activator levels (CFB and CFD) and markers of complement activation (Ba, C3d) were found to be increased in the advanced subtypes of AMD, suggesting that systemic complement activation could be associated with progression of AMD [83]. Important to note that the RPE is protected from the complement attack by complement membrane regulatory proteins such as CD46 (membrane cofactor protein, MCP), CD55 (decay accelerating factor, DAF) and CD59 (protectin) [84]. CD46 is a regulator of the alternative pathway and CD59 inhibits the formation of the membrane attack complex [85]. Decreased expression of these proteins leads to impair complement regulation on RPE cells and in effect to the formation of lesions in the outer retina and Bruch's membrane, thus contributes to the pathogenesis of AMD [86, 87].
In addition, the complement system can be a target for developing new therapies, preventing progression from an early to the late form of AMD and treating the late stages of AMD. In fact, several treatments acting on the complement pathway are currently in clinical trials [77, 88, 89]. Targeting the complement system, however, needs to balance its inhibition with protection of the immune responses and tissue homeostasis.
In addition to measurements of complement factors in plasma, genetic studies of AMD patients further confirmed the relationship between disease and the complement system by detecting multiple genetic related polymorphisms. In 2005, several groups concurrently reported on an association of a non-synonymous variant in the CFH gene with AMD risk, which showed that possession of the variant Y402H polymorphism significantly increases the risk for AMD [78, 90], [91, 92]. Individuals caring the CFH Y402H polymorphism progressed to a higher stage of AMD and about 6% developed late disease [79]. A large number of independent genetic studies have consistently confirmed the association of AMD with the risk or protective variants in genes encoding for complement proteins, including CFH, CFH-related proteins 1 and 3, factor B/C2, C3 and factor I [88, 93, 94]. CFB and C2 have been implicated as major risk or protective factors for the development of AMD [95].
4. Biomarkers for AMD and Aging
AMD affects mostly elderly people and aging is the major contributing factor to the disease. Thus, it is important to recognize how the body controls the regular age-related changes and age-related pathology in AMD. The combination of environmental and genetic risk factors target retinal homeostasis, which may lead to age-related pathology with advanced age [18]. The genes linked to immune responses and to tissue stress/injury responses are the most likely to be modified by aging [96]. Furthermore, oxidized lipoproteins and free radicals contribute to tissue stress and low grade inflammation in retina [97]. Retinal damage, activation of local macrophages/microglia, and accumulation of byproducts in subretinal space may induce a local inflammation, causing infiltration of blood macrophages, T lymphocytes, and mast cells, and initiation of the drusen formation [20, 96, 98] The low grade inflammation can persist for decades and increases with advancing age leading to pathologic changes that destabilize ocular homeostasis, particularly microglia and the complement system, and promote AMD [16]. Oxidative stress causes the RPE and, possibly, choriocapillaris injury in aging and AMD [99]. Not only in the AMD retina but also in the aging retina a large number of inflammatory genes, including genes involved in complement activation and inflammatory cytokine/chemokine production was identified [96]. Although findings of plasma proteins in drusen during an inflammatory response and complement activation suggest their causal involvement in the formation of drusen in disease, we still do not know whether inflammation is a causative or a secondary contributory event in AMD or it is just part of aging process [47]. Disease association with serological biomarkers or genetic biomarkers can be useful if we can distinguish the disease-associated biomarkers from the naturally occurring events since not every elderly person will develop AMD. Therefore, the search for biomarkers is important and helps not only with understanding the relationship between aging physiology and age-related pathology, but it is also essential for designing new therapeutic approaches for this devastating degenerative disease.
Take-home messages.
Pathology of AMD involves the disruption of many physiological pathways, but chronic inflammation plays a central role in AMD progression
Complement proteins, autoantibodies, and cytokines/chemokines can be measured in the blood and in diseased tissue
IL17 is the major inflammatory mediator involved in AMD
Anti-retinal autoantibodies persist in many AMD patients in much greater frequencies than age-matched controls
The complement-related proteins C3a, C5a, C5, C5b-9, complement factor H, CD35, and CD46 are involved in pathogenic process of AMD
ACKNOWLEDGMENT
This work was supported by the NIH grant P30 EY010572 and unrestricted grant from Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
The author has no financial conflicts of interest.
