Abstract
There are substantial variations in arteriovenous fistula (AVF) use among hemodialysis patients in different countries, in different regions of the U.S., and even in different hemodialysis units within a single metropolitan area. These variations persist after adjustment for patient demographics and co-morbidities, suggesting that practice patterns play a major role in determining the frequency of AVF use. These observations led to vascular access guidelines urging nephrologists and surgeons to increase AVF creation in patients with chronic kidney disease. Over the past twenty years, as clinicians have adopted these guidelines, the prevalence of AVF use in hemodialysis patients has increased substantially. At the same time, clinicians have recognized important limitations of an unwavering “Fistula First” approach. First, a substantial proportion of AVFs fail to mature even when routine preoperative vascular mapping is used, leading to prolonged catheter dependence. Second, certain patient subgroups are at high risk for AVF non-maturation. Third, non-maturing AVFs frequently require interventions to promote their maturation. Fourth, AVFs that require such interventions have shortened cumulative patency. Fifth, arteriovenous grafts (AVG) have several advantages over AVFs, including lower primary failure rates, fewer interventions prior to successful cannulation, and shorter duration of catheter dependence with its associated risk of bacteremia. All these observations have led nephrologists to propose an individualized approach to vascular access, with AVG being preferred in patients who initiate hemodialysis with a catheter, particularly if they are at high risk for AVF non-maturation and have a relatively short life expectancy.
What considerations led to promotion of the current “Fistula First” initiative? Several key publications in the late 1990s and early 2000s highlighted substantial variations in AVF use, and suggested that practice patterns were a major determinant of the differences in the frequency of AVF use. A seminal study by Hirth et al analyzed a large national random sample of 4,150 incident U.S hemodialysis patients in the late 1980s, and evaluated factors associated with the type of vascular access in place 30 days after initiation of hemodialysis1. Overall, 56% of the patients had an arteriovenous graft (AVG) and 44% had an arteriovenous fistula (AVF). In a multivariate logistic regression model, female sex, older age, peripheral vascular disease, ESRD due to diabetes, poverty, and lower educational level were each associated with a lower likelihood of AVF use. However, the patient's geographic region was by far the strongest predictor of AVF use. The proportion of incident hemodialysis patients in 1990 with an AVF use ranged from a low of 15% in the East South Central region of the U.S. to a high of 77% in New England. As compared to the national sample, the adjusted odds ratio for having an AVF ranged from 0.37 in the East South Central region to 5.0 in New England. Finally, the proportion of U.S. patients with an AVF in the national sample actually decreased from 49% in 1986-87 to 35% in 1990.
A subsequent study evaluated factors associated with AVF use in a cohort of 1824 prevalent U.S. hemodialysis patients enrolled in the HEMO Study between 1995 and 19992. Overall, 34% of the patients used an AVF. Using multiple regression analysis, the investigators found a lower likelihood of AVF use associated with female sex, older age, black race, peripheral vascular disease, and obesity. However, even after adjustment for demographic and clinical factors, differences in the prevalence of AVF among dialysis units persisted. Remarkably, there were substantial differences in AVF use even between hemodialysis units at a single metropolitan area. Thus, for example, the frequencies of AVF use at five individual hemodialysis units at one metropolitan region were 29, 44, 50, 59, and 77%, respectively.
Finally, the Dialysis Outcomes and Practice Patterns Study (DOPPS) compared vascular access utilization in a random sample of 6,479 hemodialysis patients in the U.S. and in several European countries (France, Germany, Italy, Spain, and the United Kingdom) between 1996 and 20003. In agreement with the two previous studies, DOPPS reported that in a multivariate logistic regression model, a lower frequency of AVF use was associated with female sex, older age, peripheral vascular disease, diabetes, coronary artery disease, and obesity. However, the most powerful predictor of AVF use was the country in which the patient was dialyzing. Among the prevalent hemodialysis population, AVFs were used by 80% of patients in Europe, as compared to 24% of U.S patients. Among the incident hemodialysis population, AVFs were used in 66% of European patients, as compared to 15% of American patients. In addition, AVF use in prevalent patients varied markedly among individual dialysis facilities, ranging from 0 to 87% in the U.S. and from 39 to 100% in Europe. These large differences could not be explained by differences in patient demographics or co-morbidities.
In an attempt to explore the differences in vascular access use among countries, a subsequent DOPPS analysis explored vascular access processes of care in the U.S., Europe, and Japan4. The proportion of patients with pre-dialysis nephrology care was similar among the countries studied. In contrast, there were substantial differences between countries in the usual time from referral for vascular access surgery to AVF creation. Additionally, the time from AVF creation to first cannulation varied greatly among countries, with the median time being 25 days in Japan, 27 days in Italy, 41 days in Germany, and 98 days in the U.S. Importantly, the adjusted risk of AVF failure was similar for patients whose AVF was cannulated at 2-4 weeks, 4-6 weeks, 6-12 weeks, and >12 weeks after its creation.
Collectively, these studies highlighted two important points. First, several demographic and clinical patient characteristics affected the likelihood of AVF use. Second, independently of these patient characteristics, practice patterns were a key determinant of the frequency of AVF use in a given hemodialysis unit. Regardless of the patient characteristics in a given practice, global improvements in practice patterns should lead to an increase in AVF use across the board. At the same time, even when optimal practice patterns are implemented to improve vascular access outcomes, individual patient characteristics will still influence the likelihood of achieving AVF use. Recognition of the importance of practice patterns in maximizing AVF use led to national consensus vascular access guidelines promoting AVFs.
The original Dialysis Outcomes Quality Initiative (DOQI) vascular access guidelines issued in 1997 encouraged nephrologists to make concerted efforts to increase AVF use in their HD patients, and set a target of 40% AVF use in the prevalent hemodialysis population5. A conscious decision was made by the guideline authors to avoid setting a target for AVF non-maturation, so as not to discourage surgeons from attempting AVF creation. Of note, however, the few relevant publications from the 1980s had reported a relatively low (∼10%) primary AVF failure rate, suggesting that AVF non-maturation might be a relatively minor obstacle to increasing AVF use6-9.
