Abstract
Estrogen receptor beta (ERβ) has been shown to play a therapeutic role in inflammatory bowel disease (IBD). However, the mechanism underlying how ERβ exerts therapeutic effects and its relationship with P2X3 receptors (P2X3R) in rats with inflammation is not known. In our study, animal behavior tests, visceromotor reflex recording, and Western blotting were used to determine whether the therapeutic effect of ERβ in rats with inflammation was related with P2X3R. In complete Freund adjuvant (CFA)-induced chronic inflammation in rats, paw withdrawal threshold was significantly decreased which were then reversed by systemic injection of ERβ agonists, DPN or ERB-041. In 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats, weight loss, higher DAI scores, increased visceromotor responses, and inflammatory responses were reversed by application of DPN or ERB-041. The higher expressions of P2X3R in dorsal root ganglia (DRG) of CFA-treated rats and those in rectocolon and DRG of TNBS-treated rats were all decreased by injection of DPN or ERB-041. DPN application also inhibited P2X3R-evoked inward currents in DRG neurons from TNBS rats. Mechanical hyperalgesia and increased P2X3 expression in ovariectomized (OVX) CFA-treated rats were reversed by estrogen replacements. Furthermore, the expressions of extracellular signal-regulated kinase (ERK) in DRG and spinal cord dorsal horn (SCDH) and c-fos in SCDH were significantly decreased after estrogen replacement compared with those of OVX rats. The ERK antagonist U0126 significantly reversed mechanical hyperalgesia in the OVX rats. These results suggest that estrogen may play an important therapeutic role in inflammation through down-regulation of P2X3R in peripheral tissues and the nervous system, probably via ERβ, suggesting a novel therapeutic strategy for clinical treatment of inflammation.
Keywords: Estrogen receptor β, P2X3 receptor, CFA, TNBS, Inflammation
Introduction
Studies have shown that human pain differs between the sexes. For example, some chronic diseases, including migraine, temporomandibular disorders, fibromyalgia, arthritis, and interstitial cystitis, are more prevalent in females [1, 2]. Women usually display stronger pain perception and request more analgesics [3, 4], while in pregnancy, increasing estrogen and progestogen have an anti-nociceptive role [5, 6]. Experimental studies have shown that after injection of estradiol, female ovariectomized (OVX) rats tended to have a longer latency in hot plate and tail-flick experiments [7]. Mechanical hyperalgesia and allodynia in the abdomen, hind limbs, and proximal tail of OVX rats, which result from a lack of estrogen in the body, can be reversed by sustaining exogenous estradiol replacement [8]. During several days or weeks after inflammation or injury, estrogen promotes the development of heat hyperalgesia and mechanical hyperalgesia [9, 10]. The estrogen effects depend on the type, time course of pain or inflammation models, and also the level and stability of estrogens [7].
It has been reported that the two classic receptors for estrogen, estrogen receptor α (ERα), and estrogen receptor β (ERβ) are closely related with inflammation [11–13]. In contrast to the long association of ERα with pain, the association of ERβ with pain may have a totally contradictory role to that mediated by ERα [14–16]. In nociceptive neurons of adult rats, 17β-estradiol inhibits transient receptor potential vanilloid subtype 1 (TRPV1) by activating the ERβ signal pathway [17]. In an inflammation model induced by complete Freund’s adjuvant (CFA), the selective ERβ agonist ERB-131 significantly reduced the extent of inflammation and pain hypersensitivity [18, 19]. The specific ERβ agonist ERB-041 efficiently inhibited arthritis induced by Freund’s adjuvant and has an anti-inflammatory effect in both arthritis and colitis [20]. Further, down-regulating the expression of ERβ by gene knockout exacerbated visceral hypersensitivity [14–16]. Although ERβ has a significant therapeutic effect in chronic inflammation related to pain transmission, the underlying mechanism is still unknown.
ATP and P2X3R have a long history of involvement with pain and inflammation [21, 22]. In colitis, more internal ATP was produced and expression of P2X3R at the end of colorectal nerves was also increased, thus leading to hypersensitivity of rectocolon [23–25]. When using the antagonists of P2X3R, 2′(3′)-O-(2,4,6-trinitrophenyl) ATP (TNP-ATP) or A317491, the expression and function of P2X3R in DRG were inhibited, resulting in the reduction of hypersensitivity in chronic inflammatory pain [26, 27]. Also, after knockout of P2X3R in a mouse model of inflammatory bowel disease (IBD), visceral motor responses were decreased, improving inflammatory symptoms [24]. Likewise, in acute inflammatory pain, activation of P2X3R at the spinal cord level and on the endings of primary sensory afferent nerves also promoted the development of inflammatory hypersensitivity 1 [26].
Therefore, the aim of this study is to investigate the role and the underlying mechanisms of ERβ activation in the treatment of chronic inflammation, including IBD and its relationship with P2X3R.
Materials and methods
Animals
Adult Sprague-Dawley female rats were used (200–250 g). As previously reported [28], rats were randomly divided into three groups: a group ovariectomized bilaterally (OVX), a group receiving the same surgical procedure without removing the ovaries (sham surgery) with both groups receiving vehicle injection, and a group ovariectomized bilaterally and receiving estrogen treatment. E2 replacement therapy was carried out in the ovariectomized rats 1 week after surgery by daily subcutaneous injection of 17β-estradiol (30 μg/kg, 0.4 mL/day); the same volume of vehicle was injected into sham and OVX rats daily. In these three groups, the studies of chronic inflammatory pain by CFA injection were carried out in the 3 weeks after estradiol replacement or vehicle treatment.
In the study on a rat model of CFA-induced chronic inflammatory pain, the rats were randomly divided into four groups, namely a saline group, a CFA (50 μg) group, a DPN (3 mg/kg) group, and an ERB-041 (1 mg/kg) group. In the study on a rat model of 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis model, the rats were randomly divided into four groups, namely a saline group, a TNBS (40 mg/kg) group, a DPN (3 mg/kg) group, and an ERB-041 (1 mg/kg) group. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Second Military Medical University.
