Abstract
We aim to investigate whether A2A/nitric oxide-mediated regulation of vascular endothelial growth factor (VEGF) expression is impaired in feto-placental endothelial cells from late-onset pre-eclampsia. Cultures of human umbilical vein endothelial cells (HUVECs) and human placental microvascular endothelial cells (hPMECs) from normal and pre-eclamptic pregnancies were used. Assays by using small interference RNA (siRNA) for A2A were performed, and transfected cells were used for estimation of messenger RNA (mRNA) levels of VEGF, as well as for cell proliferation and angiogenesis in vitro. CGS-21680 (A2A agonist, 24 h) increases HUVEC and hPMEC proliferation in a dose response manner. Furthermore, similar to CGS-21680, the nitric oxide donor, S-nitroso-N-acetyl-penicillamine oxide (SNAP), increased cell proliferation in a dose response manner (logEC50 10−9.2 M). In hPMEC, CGS-21680 increased VEGF protein levels in both normal (∼1.5-fold) and pre-eclamptic pregnancies (∼1.2-fold), an effect blocked by the A2A antagonist, ZM-241385 (10−5 M) and the inhibitor of NO synthase, N ω-nitro-L-arginine methyl ester hydrochloride (L-NAME). Subsequently, SNAP partially recovered cell proliferation and in vitro angiogenesis capacity of cells from normal pregnancies exposed to siRNA for A2A. CGS-21680 also increased (∼1.5-fold) the level of VEGF mRNA in HUVEC from normal pregnancies, but not in pre-eclampsia. Additionally, transfection with siRNA for A2A decrease (∼30 %) the level of mRNA for VEGF in normal pregnancy compared to untransfected cells, an effect partially reversed by co-incubation with SNAP. The A2A-NO-VEGF pathway is present in endothelium from microcirculation and macrocirculation in both normal and pre-eclamptic pregnancies. However, NO signaling pathway seems to be impaired in HUVEC from pre-eclampsia.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-016-9538-z) contains supplementary material, which is available to authorized users.
Keywords: Adenosine, A2A, Angiogenesis, Pre-eclampsia, Nitric oxide, VEGF
Introduction
Adenosine is a nucleoside, which activates a family of G-coupled protein receptors, named adenosine receptor (AR), type 1 (A1), 2A (A2A), 2B (A2B), and 3 (A3), and can control expression of pro-angiogenic factors such as vascular endothelial growth factor (VEGF) [1–6]. However, depending on the tissue or cell approach, A2A and A2B may play a dominant role in regulating pro-angiogenic processes [2, 4, 7, 8]. In particular, A2A can mediate cell proliferation/migration and synthesis of pro-angiogenic factors in many human, porcine, and rat endothelial cells [1]. These and other observations [9] have linked adenosine and adenosine receptor with upregulation of angiogenesis processes.
On the other hand, pre-eclampsia, a maternal syndrome characterized by placental hypoxia, new onset of hypertension, and proteinuria, is associated with high adenosine levels in umbilical blood [10, 11]. Causes and consequences of this elevated adenosine extracellular levels are not well established. However, other [12] and we [13] have suggested that it may impact placental angiogenesis. In particular, Iriyama et al. [12] described a mice model of increased placental adenosine levels, which results in a pre-eclampsia-like syndrome. This animal model also presented elevation of soluble vascular endothelial growth factor receptor type 1 (sFLT-1) and reduced expression of endothelial markers in the placenta, suggesting that high adenosine levels may instead have detrimental effect on placental angiogenesis.
Contrary to this last view, stimulation of A2A triggers nitric oxide (NO) synthesis, enhancing VEGF protein expression in human umbilical vein endothelial cells (HUVECs) from normal pregnancy [14]. Also, the inhibitor of NO synthase, N ω-nitro-L-arginine methyl ester hydrochloride (L-NAME), blocked A2A-mediated upregulation of VEGF expression (protein and messenger RNA (mRNA)) suggesting that NO is down stream of A2A-mediated VEGF in HUVEC from normal pregnancies. Nevertheless, pharmacological studies suggest that HUVECs derived from pre-eclampsia diagnosed after 34 weeks of gestation (late-onset pre-eclampsia (LOPE)) present a basal upregulation of A2A-NO-VEGF signaling pathway.
Changes in vessel formation within the human pre-eclamptic placenta are controversial [15–21]. In particular in LOPE, stereological studies evidenced no changes [22], while estimation of endothelial markers such as CD31 [18] or CD34 [17] denoted elevated placental angiogenesis compared to normotensive controls. Despite this discrepancy, previous in vitro studies also supported a pro-angiogenic behavior of HUVEC isolated from LOPE [14]. Then, it is feasible that increased vessel formation in placentas from LOPE would facilitate oxygen/nutrient transfer between mother and fetus [23] as a compensatory mechanism looking for adequate fetus growth. Knowledge about A2A-NO-VEGF signaling pathway in this potential placental compensatory mechanism is limited.
Taken all these evidences into account, we aim to investigate whether A2A-NO-mediated regulation of VEGF expression is impaired in feto-placental endothelial cells from LOPE pregnancies.
Methods
Patients
Pregnant women who attended to the Obstetric and Gynecology Department of the Herminda Martin Clinical Hospital (HCHM), Chillan, Chile, for their delivery were included as described previously by our group [14]. Exclusion criteria included chronic hypertension, altered renal function, diabetes, chronic disease, twin pregnancies, recurrent miscarriages, and abruption placenta. Women were classified into normal pregnancy (maternal blood pressure < 140/90 mmHg, absence of proteinuria, and no medical complications) and late-onset pre-eclampsia (new onset hypertension defined as blood pressure ≥ 140/90 mmHg with at least two measurements 6 h apart and proteinuria >300 mg/24 h) developed after 34 weeks of gestation. Each woman signed a written informed consent in the previous enrolment. The Ethical Committee from the Universidad del Bío-Bío approved this cohort study (Fondecyt 1140586).
Placentas and their umbilical cords were collected aseptically after birth by qualified Gynecology Service HCHM staff and then were processed in our laboratory at Department of Basic Sciences at the University of Bio Bio.