The Centers for Medicare and Medicaid Services (CMS), in partnership with the dialysis networks, sponsored the Fistula First Initiative in 2002 to help clinicians increase AVF utilization. The dialysis networks were charged with collecting information about AVF use at individual HD units, and providing regular feedback to nephrologists and dialysis units about how their AVF prevalence compared to that of other units in the network. Nephrologists and surgeons adopted these guidelines enthusiastically, and the initial goal of 40% AVF use among prevalent U.S. hemodialysis patients was achieved within a few years. Subsequently, CMS raised the bar, increasing the goal to 66% AVF utilization among the prevalent population by June 2009. By April 2010, 55.5% of U.S. prevalent hemodialysis patients were using an AVF, and 36% of facilities were achieving the threshold of 66% AVFs10. However, the proportion of dialysis facilities achieving this goal varied substantially, from a low of 22% in Dialysis Network 5 to a high of 79% in Network 16.
As clinicians gained experience with optimizing these vascular access practice patterns, several publications began to highlight unanticipated problems and challenges11. First, implementing an increase in AVF placement required close collaboration with the surgeons. It quickly became evident that routine preoperative vascular mapping was necessary, with detailed information about vascular diameters, stenosis, and thrombosis provided to the surgeons to assist them in planning the optimal vascular access for each patient12-14. However, even though routine preoperative vascular mapping helped surgeons select the optimal type and location of access, it often did not substantially reduce the likelihood of AVF non-maturation15.
Second, numerous publications highlighted the substantial rate of AVF non-maturation, that far exceeded the low rates (∼10%) reported in the 1980s6-9. A comprehensive review of studies reported between 1996 and 2002 documented a range of AVF non-maturation between 20 and 50%15. Subsequently, the NIH-sponsored Dialysis Access Consortium (DAC) Fistula Trial of nearly 900 patients undergoing creation of a new AVF observed a 60% non-maturation rate16. The high rate of AVF non-maturation was particularly striking among some high-risk patient groups. In particular, AVF non-maturation was more common in older patients, females, blacks, and those with peripheral vascular disease17-21. Despite purportedly guiding the surgeons in optimal vascular access planning, the use of preoperative vascular mapping did not abolish the differences in AVF non-maturation among patient subgroups20. In particular, there was tremendous overlap in the preoperative arterial and venous diameters between successful AVFs and those that failed to mature22.
Third, postoperative imaging studies of immature AVFs frequently identified one or more anatomic problems (juxta-anastomotic stenosis, large accessory veins or excessive depth) that required interventions to promote AVF maturation23-26. As a consequence, a substantial proportion of new AVFs required surgical or percutaneous interventions (angioplasty, stenosis revision, ligation of accessory veins or transposition) before they could be used for dialysis23,27-29. Not surprisingly, the frequent need for subsequent interventions to salvage immature AVFs or to place a second access when the first AVF failed to mature delayed the time to first successful AVF cannulation and prolonged the duration of catheter dependence.
Fourth, as compared to AVFs that matured without an intervention, those that required an intervention to achieve maturation had a shortened cumulative access survival and required more frequent interventions after maturation to maintain their long-term patency for dialysis30. In fact, a recent large, single-center observational study reported that AVFs requiring an intervention to achieve maturation actually had a shorter cumulative patency than AVGs that were cannulated successfully without prior intervention (Figure 1)28.
Figure 1. Cumulative access patency of AVFs that matured with or without prior interventions and AVGs that did or did not require intervention prior to successful cannulation.
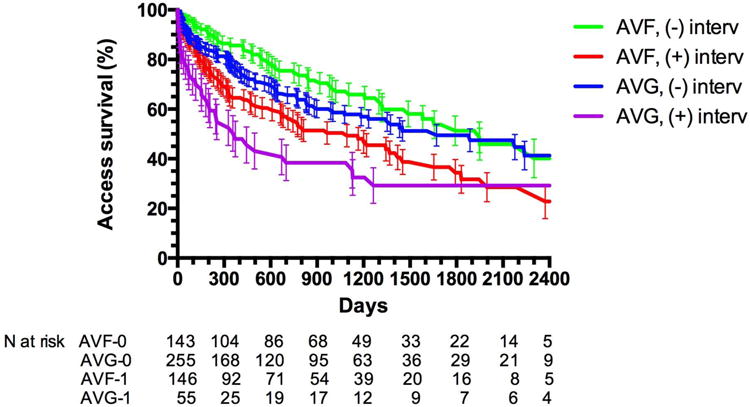
Access patency was shorter for AVF with prior intervention than AVF without interventions (P < 0.0001). Access patency was shorter for AVG with prior interventions than AVG without intervention (P < 0.0001). Access patency was similar for AVF and AVG without prior interventions (P =0.16). Cumulative access patency was worse for AVF with prior interventions than for AVG without prior interventions (P = 0.01). Reproduced with permission from28.
As a consequence of recognizing the unanticipated consequences of an unwavering “Fistula First” strategy, many nephrologists began to question the rationale for an indiscriminate preference of AVFs over AVGs. The KDOQI guidelines state categorically that AVFs have superior long-term patency for dialysis, as compared to AVGs31. Is this an accurate statement? Most studies reporting on the superiority of AVF over AVG have utilized an “as treated” analysis, focusing on access survival only from the time of successful use of the vascular access for hemodialysis. Unfortunately, this type of analysis is misleading, as it excludes vascular accesses that fail prior to successful use for hemodialysis (primary access failure), an event that occurs much more commonly with AVFs than with AVGs.
In three large head-to-head single-center observational studies, the primary failure rate of AVFs (32-40%) was considerably higher than that obtained with AVGs (12-19%)(Table 1)22,32,33. When primary failures were excluded, AVFs clearly had longer cumulative survival than did AVGs. In contrast, the comparisons looked quite different when the access outcomes were determined from the time of surgery in an “intent to treat” analysis. The four largest observational studies comparing outcomes of AVFs and AVGs observed virtually identical cumulative survivals of AVFs and AVGs when primary access failures were included (Table 1) 22,34,35,34. These analyses included all comers. It is quite possible, however, that the cumulative survival of AVFs may actually be superior to that of AVGs in patients at low risk for AVF non-maturation, but inferior to that of AVGs in patients at high risk for AVF non-maturation.