Model of CFA-induced chronic inflammation
As previously reported [29], CFA (100 μL) was injected into the left hind paw. The paw withdrawal threshold (PWT) and paw size were assessed before (baseline) and every 1 day after CFA injection. Before CFA was given, their basic mechanical pain threshold and volume of their left paws were measured by a draining method. In brief, 100 μl CFA was applied to the planta of the left hind paw to induce inflammatory hyperalgesia which then developed into sustained chronic hyperalgesia, which was characterized by intense local redness, edema, and hotness. After that, pain behavioral experiments were conducted on rats from the different groups every 24 h and the volumes of their left hind paws were recorded; the experiments lasted for at least 8 days.
Model of TNBS-induced colitis
All experiments were performed in the diestrus stage of the rat estrous cycle when the least cells were seen following vaginal smear examination. As previously reported [30], experimental colitis was induced by administration of an intrarectal enema (8 cm from the anus) of 30 % TNBS in ethanol at a dose of 40 mg/kg body weight. The enemas were given through a 6-Fr medical-grade polyurethane enteral feeding tube while the rats were under light ether anesthesia. Assessment of colitis was based on body weight and DAI scores (a disease activity index mainly based on weight loss, stool consistency, and bleeding).
Behavioral experiments
Testing of PWT
As we have reported, on the day of experimentation, rats were allowed to settle for at least 30 min before behavioral experiments. For mechanical hyperalgesia testing, rat PWT in response to stimulation of Von-Frey filaments (ranging from 0.0174 to 263 g, Autonomic Neuroscience Centre, London) to the lateral plantar surface of the tested paw until it withdrew [31]. Each monofilament was applied ten times. The PWT was taken as the force at which the animal withdrew the paw from at least five consecutive stimuli. The person conducting the behavioral measurements was blind to the treatments.
Measuring of hind paws’ volumes
According to the methods reported previously [31], the paw size of rats were assessed by using Drain-age Act [32].
Visceromotor reflex recording
Rats were implanted with electromyogram (EMG) electrodes 5–7 days before testing. On the day of experiment, a balloon was lubricated and placed into the distal colon so that the tip of the balloon was 2 cm proximal to the anus. The intracolonic balloon was manually inflated using an air-filled syringe which allowed slow, continuous inflation. The visceromotor response was measured using 20, 40, 60, 80 mmHg CRD (four distensions at each pressure, 20 s each, 3 min interstimulus interval) [19, 30]. The intracolonic and balloon pressures and the EMG trace were continuously recorded after instrument calibration. The visceromotor response (the EMG in response to CRD) was recorded with a CED 1401plus and analyzed using Spike 2 for Windows software (Cambridge Electronic Design, UK). The EMG was rectified offline and the area under the curve (AUC) determined. The baseline visceromotor response was defined as the AUC 10 s prior to distension.
Western blotting
Rats were sacrificed, and then lumbosacral DRG, spinal cord dorsal horn (SCDH), and rectocolon were obtained. Frozen rectocolonic tissues were weighed and homogenized in ice-cold buffer (50 mM Tris-HCl, pH 7.5, 8 mM MgCl2, 5 mM ethylene glycol bis (2-aminoethyl ether)-N,N,N′N′-tetraacetic acid (EGTA), 0.5 mM EDTA, 0.01 mg/ml leupeptin, 0.01 mg/ml pepstatin, 0.01 mg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 250 mM NaCl). Homogenates were centrifuged (12,000×g, 15 min, 4 °C), and the supernatants were collected and stored at −80 °C. Protein concentration of the homogenate was determined following Bradford’s colorimetric method. Aliquots of supernatants containing equal amounts of protein (50 μg) were separated on 10 % acrylamide gel by sodium dodecyl sulfate polyacrylamide gel electrophoresis. In the next step, the proteins were electrophoretically transferred onto a nitrocellulose membrane and incubated with specific primary antibodies: P2X3R (1:1000, Abcam, USA), c-fos (1:200, Santa Cruz, USA), and extracellular signal-regulated kinase (ERK) (1:200, Santa Cruz, USA). Each filter was washed three times for 15 min and incubated with the anti-rabbit immunoglobulin G antibodies for P2X3R and for c-fos and ERK. To prove equal loading, the blots were analyzed for β-actin expression using an anti-β-actin antibody (1:8000, Sigma-Aldrich, MO, USA). Immunodetection was performed using enhanced chemiluminescence light-detecting kit (Santa Cruz, USA). Densitometric data were studied following normalization to the control (housekeeping gene). The signals were analyzed and quantified by a Scientific Imaging Systems (KODAK 1D, Image Analysis Software).
Chemicals
CFA, TNBS, ERB-041, and U0126 were all purchased from Sigma-Aldrich, St. Louis, USA. DPN and NF-110 was purchased from Tocris. 17β-Estradiol was prepared in deionized water and stored frozen, while DPN and ERB-041 were dissolved in ethanol to 1 mM. U0126 were dissolved in DMSO to 1 mM.
Statistical analysis
Data are expressed as means ± SEM. Data in the behavior test were analyzed using two-way ANOVA. Unpaired Student’s t test analysis in SPSS11 was performed in c-fos expression between control and CFA groups. One-way ANOVA analysis was performed in the P2X3, c-fos, and ERK expression among control, OVX, and estrogen-treated OVX groups. Unpaired Student’s t test analysis in SPSS16 was performed in the experiments using Western blotting techniques. Differences among the groups were considered statistically significant when the p value was lower than 0.05 (p < 0.05).
