Abstract
Episodic memory involves flexible retrieval processes that allow us to link together distinct episodes, make novel inferences across overlapping events, and recombine elements of past experiences when imagining future events. However, the same flexible retrieval and recombination processes that underpin these adaptive functions may also leave memory prone to error or distortion, such as source misattributions in which details of one event are mistakenly attributed to another related event. To determine whether the same recombination-related retrieval mechanism supports both successful inference and source memory errors, we developed a modified version of an associative inference paradigm in which participants encoded everyday scenes comprised of people, objects, and other contextual details. These scenes contained overlapping elements (AB, BC) that could later be linked to support novel inferential retrieval regarding elements that had not appeared together previously (AC). Our critical experimental manipulation concerned whether contextual details were probed before or after the associative inference test, thereby allowing us to assess whether a) false memories increased for successful versus unsuccessful inferences, and b) any such effects were specific to after as compared to before participants received the inference test. In each of four experiments that used variants of this paradigm, participants were more susceptible to false memories for contextual details after successful than unsuccessful inferential retrieval, but only when contextual details were probed after the associative inference test. These results suggest that the retrieval-mediated recombination mechanism that underlies associative inference also contributes to source misattributions that result from combining elements of distinct episodes.
Keywords: Inference, False Memory, Episodic Memory, Memory, Associative Processes
Episodic memory allows individuals to recollect particular past experiences (Tulving, 2002). It has been well established that episodic memories are not literal representations of past experiences, but instead depend on constructive processes that are sometimes prone to error and distortion (cf., Bartlett, 1932; Brainerd & Reyna, 2005; Loftus, Miller, & Burns, 1978; McClelland, 1995; Roediger, 1996; Schacter, 1996). Such memory errors can arise as a consequence of multiple processes, including: knowledge- or schema-based inferences made about the meaning of observed actions or events, which are later integrated into memories of presented materials, such as sentences and stories (e.g., Alba & Hasher, 1983; Bransford, Barclay, & Franks, 1972; Bransford & Franks, 1971); activation of associations to semantically related words that may produce subsequent false recognition of a nonpresented word that is strongly associated to the list items that were presented (e.g., Gallo, 2006; Roediger & McDermott, 1995); and a variety of influences that operate during retrieval of past experiences, such as misleading suggestions or instructions to imagine what might have happened earlier (Loftus, 2003, 2005; Shaw & Porter, 2015).
While these and other forms of memory distortion could be viewed as flaws or defects in episodic memory, a number of researchers have built on Bartlett’s (1932) seminal insights and suggest instead that such errors can be viewed as byproducts of adaptive constructive processes (Schacter, 2012) that play a functional role in memory but produce errors or distortions as a direct consequence of doing so (cf., Howe, 2011; Howe, Wilkinson, Garner, & Ball, 2016; Newman & Lindsay, 2009; Schacter, 2001; Schacter, Guerin, & Jacques, 2011). Bartlett (1932), of course, focused on the functional role of schemata in guiding constructive retrieval, which he maintained “must always be supposed to be operating in any well-adapted organic response (1932, p. 201)” but also contributed to the memory distortions that he documented. Others have argued that such well established memory errors as the misinformation effect and associative false recognition may reflect, respectively, the operation of adaptive memory updating processes and retention of themes and meanings (for review, see Schacter et al., 2011). More recently, it has become increasingly clear that episodic memory supports a variety of cognitive functions, including imagining future experiences (e.g., Schacter et al., 2012; Szpunar, 2010), inferential processing (e.g., Zeithamova, Dominick & Preston, 2012; Zeithamova & Preston, 2010), means-end problem solving (e.g., Madore & Schacter, 2014; Sheldon, McAndrews, & Moscovitch, 2011), and divergent creative thinking (e.g., Madore, Addis, & Schacter, 2015). An important feature of episodic memory that supports these and other adaptive functions is the capacity to flexibly retrieve and recombine information from distinct past experiences into novel representations. For example, according to the constructive episodic simulation hypothesis (Schacter & Addis, 2007a, 2007b) the capacity to flexibly recombine elements of past experiences is crucial for our ability to imagine or simulate new situations that might occur in the future. Similarly, recent evidence suggests that flexible recombination plays a key role in our capacity to make inferences based on distinct past events that share a common feature (Zeithamova, Dominick & Preston, 2012; Zeithamova & Preston, 2010).
In line with the theoretical perspectives noted earlier that emphasize the close link between adaptive aspects of episodic memory and susceptibility to memory errors, the constructive episodic simulation hypothesis also holds that the functional benefits of flexible retrieval and recombination are accompanied by a cost: vulnerability to memory errors such as source misattribution and false recognition that can result from mistakenly combining elements of distinct past experiences (Schacter & Addis, 2007a, 2007b; for related views, see Dudai & Carruthers, 2005; Suddendorf & Corballis, 2007). There is indeed evidence that memory errors can result from mistakenly combining features of distinct episodic or autobiographical memories (e.g., Burt, Kemp, & Conway, 2004; Devitt, Monk-Fromont, Schacter, & Addis, 2015; Odegard & Lampinen, 2004). However, we are not aware of any study that has directly tested the central idea of the constructive episodic simulation hypothesis that the same flexible recombination process that supports an adaptive cognitive process can also produce memory errors that result from miscombining elements of distinct past experiences.
To test this idea, we required a task that both requires flexible recombination and supports an adaptive cognitive process. The associative inference task used by Preston and colleagues fits these requirements (e.g., Preston, Shrager, Dudukovic, & Gabrieli, 2004; Zeithamova, Dominick & Preston, 2012; Zeithamova & Preston, 2010). Associative inference is an adaptive process that allows people to link together related information acquired in distinct episodes to make novel connections that they have not directly experienced (Zeithamova, Schlichting, & Preston, 2012). For example, if one sees two different individuals entering the same house on different days, retrieving and recombining details of the two episodes allows one to infer that the two individuals are related in some way by their relationship with the house. This kind of flexible recombination is quite similar to the kind of flexible recombination that is required to draw on elements of past experiences in order to construct simulations of novel future events, as discussed by Schacter and Addis (2007a, 2007b). In previous studies using the associative inference procedure, participants learned direct associations between two items (AB) and then learned direct associations between two items that included one member of the previously studied pair (BC) and also learned indirect associations based on the overlapping pairs (AC). Later, participants received a memory test for both the direct AB and direct BC associations. In addition, participants received an associative inference test for the indirect association (AC). Here, they are told that the link between the two items is mediated by a third item (B) that was previously associated with both the A and C items, and to choose which of two items was linked to A via the shared B association.
There are two ways that participants can perform successfully on the associative inference test. First, during study of BC, participants may bring to mind the related AB pair and encode an integrated representation (ABC) that is later retrieved during the associative inference test (integrative encoding; e.g., Shohamy & Wagner, 2008). Second, participants may engage in flexible recombination at the time of retrieval, bringing to mind and combining the previously studied AB and BC pairs during the associative inference task. Neuroimaging evidence suggests that both mechanisms contribute to associative inference (Zeithamova, Dominick & Preston, 2012; Zeithamova & Preston, 2010). In the present study, we adapted the associative inference paradigm developed by Zeithamova and Preston (2010) to assess whether mechanisms linked with inferential processing (i.e., retrieval-related recombination and encoding-related integration) also contribute to source memory errors. As noted earlier, pioneering studies on memory distortion have already shown that knowledge- or schema-driven inferences about sentences and stories can contribute to memory errors (e.g., Alba & Hasher, 1983; Branford & Franks, 1971) but the kind of inferential processing tapped by Zeithamova and Preston’s associative inference task focuses specifically on combining elements from distinct episodes that are not linked by pre-existing knowledge or schemas, and thus likely draws on different processes than the meaning-based inferences elicited in classic studies of sentence and story processing. Indeed, it is precisely because the associative inference paradigm developed by Zeithamova and Preston (2010) targets flexible recombination processes which link elements of distinct episodes that their paradigm is well suited for testing the key claim of the constructive episodic simulation hypothesis - that the same flexible retrieval processes that are used to combine elements of distinct episodes into functionally useful, novel representations can also produce memory errors that result from mixing up elements of these episodes. More generally, we attempt to determine whether the domain of adaptive memory distortions, where a memory error results from carrying out a cognitive operation that has demonstrably beneficial consequences on another aspect of performance, extends to associative inference. Although the literature on associative inference has grown considerably during the past decade (for review, see Schlichting & Preston, 2015), we are not aware of any studies using the associative inference paradigm, which requires combining elements of distinct episodes, that have linked successful associative inference with memory errors.