Table 1. Cumulative survival of AVF and AVG, with or without inclusion of primary failures.
| Reference | N of pts | Primary failure | Median access survival in months (excluding primary failures) | Median access survival in months (including primary failures) | ||||
|---|---|---|---|---|---|---|---|---|
| AVF | AVG | AVF | AVG | AVF | AVG | AVF | AVG | |
| Schild, 200834 | 998 | 702 | -- | -- | -- | -- | 10 | 10 |
| Maya, 200922 | 322 | 289 | 38%* | 15% | 42 * | 20 | 13 | 13 |
| Lok, 201333 | 1012 | 128 | 40%* | 19% | 62 * | 24 | 7 | 15 |
| Allemang, 2014 32 | 390 | 265 | 32% * | 12% | -- | -- | 26 | 29 |
Primary failure, access never usable for dialysis.
p<0.001 vs AVG
Of course, cumulative access survival is not the only clinically relevant vascular access outcome. Other important outcomes include: (1) the number of interventions required prior to successful use of the access for dialysis; (2) the duration of CVC dependence before the access can successfully be used for dialysis; (3) the frequency of CRB during the period of catheter-dependence; and (4) the frequency of access interventions (angioplasty, thrombectomy or surgical revision) required to maintain the long-term access patency after successful use (Table 2). A comprehensive comparison of AVFs and AVGs should consider all of these outcomes.
Table 2. Relative advantages of AVFs and AVGs.
How are AVGs better than AVFs?
|
How are AVFs better than AVGs?
|
A retrospective study compared multiple clinical outcomes of upper arm AVFs and AVGs in patients with a prior history of a failed forearm AVF35. AVGs were superior to AVFs in that they had a significantly lower rate of primary access failure, required fewer interventions prior to successful use for dialysis, were associated with a shorter duration of catheter dependence, and were associated with fewer CRBs prior to successful access use. However, once an access was successfully used for dialysis, AVFs were superior to AVGs in that they had greater cumulative access patency for dialysis, and required substantially fewer interventions to maintain this patency. This analysis suggests that the relative advantages of AVFs vs AVGs should be considered as tradeoffs, with the weight of evidence supporting placement of an AVF in some patients and an AVG in others.
Collectively, these observations led some nephrologists to question the wisdom of a “one-size-fits-all” approach to vascular access 21,35-38. Rather than attempting to maximize AVF placement and accept a relatively high AVF non-maturation rate, they proposed a “catheter last” philosophy. According to these proposals, the decision about type of vascular access in a given patient would be predicated on four factors: (1) whether the patient had already initiated dialysis, (2) the expected patient survival, (3) the likelihood of AVF non-maturation, and (4) whether the patient had a previous non-maturing AVF (Figure 2) 37. Younger patients with a relatively high life expectancy and low likelihood of AVF non-maturation would receive an AVF, particularly if they had not yet initiated dialysis. In contrast, elderly patients who were already on dialysis, with relatively short life expectancy and high likelihood of AVF maturation, would receive an AVG, particularly if they had had a previous non-maturing AVF. Such an approach would likely result in fewer patients dialyzing with an AVF, but also fewer catheter-dependent patients. AVG would be considered a catheter-sparing procedure 39. A patient-centered approach to vascular access should weigh the relative likelihood of each of these outcomes, and determine what type of access is right for that patient. Ideally, a thorough analysis should consider several key factors:
-
Has the patient started hemodialysis? At present, 81% of U.S. patients initiate hemodialysis with a central vein catheter (CVC).40 Once dialysis has been started, concerted efforts are made to transition these patients to a permanent vascular access, arteriovenous fistula (AVF) or arteriovenous graft (AVG). The patients remain catheter dependent until a new AVF or AVG is ready to be used. The duration of CVC dependence is increased if an AVF fails to mature or if it requires an intervention to promote AVF maturation.
In a large observational study, catheter duration from AVF creation to initial AVF use was 99 days if the AVF matured without an intervention, as compared to 159 days if the AVF required an intervention prior to maturation28. In other words, the need for an intervention to promote AVF maturation prolonged catheter dependence by an additional two months. In contrast, the duration of catheter dependence was only 38 days in patients with an AVG that was used without a prior intervention and 79 days in those with an AVG that required a prior intervention. In the DOPPS Study, only 2% of new AVFs placed in the U.S. were cannulated within one month, as compared to 78% of AVGs41.
Under the best of circumstances, a new AVF matures without requiring any interventions, and is suitable for use within 2 to 4 months. However, in many cases, the new AVF requires one or more interventions to achieve maturation, or if it fails, a second AVF has to be placed and mature. In contrast, primary AVG failure is much lower (10 to 20%) even in the present era (Table 1), and AVGs are frequently ready to use in less than one month, thereby requiring a shorter duration of CVC-dependence. As a consequence of these differences, many patients who initiate hemodialysis with a CVC and receive an AVF remain CVC-dependent for six months or longer. At our medical center, among patients who were CVC-dependent, the median time from AVF surgery to AVF use was 206 days.42 In contrast, in CVC-dependent patients who received an AVG, the median duration of CVC-dependence was 48 days.39
What are the consequences of the prolonged CVC dependence? Unlike AVF and AVG, CVC use often results in bacteremia, with a frequency of 1 to 2 episodes per catheter-year.43-48 The risk of catheter-related bacteremia (CRB) is proportionate to the duration of catheter dependence. A recent, large observational study of 472 patients initiating HD with a CVC observed CRB in 35% of patients at 3 months, 54% at 6 months, and 79% at 1 year.49 CRB is a major source of morbidity and hospitalization in HD patients. It results in serious complications (e.g., endocarditis, septic arthritis, epidural abscess, septic shock, or death) in approximately 10% of patients. 50-53 CVCs also frequently cause central vein stenosis,54,55 and in patients developing this complication, they can adversely affect the longevity of subsequent AVF and AVG.56
What is the likelihood of AVF non-maturation? As discussed previously, several demographic and clinical factors can be used to predict the likelihood of AVF non-maturation. The most comprehensive study in this regard developed an FTM (failure to mature) score using a derivation cohort of 422 patients undergoing a new AVF creation17. The predictive value of this score was prospectively validated in a second cohort of 445 patients undergoing AVF creation at five other North American dialysis centers. The FTM score, which could range from 0 to 10.5, was calculated from patient age, race, peripheral vascular disease and coronary artery disease. There was a highly significant correlation in the validation cohort between AVF non-maturation rate and the FTM score (24, 34, 50, and 69% in patients with an FTM score of <2, 2-3, 3.1-7.9, and >8, respectively). While the FTM score should certainly not be used as the sole criterion to determine whether a patient should receive an AVF or an AVG, it is one of several factors that should be considered.