Results
The ERβ agonist DPN reversed the decrease of PWT induced by CFA injection in normal female rats
Compared with the control (saline + saline) group, plantar injection of CFA could significantly decrease the mechanical PWT (Fig. 1a, n = 8, p < 0.01). ERβ agonists DPN and ERB-041 were peripherally applied to study the effect on the PWT of CFA rats. On the 4th day after plantar injection of CFA, saline (500 μl), DPN (3 mg/kg, in 500 μl saline), or ERB-041 (1 mg/kg, in 500 μl saline) were given in the following 4 consecutive days. ERβ agonist DPN significantly reversed the PWT of CFA rats (Fig. 1a, b, n =8, p < 0.05) on the 8th day after CFA injection. Another ERβ agonist ERB-041 lacked the similar effect (Fig. 1a, b, n = 8, p > 0.05).
Fig. 1.
Changes in CFA-induced paw withdrawal threshold (PWT) and volumes of the left hind-paw among the three groups of female rats after injection of saline (100 μl) or CFA (100 μl) in the left hind-paw. a PWT of the group given CFA was significantly lower than that of the saline group (n = 8). On the 8th day after CFA was applied, PWT of DPN-treated rats was significantly increased compared with that of saline-treated CFA rats (n = 8). There was no significant difference between saline-treated CFA rats and ERB-041-treated CFA rats (n = 8). *p < 0.05 b On the 8th day after CFA or saline injection, PWT of saline-treated CFA rats was significantly lower than that of saline rats (n = 8). After treatment of DPN in CFA rats, PWT was significantly reversed (n = 8), while similar changes were not observed after treatment with ERB-041, compared with that of saline-treated CFA rats (n = 8). *p < 0.05, **p < 0.01 c The volumes of left hind-paw of the groups given CFA were significantly larger than that of saline-treated rats (n = 8). However, there was no significant difference among saline-treated, DPN-treated, and ERB-041-treated CFA rats (n = 8)
The volume of the left hind paws was significantly increased after plantar injection of CFA (Fig. 1c, n =8, p < 0.05). However, neither DPN nor ERB-041 reversed the increase induced by CFA (Fig. 1c, n =8, p > 0.05; n = 8, p > 0.05).
ERβ agonists played a therapeutic role in TNBS-induced colitis rats
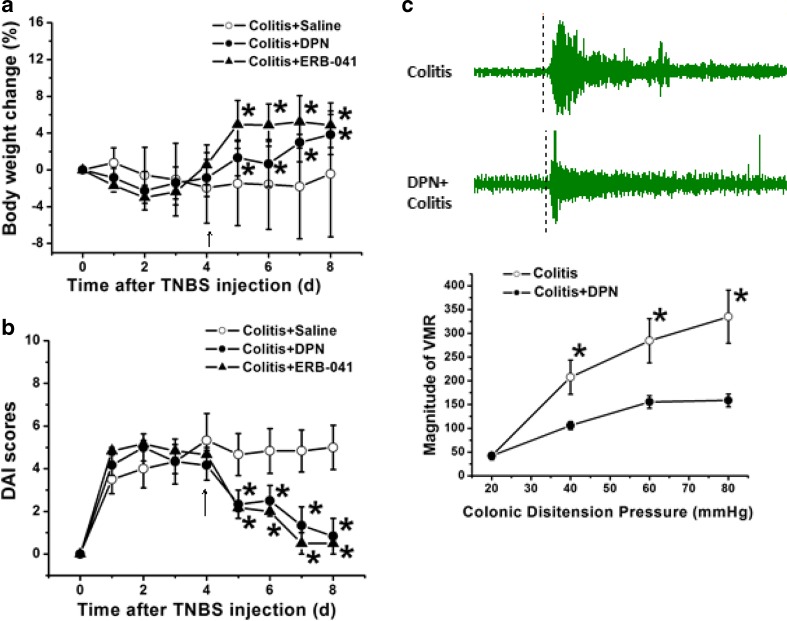
Rats with colitis induced by TNBS soon developed severe abdominal pain, diarrhea, and even bloody stools, which were characterized by a significant decrease in body weight and a significant increase in DAI scores. On the 4th day after colonic injection of TNBS, rats were given saline (500 μl), ERβ agonists DPN, or ERB-041 (1 mg/kg, in 500 μl saline) in the following 4 consecutive days. The results showed that compared with those of the colitis group, the loss of body weight was significantly reversed after DPN or ERB-041 treatment (Fig. 2a, n = 6 for each group, p < 0.05, p < 0.05). DAI scores were also decreased markedly after DPN or ERB-041 treatment (Fig. 2b, n =6 for each group, p < 0.05, p < 0.05).
Fig. 2.
Changes in body weight and DAI scores after application of DPN and ERB-041 in TNBS rats. a The body weight of DPN or ERB-041-treated colitis rats was significantly increased compared with that of saline-treated colitis rats (n = 6), which continued for at least 3 days. b The DAI scores of DPN or ERB041-treated colitis rats were significantly decreased compared with that of saline-treated colitis rats (n = 6), which also continued for at least 3 days. c Diagram showing the relationship between the magnitude of visceromotor reflex (VMR) and the colonic distension pressure in the colitis rats with or without DPN application. Compared with that in the colitis rats, the magnitude of the VMR in DPN-treated rats was significantly decreased (n = 6).*p < 0.05
As a contraction of the abdominal muscles in response to colorectal distension, the visceromotor reflex (VMR) has been reported to be related to the visceral hypersensitivity and intestinal inflammation [24, 28]. In the present study, we then tested the changes in VMR after application of DPN by using a colitis model induced by colonic injection of TNBS. Diagram showed the relationship between the magnitude of VMR and the colonic distension pressure in the colitis rats with or without DPN application. Compared with that in the colitis rats, the magnitude of the VMR in the DPN-treated rats was significantly decreased (Fig. 2c, n = 6 for each group, p < 0.05).