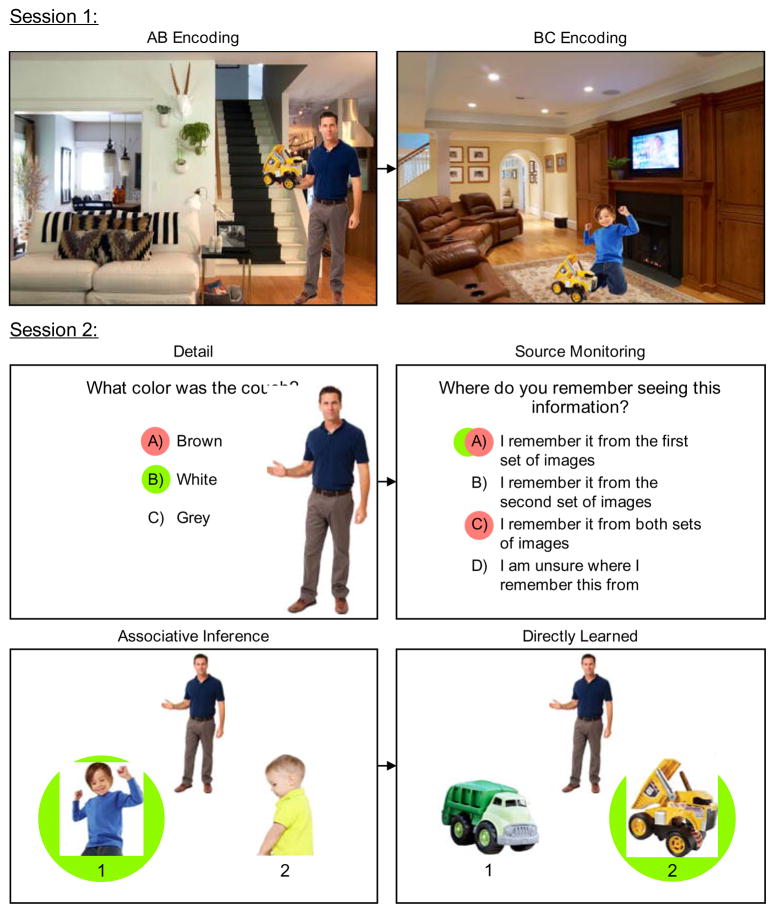
In our version of the associative inference paradigm, during an initial session participants study scenes that include AB items (e.g., a person (A) and a toy (B) in a room with a white couch; see Figure 1) and then study scenes comprised of BC items (e.g., the toy (B) and a different person (C) in a room with a brown couch). Participants are instructed to try to learn both the direct association between each person and object (AB and BC) and the indirect association between the two people based on the shared object (AC). After a delay, participants return for a second session in which they are tested for direct associations (AB, BC) and perform an associative inference test for novel combinations that are linked via the B item (AC). To test whether retrieval-related recombination processes underlying successful inference can also contribute to memory errors, memory for contextual details from both the AB and BC scenes is also probed (e.g., What color was the couch?) followed immediately by a source memory test (In which set of images do you remember seeing this information?). For one half of the AB and BC scenes, detail/source memory tests were given before the test of direct (AB, BC) and indirect (AC) associations, and for the other half, the detail/source memory tests were given after the tests of direct and indirect associations. For the detail/source test, a true memory is defined as a response in which the participants both chose the correct item and attributed the source of their memory correctly (e.g., white couch attributed to AB scene), whereas a false memory is defined as a response for which the participant both chose the item from the overlapping image (e.g., BC) and misattributed its source (e.g., brown couch attributed to AB scene; see Methods for further details).
Fig. 1.
Overview of Experimental Procedure
Illustration of materials, stimuli, and test displays from Experiments 1a and 1b. The Session 1 section shows one example of an AB image in which the man is item “A” and the toy truck is item “B” and the corresponding BC image in which the boy is item “C.” The Session 2 section shows one example of a detail and source monitoring question linked to the example AB image. For each detail question, participants saw a cutout of the “A” or “C” individual presented to the right of the question in order to indicate to which event the question referred. False memories occurred when participants chose both the misinformation detail (e.g., brown couch) during the detail question and attributed the misinformation detail incorrectly to either the original event or both events – as indicated by the red (dark) circles. True memories occurred when participants both chose the correct detail during the detail question (e.g., white couch) and attributed the correct detail correctly to the original event – as indicated by the green (light) circles. Other example detail questions for this ABC triad included: Where were the stairs located?; What color were the walls in the room?; What was this individual sitting/standing on?; What was hanging on the wall directly behind this individual?; etc. Importantly, all of these questions relate to two contradictory details from images AB and BC (e.g., stairs directly behind vs. to the far left; yellow vs. white walls; wood floors vs. carpet; potted plants vs. picture frames; etc.). The green (light) circles indicate the correct answer for the associative inference and directly learned questions. Participants saw these images without the red (dark) and green (light) circles.
The critical comparison concerns the proportions of false memories on the detail/source tests given before versus after the associative inference test, for correct as compared to incorrect associative inference trials (i.e., AC). We distinguish among three competing hypotheses:
If recombination during retrieval both enhances associative inference performance and also increases susceptibility to false memories, then the proportion of false memories should be higher for correct than incorrect inference trials, but only when the detail/source test is given after the associative inference test (during which recombination occurs); there should be no difference in the proportion of false memories for correct vs. incorrect inference trials when the detail/source test is given before the associative inference test.
If the proportion of false memories is higher for correct than incorrect inference trials both when the detail/source tests are given before and after the associative tests, then these effects would be attributable to integrative encoding processes.
If there is no link at all between source memory misattributions and associative inference, then there should be no difference between the proportion of false memories for correct and incorrect inference trials regardless of when the detail/source test is given.
To test these hypotheses, and determine the reliability of the results across variations in procedure and experimental parameters, we conducted three initial experiments that used the same basic paradigm and differed only in methodological details. Experiment 1 used a 24-hour study-test delay and a two-alternative forced choice on the associative inference test, whereas Experiment 2 used a 48-hour study-test delay and included an additional “neither” option on the forced-choice test (see below for rationale regarding these changes). In Experiment 3, we increased the delay between the directly learned (AB and BC) and associative inference trials (AC) on the one hand, and the second set of detail and source questions on the other, in order to assess the durability of the effects observed in Experiments 1 and 2. All three of these experiments provided evidence in favor of the first hypothesis outlined above: the proportion of false memories was higher for correct than incorrect inference trials, and only when the detail/source test was given after the associative inference test, during which recombination occurs. These findings implicate recombination during retrieval in both associative inference and memory misattribution, in line with the constructive episodic simulation hypothesis. To further test the hypothesis, in Experiment 4 we eliminated tests of directly learned associations (AB and BC), which in theory could have contributed to the effects that we attributed to flexible recombination. However, Experiment 4 again replicated the major findings of Experiments 1–3, providing further evidence that recombination during retrieval is responsible for the observed pattern of false memory effects.
Experiments 1 and 2
Because Experiments 1 and 2 used nearly identical procedures with only minor differences, we report the methods and results for these experiments together. To provide an overview of the basic procedure, participants came to the lab for two sessions, separated by a 24-hour (Experiment 1) or a 48-hour (Experiment 2) delay. The delay in Experiment 2 was extended from 24- to 48-hours in order to more closely replicate accuracy levels on the directly learned and associative inference test reported in the standard associative inference paradigm designed by Preston and colleagues (Preston et al., 2004; Zeithamova, Dominick & Preston, 2012; Zeithamova & Preston, 2010; Zeithamova, Schlichting & Preston, 2012). Participants completed a modified version of an associative inference paradigm based on prior studies by the Preston group (Preston et al., 2004; Zeithamova, Dominick & Preston, 2012; Zeithamova & Preston, 2010; Zeithamova, Schlichting & Preston, 2012). In the first session, participants intentionally encoded directly learned associations between individual “A” and object “B” followed by a second list with overlapping associations between object “B” and individual “C” (see Fig. 1); participants were also presented with non-overlapping X-Y individual-object pairs in order to reduce performance for directly learned associations below ceiling levels. A total of 24 ABC triads and 12 XY pairs were used in the experiment. In the second session, participants were tested on directly learned associations (i.e., AB, BC, XY) and associative inference trials consisting of novel combinations of person pairings (i.e., AC). Additionally, for one half of the ABC triads, participants answered ten detail and source monitoring questions per triad before they were tested on directly learned and associative inference trials. For the alternate half of the triads, participants answered these detail and source monitoring questions after the directly learned and associative inference trials for all items. As noted earlier, the contrast between performance on the detail and source memory tests given before as compared to after the directly learned/associative inference trials is critical to testing the three key hypotheses we outlined.
Method
Participants
For both experiments, participants were recruited via advertisements at Boston University and Harvard University. All had normal vision and no history of neurological impairment. They gave informed consent, were treated in accordance with guidelines approved by the ethics committee at Harvard University, and received either course credit or pay for completing the study. Experiment 1 included 26 young adults (mean age = 21.20, SD = 2.19; 15 female). Two participants were excluded from the true, false and foil memory analyses because they were 100 percent accurate on the associative inference trials; thus, our final sample consisted of 24 participants. Participants who were 100 percent accurate on the associative inference trials were removed from the true, false, and foil memory analyses because they did not have any trials for which they correctly recalled the directly learned relationships and incorrectly inferred the relationship between item A and item C, thereby precluding meaningful comparisons of successful inference to unsuccessful inference both before and after flexible retrieval. Experiment 2 included 25 young adults (mean age = 20, SD = 1.93; 14 female). One participant was excluded from all analyses for having prior experience with several of the task stimuli; thus, our final sample consisted of 24 participants. Prior to the experiment, we decided on a sample size of 24 based on previous work utilizing a similar source monitoring paradigm (Okado & Stark, 2005). We stopped data collection after reaching the target of 24 participants with analyzable data.
AB and BC Encoding
All experimental sessions were executed on an Apple desktop computer using PsychoPy2 (v1.80.03). Stimuli consisted of 72 still color images depicting everyday life events (e.g., walking to work). Color images of common objects (e.g., toy truck) and individuals were superimposed on outdoor and indoor scenes. Scenes were counterbalanced across participants such that each scene was used equally often for both AB and BC pairs. Using Adobe Photoshop CC 2015, 48 overlapping pairs (24 AB pairs, 24 BC pairs – 24 total ABC triads) and 24 unique, non-overlapping pairs (XY) were constructed. Overlapping AB and BC pairs were constructed such that two individuals (A and C) shared an association with an overlapping object (B; i.e., one ABC triad). XY pairs were constructed of unique individual – object pairs that did not share an overlapping association with other pairings.
Participants received one of two versions of the AB encoding task, which consisted of 36 images (i.e., AB and XY) followed by the corresponding BC encoding task, which consisted of 36 images (i.e., BC and XY; Fig. 1). Each image was randomly presented for 10 seconds within each encoding block (i.e., AB encoding and BC encoding). Participants were instructed to learn both the direct associations (i.e., AB, BC) and the indirect associations (i.e., AC) along with the contextual information presented. Following each image, participants were asked to provide a judgment of learning on a scale from 1 to 4 (1 = definitely forget, 4 = definitely remember). These judgments were collected in order to ensure participants’ attention during the encoding phase.