What is the patient's life expectancy? As mentioned previously, the cumulative survival of AVFs and AVGs is similar in an intent-to-treat analysis that includes primary access failures (Table 1). However, due to the higher primary failure rate of AVF, the cumulative survival of AVGs is actually superior to that of AVGs during the first 18 months of follow-up. As a consequence, AVG placement may actually be preferred in hemodialysis patients with a life expectancy less than two years. This is particularly relevant to older patients, those with high co-morbidity burden, those with a high frailty index, or those who reside in a nursing home.
What was the outcome of the previous AVF? It is likely that vascular properties that lead to non-maturation of an initial AVF may likewise adversely affect the outcome of a subsequent AVF. Thus, if the patient's initial AVF failed to mature, serious consideration should be given to place an AVG next, rather than a second AVF.
Fig 2. An algorithmic guide to choosing an appropriate hemodialysis vascular access for patients.
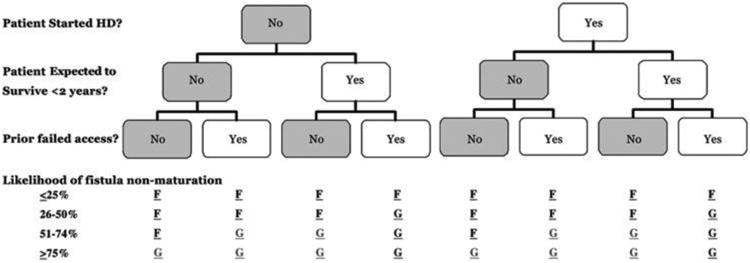
This protocol requires the nephrologist and access surgeon to consider three important clinical factors: timing of access surgery relative to initiation of hemodialysis, life-expectancy of the patient, and prior failed vascular access. This information, along with the likelihood of AVF non-maturation, is used to determine the most appropriate vascular access for that patient: fistula (F) or graft (G). Reproduced with permission from37.
The specific considerations listed above suggest that an AVG is a better choice for many older patients who initiate hemodialysis with a CVC. First, early AVF failure is much more likely in older patients. For example, a single-center study reported that the one-year cumulative AVF survival was much lower in patients ≥ 70 years of age than in younger patients (39% vs. 68%).21 Second, older patients have a high frequency of co-morbidities (coronary artery disease, peripheral vascular disease) that independently increase the likelihood of AVF non-maturation. Finally, patient survival is significantly shorter in older hemodialysis patients. The 18-month hemodialysis patient survival at one center was 50% for those ≥70 years of age, as compared to 75% in younger patients.21
Thus, not only is a new AVF less likely to be successfully used for hemodialysis in older patients, even when it matures, the patient is unlikely to live long enough to reap the rewards of prolonged AVF survival. In one study, only 35% of elderly (age ≥70 years) HD patients who died had had their AVF used prior to their death.21 All of these considerations have prompted some nephrologists to question the wisdom of indiscriminate placement of AVF in older hemodialysis patients who initiate hemodialysis with a CVC.21,36,37,57,58
Obesity may be another important factor that favors placement of an AVG over an AVF. Obesity is common in hemodialysis patients, and in one center 30% of the patients were obese 59. Obesity represents a challenge to the success of an AVF. In a large, multi-center cross-sectional analysis, a higher BMI was associated with a lower likelihood of AVF use (adjusted odds ratio 0.76, 95% CI 0.65-0.87 per 5 kg/m2)2. In obese patients the vein is frequently too deep to be cannulated safely. As a consequence, the patients typically require a second transposition procedure to bring the AVF under the skin. An analysis from UAB showed that obese patients had a similar AVF non-maturation rate as that observed in non-obese patients. However, cumulative AVF survival was shorter in this population 59. When an AVF is created in an obese patient, it frequently requires a subsequent transposition procedure to make it accessible for cannulation by the dialysis staff26. A recent analysis from our center found that, as compared to AVFs that were cannulated without a prior intervention, those that required a transposition surgery had a shorter cumulative AVF patency and required more frequent interventions to maintain long-term patency for dialysis after successful cannulation28. Placement of an AVG rather than an AVF in this population permits a single surgical procedure, faster access cannulation, and shorter catheter-dependence.
Some investigators have also evaluated the impact of access type on hemodialysis patient survival. Studies comparing the adjusted survival of incident hemodialysis patients have consistently observed inferior survival of patients initiating hemodialysis with a CVC, as compared to those initiating hemodialysis with an AVF or AVG. 60-65 The relative survival of patients initiating hemodialysis with an AVF vs an AVG was inconsistent, with some studies demonstrating no difference in patient survival, and others demonstrating modestly lower survival rates in patients initiating hemodialysis with AVG. An important limitation of these studies is that they focused on patients whose access was created and matured prior to initiating hemodialysis, in other words, those who were not CVC-dependent.