Involvement of P2X3 receptors in TNBS-induced colitis rats
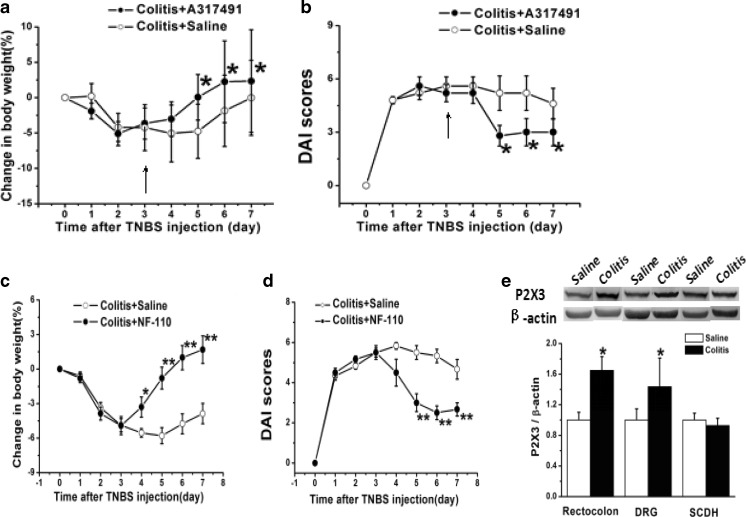
A317491, a selective antagonist of P2X3R, was used subcutaneously to treat colitis in the rats [27]. On the 3rd day after TNBS injection, rats were given saline (500 μl) or A317491 (1 mg/kg, in 500 μl saline) in the following 4 consecutive days. The results showed that compared with that of the saline group, the loss of body weight was significantly reversed in the A317491 group (Fig. 3a, n =6 for each group, p < 0.05 compared with the saline group). DAI scores were also decreased markedly after A317491 administration (Fig. 3b, n = 6 for each group, p < 0.05 compared with the saline group).
Fig. 3.
a Compared with that of the saline group, the loss of body weight was significantly reversed in the A317491-treated group (n = 6). *p < 0.05 b Compared with that of the saline group, DAI scores were also decreased markedly after A317491 administration (n = 6). *p < 0.05 c Compared with that of the saline group, the loss of body weight was significantly reversed in the NF-110-treated group (n = 6). *p < 0.05, **p < 0.01 d Compared with those of the saline group, DAI scores were also decreased significantly after NF-110 administration (n = 6). **p < 0.01 e Expression of P2X3R in rectocolon, DRG, and SCDH in TNBS-induced colitis in rats. On the 4th day after TNBS injection, the expression of P2X3R in both rectocolon and DRG was significantly increased compared with those of the saline group (n = 8). While in the SCDH, there was no marked change (n = 8). *p < 0.05 compared with the saline group
In order to further verify the role of P2X3R in colitis, another selective P2X3 antagonist, NF-110, was used subcutaneously in the colitis rats. On the 3rd day after TNBS administration, rats were given saline (500 μl) or NF-110 (1 mg/kg, in 500 μl saline) in the following 4 consecutive days. The results showed that compared with that of the saline group, the loss of body weight was significantly reversed in the NF-110 group (Fig. 3c, n = 6 for each group, p < 0.05, p < 0.01, p < 0.01, p < 0.01, compared with the saline group). DAI scores were also decreased significantly after NF-110 administration (Fig. 3d, n = 6 for each group, p < 0.01 compared with the saline group).
Evidence has shown that P2X3R was involved in the visceral hypersensitivity and intestinal inflammation [23, 24], so we further investigated P2X3R expression in rectocolon, DRG, and SCDH before and after TNBS administration. On the 4th day after TNBS injection, the expression of P2X3R in both rectocolon and DRG were significantly increased compared with those of the saline group (n = 8 for each group, p < 0.05). While in SCDH, there was no marked change (Fig. 3e, n = 8 for each group, p < 0.05 compared with the saline group).
Effects of ERβ agonists DPN and ERB-041 on P2X3R expression in both CFA and colitis rats
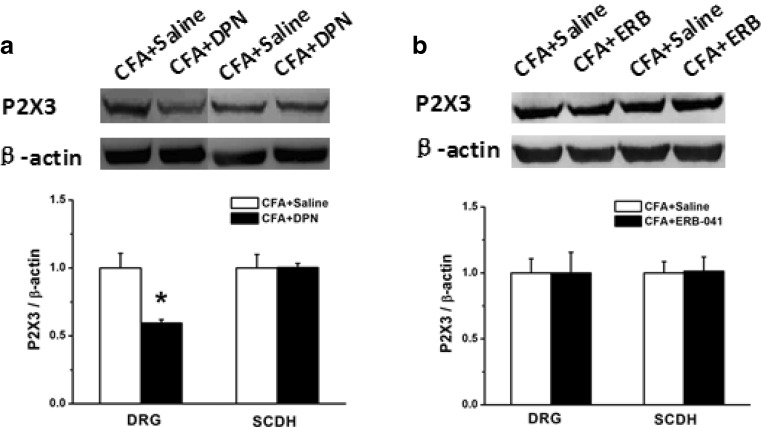
DPN and ERB-041 are selective agonists for ERβ [16, 17]. We investigated the effect of ERβ activation on P2X3R expression by using DPN and ERB-041. To further investigate the role of ERβ activation in the protein level after CFA administration, DRG and SCDH were isolated for Western blotting on the 8th day after CFA injection. The results showed that the expression of P2X3R in DRG of CFA rats was significantly increased compared with that of the saline group, while in SCDH there was no significant change (data not shown). After application of DPN, the expression of P2X3R in DRG was significantly decreased compared with that of saline-treated CFA rats (Fig. 4a, n = 8, p < 0.05). While in SCDH, there was no significant change (Fig. 4a, n = 8, p > 0.05). After application of ERB-041, there was no significant change in either DRG or SCDH (Fig. 4b, n = 8, p > 0.05, p > 0.05).
Fig. 4.