Detail and Source Monitoring
Ten detail and source monitoring questions were constructed for each of the 24 ABC triads (5 questions related to image AB and 5 questions related to image BC). Detail questions were directly related to background details that were present but contradictory in the AB and BC scenes and did not reference the overlapping “B” object (see Fig. 1). A cutout of the cue individual (i.e., either “A” or “C”) was presented to the right of each detail question in order to indicate which scene the question was referring to (Fig. 1). For each detail question, participants were given three options: the correct item, a misinformation item, and an unrelated foil item. The misinformation item consisted of information from the overlapping image in the triad (e.g., if the detail question were related to the AB image, the misinformation item would be a contradicting detail from the BC image, such as a brown couch when a white couch had appeared in the AB image). Foil items were details that were not presented in either of the overlapping images (e.g., grey couch). Following each detail question, participants indicated where they remember seeing this contextual detail (i.e., the source of the information; Fig. 1). Participants were given four possible answer choices: 1) the first set of images – AB, 2) the second set of images – BC, 3) both sets of images, or 4) unsure. Immediately following participants’ source monitoring response, they were asked to rate their confidence in their response on a scale from 1 to 4 (1 = very unsure, 4 = very sure). The presentation order of each set of questions (i.e., detail, source, confidence) was randomized for each participant and the questions were self-paced.
Participants answered the ten detail and source monitoring questions for one half of the 24 ABC triads before being tested on the directly learned and associative inference trials. After participants were tested on the directly learned and associative inference trials, they completed the ten detail and source monitoring questions for the alternate half of the 24 ABC triads.
Directly Learned and Associative Inference Trials
Following the first half of the detail and source questions, participants were tested on directly learned (AB and BC) and associative inference trials (AC). During each directly learned trial, a single cue individual (e.g., an “A” or “C” individual) was presented at the top of the screen and two choice objects were presented at the bottom of the screen (e.g., two “B” objects from different ABC triads; Fig. 1). On the associative inference trials, a cue individual (A) was presented along with two individuals at the bottom of the screen (i.e., the correct “C” individual from the ABC triad and a lure “C” individual from another triad). Participants were instructed on associative inference trials that the association between the cue (A) and the correct choice (C) was indirect, mediated through an object (B) that shared an association with both the cue and the correct choice during encoding. In Experiment 1, participants were required to make a forced-choice decision indicating which of the two choice objects/individuals was associated with the cue individual. In Experiment 2, participants were given a third option to respond “neither” when they believed that the items had not been previously paired, in order to reduce the possible influences of guessing on the associative inference task. If participants could not recall which of the two options given was paired with the cue individual, they were allowed to guess in Experiment 1, which could add noise to the source memory data by including triads for which participants were not actually able to successfully infer the relationship between item A and item C, but appeared to do so because of guessing. Thus, in an attempt to replicate the results of Experiment 1 and also control for the potential effects of guessing, a neither option was included for Experiment 2. Importantly, for both directly learned and associative inference trials, the incorrect choice was a familiar item that had been studied in the context of another individual independent from the cue. Thus, correct responses required retrieval of learned associations and could not be made based on the familiarity of the choice. The presentation order of the trials was randomized with the only constraint being that AC associative inference trials were shown before their corresponding AB and BC directly learned trials in order to ensure that participants were not able to form an association between “A” and “C” individuals during test. Following each of the directly learned and associative inference trials, participants rated their confidence in their response on a scale from 1 to 4 (1 = very unsure, 4 = very sure).
Coding of True and False Memories
Consistent with previous work using a similar detail and source monitoring paradigm (Okado & Stark, 2005), true memories were defined as detail questions for which the participant both chose the correct detail and attributed the source of their memory correctly to the currently cued image. False memories were defined as detail questions for which the participant both chose the misinformation detail and attributed the misinformation detail incorrectly to either the currently cued image or both images in the triad (Fig. 1). False memories were analyzed for ABC triads for which participants correctly inferred the relationship between “A” and “C” compared to triads for which the inference was not correctly made. Additionally, false memories were evaluated both before explicit retrieval of the inference (i.e., before AC associative inference trials) and after the retrieval of the inference in order to selectively compare the distinct effects of integration during encoding and flexible recombination at retrieval on subsequent memory errors.
Results
Directly Learned and Associative inference Trials
Experiment 1
First we evaluated overall accuracy on directly learned and associative inference trials. Performance on both directly learned and associative inference trials was generally accurate, and there was no significant difference in the proportion of directly learned (Mdirect = 0.78, SE = 0.02) as compared to associative inference trials (Massociative inference = 0.80, SE = 0.03) that participants answered correctly (t(25) = −.99, p > .250, mean difference = −0.02, 95% confidence interval (CI) = [−0.06, 0.02], d = .19). Consistent with previous research (Zeithamova & Preston, 2010), we found significantly longer reaction times on associative inference trials (Massociative inference = 4425 msec, SE = 341) as compared to directly learned trials (Mdirect = 3306 msec, SE = 314), suggesting that there may be an additional recombination-related retrieval mechanism necessary for inferential versus direct retrieval (t(25) = 9.48, p < .001, mean difference = 1.12, 95% CI = [0.88, 1.36], d = 1.85). Further, participants assigned significantly higher confidence ratings to their responses on directly learned (Mdirect = 3.34, SE = 0.07) as compared to associative inference trials (Massociative inference = 2.87, SE = 0.09), suggesting that participants were more confident in their memory for events that they had directly experienced as compared to those resulting from recombination (t(25) = 9.38, p < .001, mean difference = 0.47, 95% CI = [0.37, 0.58], d = 1.89).
Experiment 2
Again we evaluated overall accuracy on directly learned and associative inference trials. There was a trend toward a significant difference in the proportion of directly learned (Mdirect = 0.69, SE = 0.03) as compared to associative inference trials (Massociative inference = 0.64, SE = 0.03) that participants answered correctly (t(23) = 2.00, p = .057, mean difference = 0.05, 95% confidence interval (CI) = [−0.002, 0.10], d = .42). While this trend is slightly different from the results reported in Experiment 1, it does not affect the main hypotheses of interest, which are related to the false memory analyses. Consistent with results from Experiment 1, we found significantly longer reaction times on associative inference trials (Massociative inference = 4401 msec, SE = 185) compared to directly learned trials (Mdirect = 3052 msec, SE = 129), suggesting an additional recombination-related retrieval mechanism for inferential versus direct retrieval (t(23) = 5.66, p < .001, mean difference = 1.35, 95% CI = [0.99, 1.71], d = 1.62). Further, results revealed that participants were significantly more confident in their responses on directly learned (Mdirect = 3.22, SE = .09) as compared to associative inference trials (Massociative inference = 2.83, SE = .08;(t(23) = 5.67, p < .001, mean difference = 0.39, 95% CI = [0.25, 0.53], d = 1.18). Thus, results from Experiment 2 replicate those in Experiment 1.
False Memory
Experiment 1
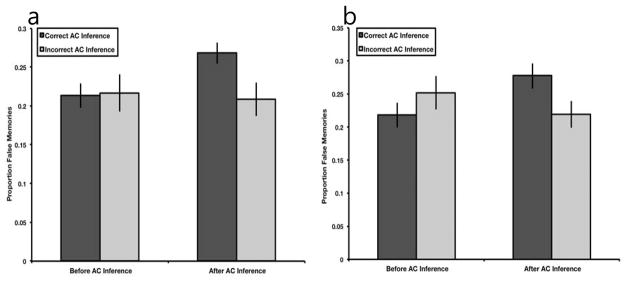
To assess the effects of integrative encoding and recombination mechanisms at retrieval on subsequent memory errors, we examined source memory errors for the detail and source monitoring questions with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures analysis of variance (ANOVA). Importantly, only trials for which participants correctly remembered the directly learned association were included in subsequent analyses. See supplementary table 1 for the raw number of trials per bin for each experiment. Results revealed a trend toward a main effect of time, F(1,23) = 3.04, p = .095, ηp2 = .12, no main effect of inference, F(1,23) = 2.40, p = .135, ηp2 = .10, and a significant time by inference interaction, F(1,23) = 7.05, p = .014, ηp2 = .24 (see Fig. 2a). Participants falsely attributed more details to the overlapping event after successful inference retrieval (Mafter = 0.27, SE = 0.01) than before successful inference retrieval (Mbefore = 0.21, SE = 0.02; t(23) = 4.05, p < .001, mean difference = 0.06, 95% CI = [0.03, 0.08], d = .83). Further, participants did not falsely attribute more details to the overlapping event after unsuccessful inference retrieval (Mafter = 0.21, SE = 0.02) than before unsuccessful inference retrieval (Mbefore = 0.22, SE = 0.02; t(23) = .385, p > .250, mean difference = −0.01, 95% CI = [−0.05, 0.04], d = .08). Participants did not falsely attribute more details to the overlapping event before successful inference retrieval (Mcorrect = 0.21, SE = 0.02) than before unsuccessful inference retrieval (Mincorrect = 0.22, SE = 0.02; t(23) = .143, p > .250, mean difference = 0.003, 95% CI = [−0.04, 0.05], d = .03). Critically, participants falsely attributed more details to the overlapping event after successful inference retrieval (Mcorrect = 0.27, SE = 0.01) than after unsuccessful inference retrieval (Mincorrect = 0.21, SE = 0.02; t(23) = 2.73, p = .012, mean difference = 0.06, 95% CI = [0.01, 0.10], d = .56), suggesting that recombination processes underlying successful inference at retrieval may also lead to source memory errors.