The situation may be quite different in those patients who initiate hemodialysis with a CVC, and subsequently receive an AVF or AVG. In the latter situation, the patients have prolonged CVC-dependence until their new AVF or AVG is suitable for dialysis. Two observational studies assessed the impact of converting catheter-dependent hemodialysis patients to an AVF or AVG. In both studies, patients who transitioned from a CVC to a permanent vascular access (AVF or AVG) had lower mortality than those who continued to use a CVC for dialysis60,62. To the extent that AVFs have a higher primary failure rate and take longer to mature (Table 1), patients undergoing AVF surgery after initiation of hemodialysis have longer CVC dependence than those receiving an AVG. In this regard, analysis of a large national database observed that the likelihood of independence from CVC 90 days after hemodialysis initiation was much higher in patients receiving an AVG vs. AVF (54 vs. 21%).66 Similarly, a recent analysis of USRDS data demonstrated a shorter mean duration of catheter dependence among incident hemodialysis patients who initiated dialysis with a CVC, and subsequently received an AVG rather than an AVF (70 vs 155 days, p<0.001)67.
The longer duration of CVC-dependence in patients receiving an AVF may translate into more episodes of CRB, and more cases of central vein stenosis, which in turn lead to shortened vascular access survival and increased patient mortality. Moreover, a recent analysis reported a similar patient survival in incident CVC-dependent incident hemodialysis patients who subsequently received an AVF or an AVG68.
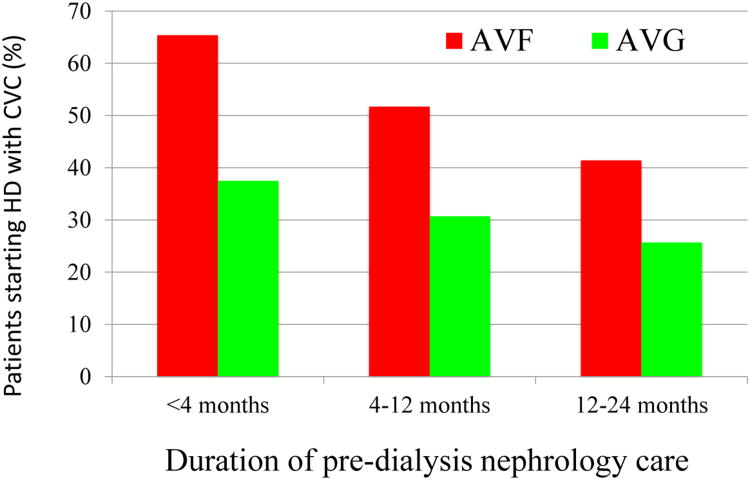
The preceding discussion has focused largely on the tradeoffs between AVFs and AVGs in patients who initiate hemodialysis with a CVC, in other words those without pre-dialysis vascular access surgery. One might assume that when the vascular access is placed in patients with advanced CKD who have not yet initiated hemodialysis, the choice of vascular access might have little impact on catheter-dependence once dialysis is initiated, as there is ample time for the AVF to mature or to undergo the necessary interventions to promote maturation. However, a recent analysis of a nationally representative random sample of elderly CKD patients undergoing pre-dialysis vascular access surgery suggested that this might not be the case29. Catheter dependence at initiation of hemodialysis was significantly higher in patients with pre-dialysis AVF surgery, as compared to those receiving an AVG (46.0 vs 28.5%, p<0.0001). As expected, catheter dependence at dialysis initiation was inversely related to the duration of pre-dialysis nephrology follow-up in both patients with pre-dialysis AVF and AVG surgery. However, regardless of the duration of pre-dialysis nephrology care, patients with pre-dialysis AVG surgery were consistently less likely to initiate dialysis with a CVC, as compared to those with AVF surgery (65.4 vs 37.5% for patients with <4 months of nephrology follow-up; 51.7 vs 30.7% for those with 4–12 months of nephrology follow-up; and 41.4 vs 25.7% for those with >12 months of nephrology follow up) (Figure 3).
Figure 3.
The likelihood of catheter dependence at initiation of hemodialysis in elderly patients undergoing pre-dialysis AVF or AVG surgery, sorted by duration of pre-dialysis nephrology follow-up. Adapted from29.
What might explain this surprising observation? It likely reflects the uncertainty in predicting time to initiation of dialysis in patients with advanced CKD, the greater need for interventions prior to successful cannulation in patients undergoing AVF creation, the fragmented medical care system in the U.S., and the absence of adequate insurance to pay for pre-dialysis vascular access surgery in a subset of patients with CKD. Most importantly, the complexity of processes of care to achieve a successful AVF are greater than those required to achieve a successful AVG, and the discrepancy is even greater among patients who have not yet initiated hemodialysis.
From a personal perspective, I've had an ongoing research interest in vascular access for the past 20 years. In the mid-1990s most hemodialysis patients at University of Alabama at Birmingham (UAB) received an AVG, whereas AVFs were reserved for young patients with low co-morbidity. CVCs were used in patients who started dialysis without a permanent access or in patients whose existing access failed. If a patient's AVG failed, a new AVG was placed. The duration of catheter-dependence was relatively short, as AVGs were typically cannulated within 2-3 weeks of their placement. Many of the patients received non-tunneled dialysis catheters due to the anticipated short duration of catheter-dependence. In February 1997 we had approximately 350 HD patients and the distribution of vascular access in use was as follows; 26% AVF, 61% AVG, and 13% CVC (Table 3) 69. Among patients with AVFs, approximately half were created in the forearm 19. This vascular access distribution at UAB was comparable to that obtained at other medical centers. Thus, for example, among ∼1800 HD patients enrolled in the HEMO Study from 15 academic medical centers across the country, only 33% were using an AVF2. Moreover, 66% of the AVF in the HEMO Study were placed in the forearm. The major vascular access issue was frequent AVG thrombosis, and the focus was on identifying underlying hemodynamically significant stenosis and scheduling preemptive angioplasty to prevent AVG thrombosis.