Expression of P2X3R protein in DRG and SCDH in saline-treated, DPN-treated, and ERB-041-treated CFA rats. a Compared with that in the saline-treated CFA group, the expression of P2X3R was significantly decreased after DPN in DRG (n = 8), while there was no significant change in SCDH (n = 8). b Compared with that in the saline-treated CFA group, the expression of P2X3R did not significantly change after ERB-041 was given in either DRG and SCDH (n = 8). *p < 0.05
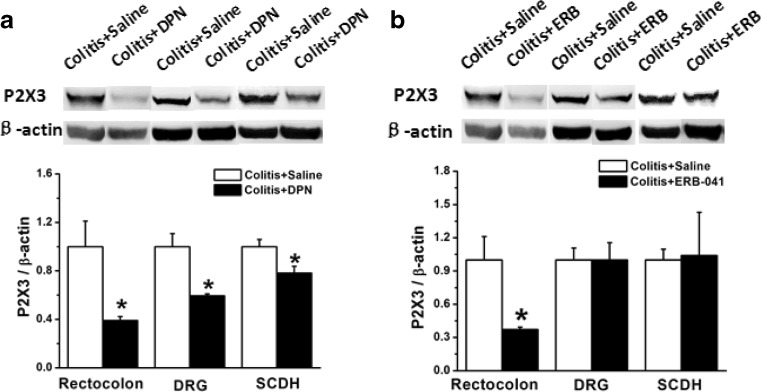
Our previous study has shown that both P2X3 expression and functions could be affected by estrogen [28–31], while the effect of ERβ activation on P2X3R in colitis is unknown. In the present study, injection of DPN (10 μg) for 4 days decreased P2X3R protein expression in colitis. The expression of P2X3R in rectocolon, DRG, and SCDH of rats with colitis were all significantly decreased compared with those of the saline group (Fig. 5a, n = 6 for each group, p < 0.05, p < 0.05, p < 0.05). However, after treatment with ERB-041, the expression of P2X3R was decreased significantly only in rectocolon, but not DRG or SCDH, compared with those of the saline group (Fig. 5b, n = 6 for each group, p < 0.05, p > 0.05, p > 0.05).
Fig. 5.
Expression of P2X3R in rectocolon, DRG, and SCDH in saline-treated, DPN-treated, and ERB-041-treated rats with colitis induced by colonic injection of TNBS. a Compared with that in the saline-treated group, the expression of P2X3R was significantly decreased in the rectocolon, DRG, and SCDH in the DPN-treated group (n = 6). b Compared with that in the saline-treated group, in the ERB-041-treated group, the expression of P2X3R was significantly decreased only in the rectocolon (n = 6), but not in DRG or SCDH (n = 8). * p < 0.05
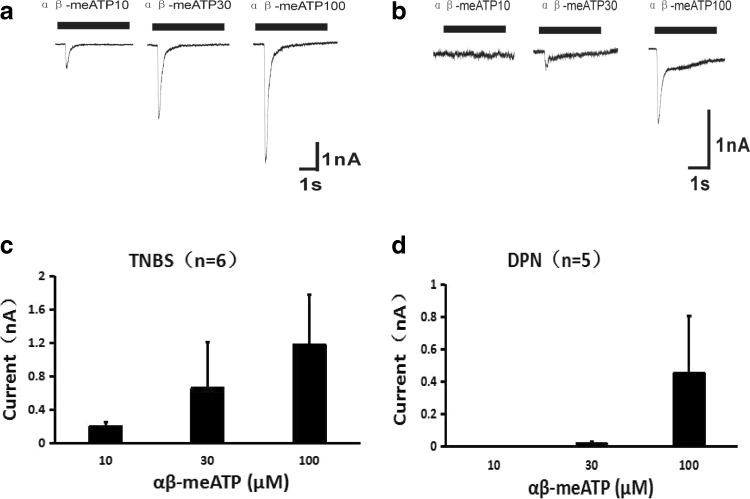
We then investigated the P2X3 activity in DRG neurons from rats in the different conditions; the DRG neurons from the TNBS rats and DPN-treated TNBS rats were isolated and cultured for the electrophysiological study. The results showed that (10–100 μM) αβ-meATP-induced transient currents were reduced in the DPN group (Fig. 6, n = 5 neurons) compared with those of TNBS group (Fig. 6, n = 6 neurons), suggesting the inhibition of DPN on P2X3R functions.
Fig. 6.
Comparison of P2X3 receptor-mediated transient currents in DRG neurons from TNBS or DPN-treated TNBS rats. The transient currents induced by αβ-me-ATP (10–100 μM) in DRG neurons from TNBS rats (a) and DPN-treated TNBS rats (b). A diagram showing the reduced transient currents induced by αβ-meATP in the DPN group (d, n = 5 neurons) compared with those of TNBS group (c, n = 6 neurons)
Estrogen replacement reversed the decrease of PWT in ovariectomized CFA rats
Evidence has shown that estrogen affected chronic inflammation [9, 10]. We then investigated the effect of estrogen on OVX CFA rats. Three weeks after replacement of estrogen or sesame oil in rats, there was a significant difference in mechanical pain threshold among all three groups (Fig. 7a, n = 8 for each group, p < 0.01). On the 1st day, mechanical PWT of the sham, OVX, and estrogen-treated groups were decreased from (10.67 ± 1.48 g), (8.16 ± 1.60 g), (10.54 ± 1.52 g) to (7.56 ± 0.96 g), (6.29 ± 1.99 g), (8.08 ± 0.00 g) after CFA injection, respectively, which were significantly different compared with that tested before CFA administration (Fig. 7a, b, p<0.01, p < 0.01, p < 0.01). Subsequently, the PWT of the three groups declined, but not significantly (Fig. 7a, p>0.05, n = 8 for each group). PWT of OVX (0.09 ± 0.02 g) was significantly lower than that of the sham group (1.08 ± 1.04 g, p < 0.05), which was then reversed by estrogen replacement (Fig. 7a, n =8 for each group, p < 0.05). The difference sustained until day 11 after CFA injection (Fig. 7a). Before injection of CFA, the PWT in OVX rats was significantly decreased compared with that of sham rat (n = 8 for each group p < 0.01), which was then reversed by estrogen-treatment (n = 8 for each group, p < 0.01) (Fig. 7b).