Fig. 2.
Proportion of false memories in Experiment 1 (a) and Experiment 2 (b). Performance on detail and source monitoring questions was examined both before and after either successful or unsuccessful inference. Importantly, only trials for which participants responded correctly to directly learned trials were included in this analysis. Results revealed a significant time by inference interaction in both Experiment 1 and 2. Subsequent t-tests confirm that false memories selectively increased only following successful associative inference. Error bars represent ± 1 SEM.
Experiment 2
Identical to Experiment 1, we examined source memory errors for the detail and source monitoring questions with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA. Results revealed no main effect of time, F(1,23) = .357, p > .250, ηp2 = .02, no main effect of inference, F(1,23) = .57, p > .250, ηp2 = .02, and a significant time by inference interaction, F(1,23) = 7.40, p = .012, ηp2 = .24 (see Fig. 2b). Participants falsely attributed more details to the overlapping event after successful inference retrieval (Mafter = 0.28, SE = 0.02) than before successful inference retrieval (Mbefore = 0.22, SE = 0.02; t(23) = 2.48, p = .021, mean difference = 0.06, 95% CI = [0.01, 0.11], d = .51). Further, participants did not falsely attribute more details to the overlapping event after unsuccessful inference retrieval (Mafter = 0.22, SE = 0.02) than before unsuccessful inference retrieval (Mbefore = 0.25, SE = 0.03; t(23) = −1.022, p > .250, mean difference = −0.03, 95% CI = [−0.10, 0.03], d = .21). Participants did not falsely attribute more details to the overlapping event before successful inference retrieval (Mcorrect = 0.22, SE = 0.02) than before unsuccessful inference retrieval (Mincorrect = 0.25, SE = 0.03; t(23) = 1.40, p = .175, mean difference = 0.03, 95% CI = [−0.02, 0.08], d = .29). Critically, participants falsely attributed more details to the overlapping event after successful inference retrieval (Mcorrect = 0.28, SE = 0.02) than after unsuccessful inference retrieval (Mincorrect = 0.22, SE = 0.02; t(23) = 2.56, p = .018, mean difference = 0.06, 95% CI = [0.01, 0.11], d = .52), replicating results from Experiment 1 and suggesting that recombination during retrieval required for successful inference may be linked to source memory errors.
True Memory
Experiment 1
To examine the effects of integrative encoding and recombination mechanisms at retrieval on successful source memory, we examined correct responses on the detail and source monitoring questions with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA. Results revealed no main effect of time, F(1,23) = 2.33, p = .141, ηp2 = .09, no main effect of inference, F(1,23) = .10, p > .250, ηp2 = .02, but a significant time by inference interaction, F(1,23) = 6.83, p = .016, ηp2 = .23. Participants attributed more details to the correct source after successful inference retrieval (Mafter = 0.23, SE = 0.02) than before successful inference retrieval Mbefore = 0.17 SE = 0.02; t(23) = 3.82, p = .001, mean difference = 0.06, 95% CI = [0.03, 0.09], d = .82). By contrast, participants did not attribute more details to the correct source after successful inference retrieval (Mcorrect = 0.23 SE = 0.02) than after unsuccessful inference retrieval (Mincorrect = 0.20 SE = 0.03; t(23) = 1.04, p > .250, mean difference = 0.03, 95% CI = [−0.03, 0.08], d = .21) and did not attribute more details to the correct source before successful inference retrieval (Mcorrect = 0.17 SE = 0.02) than before unsuccessful inference retrieval (Mincorrect = 0.22 SE = 0.03; t(23) = 1.50, p = .146, mean difference = 0.04, 95% CI = [−0.02, 0.10], d = .31). Further, participants did not attribute more details to the correct source after unsuccessful inference retrieval (Mafter = 0.20 SE = 0.03) than before unsuccessful inference retrieval (Mbefore = 0.22 SE = 0.03; t(23) < 1, p > .250, mean difference = 0.01, 95% CI = [−0.03, 0.06], d = .12). The increase in true memory from before inference retrieval to after appears to be attributable to changes in ‘unsure’ responses on the source monitoring test following recognition of the correct item: Participants were significantly less likely to respond unsure following successful inference when they correctly recognized the detail (Mafter = 0.12 SE = 0.05) than before successful inference (Mbefore = 0.26 SE = 0.06; t(23) = 3.04, p = .008, mean difference = 0.14, 95% CI = [0.04, 0.23], d = .18).
Experiment 2
A 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA on correct responses to the detail and source monitoring questions revealed no main effect of time, F(1,23) = .40, p > .250, ηp2 = .02, no main effect of inference, F(1,23) = .55, p > .250, ηp2 = .02, and no time by inference interaction, F(1,23) = 1.34, p > .250, ηp2 = .06. Thus, true memory scores were similar both before (Mbefore = .18, SE = .029) and after successful inference retrieval (Mafter = 0.21, SE = 0.03). Additionally, true memory scores were similar both before (Mbefore = 0.16, SE = 0.03) and after unsuccessful inference retrieval (Mafter = 0.20, SE = 0.03).
Foil Memory
Experiment 1
To assess whether critical patterns of source misattribution errors are specific to related items from previously studied episodes, we examined foil memories, which were defined as detail questions for which participants chose the unrelated foil option (e.g., grey couch) and attributed the information to either the currently cued image or both images in the triad. We conducted a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA to evaluate participants’ foil memory scores. Results revealed no main effect of time, F(1,23) = 1.16, p > .250, ηp2 = .05, no main effect of inference, F(1,23) = 1.71, p = .204, ηp2 = .07, and no time by inference interaction, F(1,23) = 1.59, p = .220, ηp2 = .07. Thus, foil memory scores were similar both before (Mbefore = 0.18, SE = 0.01) and after successful inference retrieval (Mafter = 0.18, SE = 0.01). Additionally, foil memory scores were similar both before (Mbefore = 0.14, SE = 0.03) and after unsuccessful inference retrieval (Mafter = 0.17, SE = 0.02).
Experiment 2
We conducted a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA to evaluate participants’ foil memory scores. Results revealed no main effect of time, F(1,23) = 1.04, p > .250, ηp2 = .04, no main effect of inference, F(1,23) = 1.28, p > .250, ηp2 = .05, and no time by inference interaction, F(1,23) = 1.22, p > .250, ηp2 = .05. Thus, foil memory scores were similar both before (Mbefore = 0.21, SE = 0.02) and after successful inference retrieval (Mafter = 0.17, SE = 0.02). Additionally, foil memory scores were similar both before (Mbefore = 0.21, SE = 0.03) and after unsuccessful inference retrieval (Mafter = 0.21, SE = 0.02).
Discussion
The results of Experiments 1 and 2 provide evidence that flexible retrieval processes required for successful associative inference also produce increases in false memories as a result of source misattributions: memory errors increased significantly after but not before successful compared to unsuccessful inferential retrieval. This pattern was observed both when the test of directly learned and associative inference items was two-alternative forced-choice (Experiment 1), and also when a third “neither” option was provided (Experiment 2); the effect was also robust across both a 24-hour study-test delay (Experiment 1) and a 48-hour study-test delay (Experiment 2).
The finding that source misattributions occurred more often for correct versus incorrect inferences constitutes evidence for a link between processes that support associative inference and those that contribute to false memories; if there were no such link, then source memory errors would not differ for correct and incorrect inferences. This finding alone, however, does not allow us to distinguish whether integrative encoding or flexible retrieval is responsible for the observed increased of source memory errors related to successful inference. However, the finding that the observed increase in false memories for correct inference occurred only after the associative inference test was given implicates flexible retrieval, rather than integrative encoding, as the key process responsible for the boost in false memories. Furthermore, foil memory scores (i.e., detail questions for which participants chose the unrelated foil option) showed no relationship to correct vs. incorrect inferences either before or after the associative inference test was given. This finding indicates that the observed source memory error effects are selective to previously experienced details, details that seemingly migrated between the AB and BC episodes as a consequence of successful inference.
Experiment 3
In Experiments 1 and 2, we replicated the key effect of successful inference on false memories across minor variations in procedure, suggesting that the effect is reliable. However, the absolute magnitude of the effect is not large, and because the critical tests in Experiments 1 and 2 were administered in immediate succession, we do not know whether the process of recombination during retrieval that supports successful inference results in only a transient change in participants’ susceptibility to source memory errors. To further assess the reliability of the key effect, and to determine whether the effects of recombination allowing for successful associative inference on subsequent source memory error lasts beyond the brief interval between the main tests and further shows a longer-lasting effect on participants susceptibility to source memory error, in Experiment 3 we introduced a 30-minute delay between the directly learned (AB and BC)/associative inference trials (AC) on the one hand and the second set of detail and source questions on the other.
Methods
Participants
Experiment 3 included 24 young adults (mean age = 20, SD = 2.07; 15 female). No participants were excluded from the analyses; thus, our final sample consisted of 24 participants.
Summary of the Procedure
Participants came to the lab for two sessions, separated by a 48-hour delay. The design parameters, stimuli, and coding of true and false memories were exactly the same in Experiment 3 as in Experiment 2 with one exception. During the second session, following the test of the directly learned (AB and BC) and associative inference trials (AC), participants completed 30-minutes of unrelated filler tasks (e.g., simple math problems) before completing the second half of the detail and source questions. As noted earlier, in Experiment 3 we introduced a 30-minute delay between the directly learned/associative inference trials and the second set of detail and source questions.