Table 3. UAB vascular access distributions in 2 time periods.
| Type of vascular access | 1997 (pre-KDOQI) | 2015 |
| AVF | 26% | 47% |
| AVG | 61% | 32% |
| CVC | 13% | 21% |
In response to the national guidelines on vascular access, we developed a multidisciplinary approach to vascular access at our medical center, incorporating close collaboration among nephrologists, surgeons and radiologists69. We created a position for two dedicated full-time vascular access coordinators, who were responsible for scheduling all vascular access procedures, tracking outcomes, and maintaining a prospective, computerized vascular access database. To assist the surgeons planning the optimal vascular access for each patient, we implemented a program of routine preoperative sonographic vascular mapping13,14. Subsequently, we began to obtain routine postoperative ultrasounds to assess AVF maturation70. We also intervened aggressively by percutaneous or surgical approaches to salvage immature AVFs23. All these measures resulted in an increase in AVF use, a decrease in AVG use, but also an increase in catheter dependence (Table 3). Over time, we came to realize some of the limitations of the Fistula First approach, and urged a more nuanced approach to vascular access. We argued that certain patient subsets were better served by getting an AVG rather than an AVF36,37.
Clearly, there is a discrepancy between the current Fistula First recommendations and the realities that most U.S. nephrologists and surgeons are facing. There is an urgent need for a randomized clinical trial of AVFs vs AVGs in patients who initiate hemodialysis with a CVC and who are at high risk for AVF non-maturation. Until such a study is completed, it is imperative that we use sound clinical judgment rather than adhere blindly to vascular access guidelines that are not supported by the published literature.
Acknowledgments
Dr. Allon was supported by grant R01-DK-085027 from the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK).
References
- 1.Hirth RA, Turenne MN, Woods JD, Young EW, Port FK, Pauly MV, et al. Predictors of type of vascular access in hemodialysis patients. JAMA. 1996;276:1303–07. [PubMed] [Google Scholar]
- 2.Allon M, Ornt D, Schwab S, Rasmussen C, Delmez JA, Greene T, et al. Factors associated with the prevalence of A-V fistulas in hemodialysis patients in the HEMO Study. Kidney Int. 2000;58:2178–85. doi: 10.1111/j.1523-1755.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 3.Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, et al. Vascular access use in Europe and in the United States: Results from the DOPPS. Kidney Int. 2002;61:305–16. doi: 10.1046/j.1523-1755.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 4.Rayner HC, Pisoni Rl, Gillespie BW, Goodkin DA, Akiba T, Azikawa T, et al. Creation, cannulation, and survival of arteriovenous fistulae: Data from the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;63:323–30. doi: 10.1046/j.1523-1755.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- 5.NKF-DOQI Clinical Practice Guidelines for Vascular Access. New York: National Kidney Foundation; 1997. pp. 22–23. [PubMed] [Google Scholar]
- 6.Bonalumi U, Civalleri D, Rovida S, Adami GF, Gianetta E, Griffanti-Bartoli F. Nine years' experience with end-to-end arteriovenous fistula at the “anatomic snuffbox” for maintenance hemodialysis. Br J Surg. 1982;69:486–88. doi: 10.1002/bjs.1800690820. [DOI] [PubMed] [Google Scholar]
- 7.Kherlakian GM, Roedersheimer LR, Arbaugh JJ, Newmark KJ, King LR. Comparison of autogenous fistula versus expanded polytetrafluoroethylene graft fistula for angioaccess in hemodialysis. Am J Surg. 1986;152:238–43. doi: 10.1016/0002-9610(86)90249-7. [DOI] [PubMed] [Google Scholar]
- 8.Palder SB, Kirkman RL, Whittemore AD, Hakim RM, Lazarus JM, Tilney NL. Vascular access for hemodialysis: patency rates and results of revisions. Ann Surg. 1985;202:235–39. doi: 10.1097/00000658-198508000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reilly DT, Wood RFM, Bell PRF. Prospective study of dialysis fistulas: problem patients and their treatment. Br J Surg. 1982;69:549–53. doi: 10.1002/bjs.1800690918. [DOI] [PubMed] [Google Scholar]
- 10.Lynch JR, Wasse H, Armistead NC, McClellan WM. Achieving the goal of the Fistula First Breakthrough Initiative for prevalent maintenance hemodialysis patients. Am J Kidney Dis. 2011;57:78–89. doi: 10.1053/j.ajkd.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allon M. Current management of vascular access. Clin J Am Soc Nephrol. 2007;2:786–800. doi: 10.2215/CJN.00860207. [DOI] [PubMed] [Google Scholar]
- 12.Silva MB, Hobson RW, Pappas PJ, Jamil Z, Araki CT, Goldberg MC, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg. 1998;27:302–08. doi: 10.1016/s0741-5214(98)70360-x. [DOI] [PubMed] [Google Scholar]
- 13.Robbin ML, Gallichio ML, Deierhoi MH, Young CJ, Weber TM, Allon M. US vascular mapping before hemodialysis access placement. Radiology. 2000;217:83–88. doi: 10.1148/radiology.217.1.r00oc2883. [DOI] [PubMed] [Google Scholar]
- 14.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, et al. Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int. 2001;60:2013–20. doi: 10.1046/j.1523-1755.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 15.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002;62:1109–24. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- 16.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis. JAMA. 2008;299:2164–71. [Google Scholar]
- 17.Lok CE, Allon M, Moist LM, Oliver MJ, Shah H, Zimmerman D. REDUCE FTM I (Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas) J Am Soc Nephrol. 2006;17:3204–12. doi: 10.1681/ASN.2006030190. [DOI] [PubMed] [Google Scholar]