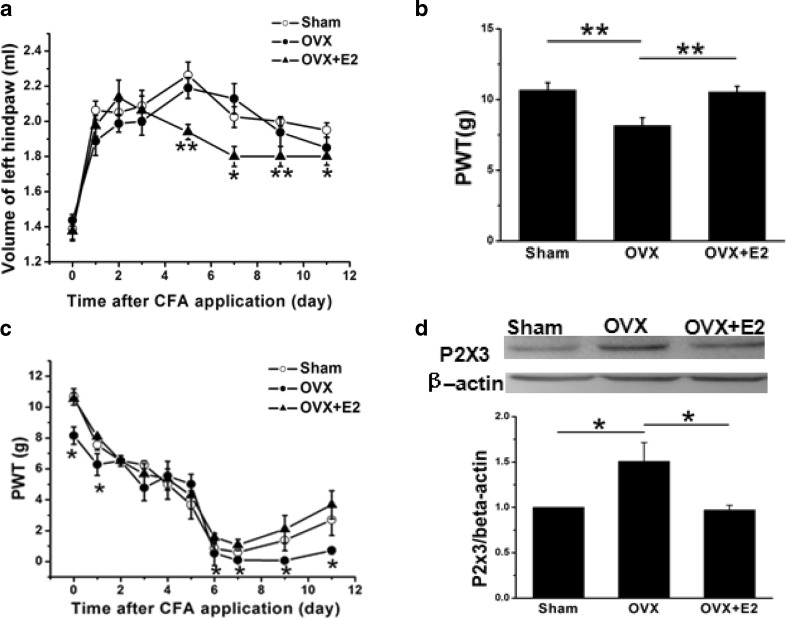
Fig. 7.
Changes in left hind paw volume and the mechanical pain threshold (PWT) and expression of P2X3R after injection of CFA in the left hind-paw. a Two days after injection of CFA, PWT was significantly decreased compared with that of pre-injection (n = 8), while there was no differences among groups (n = 8). The decline of PWT reached a maximum on day 7 after CFA injection. The PWT of OVX rats was significantly lower than that of the sham group (n = 8), which was then reversed by estrogen replacement (n = 8). The difference was sustained until day 11 after CFA injection. *p < 0.05, compared with the sham group. b Before injection of CFA, the PWT in OVX rats was significantly decreased compared with that of sham rats (n = 8), which was then reversed by estrogen treatment (n = 8). *p < 0.01 c On the 1st day after injection of CFA, the volume of the left hind paw was increased significantly compared with that of pre-injection (n = 8). The volume of the left hind paw in estrogen-treated OVX rats was significantly decreased compared with that of OVX rats (n = 8). There was no significant difference in plantar volumes between sham and OVX rats. On days 6, 7, 9, and 11 after CFA injection, the plantar volume of estrogen-treated rats was significantly less than that of OVX rats. *p < 0.05, **p < 0.01, compared with the OVX group. d In CFA rats, the total P2X3R protein obtained from DRG isolated from OVX rats was significantly increased compared with that from sham rats (n = 6), which was then reversed by estrogen replacement for 3 weeks. *p < 0.05
In order to observe the effect of CFA on the inflammatory progress of rats, Drainage Act was used to measure the volume of the left hind limb. On the 1st day after CFA injection, the volume of the left hind limbs in the sham, OVX, and estrogen-treated groups rapidly increased from (1.44 ± 0.09 ml), (1.39 ± 0.19 ml), (1.38 ± 0.13 ml) to (1.89 ± 0.23 ml), (2.06 ± 0.15 ml), (1.98 ± 0.16 ml). There were significant differences between pre-injection and injection in all three groups (Fig. 7c, p < 0.01, p < 0.01, p < 0.01, n = 8 for each group), indicating the successful induction of plantar inflammation. There was no significant difference in plantar volumes between sham and OVX rats (Fig. 7c, p > 0.05). On days 6, 7, 9, and 11 after CFA injection, the plantar volume of estrogen-treated rats was significantly less than that of OVX rats (Fig. 7c, p<0.01, p < 0.05, p < 0.01, p < 0.05).
Estrogen replacement decreased the upregulation of P2X3R expressions in DRG from ovariectomized CFA rats
As mentioned above, activation of P2X3R on the endings of primary sensory afferent nerves also promoted the development of inflammatory hypersensitivity [26]. We then investigated the expression of P2X3R in sham, OVX, and estrogen-treated CFA rats. In CFA rats, the total P2X3R protein obtained from DRG isolated from OVX rats was significantly increased compared with that from sham rats (Fig. 7d, n = 6 for each group, p < 0.05), which was then reversed by estrogen replacement (Fig. 7d, p < 0.05).
Involvement of ERK and c-fos after estrogen replacement in ovariectomized CFA rats
ERK is one member of the mitogen-activated protein kinase (MAPK) family, which is known to have different functions in sensory processing [33–35]. In the present study, the expressions of ERK and c-fos in the sham, OVX, and estrogen-treated CFA rats were investigated. The results showed that the expression of total ERK protein in lumbosacral DRG (Fig. 8a) and SCDH (Fig. 8b) of the estrogen-treated group was significantly lower than those of OVX rats (n = 8 for each group, p < 0.05, p < 0.05). However, there was no significant difference in the expression of total ERK in DRG between OVX and Sham rats (Fig. 8a, p>0.05). The ERK expression was significantly increased after ovariectomy in SCDH compared with that of the sham rats (Fig. 8b, p<0.05).