Results and Discussion
Directly Learned and Associative Inference Trials
First we evaluated overall accuracy on directly learned and associative inference trials. Performance on both directly learned and associative inference trials was generally accurate, and there was no significant difference in the proportion of directly learned (Mdirect = 0.72, SE = 0.02) as compared to associative inference trials (Massociative inference = 0.71, SE = 0.02) that participants answered correctly (t(23) = 0.62, p > .250, mean difference = 0.01, 95% confidence interval (CI) = [−0.03, 0.06], d = .13). Consistent with previous research (Zeithamova & Preston, 2010) and results from Experiment 1 and 2, we found significantly longer reaction times on associative inference trials (Massociative inference = 4186 msec, SE = 196) as compared to directly learned trials (Mdirect = 3057 msec, SE = 123), suggesting that there may be an additional recombination-related retrieval mechanism necessary for inferential versus direct retrieval (t(23) = −7.46, p < .001, mean difference = −1.13, 95% CI = [−1.44, −.82], d = 1.52). Further, participants assigned significantly higher confidence ratings to their responses on directly learned (Mdirect = 3.23, SE = 0.08) as compared to associative inference trials (Massociative inference = 2.83, SE = 0.10), suggesting that participants were more confident in their memory for events that they had directly experienced as compared to those resulting from recombination (t(23) = 8.97, p < .001, mean difference = 0.40, 95% CI = [0.31, 0.50], d = 1.83).
False Memory
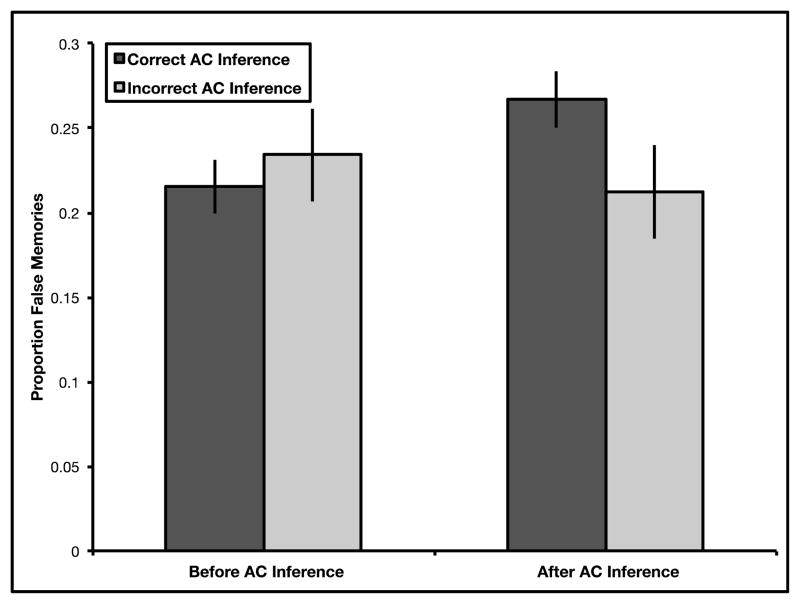
Identical to Experiments 1 and 2, we examined source memory errors for the detail and source monitoring questions with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA. Results revealed no main effect of time, F(1,23) = 0.46, p > .250, ηp2 = .02, no main effect of inference, F(1,23) = 1.17, p > .250, ηp2 = .05, and a significant time by inference interaction, F(1,23) = 5.89, p = .023, ηp2 = .20 (see Fig. 3). Participants falsely attributed more details to the overlapping event after successful inference retrieval (Mafter = 0.27, SE = 0.02) than before successful inference retrieval (Mbefore = 0.22, SE = 0.02; t(23) = 3.46, p = .002, mean difference = 0.05, 95% CI = [0.02, 0.08], d = .71). Further, participants did not falsely attribute more details to the overlapping event after unsuccessful inference retrieval (Mafter = 0.21, SE = 0.03) than before unsuccessful inference retrieval (Mbefore = 0.23, SE = 0.03; t(23) = −0.63, p > .250, mean difference = −0.02, 95% CI = [−0.09, 0.05], d = .13). Participants did not falsely attribute more details to the overlapping event before successful inference retrieval (Mcorrect = 0.22, SE = 0.02) than before unsuccessful inference retrieval (Mincorrect = 0.23, SE = 0.03; t(23) = .829, p > .250, mean difference = 0.02, 95% CI = [−0.03, 0.06], d = .17). Critically, participants falsely attributed more details to the overlapping event after successful inference retrieval (Mcorrect = 0.27, SE = 0.02) than after unsuccessful inference retrieval (Mincorrect = 0.21, SE = 0.03; t(23) = 2.49, p = .021, mean difference = 0.05, 95% CI = [0.009, 0.10], d = .51), replicating results from Experiment 1 and 2, suggesting again that recombination during retrieval required for successful inference may be linked to source memory errors.
Fig. 3.
Proportion of false memories in Experiment 3. Performance on detail and source monitoring questions was examined both before and after either successful or unsuccessful inference. Importantly, only trials for which participants responded correctly to directly learned trials were included in this analysis. Results revealed a significant time by inference interaction in Experiment 3. Subsequent t-tests confirm that false memories selectively increased only following successful associative inference. Error bars represent ± 1 SEM.
True Memory
A 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA on correct responses to the detail and source monitoring questions revealed no main effect of time, F(1,23) = 0.46, p > .250, ηp2 = .02, no main effect of inference, F(1,23) = 0.91, p > .250, ηp2 = .04, and no time by inference interaction, F(1,23) = .042, p > .250, ηp2 = .002. Thus, true memory scores were similar both before (Mbefore = .22, SE = .02) and after successful inference retrieval (Mafter = 0.24, SE = 0.02). Additionally, true memory scores were similar both before (Mbefore = 0.21, SE = 0.04) and after unsuccessful inference retrieval (Mafter = 0.22, SE = 0.02).
Foil Memory
We conducted a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA to evaluate participants’ foil memory scores. Results revealed no main effect of time, F(1,23) = 1.74, p = .200, ηp2 = .07, no main effect of inference, F(1,23) = 0.62, p > .250, ηp2 = .03, and no time by inference interaction, F(1,23) = 2.23, p = .150, ηp2 = .09. Thus, foil memory scores were similar both before (Mbefore = 0.20, SE = 0.02) and after successful inference retrieval (Mafter = 0.16, SE = 0.01). Additionally, foil memory scores were similar both before (Mbefore = 0.16, SE = 0.02) and after unsuccessful inference retrieval (Mafter = 0.17, SE = 0.02).
Overall, the pattern of results from Experiment 3 was essentially identical to that observed in Experiments 1 and 2: participants made significantly more source misattributions for correct than incorrect inferences, but only when the detail and source monitoring test was given after the test of directly learned and associative inference items. Because the second source test was administered 30 minutes after completion of the directly learned and associative inference test, Experiment 3 indicates that the effects we have attributed to flexible recombination during retrieval are not simply transient influences that disappear following a filled delay.
Experiment 4
Although our central theoretical claim of Experiments 1–3 focuses on retrieval-related recombination processes that occurred during the associative inference test for previously unpaired AC items, participants were also tested for AB and BC items that did appear together previously. Thus, it is conceivable that the increase in source memory errors following the associative inference test is attributable to direct retrieval of previously studied pairs (AB, BC) as opposed to retrieval-related recombination processes. Two key features of the data from Experiments 1–3 speak against this possibility. First, if retrieval of directly learned associations were responsible for the increase in false memories, then false memory rates should have been similar for successful and unsuccessful inferential retrieval after the associative inference test, but as noted above memory errors increased significantly following successful compared to unsuccessful inferential retrieval. Second, neither experiment revealed significant differences in the number false memories before as compared to after unsuccessful inferential retrieval. If testing of directly learned pairs during the associative inference test were responsible for the increased false memory effects after as compared to before the associative inference test, then those effects should have been observed for unsuccessful inference trials. However, no such effects were observed. While the results of Experiments 1–3 thus suggest that the testing of directly learned associations is not responsible for the key effects of successful inference on false memories, in Experiment 4 we assess this possibility empirically by testing directly learned associations only after both sets of detail and source monitoring tests were completed. If, as we have suggested, recombination during retrieval is responsible for observed increases in false memories, then the same critical pattern of results from previous experiments – more source misattributions for correct than incorrect inference items, after but not before the inference test – should be observed in Experiment 4, even though directly learned associations were not tested prior to the detail and source memory tests.
Methods
Participants
Experiment 4 included 26 young adults (mean age = 20.70, SD = 2.19; 16 female). Two participants were excluded from the true and false memory analyses. One participant was excluded from the true, false, and foil memory analyses because they were 100 percent accurate on the associative inference trials and one participant was excluded from all analyses for non-compliance during the second session (e.g., did not make responses during the detail and source monitoring questions); thus, our final sample consisted of 24 participants.
Summary of the Procedure
Participants came to the lab for two sessions, separated by a 48-hour delay. The design parameters, stimuli, and coding of true and false memories were exactly the same in Experiment 4 as in Experiment 2 with the one exception. During the second session, following the first half of the detail and source questions, participants were only tested on associative inference trials (AC), thus, eliminating the potential effect of retrieving directly learned associations on false memory following successful associative inference. However, to ensure that we still obtained a measure of performance on directly learned items, following the second half of the detail and source questions, participants were tested on directly learned trials (AB and BC).
Results and Discussion
Directly Learned and Associative Inference Trials
First we evaluated overall accuracy on directly learned and associative inference trials. Performance on both directly learned and associative inference trials was generally accurate, and there was no significant difference in the proportion of directly learned (Mdirect = 0.66, SE = 0.03) as compared to associative inference trials (Massociative inference = 0.70, SE = 0.03) that participants answered correctly (t(24) = −1.01, p > .250, mean difference = −0.04, 95% confidence interval (CI) = [−0.11, 0.04], d = .20). Consistent with previous research (Zeithamova & Preston, 2010) and results from Experiments 1–3, we found significantly longer reaction times on associative inference trials (Massociative inference = 5140 msec, SE = 242) as compared to directly learned trials (Mdirect = 3300 msec, SE = 148), suggesting that there may be an additional recombination-related retrieval mechanism necessary for inferential versus direct retrieval (t(24) = −10.18, p < .001, mean difference = −1.84, 95% CI = [−2.21, −1.47], d = 2.04). Further, participants assigned significantly higher confidence ratings to their responses on directly learned (Mdirect = 3.15, SE = 0.12) as compared to associative inference trials (Massociative inference = 2.87, SE = 0.11), suggesting that participants were more confident in their memory for events that they had directly experienced as compared to those resulting from recombination (t(24) = 3.06, p = .005, mean difference = 0.27, 95% CI = [0.89, 0.46], d = 0.61).