- 18.Miller CD, Robbin ML, Allon M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int. 2003;63:346–52. doi: 10.1046/j.1523-1755.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller PE, Tolwani A, Luscy CP, Deierhoi MH, Bailey R, Redden DT, et al. Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int. 1999;56:275–80. doi: 10.1046/j.1523-1755.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- 20.Peterson WJ, Barker J, Allon M. Disparities in fistula maturation persist despite preoperative vascular mapping. Clin J Am Soc Nephrol. 2008;3:437–41. doi: 10.2215/CJN.03480807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson AI, Leake A, Schmieder GC, Biuckians A, Stokes GK, Panneton JM, et al. Should fistulas really be first in the elderly patient? J Vasc Access. 2009;10(3):199–202. doi: 10.1177/112972980901000311. [DOI] [PubMed] [Google Scholar]
- 22.Maya ID, O'Neal JC, Young CJ, Barker-Finkel J, Allon M. Outcomes of brachiocephalic fistulas, transposed brachiobasilic fistulas, and upper arm grafts. Clin J Am Soc Nephrol. 2009;4:86–92. doi: 10.2215/CJN.02910608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh P, Robbin ML, Lockhart ME, Allon M. Clinically immature arteriovenous hemodialysis fistulas: effect of US on salvage. Radiology. 2008;246:299–305. doi: 10.1148/radiol.2463061942. [DOI] [PubMed] [Google Scholar]
- 24.Beathard GA, Arnold P, Jackson J, Litchfield T. Physician Operators Forum of RMS Lifeline. Aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487–94. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 25.Beathard GA, Settle SM, Shields MW. Salvage of the nonfunctioning arteriovenous fistula. Am J Kidney Dis. 1999;33:910–16. doi: 10.1016/s0272-6386(99)70425-7. [DOI] [PubMed] [Google Scholar]
- 26.Inkollu S, Wellen J, Beller Z, Zhang T, Vaccharajani N, Shenoy S. Successful use of minimal incision superficialization technique for arteriovenous fistula maturation. J Vasc Surg. 2016;63:1018–25. doi: 10.1016/j.jvs.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 27.Falk A. Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol. 2006;17:807–13. doi: 10.1097/01.RVI.0000217928.43396.35. [DOI] [PubMed] [Google Scholar]
- 28.Harms JC, Rangarajan S, Young CJ, Barker-Finkel J, Allon M. Outcomes of arteriovenous fistulas and grafts with and without intervention prior to successful use. J Vasc Surg. doi: 10.1016/j.jvs.2016.02.033. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee T, Thamer M, Zhang Y, Zhang Q, Allon M. Outcomes of elderly patients after predialysis vascular access creation. J Am Soc Nephrol. 2015;26:3133–40. doi: 10.1681/ASN.2014090938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee T, Ullah A, Allon M, Succop P, El-Khatib M, Munda R, et al. Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol. 2011;6:575–81. doi: 10.2215/CJN.06630810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.KDOQI clinical practice guidelines and clinical practice recommendations for vascular access 2006. Am J Kidney Dis. 2006;48(suppl 1):S176–S322. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 32.Allemang MT, Schmotzer B, Wong VL, Lakin RO, Woodside KJ, Schulak JA, et al. Arteriovenous grafts have higher secondary patency in the short term compared with autologous fistulae. Am J Surg. 2014;208:800–05. doi: 10.1016/j.amjsurg.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Lok CE, Sontrop JM, Tomlinson G, Rajan DK, Cattral M, Oreopoulos G, et al. Cumulative patency of contemporary fistulas versus graft (2000-2010) Clin J Am Soc Nephrol. 2013;8:810–18. doi: 10.2215/CJN.00730112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schild AF, Perez E, Gillaspie E, Seaver C, Livingstone J, Thibonnier A. Arteriovenous fistulae vs arteriovenous grafts: a retrospective review of 1,700 consecutive vascular access cases. J Vasc Access. 2008;9(4):231–5. [PubMed] [Google Scholar]
- 35.Lee T, Barker J, Allon M. Comparison of survival of upper arm arteriovenous fistulas and grafts after failed forearm fistulas. J Am Soc Nephrol. 2007;18:1936–41. doi: 10.1681/ASN.2006101119. [DOI] [PubMed] [Google Scholar]
- 36.Allon M, Robbin ML. Resolved: Fistulas are preferred to grafts as initial vascular access for dialysis: Con. J Am Soc Nephrol. 2008;19:1632–33. [PubMed] [Google Scholar]
- 37.Allon M, Lok CE. Dialysis fistula or graft: The role for randomized clinical trials. Clin J Am Soc Nephrol. 2010;5:2348–54. doi: 10.2215/CJN.06050710. [DOI] [PubMed] [Google Scholar]
- 38.Wish JB. Catheter last, fistula not-so-first. J Am Soc Nephrol. 2015;26:5–7. doi: 10.1681/ASN.2014060594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shingarev R, Maya ID, Barker-Finkel J, Allon M. Arteriovenous graft placement in pre-dialysis patients: A potential catheter-sparing strategy. Am J Kidney Dis. 2011;58:243–47. doi: 10.1053/j.ajkd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins AJ, Foley RN, Gilbertson DT, Chen SC. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(1):S5–11. doi: 10.2215/CJN.05980809. [DOI] [PubMed] [Google Scholar]
- 41.Saran R, Dykstra DM, Pisoni RL, Akiba T, Akizawa T, Canaud B, et al. Timing of first cannulation and vascular access failure in hemodialysis: an analysis of practice patterns at dialysis facilities in the DOPPS. Nephrol Dial Transplant. 2004;19:2334–40. doi: 10.1093/ndt/gfh363. [DOI] [PubMed] [Google Scholar]
- 42.Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: Reasons and subsequent outcomes. Am J Kidney Dis. 2005;46:501–08. doi: 10.1053/j.ajkd.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 43.Beathard GA. Management of bacteremia associated with tunneled-cuffed hemodialysis catheters. J Am Soc Nephrol. 1999;10:1045–49. doi: 10.1681/ASN.V1051045. [DOI] [PubMed] [Google Scholar]
- 44.Krishnasami Z, Carlton D, Bimbo L, Taylor ME, Balkovetz DF, Barker J, et al. Management of hemodialysis catheter related bacteremia with an adjunctive antibiotic lock solution. Kidney Int. 2002;61:1136–42. doi: 10.1046/j.1523-1755.2002.00201.x. [DOI] [PubMed] [Google Scholar]