REFERENCES
- 1.Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Ret and Eye Res. 2016;50:1–24. doi: 10.1016/j.preteyeres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38:450–71. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, et al. From the Cover: Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. PNAS. 2002;99:14682–7. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudnicka AR, Kapetanakis VV, Jarrar Z, Wathern AK, Wormald R, Fletcher AE, et al. Incidence of Late-Stage Age-Related Macular Degeneration in American Whites: Systematic Review and Meta-analysis. Am J Ophthalmol. 2015;160:85–93. e3. doi: 10.1016/j.ajo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Provis JM, Penfold PL, Cornish EE, Sandercoe TM, Madigan MC. Anatomy and development of the macula: specialisation and the vulnerability to macular degeneration. Clin and Exp Optom. 2005;88:269–81. doi: 10.1111/j.1444-0938.2005.tb06711.x. [DOI] [PubMed] [Google Scholar]
- 6.Yonekawa Y, Kim IK. Clinical Characteristics and Current Treatment of Age-Related Macular Degeneration. Cold Spring Harbor Persp Med. 2015:5. doi: 10.1101/cshperspect.a017178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Liu B, Lukas TJ, Neufeld AH. The Aged Retinal Pigment Epithelium/Choroid: A Potential Substratum for the Pathogenesis of Age-Related Macular Degeneration. PLoS ONE. 2008;3:e2339. doi: 10.1371/journal.pone.0002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seddon JM, Reynolds R, Yu Y, Daly MJ, Rosner B. Risk Models for Progression to Advanced Age-Related Macular Degeneration Using Demographic, Environmental, Genetic, and Ocular Factors. Ophthalmol. 2011;118:2203–11. doi: 10.1016/j.ophtha.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferris III FL, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Clinical Classification of Age-related Macular Degeneration. Ophthalmology. 2013;120:844–51. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Asp Med. 2012;33:295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and Choriocapillaris in Age-Related Macular Degeneration. Inves Ophthalmol Vis Sci. 2009;50:4982–91. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The Role of Inflammation in the Pathogenesis of Age-related Macular Degeneration. Sur Ophthalmol. 2006;51:137–52. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nita M, Grzybowski A, Ascaso FJ, Huerva V. Age-Related Macular Degeneration in the Aspect of Chronic Low-Grade Inflammation (Pathophysiological ParaInflammation). Med Inflammat. 2014;2014:930671. doi: 10.1155/2014/930671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camelo S. Potential Sources and Roles of Adaptive Immunity in Age-Related Macular Degeneration: Shall We Rename AMD into Autoimmune Macular Disease? Autoimmune Dis. 2014;2014:532487. doi: 10.1155/2014/532487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ardeljan D, Chan C-C. Aging is not a disease: Distinguishing age-related macular degeneration from aging. Prog Ret Eye Res. 2013;37:68–89. doi: 10.1016/j.preteyeres.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez VL, Caspi RR. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015;36:354–63. doi: 10.1016/j.it.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buschini E, Piras A, Nuzzi R, Vercelli A. Age related macular degeneration and drusen: Neuroinflammation in the retina. Prog Neurobiol. 2011;95:14–25. doi: 10.1016/j.pneurobio.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Strimbu K, Tavel JA. What are biomarker? Curr Opin HIV & AIDS. 2010;5:463–6. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landeck L, Kneip C, Reischl J, Asadullah K. Biomarkers and personalized medicine: current status and further perspectives with special focus on dermatology. Exp Dermatol. 2016;25:333–9. doi: 10.1111/exd.12948. [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Myers CE, Cruickshanks KJ, Gangnon RE, Danforth LG, Sivakumaran TA, et al. Markers of inflammation, oxidative stress, and endothelial dysfunction and the 20-year cumulative incidence of early age-related macular degeneration: the Beaver Dam Eye Study. JAMA Ophthalmol. 2014;132:446–55. doi: 10.1001/jamaophthalmol.2013.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and Aetiological Aspects of Macular Degeneration. Prog Ret Eye Res. 2001;20:385–414. doi: 10.1016/s1350-9462(00)00025-2. [DOI] [PubMed] [Google Scholar]