Use of agonists/antagonists of adenosine receptors
Specific agonist for A2A receptor, 2-(p-(2-carbonyl-ethyl)-feniletilamino)-50-N-ethylcarboxamidoadenosine) (CGS-21680; Sigma-Aldrich, MO, USA), at a maximum concentration of 10−8 M was used [24]. Also, we used the selective A2A antagonists, 4-(2-[7-amino-2-(2-furyl)(1,2,4)tri-azolo(2,3-a)(1,3,5)triazin-5-ylamino)ethyl)phenol (ZM-241385; Tocris Biosciences, MN, USA) at final concentration of 10−5 M [25] as well as 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-(4,3-e)-1,2,4-triazolo(1,5-c)pyrimidine (SCH-58261; Sigma-Adrich, MO, USA) at final concentration of 10−14 M [26]. In addition, the non-selective inhibitor of nitric oxide synthase, L-NAME (Sigma-Adrich, MO, USA), was used at final concentration of 10−5 M [27]. Nitric donor S-nitroso-N-acetyl-penicillamine oxide (SNAP) at final concentration of 10−9 M was also used.
Antibodies used were anti-A2A (Merck Millipore, Darmstadt, Germany) and anti-β-actin (Sigma-Aldrich, MO, USA).
Endothelial cell cultures
In order to test whether adenosine-mediated VEGF expression is a broadly effect on endothelial cells, we used two primary cultures of endothelial cells (macrovascular and microvascular) and one cell line of HUVEC (see supplementary information). Briefly, human placental microvascular endothelial cells (hPMECs) [28] and HUVEC [14] were isolated from normal and pre-eclamptic placentas as previously described by our group. Experiments were performed at standard culture condition (37 °C, 5 % CO2) without control of oxygen tension. Nevertheless, in parallel experiments, the cell line of HUVECs was also incubated at 2 % oxygen (i.e., hypoxia) (see supplementary information).
Small interference RNA for A2A receptor
Human umbilical vein endothelial cells from normal and pre-eclamptic pregnancies were placed in culture dish (60 mm) until 60 % confluence. Cell were transfected with small interference RNA for A2A receptor (small interference RNA (siRNA)-A2A, 0.1 μg/μl) by using transfection medium, as recommended by manufacturers (Santa Cruz Biotechnology, USA). After that, the medium was shifted by M199 containing 5 mM D-glucose, 20 % new born calf serum (NBCS), 20 % fetal calf serum (FCS), 3.2 mM L-glutamine, and 100 U/ml penicillin-streptomycin (primary culture medium (PCM)), and cells were allowed to grow for 48 h under standard conditions (37 °C, 5 % CO2). Then, cells were starving and exposed to agonists as described above.
Cell proliferation
Endothelial cells taken from normal and pre-eclamptic pregnancies (hPMEC or HUVEC) were cultured in conditions described above and used for cell proliferation analysis. Briefly, cells (7 × 103 cell/ml) were seeded in 96-well plate and maintained in growth in standard conditions (PCM, 37 °C, 5 % CO2) for 12 h. After serum deprivation, cells were then incubated with 5-bromo-2-deoxyuridine (BrdU) in the presence of adenosine deaminase (ADA, 0.1 U/ml) and CGS-21680 (10−8 M) or SNAP (10−9 M) during additional 24 h. Cells were fixed, and ELISA for detection of BrdU was performed according to the manufacturer’s instructions (Roche Diagnostics, IN, USA).
In vitro angiogenesis
In vitro angiogenesis assay on matrigel was performed by using primary culture of HUVEC from normal and pre-eclamptic pregnancies exposed or not to siRNA for A2A receptor (Santa Cruz Biotechnology, CA, USA). Briefly, HUVECs (4 × 104 cell) previously transfected or not to siRNA for A2A were cultured in 96-well plates coated with 40 μl of Matrigel basement membrane matrix (Corning, MA, USA). Under these conditions, cells were stimulated with CGS-21680 (10−8 M, 6 h) or SNAP (10−9 M, 6 h) and incubated under standard conditions (37 °C and 5 % CO2). Tube length was quantified by using the “Analyzer Angiogenesis” plugin from the ImageJ (NIH, USA) software.
RNA extraction and reverse transcription
Total RNA was isolated by using Trizol (Thermo Fisher Scientific, MA, USA). RNA was quantified by spectrophotometry (A260 nm), and its integrity was assessed by the A260/280 nm ratio and directly observed in an agarose gel (2 %). Aliquots of 1 μg of total RNA were used in the reverse transcription reaction by using oligo dT, random primers, and the enzyme Moloney Murine Leukemia Virus-reverse transcriptase (MMLV-RT, Promega, USA) in a cycle of reverse transcription at 37 °C as described elsewhere [14].
Non-quantitative PCR
Non-quantitative PCR was performed by using commercially available kits (Thermo Fisher Scientific, MA, USA) and sequence-specific oligonucleotide primers for human A2A: sense 5′-AGCTCCATCTTCAGTCTCCT-3′; antisense 5′-ACCGCAGTTGTTCCAACCTA-3′ (size 174 bp); VEGF: sense 5′-CTTGCCTTGCTGCTCTACCT-3′; antisense 5′-TCTCTCCTATGTGCTGGCCT-3′ (size 325 bp); or 18S: sense 5′-TCAAGAACGAAAGCTGGAGG-3′, antisense 5′-GGACATCTAAGGGCATCACA-3′ (size 489 bp) (0.5 μM). Cycles for PCR include 4 min at 95 °C, followed by 35 cycles of 30 s at 95 °C then 30 s at 58 °C or 60 °C (for A2A, VEGF, or 18S, respectively) and later 30 s at 72 °C, and finally 7-min extension at 72 °C. 18S mRNA was used as internal reference.
Statistical analysis
Comparisons between studied groups were performed by non-parametric Kruskal and Wallis test, and in cases in which there were significant differences, the Mann-Whitney test was performed. We used X 2 test to analyze proportions. Except when is indicated, values are medians and interquartile ranges, where n indicates the number of different cell cultures (in duplicated). p < 0.05 is considered statistically significant. The statistical software GraphPad Prism 5.00 (GraphPad Software Inc., CA, USA) was used for data analysis.
Results
Patients
Eighteen pregnant women were included (normotensive, n = 10; pre-eclampsia, n = 8). Clinical characteristics are included in Table 1. According to inclusion criteria, pre-eclamptic women had higher systolic and diastolic blood pressure than women with normal pregnancy (p < 0.002, in both cases). Pre-eclamptic women had proteinuria (460.0 ± 60.0 mg/day), while it was absent in normotensive women. No other statistically significant differences were observed in maternal, newborn, or placental parameters.
Table 1.