False Memory
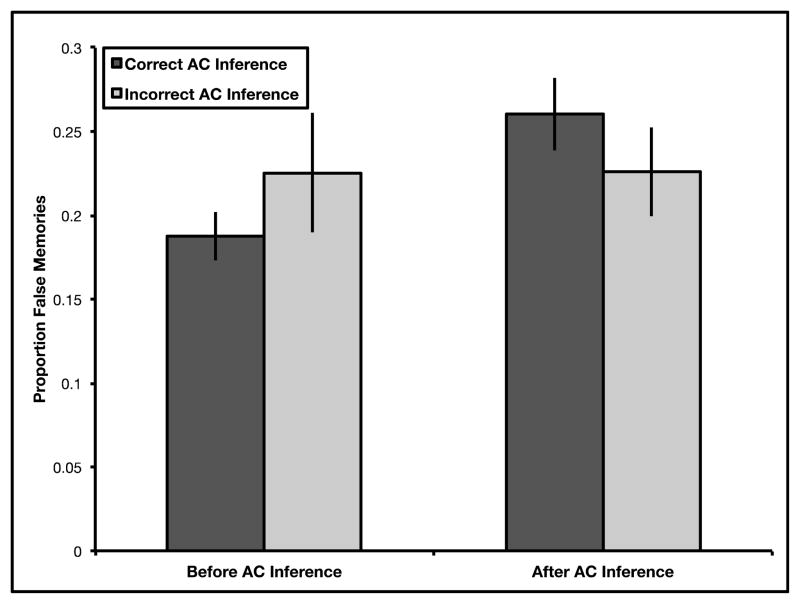
Identical to Experiments 1–3, we examined source memory errors for the detail and source monitoring questions with a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA. Results revealed no main effect of time, F(1,23) = 1.60, p = .219, ηp2 = .07, no main effect of inference, F(1,23) = .011, p > .250, ηp2 = .00, and a significant time by inference interaction, F(1,23) = 4.79, p = .039, ηp2 = .17 (see Fig. 4). Participants falsely attributed more details to the overlapping event after successful inference retrieval (Mafter = 0.26, SE = 0.02) than before successful inference retrieval (Mbefore = 0.19, SE = 0.01; t(23) = 3.20, p = .004, mean difference = 0.07, 95% CI = [0.03, 0.12], d = .65). Further, participants did not falsely attribute more details to the overlapping event after unsuccessful inference retrieval (Mafter = 0.23, SE = 0.03) than before unsuccessful inference retrieval (Mbefore = 0.23, SE = 0.04; t(23) = .010, p > .250, mean difference = 0.0004, 95% CI = [−0.09, 0.09], d = .002). Participants did not falsely attribute more details to the overlapping event before successful inference retrieval (Mcorrect = 0.19, SE = 0.01) than before unsuccessful inference retrieval (Mincorrect = 0.23, SE = 0.04; t(23) = 1.22, p = .231, mean difference = 0.04, 95% CI = [−0.03, 0.10], d = .25). Critically, participants falsely attributed more details to the overlapping event after successful inference retrieval (Mcorrect = 0.26, SE = 0.02) than after unsuccessful inference retrieval (Mincorrect = 0.23, SE = 0.03; t(23) = 2.37, p = .027, mean difference = 0.03, 95% CI = [0.004, 0.64], d = .48), replicating results from Experiments 1–3, suggesting that recombination during retrieval required for successful inference may be linked to source memory errors.
Fig. 4.
Proportion of false memories in Experiment 4. Performance on detail and source monitoring questions was examined both before and after either successful or unsuccessful inference. Importantly, only trials for which participants responded correctly to directly learned trials were included in this analysis. Results revealed a significant time by inference interaction in Experiment 4. Subsequent t-tests confirm that false memories selectively increased only following successful associative inference. Error bars represent ± 1 SEM.
True Memory
A 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA on correct responses to the detail and source monitoring questions revealed no main effect of time, F(1,23) = 2.53, p = .13, ηp2 = .10, no main effect of inference, F(1,23) = 1.68, p = .21, ηp2 = .07, and no time by inference interaction, F(1,23) = .43, p > .250, ηp2 = .02. Thus, true memory scores were similar both before (Mbefore = .22, SE = .031) and after successful inference retrieval (Mafter = 0.17, SE = 0.02). Additionally, true memory scores were similar both before (Mbefore = 0.18, SE = 0.03) and after unsuccessful inference retrieval (Mafter = 0.16, SE = 0.03).
Foil Memory
We conducted a 2 (time: before vs. after inference retrieval) X 2 (inference: correct vs. incorrect inference) repeated measures ANOVA to evaluate participants’ foil memory scores. Results revealed no main effect of time, F(1,23) = 3.67, p = .068, ηp2 = .14, no main effect of inference, F(1,23) = 2.47, p = .130, ηp2 = .10, and no time by inference interaction, F(1,23) = 0.59, p > .250, ηp2 = .03. Thus, foil memory scores were similar both before (Mbefore = 0.18, SE = 0.02) and after successful inference retrieval (Mafter = 0.19, SE = 0.02). Additionally, foil memory scores were similar both before (Mbefore = 0.14, SE = 0.02) and after unsuccessful inference retrieval (Mafter = 0.18, SE = 0.02).
In summary, the results of Experiment 4 replicated the results of Experiment 1–3, while also providing additional evidence that testing of directly learned pairs during the associative inference test was not responsible for the increased false memory effects after as compared to before successful associative inference. During the associative inference test for Experiment 4, participants were only tested on previously unpaired AC items (i.e., inference items) before the second set of detail and source questions. Thus, the increase in source memory errors following the associative inference test in Experiment 4 cannot be attributable to direct retrieval of previously studied pairs; rather, the current results support the role of recombination-related retrieval processes in subsequent source memory errors.
General Discussion
The four experiments reported here each provide evidence that flexible retrieval processes that support successful associative inference also produce increases in false memories that result from source misattributions: memory errors increased significantly after but not before successful compared to unsuccessful inferential retrieval. Experiments 1 and 2 provided evidence that flexible retrieval processes required for successful associative inference also produce increases in source misattributions when the detail/source memory test immediately followed the test of directly learned and associative inference items, whereas Experiment 3 revealed that these effects persisted across a 30-minute delay between the associative inference tests and the second source memory test. Experiment 4 revealed the same significant increase in source memory error after but not before successful compared to unsuccessful inferential retrieval as observed in Experiment 1–3 even when directly learned associations were not tested until participants completed all of the detail and source monitoring questions. Further, across all four experiments results revealed that both foil memory and correct memory scores showed no relationship to correct as compared to incorrect inference either before or after the directly learned and associative inference test, thereby indicating that the observed effects are specific to the misattribution of previously experienced details to the related event rather than to a general decrease of detail with which the original event was remembered. Thus, the results of all four experiments provide direct evidence supporting the role of flexible retrieval and recombination processes in both successful associative inference and subsequent source memory error. These data thus provide, for the first time, direct experimental support for a key claim of the constructive episodic simulation hypothesis (Schacter & Addis, 2007a, 2007b), namely that the same flexible recombination process that supports an adaptive cognitive process can also increase memory errors that result from combining elements of distinct episodes. More generally, our results add to the mounting evidence that certain kinds of memory errors result from the operation of adaptive constructive processes that are linked to beneficial effects (for reviews, see Howe, 2011; Howe et al., 2016; Newman & Lindsay, 2009; Schacter, 2012; Schacter et al., 2011).
As noted in the Introduction, previous research (cf., Shohamy & Wagner, 2008; Zeithamova & Preston, 2010) indicates that successful associative inference in the AB, BC paradigm used here can result from flexible retrieval and/or integrative encoding (i.e., during study of BC, participants recall the related AC pair and encode an integrated representation (ABC) that they later retrieve on the associative inference test). If integrative encoding contributes to false memories in our paradigm, then there should be more false memories for successful than unsuccessful inference trials before the associative inference test, but such effects were observed only after the associative inference test. Note, however, that previous research suggests that integrative encoding primarily supports associative inference when learning occurs across multiple repetitions, by affording multiple opportunities for cross-episode binding (Shohamy & Wagner, 2008; Zeithamova & Preston, 2010). By contrast, our experimental design utilized a single-trial learning paradigm that elicits an additional recombination mechanism during successful inference retrieval (Zeithamova & Preston, 2010). It is thus possible that when there are multiple repetitions during the learning phase, or under other experimental conditions that heighten the contribution of integrative encoding to associative inference, integrative encoding processes contribute to the type of source memory errors observed here. Thus, while the present data provide evidence for a link between flexible retrieval and false memories, they by no means rule out a similar link to integrative encoding under a different set of experimental parameters that are more likely to elicit successful associative inference as a result of integration during encoding. Future research should aim to examine the role of integration during encoding on subsequent source memory error.