- 25.Cherepanoff S, Mitchell P, Wang JJ, Gillies MC. Retinal autoantibody profile in early age-related macular degeneration: preliminary findings from the Blue Mountains Eye Study. Clin Exp Ophthalmol. 2006;34:590–5. doi: 10.1111/j.1442-9071.2006.01281.x. [DOI] [PubMed] [Google Scholar]
- 26.Adamus G, Chew EY, Ferris FL, Klein ML. Prevalence of anti-retinal autoantibodies in different stages of Age-related macular degeneration. BMC Ophthalmol. 2014;14:154. doi: 10.1186/1471-2415-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73:1765–86. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ezzat MK, Hann CR, Vuk-Pavlovic S, Pulido JS. Immune cells in the human choroid. Br J Ophthalmol. 2008;92:976–80. doi: 10.1136/bjo.2007.129742. [DOI] [PubMed] [Google Scholar]
- 29.Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol. 2004;122:1013–8. doi: 10.1001/archopht.122.7.1013. [DOI] [PubMed] [Google Scholar]
- 30.Caicedo A, Espinosa-Heidmann DG, Piña Y, Hernandez EP, Cousins SW. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Exp Eye Res. 2005;81:38–47. doi: 10.1016/j.exer.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Cruz-Guilloty F, Saeed AM, Duffort S, Cano M, Ebrahimi KB, Ballmick A, et al. T Cells and Macrophages Responding to Oxidative Damage Cooperate in Pathogenesis of a Mouse Model of Age-Related Macular Degeneration. PLoS One. 2014;9:e88201. doi: 10.1371/journal.pone.0088201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuppner MC, McKillop-Smith S, Forrester JV. TGF-beta and IL-1 beta act in synergy to enhance IL-6 and IL-8 mRNA levels and IL-6 production by human retinal pigment epithelial cells. Immunol. 1995;84:265–71. [PMC free article] [PubMed] [Google Scholar]
- 33.Planck SR, Huang XN, Robertson JE, Rosenbaum JT. Retinal pigment epithelial cells produce interleukin-1 beta and granulocyte-macrophage colony-stimulating factor in response to interleukin-1 alpha. Curr Eye Res. 1993;12:205–12. doi: 10.3109/02713689308999465. [DOI] [PubMed] [Google Scholar]
- 34.Bian ZM, Elner SG, Yoshida A, Elner VM. Differential involvement of phosphoinositide 3-kinase/Akt in human RPE MCP-1 and IL-8 expression. Invest Ophthalmol Vis Sci. 2004;45:1887–96. doi: 10.1167/iovs.03-0608. [DOI] [PubMed] [Google Scholar]
- 35.Nassar K, Grisanti S, Elfar E, Lüke J, Lüke M, Grisanti S. Serum cytokines as biomarkers for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2014;253:699–704. doi: 10.1007/s00417-014-2738-8. [DOI] [PubMed] [Google Scholar]
- 36.Ardeljan D, Wang Y, Park S, Shen D, Chu XK, Yu C-R, et al. Interleukin-17 Retinotoxicity Is Prevented by Gene Transfer of a Soluble Interleukin-17 Receptor Acting as a Cytokine Blocker: Implications for Age-Related Macular Degeneration. PLoS One. 2014;9:e95900. doi: 10.1371/journal.pone.0095900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin JI, Bayry J. A role for IL-17 in age-related macular degeneration. Nat Rev Immunol. 2013;13:701. doi: 10.1038/nri3459-c1. [DOI] [PubMed] [Google Scholar]
- 38.Wei L, Liu B, Tuo J, Shen D, Chen P, Li Z, et al. Hypomethylation of IL17RC Promoter Associates with Age-related Macular Degeneration. Cell reports. 2012;2:1151–8. doi: 10.1016/j.celrep.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan C-C, Ardeljan D. Molecular Pathology of Macrophages and Interleukin-17 in Age-Related Macular Degeneration. In: Ash JD, Grimm C, Hollyfield JG, Anderson RE, LaVail MM, Bowes Rickman C, editors. Retinal Degenerative Diseases. Springer; New York: 2014. pp. 193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan B, Shen J, Cao J, Zhou Y, Shang L, Jin S, et al. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Scientific Reports. 2015;5:16053. doi: 10.1038/srep16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu B, Wei L, Meyerle C, Tuo J, Sen HN, Li Z, et al. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. J Transl Med. 2011;9:1–12. doi: 10.1186/1479-5876-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jonas JB, Tao Y, Neumaier M, Findeisen P. Monocyte chemoattractant protein 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in exudative age-related macular degeneration. Arch Ophthalmol. 2010;128:1281–6. doi: 10.1001/archophthalmol.2010.227. [DOI] [PubMed] [Google Scholar]