Clinical characteristics of included patients
| Normal (n = 10) | Pre-eclampsia (n = 8) | |
|---|---|---|
| Maternal characteristics | ||
| Age, years | 24.5 ± 1.9 | 22.5 ± 2.6 |
| BMI1, kg/m2 | 27.0 ± 0.8 | 26.3 ± 1.4 |
| BMI2, kg/m2 | 32.5 ± 0.7 | 33.3 ± 1.5 |
| Gest. age delivery, weeks | 38.9 ± 0.4 | 38.3 ± 0.6 |
| SBP, mmHg | 115.6 ± 3.7 | 146.1 ± 2.5* |
| DBP, mmHg | 68.4 ± 2.7 | 90.9 ± 2.8* |
| Proteinuria, mg × 24 h | – | 460.0 ± 60.0 |
| Platelets, #1000/ml | 182.0 ± 13.0 | 197.0 ± 16.0 |
| Hemoglobin, mg/dl | 12.6 ± 0.2 | 12.3 ± 0.6 |
| Hematocrit, % | 36.0 ± 0.7 | 35.4 ± 1.3 |
| Leucocytes, #1000/ml | 11.0 ± 0.7 | 13.0 ± 1.5 |
| Newborn characteristics | ||
| Weight, g | 3291.0 ± 112.0 | 3365.0 ± 195.1 |
| Height, cm | 48.7 ± 0.4 | 48.8 ± 0.8 |
| Cephalic perimeter, cm | 34.3 ± 0.3 | 34.4 ± 0.7 |
| Placenta characteristics | ||
| Weight, g | 532.0 ± 32.6 | 547.5 ± 38.2 |
| NBW/PW, g | 6.4 ± 0.4 | 6.3 ± 0.3 |
Values are mean ± SEM
BMI 1 body mass index at first trimester, BMI 2 body mass index at third trimester, SBP systolic blood pressure, DBP diastolic blood pressure, NBW/PW newborn weight/placental weight ratio
*p < 0.0002 vs normal pregnancy
Characterization of the A2A-NO pathway in feto-placental endothelium
Figure 1 shows functional characterization of A2A-NO pathway in HUVEC and hPMEC. Thus, in HUVEC, stimulation of A2A receptor by CGS-21680 increases proliferation in normal pregnancies (Fig. 1a) with a logEC50 of 10−9.6 M (95 % CI 10−10 to 10−9.2 M). Then, we used 10−8 M of CGS-21680 for all next experiments.
Fig. 1.
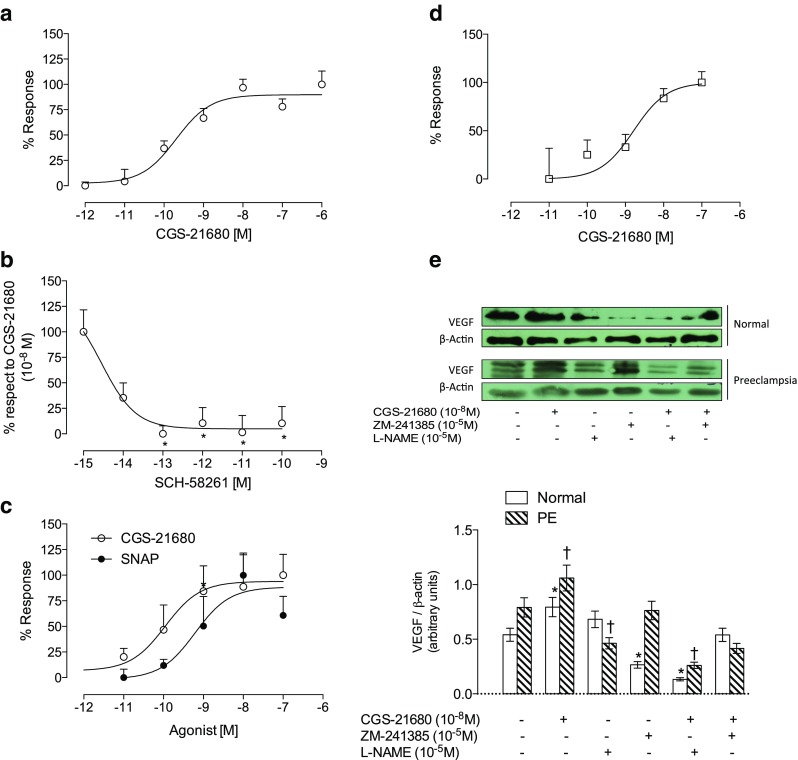
A2A-NO-VEGF pathway in feto-placental endothelial cells. Endothelial cells from human umbilical vein (HUVEC) were isolated from normal pregnancies and used for cell proliferation analysis by incorporation of bromouridine. Cells were incubated for 24 h with a CGS-21680 (10−6 to 10−12 M) and b co-incubated with CGS-21680 (10−8 M, maximum dose) and SCH-58261 in dose response (10−15 to 10−10 M) or c CGS-21680 (10−7 to 10−12 M, white circles) or SNAP (10−7 to 10−11 M, black circles). d Placental microvascular endothelial cells (hPMEC) taken from normal pregnancies (white bar) and pre-eclamptic (hatched bar) were extracted as described in Methods section. Cell proliferation quantified by incorporating bromouridine in the presence of CGS-21896 (10−6 to 10−11 M). e Representative image of western blot analysis for VEGF and its respective load control β-actin in the presence of CGS-21680 (10−8 M) and/or ZM-241385 (10−6 M) and/or L-NAME (10−5 M) in cells from normal and pre-eclamptic pregnancies (top panel). In the lower panel, densitometry of VEGF/β-actin ratio treated as indicated in the top panel. The presence of drugs is marked with (+) and absence with (−). Results are expressed as medians and interquartile ranges. In b, *p < 0.05 vs 100 % response. n = 5 for each assay in duplicate. In d, n = 4. In e, n = 3 per group in duplicated
Stimulatory effect of CGS-21680 (10−8 M) on HUVEC proliferation was blocked in a dose response manner with selective A2A antagonist, SCH-58261 (logIC50 10−14 M; 95 % CI 10−15 to 10−13 M) (Fig. 1b). In parallel experiments, cells were exposed to SNAP (Fig. 1c). logEC50 of SNAP (10−9.2 M; 95 % CI 10−10 to 10−8.2 M) was similar to CGS-21680 (logEC50 10−9.9 M (95 % CI 10−11 10−8.8 M).