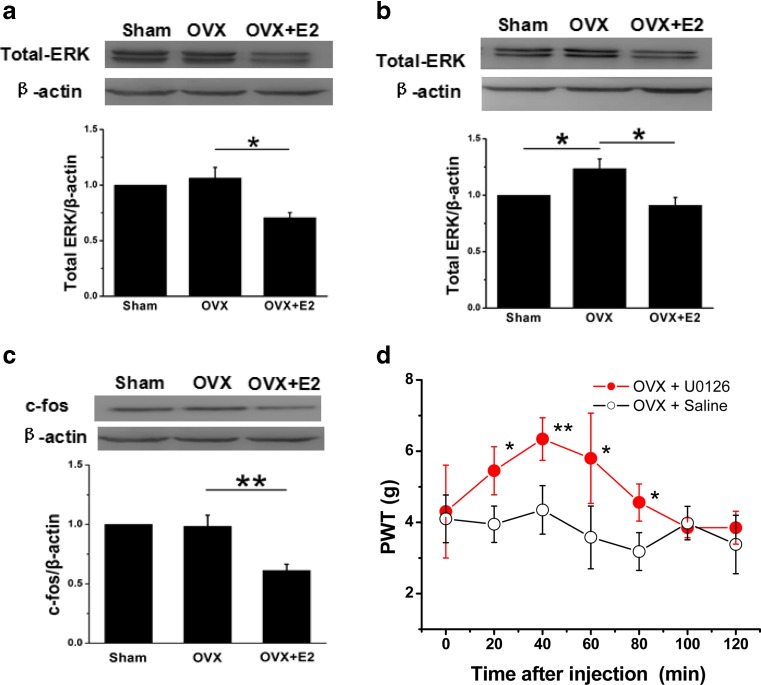
Fig. 8.
Expressions of ERK and c-fos proteins in DRG and SCDH from sham, OVX, and estrogen-treated OVX rats. a The total expression of ERK in DRG, expressed as blot density relative to OVX rats, was decreased significantly in estrogen-treated rats (n = 8). There was no significant difference between the OVX and sham group (n = 8). b The total expression of ERK in SCDH, expressed as blot density relative to sham rats, was increased significantly in OVX group (n = 8) and then reversed in the estrogen-treated group (n = 8). c Relative to OVX rats, the expression of c-fos was decreased significantly in estrogen-treated rats (n = 8). There was no significant difference between the OVX and sham groups (n = 8). *p < 0.05, **p < 0.01 d PWT changes following plantar injection of U0126 in OVX rats. PWT was significantly increased 20 min after injection and was maintained until 80 min (n = 6), compared with control rats. *p < 0.05, **p < 0.01
Western blot results also showed that the expression of c-fos protein in SCDH of the estrogen-treated group was significantly lower than that of OVX rats (Fig. 8c, n = 8 for each group, p < 0.01). However, there was no significant change in the expression of c-fos protein of OVX group compared with that of the sham group (Fig. 8c, p > 0.05). We finally tested the PWT changes after plantar injection of U0126 in OVX rats. The results showed that the PWT was significantly increased 20 min after injection and maintained until 80 min (Fig. 8D, n = 6 for each group, p < 0.05, p < 0.01, p < 0.05, p < 0.05), compared with those time points of control rats.
Discussion
In the present study, ERβ agonists, DPN, or ERB-041 significantly decreased mechanical hypersensitivity in CFA-treated rats and inflammatory pain and responses in rats with colitis. The higher expressions of P2X3R in DRG of CFA-treated rats and those in rectocolon and DRG of TNBS-treated rats were all decreased by injection of DPN or ERB-041. Mechanical hyperalgesia and increased P2X3 expression in ovariectomized CFA-treated rats were then reversed by estrogen replacement. Furthermore, the expressions of ERK in DRG and SCDH and c-fos in SCDH were significantly decreased after estrogen replacement compared with those of OVX rats. The ERK antagonist U0126 significantly reversed mechanical hyperalgesia in the OVX rats. These results suggest that activation of ERβ may play an important therapeutic role in inflammation through down-regulation of P2X3R in peripheral tissues and the nervous system.
Estrogen modulates inflammatory pain via ERβ
Evidence has shown that estradiol replacement has an anti-nociceptive effect in OVX rats [23, 36, 37]. Here, we found that during inflammation, estrogen replacement significantly reversed hyperalgesia in the ovariectomized CFA-treated rats. ERβ is widely expressed on small-sized nociceptive DRG neurons and the colon, suggesting modulation in peripheral pain signal transduction [38–40]. Reports have shown that the selective ERβ agonist ERB-131 regulated nociception during the inflammatory state [18, 19, 41]. In the present study, the ERβ agonist DPN inhibited mechanical pain sensitivity in CFA-treated rats and visceral pain in rats with colitis. These results indicate that ERβ activation might play a specific role in alleviating chronic nociception. Our previous study showed that application of estradiol in rat hind-paws rapidly inhibited ATP-induced peripheral hyperalgesia via ERα and GPR30 [29, 31]. Therefore, we suggest that the anti-nociceptive effect of estrogen on OVX rats is probably via ERβ rather than ERα.
Estrogen modulates P2X3R via ERβ in inflammatory pain
It is well accepted that up-regulated expression and enhanced activity of P2X3R in DRG neurons is closely associated with the development and maintenance of acute and chronic pain [42, 43]. Activation of P2X3R stimulates different cells, such as neurons or immunocytes, to cause abnormal excitability [23, 25] or release inflammatory mediators, including TNFα, to induce inflammation [26]. Our results show that the expression of P2X3R in neurons was significantly increased in CFA-treated rat [44] and in TNBS-induced colitis in rats [23, 25], indicating a vital role of P2X3R in inflammatory reactions. It has been reported that estrogen replacement decreased P2X3 upregulation in DRG after ovariectomy [30]. In the present study, estrogen replacement significantly decreased peripheral pain sensitivity and P2X3R expression in DRG from CFA-treated OVX rats. The higher expressions of P2X3R in DRG of CFA-treated rats and those in rectocolon and DRG of TNBS-treated rats were all decreased by injection of ERβ agonists DPN or ERB-041. Furthermore, results in the electrophysiological study showed that the inhibition of ERβ agonists DPN on P2X3 receptors in TNBS rats was not only in the protein expression but also at functional level. These results suggest that, in chronic inflammatory pain, estrogen might inhibit P2X3R expression and function in primary sensory neurons through ERβ. Although the two agonists were both beneficial to colitis, some minor differences between DPN and ERB-041 in their modulatory function on P2X3R were found. After using DPN, the expression of P2X3R was decreased in rectocolon, DRG, and SCDH, while a similar decrease was observed only in rectocolon after ERB-041 application. Based on the fact that DPN may easily pass through the blood–brain barrier to develop its role in the central nervous system while ERB-041 cannot [33, 45], we suggest that the action of ERB-041 might be limited to the rectocolon, while DPN might have roles in both the peripheral and central nervous systems. The underlying mechanisms of differences between DPN and ERB-041 need further study.