Why does successful inferential retrieval result in heightened susceptibility to source memory errors? We suggest that the effects that we have documented here reflect the joint operation of two related but distinct mechanisms: cross-episode binding (e.g., Bridge & Voss, 2014a, 2014b) and retrieval-based reactivation and recombination (e.g., Bridge & Voss, 2014a, 2014b; Hupbach, Gomez, Hardt, & Nadel, 2007; St. Jacques & Schacter, 2013). Binding processes that link disparate elements of an episode into a unified representation have been extensively studied in recent years, and have been linked closely to the operation of the hippocampus (e.g., Eichenbaum & Cohen, 2001; Hannula & Ranganath, 2008; Shimamura, 2010). As Bridge and Voss (2014b) point out, however, most such studies have focused on binding of elements within an episode. Bridge and Voss (2014b) studied cross-episode binding processes, and provided evidence that participants sometimes bind elements from distinct episodes (e.g., a face from one episode and a scene from another), resulting in memory error (for additional evidence linking binding processes to memory distortions, see Lew & Howe, in press). We suggest that such cross-episode binding in our paradigm occurs most often and most extensively for episodes that result in successful, as opposed to unsuccessful, associative inference. That is, when people make a correct inference about the relationship between elements of events that have not been experienced together previously (i.e., AC), they may more fully bind details from the two episodes, such that details from one episode (AB) migrate to and become incorporated in the overlapping (BC) episode.
However, this binding account alone cannot explain the key finding from our experiments that increased false memories were observed for successful compared to unsuccessful inference trials only when the detail/source memory test was given after the associative inference test, and it is this finding that has led us to implicate a role for flexible retrieval and recombination processes in increased source memory errors. These observations fit well with prior findings that reactivating or retrieving memories can be a potent source of memory distortion if novel information is incorporated into a memory during the retrieval process (e.g., Chan, Thomas, & Bulevich, 2009; Gerschman, Schapiro, Hupbach, Norman, 2013; Gordon, Thomas, & Bulevich, 2015; Hupbach et al., 2007; Hupbach, Gomez, & Nadel, 2011; St. Jacques, Olm, & Schacter, 2013; St. Jacques & Schacter, 2013), possibly related to processes associated with memory reconsolidation that render a memory labile and prone to distortion during retrieval (Chan & Lapaglia, 2013; Dudai, 2012; Hardt, Einarsson, & Nader, 2010). From this perspective, in our experimental paradigm source memory errors arise when overlapping AB and BC relationships (along with their corresponding contextual details) are reactivated and flexibly recombined in order to encode the novel inference between the previously unrelated A and C items. Indeed, and consistent with our results, Bridge and Voss (2014b) only observed evidence for memory distortion associated with cross-episode binding following an active (versus passive) retrieval condition. In line with the current results, retrieval-related recombination may thus result in heightened rates of source memory error following successful compared to unsuccessful inference because inferring the relationship between the nonoverlapping A and C items requires both a) reactivating distinct AB and BC episodes and b) flexibly recombining the nonoverlapping A and C items – during which contextual details from the AB episode are more fully bound to the BC episode and visa versa – resulting in memory distortions associated with cross-episode binding as a consequence of flexible retrieval and recombination processes. An important task for future research is to explore and clarify exactly how the recombination process supporting successful inference produces such erroneous memories. While previous evidence supports the idea that memory errors can result from erroneously combining details of individual episodic or autobiographical memories (e.g., Burt et al., 2004; Devitt et al., 2015; Odegard & Lampinen, 2004), the present studies provide novel evidence that the same flexible recombination mechanism that supports an adaptive cognitive process, such as associative inference, also increases subsequent memory errors.
Although we are not aware of any prior studies that have specifically linked successful associative inference with memory errors, as noted earlier previous research has linked memory reactivation processes with source misattributions and related kinds of memory errors. The studies noted earlier by Bridge and Voss (2014a, 2014b) suggest that simply co-activating memories during retrieval can lead to source misattributions, wherein co-activation of existing memory traces produces cross-episode binding of peripheral features from each episode. Although these results are consistent with the results reported here, it is unlikely that simple co-activation of elements from different episodes is sufficient to account for our key results. Our data speak against a simple co-activation hypothesis specifically because only trials for which participants were able to successfully reactivate both AB and BC episodes (as assessed by the test for directly learned associations) were used in the false memory analyses. Thus, both AB and BC events should have been successfully reactivated during the inference test. Accordingly, if co-activation of AB and BC events accounted for the increase in source memory error, we would not expect to see a significant difference between successful inference and unsuccessful inference after the associative inference test. Because we observed such a difference, we suggest that successful inference requires an additional retrieval-related recombination process that results in increased source memory error. Indeed, in each of our experiments we observed significantly longer reaction times on associative inference trials than on directly learned trials, which is in line with the arguments of Zeithamova and Preston (2010), suggesting that there is an additional retrieval mechanism necessary for inferential versus direct retrieval following single-trial learning. Co-activation of memories at test clearly can lead to source misattributions (Bridge & Voss, 2014a, 2014b), and it may be a contributing factor and perhaps even a necessary condition for increased source memory errors in the current paradigm. Nonetheless, co-activation of elements from distinct episodes during retrieval does not appear to a sufficient condition for producing the increase in source misattributions in the current paradigm. Alternatively, co-activation may have an effect during encoding such that participants bring to mind overlapping AB pairs during BC encoding thereby linking the two related events. However, if this were the case we would expect to see elevated source memory error before successful inference, which was not the case in our experiments, as we emphasized in the discussion of integrative encoding.
Although we have emphasized throughout the distinction between integrative encoding and flexible retrieval, and provided in the Introduction explicit predictions regarding outcomes that distinguish between these processes, it is important to emphasize we are not advocating that a simple encoding-retrieval dichotomy can account for the results observed here. Students of memory have long recognized that that encoding processes involve retrieval and vice versa. With respect to the present paradigm, integrative encoding requires some amount of retrieval (i.e., during study of BC, participants retrieve an overlapping AB pair in order to encode an integrated ABC representation), and flexible retrieval results in some degree of encoding (i.e., cross-episode binding). Nonetheless, the pattern of results observed here indicates a sharp difference in patterns of false memory before and after the associative inference test, which we have attempted to characterize in terms of the joint operation of cross-episode binding and flexible retrieval processes.
We have emphasized throughout that the current results fit well with an emerging theoretical picture in which various kinds of memory distortions are viewed as products of adaptive constructive processes (Schacter, 2012) that serve a range of cognitive functions, including simulating future experiences (Dudai & Carruthers, 2005; Schacter & Addis, 2007a, 2007b; Suddendorf & Corballis, 2007), solving problems (Howe, Garner, Charlesworth, & Knott, 2011), memory updating (Hardt et al., 2010; Hupbach et al., 2007, 2011; St. Jacques et al., 2013), and extracting gist or meaning (Brainerd & Reyna, 1990; Koutstaal, 2006; Schacter, 2001; for recent reviews, see Howe, 2011; Howe et al., 2016; Newman & Lindsay, 2009; Schacter et al, 2011; Schlichting & Preston, 2015). Here we have focused on associative inference, which serves the adaptive function of allowing us to make new connections, and decisions about novel situations, based on flexibly retrieving and recombining information acquired in distinct though related prior experiences (Zeithamova, Schlichting, & Preston, 2012). Neuroimaging studies have linked the retrieval-based recombination process that supports associative inference to hippocampal function (Zeithamova & Preston, 2010; Zeithamova, Dominick, & Preston, 2012), but the nature of hippocampal contributions to the kinds of false memories associated with successful inference is unknown. Future research aimed at delineating the neural basis of the costs and benefits associated with flexible retrieval and recombination would enhance our understanding of the nature and functions of episodic memory.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Mental Health grant MH060941 and National Institute on Aging grant AG08441 to D.L.S. We thank Karen Campbell, Aleea Devitt, Alison Preston, and Preston Thakral for helpful comments on an earlier draft of the manuscript.