Also, CGS-21680 increases cell proliferation in hPMEC isolated from normal pregnancies in a dose response manner (logEC50 of 10−8.7 M; 95 % CI 10−9.3 to 10−8.2 M) (Fig. 1d).
In addition, CGS-21680 increased protein levels of VEGF in hPMEC from normal (∼1.5-fold) and pre-eclamptic pregnancies (∼1.2 times) (Fig. 1e). This increase in VEGF protein levels was blocked by the A2A antagonist, ZM-241385 (10−5 M), or by the inhibitor of NO synthase, L-NAME, in cells from normal and pre-eclamptic pregnancies. Interestingly, incubation with ZM-241385 or L-NAME alone reduced the abundance of VEGF in hPMEC from normal pregnancies (40 and 80 %, respectively). Meanwhile, in pre-eclampsia, this reduction was less evident, in particular with L-NAME in which it reached only 15 % with respect to basal conditions without stimulation.
A2A is required for angiogenesis in vitro
The expression and function of A2A were diminished by siRNA without affecting cell viability (see Fig. 2). Transfected cells with siRNA for A2A exhibited a lower increase in the cAMP intracellular levels after stimulation with CGS-21680 than non-transfected cells (7.8-fold vs 31.5-fold, respectively) (Fig. 2d). Similarly, nitrite levels in the culture medium were increased (2.6-fold) in the presence of CGS-21680, which was reversed when the cells were exposed to siRNA for A2A (Fig. 2e).
Fig. 2.
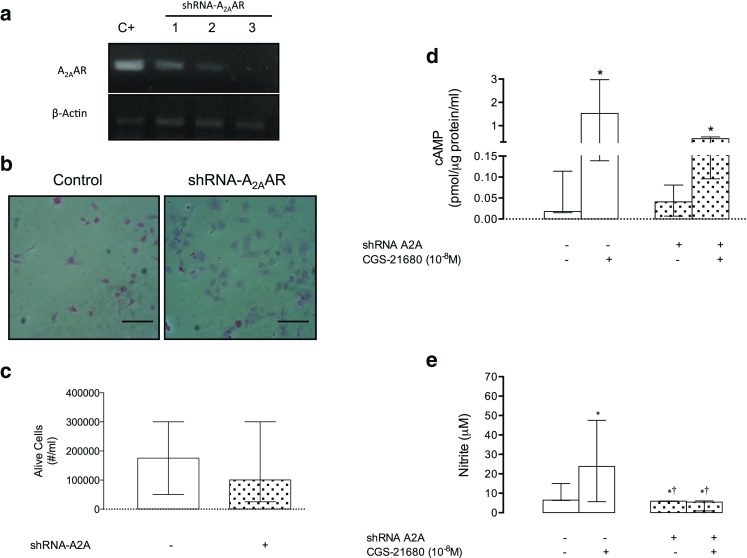
Characterization of siRNA for A2A. HUVECs were isolated from normal pregnancies and exposed to siRNA for A2A (siRNA-A2A) for 48 h as described in Methods section. a Representative image of semiquantitative PCR assays for A2A (A2A) and their respective control loading β-actin. Positive control (C+, HUVEC without transfection). Lanes 1, 2, and 3 show different doses used in RNA interference (0.3 to 1 μg/μl). b HUVEC immunocytochemistry for the presence of protein A2A, in the absence (Control) or presence of siRNA-A2A. c Testing of survival determined by cell counting with trypan blue in the absence (−) or presence (+) of siRNA-A2A. d Quantification of intracellular cAMP in HUVEC cells in the absence (−, white bar) or presence (+, hatched bar) of siRNA-A2A and CGS-21680 (10−8 M). e Quantification of nitrite in culture medium as described in d. The results are expressed as medians and interquartile ranges. In b, black bar represents 70 μm. d *p < 0.05 vs respective control in the absence of CGS-21680. In e, *p < 0.05 compared with control without CGS-21680 or siRNA for A2A. †p < 0.05 vs CGS-21680 in the absence siRNA for A2A. n = 3–5 for each assay in duplicate
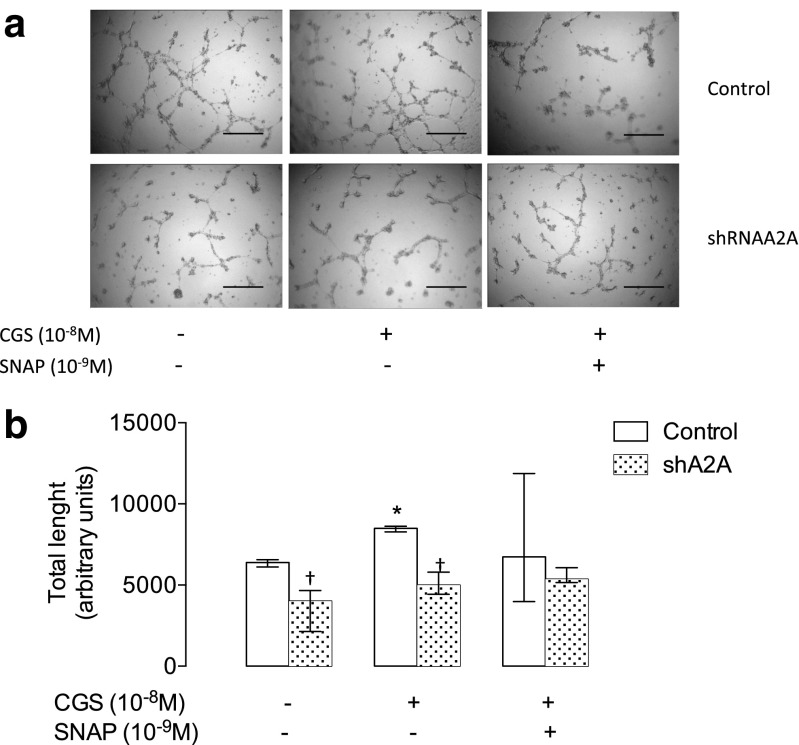
Also, CGS-21680 increased in vitro angiogenesis in non-transfected cells, while cells exposed to siRNA for A2A showed a reduction in this ability either in the absence or presence of CGS-21680 (Fig. 3). It is notable that SNAP partially reverted the reduced in vitro angiogenesis in cells exposed to siRNA for A2A (Fig. 3).
Fig. 3.