- 43.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, et al. Abnormalities in Monocyte Recruitment and Cytokine Expression in Monocyte Chemoattractant Protein 1-deficient Mice. J Exp Med. 1998;187:601–8. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nature Immunol. 2008;9:970–80. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 45.Raoul W, Auvynet C, Camelo S, Guillonneau X, Feumi C, Combadière C, et al. CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration. J Neuroinflamm. 2010;7:87. doi: 10.1186/1742-2094-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu F, Ding X, Yang Y, Li J, Tang M, Yuan M, et al. Aqueous humor cytokine profiling in patients with wet AMD. Mol Vision. 2016;22:352–61. [PMC free article] [PubMed] [Google Scholar]
- 47.Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–7. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 48.Combadi C, Feumi C, Raoul W, Keller N, Rodro M, Pezard A, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clinical Invest. 2007;117:2920–8. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan CC, Ross RJ, Shen D, Ding X, Majumdar Z, Bojanowski CM, et al. Ccl2/Cx3cr1-deficient mice: an animal model for age-related macular degeneration. Ophthalmic Res. 2008;40:124–8. doi: 10.1159/000119862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Škuljec J, Sun H, Pul R, Bénardais K, Ragancokova D, Moharregh-Khiabani D, et al. CCL5 induces a pro-inflammatory profile in microglia in vitro. Cell Immunol. 2011;270:164–71. doi: 10.1016/j.cellimm.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 51.Gurne DH, Tso MOM, Edward DP, Ripps H. Antiretinal antibodies in serum of patients with age-related macular degeneration. Ophthalmol. 1991;98:602–7. doi: 10.1016/s0161-6420(91)32252-8. [DOI] [PubMed] [Google Scholar]
- 52.Gu X, Meer SG, Miyagi M, Rayborn ME, Hollyfield JG, Crabb JW, et al. Carboxyethylpyrrole Protein Adducts and Autoantibodies, Biomarkers for Age-related Macular Degeneration. J Biol Chem. 2003;278:42027–35. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 53.Patel N, Ohbayashi M, Nugent AK, Ramchand K, Toda M, Chau KY, et al. Circulating anti-retinal antibodies as immune markers in age-related macular degeneration. Immunol. 2005;115:422–30. doi: 10.1111/j.1365-2567.2005.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Majka DS, Deane KD, Parrish LA, Lazar AA, Baron AE, Walker CW, et al. The Duration of Pre-Clinical Rheumatoid Arthritis-Related Autoantibody Positivity Increases in Subjects with Older Age at Time of Disease Diagnosis. Ann Rheum Dis. 2008;67:801–7. doi: 10.1136/ard.2007.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deane KD. Preclinical Rheumatoid Arthritis (Autoantibodies): An Updated Review. Curr Rheumatol Rep. 2014;16:419. doi: 10.1007/s11926-014-0419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morohoshi K, Patel N, Ohbayashi M, Chong V, Grossniklaus HE, Bird AC, et al. Serum autoantibody biomarkers for age-related macular degeneration and possible regulators of neovascularization. Exp Mol Path. 2012;92:64–73. doi: 10.1016/j.yexmp.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 57.Joachim S, Bruns K, Lackner K, Pfeiffer N, Grus F. Analysis of IgG antibody patterns against retinal antigens and antibodies to a-crystallin, GFAP, and a-enolase in sera of patients with “wet” age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007;245:619–26. doi: 10.1007/s00417-006-0429-9. [DOI] [PubMed] [Google Scholar]
- 58.Morohoshi K, Ohbayashi M, Patel N, Chong V, Bird AC, Ono SJ. Identification of anti-retinal antibodies in patients with age-related macular degeneration. Experimental and Mol Pathol. 2012;93:193–9. doi: 10.1016/j.yexmp.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Iannaccone A, Giorgianni F, New DD, Hollingsworth TJ, Umfress A, Alhatem AH, et al. Circulating Autoantibodies in Age-Related Macular Degeneration Recognize Human Macular Tissue Antigens Implicated in Autophagy, Immunomodulation, and Protection from Oxidative Stress and Apoptosis. PLoS One. 2015;10:e0145323. doi: 10.1371/journal.pone.0145323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doycheva D, Preuss B, Deuter C, Zierhut M, Klein R. Identification of immunodominant epitopes of alpha-crystallins recognized by antibodies in sera of patients with uveitis. Graefes Arch Clin Exp Ophthalmol. 2012;250:297–305. doi: 10.1007/s00417-011-1758-x. [DOI] [PubMed] [Google Scholar]