References
- Alba JW, Hasher L. Is memory schematic? Psychological Bulletin. 1983;93(2):203–231. http://doi.org/10.1037/0033-2909.93.2.203. [Google Scholar]
- Bartlett FC. Remembering: A study in experimental and social psychology. Cambridge: Cambridge University Press; 1932. [Google Scholar]
- Brainerd CJ, Reyna VF. Gist is the grist: Fuzzy-trace theory and the new intuitionism. Developmental Review. 1990;10:3–47. http://doi.org/10.1016/0273-2297(90)90003-M. [Google Scholar]
- Brainerd CJ, Reyna VF. The science of false memory. Oxford University Press; 2005. http://doi.org/10.1093/acprof:oso/9780195154054.001.0001. [Google Scholar]
- Bransford JD, Barclay JR, Franks JJ. Sentence memory: A constructive versus interpretive approach. Cognitive Psychology. 1972;3(2):193–209. http://doi.org/10.1016/0010-0285(72)90003-5. [Google Scholar]
- Bransford JD, Franks JJ. The abstraction of linguistic ideas. Cognitive Psychology. 1971;2(4):331–350. http://doi.org/10.1016/0010-0285(71)90019-3. [Google Scholar]
- Bridge DJ, Voss JL. Hippocampal binding of novel information with dominant memory traces can support both memory stability and change. Journal of Neuroscience. 2014a;34:2203–13. doi: 10.1523/JNEUROSCI.3819-13.2014. http://doi.org/10.1523/JNEUROSCI.3819-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge DJ, Voss JL. Active retrieval facilitates across-episode binding by modulating the content of memory. Neuropsychologia. 2014b;63:154–64. doi: 10.1016/j.neuropsychologia.2014.08.024. http://doi.org/10.1016/j.neuropsychologia.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt CDB, Kemp S, Conway M. Memory for true and false autobiographical event descriptions. Memory. 2004;12:545–52. doi: 10.1080/09658210344000071. http://doi.org/10.1080/09658210344000071. [DOI] [PubMed] [Google Scholar]
- Chan JCK, LaPaglia JA. Impairing existing declarative memory in humans by disrupting reconsolidation. Proceedings of the National Academy of Sciences. 2013;110(23):9309–9313. doi: 10.1073/pnas.1218472110. http://doi.org/10.1073/pnas.1218472110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JCK, Thomas AK, Bulevich JB. Recalling a witnessed event increases eyewitness suggestibility: The reversed testing effect. Psychological Science. 2009;20:66–73. doi: 10.1111/j.1467-9280.2008.02245.x. http://doi.org/10.1111/j.1467-9280.2008.02245.x. [DOI] [PubMed] [Google Scholar]
- Devitt AL, Monk-Fromont E, Schacter DL, Addis DR. Factors that influence the generation of autobiographical memory conjunction errors. Memory. 2015;24:204–22. doi: 10.1080/09658211.2014.998680. http://doi.org/10.1080/09658211.2014.998680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. The restless engram: Consolidations never end. Annual Review of Neuroscience. 2012;35(1):227–247. doi: 10.1146/annurev-neuro-062111-150500. http://doi.org/10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Carruthers M. The Janus face of Mnemosyne. Nature. 2005;434:567. doi: 10.1038/434567a. http://doi.org/10.1038/434567a. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. Oxford: Oxford University Press; 2001. [Google Scholar]
- Gallo DA. Associative illusions of memory: False memory research in DRM and related tasks. New York, NY, US: Psychology Press; 2006. [Google Scholar]
- Gershman SJ, Schapiro AC, Hupbach A, Norman KA. Neural context reinstatement predicts memory misattribution. Journal of Neuroscience. 2013;33(20):8590–5. doi: 10.1523/JNEUROSCI.0096-13.2013. http://doi.org/10.1523/JNEUROSCI.0096-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon LT, Thomas AK, Bulevich JB. Looking for answers in all the wrong places: How testing facilitates learning of misinformation. Journal of Memory and Language. 2015;83:140–151. http://doi.org/10.1016/j.jml.2015.03.007. [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. Journal of Neuroscience. 2008;28(1):116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. http://doi.org/10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt O, Einarsson EO, Nader K. A bridge over troubled water: Reconsolidation as a link between cognitive and neuroscientific memory research traditions. Annual Review of Psychology. 2010;61:141–67. doi: 10.1146/annurev.psych.093008.100455. http://doi.org/10.1146/annurev.psych.093008.100455. [DOI] [PubMed] [Google Scholar]
- Howe ML. The adaptive nature of memory and its illusions. Current Directions in Psychological Science. 2011;20:312–315. http://doi.org/10.1177/0963721411416571. [Google Scholar]
- Howe ML, Garner SR, Charlesworth M, Knott L. A brighter side to memory illusions: False memories prime children’s and adults’ insight-based problem solving. Journal of Experimental Child Psychology. 2011;108:383–393. doi: 10.1016/j.jecp.2010.08.012. http://doi.org/10.1016/j.jecp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Howe ML, Wilkinson S, Garner SR, Ball LJ. On the adaptive function of children’s and adults’ false memories. Memory. 2016;24(8):1062–77. doi: 10.1080/09658211.2015.1068335. http://doi.org/10.1080/09658211.2015.1068335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learning and Memory. 2007;14:47–53. doi: 10.1101/lm.365707. http://doi.org/10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Nadel L. Episodic memory updating: The role of context familiarity. Psychological Bulletin & Review. 2011;18:787–797. doi: 10.3758/s13423-011-0117-6. http://doi.org/10.3758/s13423-011-0117-6. [DOI] [PubMed] [Google Scholar]
- Koutstaal W. Flexible remembering. Psychonomic Bulletin & Review. 2006;13:84–91. doi: 10.3758/bf03193817. http://doi.org/10.3758/BF03193817. [DOI] [PubMed] [Google Scholar]
- Lew AR, Howe ML. Out of place, out of mind: Schema-driven false memory effects for object-location bindings. Journal of Experimental Psychology: Learning, Memory, and Cognition. doi: 10.1037/xlm0000317. in press. http://dx.doi.org/10.1037/xlm0000317. [DOI] [PubMed]
- Loftus E. Make-believe memories. American Psychologist. 2003;58:555–583. doi: 10.1037/0003-066X.58.11.867. http://dx.doi.org/10.1037/0003-066X.58.11.867. [DOI] [PubMed] [Google Scholar]
- Loftus EF. Planting misinformation in the human mind: A 30-year investigation of the malleability of memory. Learning and Memory. 2005:361–366. doi: 10.1101/lm.94705. http://doi.org/10.1101/lm.94705. [DOI] [PubMed]
- Loftus EF, Miller DG, Burns HJ. Semantic integration of verbal information into a visual memory. Journal of Experimental Psychology: Human Learning and Memory. 1978;4(1):19–31. http://dx.doi.org/10.1037/0278-7393.4.1.19. [PubMed] [Google Scholar]
- Madore KP, Addis DR, Schacter DL. Creativity and memory: Effects of an episodic-specificity induction on divergent thinking. Psychological Science. 2015;26:1461–8. doi: 10.1177/0956797615591863. http://doi.org/10.1177/0956797615591863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Schacter DL. An episodic specificity induction enhances means-end problem solving in young and older adults. Psychology and Aging. 2014;29:913–24. doi: 10.1037/a0038209. http://doi.org/10.1037/a0038209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL. Constructive memory and memory distortions: A parallel-distributed processing approach. In: Schacter DL, editor. Memory distortion. Cambridge, MA: Harvard University Press; 1995. pp. 69–90. [Google Scholar]
- Newman EJ, Lindsay SD. False memories: What the hell are they for? Applied Cognitive Psychology. 2009;23:1105–1121. http://doi.org/10.1002/acp.1613. [Google Scholar]
- Odegard TN, Lampinen JM. Memory conjunction errors for autobiographical events: More than just familiarity. Memory. 2004;12:288–300. doi: 10.1080/09658210244000621. http://doi.org/10.1080/09658210244000621. [DOI] [PubMed] [Google Scholar]
- Okado Y, Stark CEL. Neural activity during encoding predicts false memories created by misinformation. Learning and Memory. 2005;12:3–11. doi: 10.1101/lm.87605. http://doi.org/10.1101/lm.87605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JDE. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus. 2004;14:148–52. doi: 10.1002/hipo.20009. http://doi.org/10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- Roediger HL. Memory illusions. Journal of Memory and Language. 1996;35:76–100. http://doi.org/10.1006/jmla.1996.0005. [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1995;21(4):803–814. http://doi.org/10.1037/0278-7393.21.4.803. [Google Scholar]
- Shaw J, Porter S. Constructing rich false memories of committing crime. Psychological Science. 2015;26(3):291–301. doi: 10.1177/0956797614562862. http://doi.org/10.1177/0956797614562862. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Searching for memory: The brain, the mind, and the past. New York: Basic Books; 1996. [Google Scholar]
- Schacter DL. The seven sins of memory: How the mind forgets and remembers. Boston and New York: Houghton Mifflin; 2001. [Google Scholar]
- Schacter DL. Adaptive constructive processes and the future of memory. American Psychologist. 2012;67:603–613. doi: 10.1037/a0029869. http://doi.org/10.1037/a0029869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007a;362:773–786. doi: 10.1098/rstb.2007.2087. http://doi.org/10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The ghosts of past and future. Nature. 2007b;445:27. doi: 10.1038/445027a. [DOI] [PubMed] [Google Scholar]
- Schacter DK, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: Remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. http://doi.org/10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Guerin SA, St Jacques PL. Memory distortion: An adaptive perspective. Trends in Cognitive Sciences. 2011;15:467–474. doi: 10.1016/j.tics.2011.08.004. http://doi.org/10.1016/j.tics.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Preston AR. Memory integration: Neural mechanisms and implicatioms for beheavior. Current Opinion in Behavioral Sciences. 2015;1:1–8. doi: 10.1016/j.cobeha.2014.07.005. http://doi.org/10.1016/j.cobeha.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon S, McAndrews MP, Moscovitch M. Episodic memory processes mediated by the medial temporal lobes contribute to open-ended problem solving. Neuropsychologia. 2011;49:2439–47. doi: 10.1016/j.neuropsychologia.2011.04.021. http://doi.org/10.1016/j.neuropsychologia.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Hierarchical relational binding in the medial temporal lobe: The strong get stronger. Hippocampus. 2010;20(11):1206–16. doi: 10.1002/hipo.20856. http://doi.org/10.1002/hipo.20856. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: Hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–89. doi: 10.1016/j.neuron.2008.09.023. http://doi.org/10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Olm C, Schacter DL. Neural mechanisms of reactivation-induced updating that enhance and distort memory. Proceedings of the National Academy of Sciences. 2013;110:19671–19678. doi: 10.1073/pnas.1319630110. http://doi.org/10.1073/pnas.1319630110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Schacter DL. Modifying memory: Selectively enhancing and updating personal memories for a museum tour by reactivating them. Psychological Science. 2013;24:537–543. doi: 10.1177/0956797612457377. http://doi.org/10.1177/0956797612457377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behavioral and Brain Sciences. 2007;30:299–313. doi: 10.1017/S0140525X07001975. discussion 313–51. http://doi.org/10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Szpunar KK. Episodic future thought: An emerging concept. Perspectives on Psychological Science. 2010;5(2):142–62. doi: 10.1177/1745691610362350. http://doi.org/10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: From mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. http://doi.org/10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–79. doi: 10.1016/j.neuron.2012.05.010. http://doi.org/10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Preston AR. Flexible memories: Differential roles for medial temporal lobe and prefrontal cortex in cross-episode binding. Journal of Neuroscience. 2010;30:14676–84. doi: 10.1523/JNEUROSCI.3250-10.2010. http://doi.org/10.1523/JNEUROSCI.3250-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Schlichting ML, Preston AR. The hippocampus and inferential reasoning: Building memories to navigate future decisions. Frontiers in Human Neuroscience. 2012;6:70. doi: 10.3389/fnhum.2012.00070. http://doi.org/10.3389/fnhum.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.