A2A is required for in vitro angiogenesis. HUVECs were isolated from normal pregnancies and used for in vitro angiogenesis assays. a Representative images of in vitro angiogenesis in the presence (+) of CGS-21680 (10−8 M) and/or SNAP (10−9 M) in control conditions or in cells exposed to siRNA for adenosine receptor (siRNA-A2A). b Quantification of the length of the tubes in basal conditions (open bar) and in the presence of siRNA-A2A (hatched bar). Results are expressed as medians and interquartile ranges. In b, *p < 0.05 vs baseline. †p < 0.05 vs respective values in non-transfected cells, n = 3 for each assay in duplicate
A2A-NO pathway is required for HUVEC proliferation
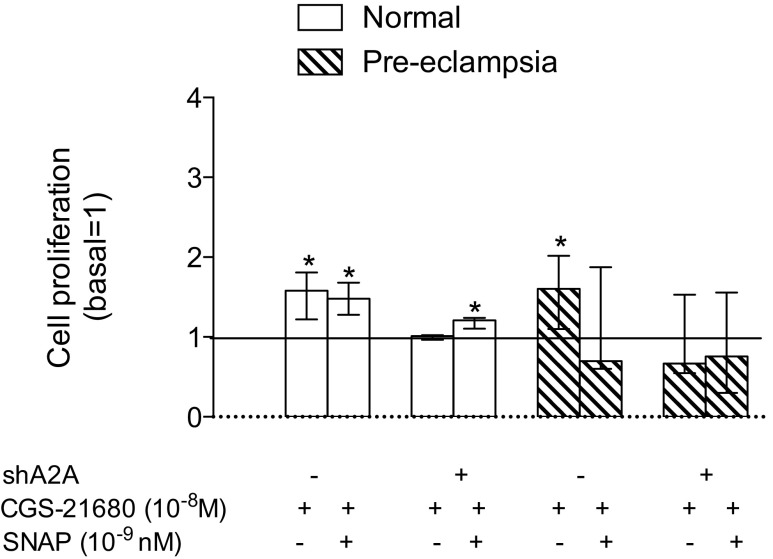
Incubation with CGS-21680 (10−8 M, 24 h) in HUVEC from normal pregnancies in the presence or absence of SNAP (10−9 M) increased cell proliferation (∼1.5-fold) (Fig. 4). In turn, the stimulatory effect of CGS-21680 was absent in cells transfected with siRNA for A2A. However co-incubation of SNAP and CGS-21680 partially recovered the inhibitory effect of siRNA for A2A in HUVEC from normal pregnancies. Similarly, in cells from pre-eclampsia, the presence of siRNA for A2A blocked the stimulatory effect of CGS-21680 on cell proliferation. But contrary to what was observed in cells from normal pregnancies, co-incubation of CGS-21680 and SNAP, either in the presence or absence of siRNA for A2A, was not be able to modify significantly cell proliferation (Fig. 4).
Fig. 4.
A2A is required for cell proliferation in normal and pre-eclamptic pregnancies. HUVECs were isolated from normal pregnancies (white bars) and pre-eclamptic (hatched bars) to estimate cell proliferation by incorporation of bromouridine. Cells were incubated for 24 h in the absence (−) or presence (+) of CGS-21680 (10−8 M), SNAP (10−9 M), and/or siRNA-A2A. Line corresponds to the baseline without stimulation. The results are expressed relative to baseline in medians and interquartile ranges. *p < 0.05 vs baseline. n = 3 for each assay in duplicate
Loss of A2A affects mRNA levels of VEGF
In HUVEC from normal pregnancies, CGS-21680 increased (∼1.5-fold) mRNA level of VEGF, which was avoided in cells transfected with siRNA for A2A (Fig. 5). Similar to what was observed in cell proliferation, co-incubation of SNAP and CGS-21680 partially recovered the inhibitory effect of siRNA for A2A on VEGF mRNA levels in HUVEC from normal pregnancies. Contrarily, in HUVEC from pre-eclampsia, differences did not reach statistical significance.
Fig. 5.
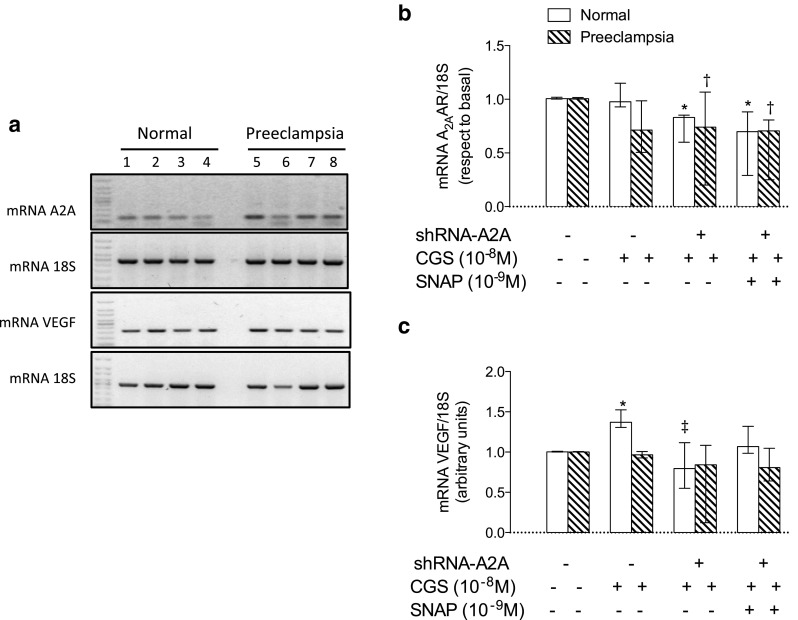
A2A increases mRNA levels of VEGF. HUVECs were isolated from normal and pre-eclamptic pregnancies. a Representative image of semiquantitative PCR for A2A, VEGF, and their respective loading control 18S. Cells from normal pregnancies (1 to 4) and cells pre-eclamptic pregnancies (5 to 8). Lines 1 and 5, in the absence of CGS-21680 or SNAP; 2 and 6, in the presence of CGS-21680; 3 and 7, in the presence of CGS-21680 and siRNA-A2A; 4 and 8, in the presence of CGS-21860, SNAP and siRNA-A2A. b Densitometry of A2A/18S ratio in normal cells (white bars) and pre-eclamptic (hatched bars) that were co-incubated with CGS and/or SNAP and/or siRNA-A2A, whose presence (+) and/or absence (−) is marked with respective symbols. c Densitometry of VEGF/18S ratio in normal and pre-eclampsia-derived cells as described in b. The results are expressed as medians and interquartile ranges. In b, *p < 0.05 vs cells from normal pregnancy at baseline. †p < 0.05 vs cells from pre-eclampsia at baseline. In c, *p < 0.05 vs cells from normal pregnancy at baseline. ‡p < 0.05 vs CGS-21680 in normal pregnancy. n = 4 for each group in duplicate
Discussion
Results confirm the functional presence of A2A-NO-VEGF pathway in feto-placental endothelial cells in normal pregnancies. Also, we found that bioavailability of NO generated by activation of A2A in cells from pre-eclampsia might be altered, thwarting its regulatory function on VEGF expression and thereby its pro-angiogenic capacity.