- 45.Marr KA, Sexton DJ, Conlon PJ, Corey GR, Schwab SJ, Kirkland KB. Catheter-related bacteremia and outcome of attempted catheter salvage in patients undergoing hemodialysis. Ann Intern Med. 1997;127:275–80. doi: 10.7326/0003-4819-127-4-199708150-00003. [DOI] [PubMed] [Google Scholar]
- 46.Poole CV, Carlton D, Bimbo L, Allon M. Treatment of catheter-related bacteremia with an antibiotic lock protocol: effect of bacterial pathogen. Nephrol Dial Transplant. 2004;19:1237–44. doi: 10.1093/ndt/gfh041. [DOI] [PubMed] [Google Scholar]
- 47.Saad TF. Bacteremia associated with tunnneled, cuffed hemodialysis catheters. Am J Kidney Dis. 1999;34:1114–24. doi: 10.1016/S0272-6386(99)70018-1. [DOI] [PubMed] [Google Scholar]
- 48.Schwab SJ, Weiss MA, Rushton F, Ross Jp, Jackson J, Kapoian T, et al. Multicenter clinical trial results with the Lifesite hemodialysis access system. Kidney Int. 2002;62:1026–33. doi: 10.1046/j.1523-1755.2002.00540.x. [DOI] [PubMed] [Google Scholar]
- 49.Shingarev R, Barker-Finkel J, Allon M. Natural history of tunneled dialysis catheters placed for hemodialysis initiation. J Vasc Interv Radiol. 2013;24:1289–94. doi: 10.1016/j.jvir.2013.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allon M. Dialysis catheter-related bacteremia: Treatment and prophylaxis. Am J Kidney Dis. 2004;44:779–91. [PubMed] [Google Scholar]
- 51.Maya ID, Carlton D, Estrada E, Allon M. Treatment of dialysis catheter-related Staphylococcus aureus bacteremia with an antibiotic lock: A quality improvement report. Am J Kidney Dis. 2007;50:289–95. doi: 10.1053/j.ajkd.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 52.Peterson WJ, Maya ID, Carlton D, Estrada E, Allon M. Treatment of dialysis catheter-related Enterococcus bacteremia with an antibiotic lock: A quality improvement report. Am J Kidney Dis. 2009;53:107–11. doi: 10.1053/j.ajkd.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lok CE, Mokrzycki MH. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011;79:587–98. doi: 10.1038/ki.2010.471. [DOI] [PubMed] [Google Scholar]
- 54.MacRae JM, Ahmed A, Johnson N, Levin A, Kiaii M. Central vein stenosis: a common problem in patients on hemodialysis. ASAIO Journa. 2005;51:77–81. doi: 10.1097/01.mat.0000151921.95165.1e. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal AK, Patel BM, Haddad NJ. Central vein stenosis: A nephrologist's perspective. Semin Dial. 2007;20:53–62. doi: 10.1111/j.1525-139X.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 56.Shingarev R, Barker-Finkel J, Allon M. Association of hemodialysis central venous catheter use with ipsilateral arteriovenous access survival. Am J Kidney Dis. 2012;60:510–13. doi: 10.1053/j.ajkd.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Besarab A. Resolved: Fistulas are preferred to grafts as initial vascular access for dialysis: Pro. J Am Soc Nephrol. 2008;19:1629–31. doi: 10.1681/ASN.2008020172. [DOI] [PubMed] [Google Scholar]
- 58.Lacson E, Lazarus JM, Himmelfarb J, Ikizler TA, Hakim RM. Balancing fistula first with catheters last. Am J Kidney Dis. 2007;50:379–95. doi: 10.1053/j.ajkd.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 59.Kats M, Hawxby AM, Barker J, Allon M. Impact of obesity on arteriovenous fistula outcomes in dialysis patients. Kidney Int. 2007;71:39–43. doi: 10.1038/sj.ki.5001904. [DOI] [PubMed] [Google Scholar]
- 60.Allon M, Daugirdas JT, Depner TA, Greene T, Ornt D, Schwab SJ. Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis. 2006;47:469–77. doi: 10.1053/j.ajkd.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 61.Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001;60:1443–51. doi: 10.1046/j.1523-1755.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 62.Lacson E, Jr, Wang W, Lazarus JM, Hakim RM. Change in vascular access and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2009;54(5):912–21. doi: 10.1053/j.ajkd.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Pastan S, Soucie M, McClellan WM. Vascular access and increased risk of death among hemodialysis patients. Kidney Int. 2002;62:620–26. doi: 10.1046/j.1523-1755.2002.00460.x. [DOI] [PubMed] [Google Scholar]
- 64.Polkinghorne KR, McDonald SP, Atkinns RC, Kerr PG. Vascular access and all-cause mortality: A propensity score analysis. J Am Soc Nephrol. 2004;15:477–86. doi: 10.1097/01.asn.0000109668.05157.05. [DOI] [PubMed] [Google Scholar]
- 65.Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42:1013–19. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Lacson EK, Wang W, Lazarus M, Hakim R. Arteriovenous vascular access maturation and failure rates in the 1st 90 days. Proceedings of the American Society of Nephrology meeting in San Diego, October 27-November 1, 2009. 2009:F–FC296. [Google Scholar]
- 67.Leake AE, Yuo TH, Wu TH, Fish L, Dillavou ED, Chaer RA, et al. Arteriovenous grafts are associated with earlier catheter removal and fewer catheter days in the United States Renal Data System population. J Vasc Surg. 2015;62:123–27. doi: 10.1016/j.jvs.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Yuo TH, Chaer RA, Dillavou ED, Leers SA, Makaroun MS. Patients started on hemodialysis with tunneled dialysis catheter have similar survival after arteriovenous fistula and arteriovenous graft creation. J Vasc Surg. 2015 doi: 10.1016/j.jvs.2015.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allon M, Bailey R, Ballard R, Deierhoi MH, Hamrick K, Oser R, et al. A multidisciplinary approach to hemodialysis access: prospective evaluation. Kidney Int. 1998;53:473–79. doi: 10.1046/j.1523-1755.1998.00761.x. [DOI] [PubMed] [Google Scholar]
- 70.Robbin ML, Chamberlain NE, Lockhart ME, Gallichio MH, Young CJ, Deierhoi MH, et al. Hemodialysis arteriovenous fistula maturity: US evaluation. Radiology. 2002;225:59–64. doi: 10.1148/radiol.2251011367. [DOI] [PubMed] [Google Scholar]