- 61.Joachim SC, Bruns K, Lackner KJ, Pfeiffer N, Grus FH. Antibodies to alpha B-crystallin, vimentin, and heat shock protein 70 in aqueous humor of patients with normal tension glaucoma and IgG antibody patterns against retinal antigen in aqueous humor. Curr Eye Res. 2007;32:501–9. doi: 10.1080/02713680701375183. [DOI] [PubMed] [Google Scholar]
- 62.Celet B, Akman-Demir G, Serdaroglu P, Yentur SP, Tasci B, van Noort JM, et al. Anti-aB-crystallin immunoreactivity in inflammatory nervous system diseases. J Neurol. 2000;247:935–9. doi: 10.1007/s004150070049. [DOI] [PubMed] [Google Scholar]
- 63.Díaz-Ramos À , Roig-Borrellas A, García-Melero A, López-Alemany R. α-Enolase, a Multifunctional Protein: Its Role on Pathophysiological Situations. J Biomed Biotech. 2012;2012:156795. doi: 10.1155/2012/156795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adamus G. Autoantibody Targets and their Cancer Relationship in the Pathogenicity of Paraneoplastic Retinopathy. Autoimmun Rev. 2009;8:410–4. doi: 10.1016/j.autrev.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol. 2004;4:5. doi: 10.1186/1471-2415-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adamus G, Amundson D, Seigel GM, Machnicki M. Anti-enolase alpha autoantibodies in cancer-associated retinopathy: epitope mapping and cytotoxicity on retinal cells. J Autoimmun. 1998;11:671–7. doi: 10.1006/jaut.1998.0239. [DOI] [PubMed] [Google Scholar]
- 67.Magrys A, Anekonda T, Ren G, Adamus G. The role of anti-alpha-enolase autoantibodies in pathogenicity of autoimmune-mediated retinopathy. J Clin Immunol. 2007;27:181–92. doi: 10.1007/s10875-006-9065-8. [DOI] [PubMed] [Google Scholar]
- 68.Ramkumar HL, Zhang J, Chan C-C. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD). Prog Ret Eye Res. 2010;29:169–90. doi: 10.1016/j.preteyeres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saeed AM, Duffort S, Ivanov D, Wang H, Laird JM, Salomon RG, et al. The oxidative stress product carboxyethylpyrrole potentiates TLR2/TLR1 inflammatory signaling in macrophages. PLoS One. 2014;9:e106421. doi: 10.1371/journal.pone.0106421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salomon RG, Hong L, Hollyfield JG. The Discovery of Carboxyethylpyrroles (CEPs): Critical Insights into AMD, Autism, Cancer, and Wound Healing from Basic Research on the Chemistry of Oxidized Phospholipids. Chem Res Tox. 2011;24:1803–16. doi: 10.1021/tx200206v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13:438–51. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-Related Macular Degeneration: Etiology, Pathogenesis, and Therapeutic Strategies. Sur Ophthalmol. 2003;48:257–93. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 73.Khandhadia S, Cipriani V, Yates JRW, Lotery AJ. Age-related macular degeneration and the complement system. Immunobiol. 2012;217:127–46. doi: 10.1016/j.imbio.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 74.McHarg S, Clark SJ, Day AJ, Bishop PN. Age-related macular degeneration and the role of the complement system. Mol Immunol. 2015;67:43–50. doi: 10.1016/j.molimm.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 75.Bhutto IA, Baba T, Merges C, Juriasinghani V, McLeod DS, Lutty GA. C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Bri J Ophthalmol. 2011;95 doi: 10.1136/bjo.2010.199216. doi:10.1136/bjo.2010.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Molins B, Fuentes-Prior P, Adán A, Antón R, Arostegui JI, Yagüe J, et al. Complement factor H binding of monomeric C-reactive protein downregulates proinflammatory activity and is impaired with at risk polymorphic CFH variants. Sci Reports. 2016;6:22889. doi: 10.1038/srep22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Lookeren Campagne M, Strauss EC, Yaspan BL. Age-related macular degeneration: Complement in action. Immunobiol. 2015;221:733–9. doi: 10.1016/j.imbio.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 78.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. PNAS. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Despriet DDG, Klaver CCW, Witteman JCM, Bergen AAB, Kardys I, de Maat MPM, et al. Complement Factor H Polymorphism, Complement Activators, and Risk of Age-Related Macular Degeneration. JAMA. 2006;296:301–9. doi: 10.1001/jama.296.3.301. [DOI] [PubMed] [Google Scholar]