We have included a group of pre-eclamptic patients whose disease was present after 34 weeks of gestation, defined as LOPE. Rationality for using this pathology was because we have previously described elevated basal (i.e., without stimulation) activity of A2A-NO-VEGF signaling pathway in HUVEC derived from this disease [14]. Since LOPE is associated with less maternal and fetal complication than early onset pre-eclampsia (EOPE) [29, 30], it is feasible that elevation of A2A-NO-VEGF observed in fetal endothelium derived from LOPE might constitute a compensatory pro-angiogenic mechanism for normal fetal development. Compatible with this last hypothesis, fetal and placental weights were similar in LOPE compared to normal pregnancy, but these parameters were reduced in EOPE [14].
Nevertheless, placenta hypoxia can be present in LOPE as suggested for increased placental expression of hypoxia inducible factor [31]. Then, even increased angiogenesis observed in LOPE may be insufficient to overcome both the reduced oxygen delivery that is due to the infarcted placenta and the reduced oxygen delivery that would be predicted with failed remodeling of the maternal spiral arteries supplying the intervillous space [32]. More work is needed to better assess the role of hypoxia in the increased placental angiogenesis present in LOPE.
Regarding feto-placental endothelial cells used in this study, it has been shown that cells from microcirculation (i.e., hPMEC) are more reactive to pro-angiogenic signals than macrovascular endothelium (i.e., HUVEC) [2, 33, 34]. Despite that, hPMEC and HUVECs have similar response to CGS-21680 when cell proliferation was analyzed (logEC50 10−8.7 M vs logEC50 10−9.6 M, respectively). Similar to what was observed in HUVEC [14], CGS-21680 also increased VEGF protein levels in hPMEC, which, in turn, was reverted by the selective antagonist ZM-241385 or by the NOS inhibitor (L-NAME) in cells from either normal or pre-eclamptic pregnancies. Therefore, A2A-NO-VEGF pathway seems to be a common pathway present along the placental circulation.
Elevated circulating levels of adenosine in feto-placental circulation have been described in pre-eclamptic pregnancies [10, 11, 28, 35]. However, causes and consequences of this elevation are not well understood. Although, high placental adenosine levels were associated with either reduction in placental angiogenesis [12] or pro-angiogenic behavior in HUVEC [14].
How adenosine may control placental angiogenesis is not entirely known. However, it has been described that adenosine A2A (and A2B) receptors may increase endothelial proliferation/migration and in vitro angiogenesis capacity, as well as induce vasodilatation, increase the synthesis of pro-angiogenic factors (i.e., VEGF), or decrease expression of antiangiogenic factors such as sFLT-1 [see details in 1]. In particular, other [2, 4, 6] and we [14, 36] have previously showed that stimulation of A2A receptor increased the expression of VEGF in endothelial cells, as confirmed in the current work.
For instance, in HUVEC cell line exposed to normoxia (21 % oxygen), situation in which A2A exceeds to A2B expression, the non-selective adenosine receptor agonist, N-ethylcarboxamidoadenosine (NECA), increases (1.5 times) the expression of VEGF. But, when those cells were incubated in hypoxia (4.6 % oxygen, 1 or 3 h), there was a shift in the expression of A2B over A2A, associated with a significant increase in the expression and release of VEGF (eight times) [4]. Although we have not investigated expression of A2B in this manuscript, previously, we have described high expression of A2B in HUVEC from LOPE [36]. But contrary to what was found in hypoxia [4], in HUVEC from pre-eclampsia, we did not find an increase in the VEGF protein levels after stimulation with NECA. Also, we found that both repression and overexpression of A2A lead to reduction in VEGF mRNA levels in normoxic (21 % oxygen) conditions without significant changes in hypoxia (2 % oxygen, overnight) in a HUVEC cell line (see supplementary information). Then, future studies should block both A2A and A2B receptors in order to better characterize its participation for controlling VEGF expression in fetal endothelium from pre-eclampsia. Also, potential cross talk between A2A and VEGF needs to be clarified.
Intracellular pathway involved in A2-mediated VEGF expression is not completely elucidated; however, we have found participation of NO in this process [14, 36]. Despite the A2A-NO-VEGF pathway is present in pre-eclamptic pregnancies, participation of NO seems to be associated with gestational age of pre-eclampsia onset. Thus, while LOPE is characterized by elevation of A2A-mediated NO-VEGF signaling, in EOPE (diagnosed <34 weeks of gestation), this particular pathway is decreased [14]. These results partially agree with those presented by Salsoso and colleagues [37], since these authors found reduced expression of A2A receptor in HUVEC isolated from LOPE, associated with elevation in the L-arginine transport, the substrate for NO synthesis.
Also, results presented in this manuscript suggest that contrary to what was observed in cells from normal pregnancy, in those from LOPE, NO donor did not recover the reduction in mRNA levels of VEGF or cell proliferation induced by siRNA for A2A. Mechanisms for this effect were not addressed in this manuscript; however, at least two possibilities can be noted. First, HUVEC from LOPE exhibited elevated nitrite levels in the culture medium, which was not further elevated in the presence of CGS-21680 [14]. Then, additional NO given by SNAP might not be useful since cells would be on saturated conditions. Second, high oxidative stress is well described in HUVEC from pre-eclampsia [see details in 38]; then, NO bioavailability would be impaired. Additional studies are required to fully understand this phenomenon.
In conclusion, we demonstrated that A2A-NO-VEGF pathway controls VEGF expression and cell proliferation in HUVEC and hPMEC (Fig. 6). In LOPE, basal elevation of A2A-NO-VEGF impaired cell response to NO donor, SNAP. We believe that the results may contribute in the understanding of adaptive processes in the fetal-placental circulation pregnancy with pre-eclampsia.