- 80.Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. PNAS. 2006;103:2328–33. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma Complement Components and Activation Fragments: Associations with Age-Related Macular Degeneration Genotypes and Phenotypes. Invest Ophthalmol Vis Sci. 2009;50:5818–27. doi: 10.1167/iovs.09-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scholl HPN, Issa PC, Walier M, Janzer S, Pollok-Kopp B, Börncke F, et al. Systemic Complement Activation in Age-Related Macular Degeneration. PLoS One. 2008;3:e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hecker LA, Edwards AO, Ryu E, Tosakulwong N, Baratz KH, Brown WL, et al. Genetic control of the alternative pathway of complement in humans and age-related macular degeneration. Hum Mol Gen. 2010;19:209–15. doi: 10.1093/hmg/ddp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vogt SD, Curcio CA, Wang L, Li CM, McGwin G, Medeiros NE, et al. Retinal pigment epithelial expression of complement regulator CD46 is altered early in the course of geographic atrophy. Exp Eye Res. 2011;93:413–23. doi: 10.1016/j.exer.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bora NS, Gobleman CL, Atkinson JP, Pepose JS, Kaplan HJ. Differential expression of the complement regulatory proteins in the human eye. Invest Ophthalmol Vis Sci. 1993;34:3579–84. [PubMed] [Google Scholar]
- 86.Singh A, Faber C, Falk M, Nissen MH, Hviid TVF, Sørensen TL. Altered Expression of CD46 and CD59 on Leukocytes in Neovascular Age-Related Macular Degeneration. Am J Ophthalmol. 2012;154:193–9. e2. doi: 10.1016/j.ajo.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 87.Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Decreased Membrane Complement Regulators in the RPE Contributes to AMD. J Pathol. 2013;229 doi: 10.1002/path.4128. 10.1002/path.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Charbel Issa P, Victor Chong N, Scholl H. The significance of the complement system for the pathogenesis of age-related macular degeneration — current evidence and translation into clinical application. Graefes Arch Clin Exp Ophthalmol. 2011;249:163–74. doi: 10.1007/s00417-010-1568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gemenetzi M, Lotery AJ. Complement pathway biomarkers and age-related macular degeneration. Eye. 2016;30:1–14. doi: 10.1038/eye.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 92.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 93.Haddad S, Chen CA, Santangelo SL, Seddon JM. The Genetics of Age-Related Macular Degeneration: A Review of Progress to Date. Sur Ophthalmol. 2006;51:316–63. doi: 10.1016/j.survophthal.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Fritsche LG, Igl W, Bailey JNC, Grassmann F, Sengupta S, Bragg-Gresham JL, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48:134–43. doi: 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Montezuma SR, Sobrin L, Seddon JM. Review of Genetics in Age Related Macular Degeneration. Sem Ophthalmol. 2007;22:229–40. doi: 10.1080/08820530701745140. [DOI] [PubMed] [Google Scholar]
- 96.Chen M, Muckersie E, Forrester JV, Xu H. Immune Activation in Retinal Aging: A Gene Expression Study. Investigative Ophthalmology & Visual Science. 2010;51:5888–96. doi: 10.1167/iovs.09-5103. [DOI] [PubMed] [Google Scholar]
- 97.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Ret Eye Res. 2009;28:348–68. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 98.Xu H, Chen M, Manivannan A, Lois N, Forrester JV. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008;7:58–68. doi: 10.1111/j.1474-9726.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 99.Zarbin MA. Current Concepts in the Pathogenesis of Age-Related Macular Degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]