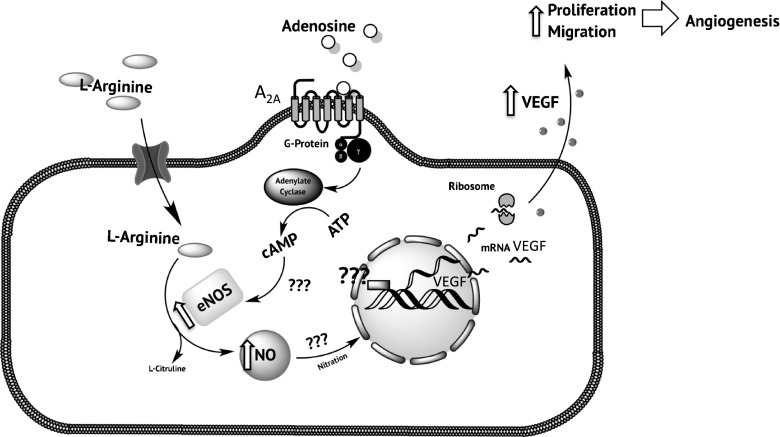
Fig. 6.
A2A and NO signaling pathway regulate the expression of VEGF in normal and late-onset pre-eclampsia. In human feto-placental endothelium from normal pregnancy, activation of A2A adenosine receptor triggers an intracellular pathway involved increased generation of cAMP, activation (serine1175 phosphorylation) of nitric oxide synthase (eNOS), and generation of nitric oxide (NO) from L-arginine. Nitric oxide increases mRNA levels of VEGF via unknown (?) mechanism(s), which may include protein nitration. This A2A-NO-VEGF pathway can lead to a pro-angiogenic behavior of feto-placental endothelium, since it can stimulate cell proliferation/migration and tube formation. In LOPE, this A2A-mediated intracellular pathway is elevated (⇪), in particular eNOS activation, NO synthesis, and VEGF expression. Upregulation of A2A-NO-VEGF pathway observed in LOPE may constitute a compensatory mechanism to hypoxia in the placenta
Electronic supplementary material
(DOCX 5387 kb)
Acknowledgments
We would like to thank all research staff at the Vascular Physiology Laboratory and the Group of Investigation of Tumor Angiogenesis (GIANT) of the Universidad del Bío-Bío for their technical support. We also give thanks to researchers in the GRIVAS Health group for outstanding discussion regarding ideas presented in this manuscript. We thank Dr. Igor Feoktistov from University of Vanderbilt for active discussion and molecular tools kindly shared with our lab. This study was financed by Fondecyt Regular 1140586, Fondequip EQM140104, DIUBB GI153109/EF, and GI 152920/EF.
Individual contributions
This work was carried out as a full collaboration among all the authors. CE defined the research topic. JA, KH, FT, and CA prepared draft of the manuscript. PB is the clinical responsible for patient inclusion. CE, JA, and CA co-wrote the manuscript. All authors approved the final version of the manuscript.
Abbreviations
- CGS-21680
2-(p-(2-Carbonyl-ethyl)-feniletilamino)-50-N-ethylcarboxamidoadenosine)
- BrdU
5-Bromo-2-deoxyuridine
- SCH-58261
7-(2-Phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-(4,3-e)-1,2,4-triazolo(1,5-c)pyrimidine
- AR
Adenosine receptors
- HMEC-1
Dermal microvascular endothelial cells
- EOPE
Early onset pre-eclampsia
- hPMEC
Human placental microvascular endothelial cells
- HUVEC
Human umbilical vein endothelial cells
- LOPE
Late-onset pre-eclampsia
- NECA
N-ethylcarboxamidoadenosine
- NO
Nitric oxide
- L-NAME
N ω-nitro-L-arginine methyl ester hydrochloride
- VEGF
Vascular endothelial growth factor
Compliance with ethical standards
Financial disclosure
None.
Conflict of interest
None
References
- 1.Escudero C, Roberts JM, Myatt L, Feoktistov I. Impaired adenosine-mediated angiogenesis in preeclampsia: potential implications for fetal programming. Front Pharmacol. 2014;5:134. doi: 10.3389/fphar.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res. 2002;90(5):531–538. doi: 10.1161/01.RES.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- 3.Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I. Mast cell-mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ Res. 2003;92(5):485–492. doi: 10.1161/01.RES.0000061572.10929.2D. [DOI] [PubMed] [Google Scholar]
- 4.Feoktistov I, Ryzhov S, Zhong H, Goldstein AE, Matafonov A, Zeng D, Biaggioni I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension. 2004;44(5):649–654. doi: 10.1161/01.HYP.0000144800.21037.a5. [DOI] [PubMed] [Google Scholar]
- 5.Grant MB, Davis MI, Caballero S, Feoktistov I, Biaggioni I, Belardinelli L. Proliferation, migration, and ERK activation in human retinal endothelial cells through a(2B) adenosine receptor stimulation. Invest Ophthalmol Vis Sci. 2001;42(9):2068–2073. [PubMed] [Google Scholar]
- 6.Grant MB, Tarnuzzer RW, Caballero S, Ozeck MJ, Davis MI, Spoerri PE, Feoktistov I, Biaggioni I, Shryock JC, Belardinelli L. Adenosine receptor activation induces vascular endothelial growth factor in human retinal endothelial cells. Circ Res. 1999;85(8):699–706. doi: 10.1161/01.RES.85.8.699. [DOI] [PubMed] [Google Scholar]
- 7.Feoktistov I, Biaggioni I, Cronstein BN. Adenosine receptors in wound healing, fibrosis and angiogenesis. Handb Exp Pharmacol. 2009;193:383–397. doi: 10.1007/978-3-540-89615-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol. 2005;67(5):1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- 9.Ryzhov S, McCaleb JL, Goldstein AE, Biaggioni I, Feoktistov I. Role of adenosine receptors in the regulation of angiogenic factors and neovascularization in hypoxia. J Pharmacol Exp Ther. 2007;320(2):565–572. doi: 10.1124/jpet.106.114850. [DOI] [PubMed] [Google Scholar]
- 10.Espinoza J, Espinoza AF, Power GG. High fetal plasma adenosine concentration: a role for the fetus in preeclampsia? Am J Obstet Gynecol. 2011;205(5):485. doi: 10.1016/j.ajog.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Yoneyama Y, Sawa R, Suzuki S, Shin S, Power GG, Araki T. The relationship between uterine artery Doppler velocimetry and umbilical venous adenosine levels in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 1996;174(1 Pt 1):267–271. doi: 10.1016/S0002-9378(96)70406-4. [DOI] [PubMed] [Google Scholar]
- 12.Iriyama T, Sun K, Parchim NF, Li J, Zhao C, Song A, Hart LA, Blackwell SC, Sibai BM, Chan LL, Chan TS, Hicks MJ, Blackburn MR, Kellems RE, Xia Y. Elevated placental adenosine signaling contributes to the pathogenesis of preeclampsia. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.114.013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escudero C, Puebla C, Westermeier F, Sobrevia L. Potential cell signalling mechanisms involved in differential placental angiogenesis in mild and severe pre-eclampsia. Curr Vasc Pharmacol. 2009;7(4):475–485. doi: 10.2174/157016109789043865. [DOI] [PubMed] [Google Scholar]
- 14.Escudero C, Bertoglia P, Hernadez M, Celis C, Gonzalez M, Aguayo C, Acurio J. Impaired A2A adenosine receptor/nitric oxide/VEGF signaling pathway in fetal endothelium during late- and early-onset preeclampsia. Purinergic Signal. 2013;9(2):215–226. doi: 10.1007/s11302-012-9341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92(1):35–43. doi: 10.1016/S0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 16.Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18(8):613–621. doi: 10.1016/S0143-4004(97)90000-X. [DOI] [PubMed] [Google Scholar]
- 17.Resta L, Capobianco C, Marzullo A, Piscitelli D, Sanguedolce F, Schena FP, Gesualdo L. Confocal laser scanning microscope study of terminal villi vessels in normal term and pre-eclamptic placentas. Placenta. 2006;27(6–7):735–739. doi: 10.1016/j.placenta.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Escudero C, Celis C, Saez T, San Martin S, Valenzuela FJ, Aguayo C, Bertoglia P, Roberts JM, Acurio J. Increased placental angiogenesis in late and early onset pre-eclampsia is associated with differential activation of vascular endothelial growth factor receptor 2. Placenta. 2014;35(3):207–215. doi: 10.1016/j.placenta.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Mayhew TM. A stereological perspective on placental morphology in normal and complicated pregnancies. [review] [97 refs. J Anat. 2009;215(1):77–90. doi: 10.1111/j.1469-7580.2008.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayhew TM, Manwani R, Ohadike C, Wijesekara J, Baker PN. The placenta in pre-eclampsia and intrauterine growth restriction: studies on exchange surface areas, diffusion distances and villous membrane diffusive conductances. Placenta. 2007;28(2–3):233–238. doi: 10.1016/j.placenta.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Mayhew TM, Ohadike C, Baker PN, Crocker IP, Mitchell C, Ong SS. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta. 2003;24(2–3):219–226. doi: 10.1053/plac.2002.0900. [DOI] [PubMed] [Google Scholar]
- 22.Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG. 2006;113(5):580–589. doi: 10.1111/j.1471-0528.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 23.Gill JS, Salafia CM, Grebenkov D, Vvedensky DD. Modeling oxygen transport in human placental terminal villi. J Theor Biol. 2011;291:33–41. doi: 10.1016/j.jtbi.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, Franklin WA, Bridges JP, Schaack JB, Colgan SP, White CW. Adenosine A2 A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci U S A. 2009;106(26):10684–10689. doi: 10.1073/pnas.0901326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai A, Victor-Vega C, Gadangi S, Montesinos MC, Chu CC, Cronstein BN. Adenosine A2 A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005;67(5):1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- 26.Dionisotti S, Ongini E, Zocchi C, Kull B, Arslan G, Fredholm BB. Characterization of human A2 A adenosine receptors with the antagonist radioligand [3H]-SCH 58261. Br J Pharmacol. 1997;121(3):353–360. doi: 10.1038/sj.bjp.0701119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escudero C, Casanello P, Sobrevia L. Human equilibrative nucleoside transporters 1 and 2 may be differentially modulated by A2B adenosine receptors in placenta microvascular endothelial cells from pre-eclampsia. Placenta. 2008;29(9):816–825. doi: 10.1016/j.placenta.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 29.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 30.Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–192. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 31.Sezer SD, Kucuk M, Doger FK, Yuksel H, Odabasi AR, Turkmen MK, Cakmak BC, Omurlu IK, Kinas MG. VEGF, PIGF and HIF-1alpha in placentas of early- and late-onset pre-eclamptic patients. Gynecol Endocrinol. 2013;29(8):797–800. doi: 10.3109/09513590.2013.801437. [DOI] [PubMed] [Google Scholar]
- 32.Roberts JM, Escudero C. The placenta in preeclampsia. Hypertension Pregnancy. 2012;2(2):72–83. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dye J, Lawrence L, Linge C, Leach L, Firth J, Clark P. Distinct patterns of microvascular endothelial cell morphology are determined by extracellular matrix composition. Endothelium. 2004;11(3–4):151–167. doi: 10.1080/10623320490512093. [DOI] [PubMed] [Google Scholar]
- 34.Escudero CGM, Acurio J, Valenzuela F, Sobrevia L (2013) The role of placenta in the fetal programming associated to gestational diabetes. Gestational Diabetes - Causes, Diagnosis and Treatment, vol 1. InTeach, Croacia
- 35.Escudero C, Sobrevia L. Understanding physiological significance of high extracellular adenosine levels in feto-placental circulation in preeclamptic pregnancies. In: Sobrevia L, Casanello P, editors. Membrane transporters and receptors in disease, vol 1. Kerala, India: Research Signpost; 2009. pp. 27–51. [Google Scholar]
- 36.Acurio J, Troncoso F, Bertoglia P, Salomon C, Aguayo C, Sobrevia L, Escudero C. Potential role of A2B adenosine receptors on proliferation/migration of fetal endothelium derived from preeclamptic pregnancies. Biomed Res Int. 2014;2014:274507. doi: 10.1155/2014/274507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salsoso R, Guzman-Gutierrez E, Saez T, Bugueno K, Ramirez MA, Farias M, Pardo F, Leiva A, Sanhueza C, Mate A, Vazquez C, Sobrevia L. Insulin restores L-arginine transport requiring adenosine receptors activation in umbilical vein endothelium from late-onset preeclampsia. Placenta. 2015;36(3):287–296. doi: 10.1016/j.placenta.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Myatt L. Review: reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31(Suppl):S66–S69. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 5387 kb)