Functions of ERK in the modulation of estrogen in inflammatory pain
ERK functions as threonine/tyrosine double kinases and thus phosphorylates a series of transcription factors, including c-fos [46, 47], and thus modulates protein synthesis [48]. Evidence has also shown that ERα and ERβ activate different signal transduction pathways, especially the MAPK system, which is usually determined by intracellular protein phosphorylation or calcium increase [49, 50]. We found that after CFA application, expression of ERK in SCDH from OVX rats was significantly higher than that of controls, which was reversed by estrogen replacement. These results indicate that the inhibitory role of estrogen in pain perception might be related to the inhibition of ERK, probably with subsequent genetic transcription and protein synthesis. In DRG, the expression of ERK did not change after OVX compared with that of the sham group, but was significantly decreased in the estrogen-treated OVX group. The possible contribution of progesterone and other associated steroids from intact gonads should probably also be taken into account.
Reports have shown that phosphorylated ERK-positive neurons co-express P2X3R [51]. Injection of TNP-ATP, which antagonizes P2X3 and P2X2/3 receptors, into the inflamed joint reduced the phosphorylated ERK and the struggle behavior in CFA rats [52]. These results suggest the possible contribution of ERK in the hypersensitivity of P2X3R-induced inflammation. In the present study, although we could not demonstrate a direct relationship between ERK and P2X3R, the results support the views that estrogen replacement might inhibit the expression of P2X3R in DRG neurons through ERβ, as well as the expression of ERK, thus playing a regulatory role in inflammatory pain.
Functions of c-fos in the modulation of estrogen in inflammatory pain
Being an immediate early gene, c-fos can characterize neuronal excitation after certain stimulations and is an ideal location marker for central nervous activity [53, 54]. Activation of ERK could transcriptionally activate c-fos and lead to increased expression [55]. In this study, we found that in SCDH from estrogen-treated OVX rats, the expression of c-fos was significantly lower than that of OVX rats. Combined with the correlation between ERK and c-fos, the results in the present study indicate that, at least at the level of SCDH, the regulatory role of estrogen in pain might be through regulation of the expression of ERK, then that of c-fos, and subsequently the regulation of gene transcription.
In conclusion, our results demonstrate that estradiol might inhibit CFA-induced chronic inflammatory pain through inhibiting the expression of P2X3R and ERK in the DRG and the expression of c-fos and ERK in the spinal cord. Similarly, ERβ agonists improved TNBS-induced colitis through down-regulating the expression of P2X3R. Our study further supports the view that the inhibitory effect of estrogen in inflammatory pain indicates that the effect of estrogen is probably via ERβ and provides clues for clarifying the regulatory mechanism of estrogen in inflammatory pain perception.
Acknowledgments
This study is supported by National Natural Science Foundation of China (No, 31471103). We are deeply grateful to Dr. Gillian E. Knight (from the Autonomic Neuroscience Centre, University College Medical School, UK) for her kind assistance in the written English.
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Qian Jiang, Email: jq880117@126.com.
Wen-xin Li, Email: 995226387@qq.com.
Jia-run Sun, Email: 446265013@qq.com.
Tian-tian Zhu, Email: 15221010849@163.com.
Hua Yang, Email: Yanghua200433@126.com.
Bei Ma, Email: mmbb98@sohu.com.
References
- 1.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20(3):371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 2.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 3.Chesterton LS, Barlas P, Foster NE, Baxter GD, Wright CC. Gender differences in pressure pain threshold in healthy humans. Pain. 2003;101(3):259–266. doi: 10.1016/S0304-3959(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 4.Craft RM, Ulibarri C, Leitl MD, Sumner JE. Dose- and time-dependent estradiol modulation of morphine antinociception in adult female rats. Eur J Pain. 2008;12:472–479. doi: 10.1016/j.ejpain.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Gintzler AR, Bohan MC, Gintzler AR, Bohan M. Pain thresholds are elevated during pseudopregnancy. Brain Res. 1990;507(2):312–316. doi: 10.1016/0006-8993(90)90288-M. [DOI] [PubMed] [Google Scholar]
- 6.Dawson-Basoa M, Gintzler AR. Gestational and ovarian sex steroid antinociception: synergy between spinal kappa and delta opioid systems. Brain Res. 1998;794(1):61–67. doi: 10.1016/S0006-8993(98)00192-9. [DOI] [PubMed] [Google Scholar]
- 7.Craft RM. Modulation of pain by estrogens. Pain Suppl. 2007;132(1):S3–S12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Sanoja R, Cervero F. Estrogen-dependent abdominal hyperalegsia induced by ovariectimy in adult mice: a model of functional abdominal pain. Pain. 2005;118:243–253. doi: 10.1016/j.pain.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Aloisi AM, Ceccarelli I. Role of gonadal hormones in formalin-induced pain responses of male rats: modulation by estradiol and naloxone administration. Neuroscience. 2000;95:559–566. doi: 10.1016/S0306-4522(99)00445-5. [DOI] [PubMed] [Google Scholar]
- 10.Bradshaw H, Miller J, Ling Q, Malsnee K, Ruda MA. Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain. 2000;85:93–99. doi: 10.1016/S0304-3959(99)00253-5. [DOI] [PubMed] [Google Scholar]
- 11.De Marinis E, Acaz-Fonseca E, Arevalo MA, Ascenzi P, Fiocchetti M, Marino M, Garcia-Segura LM. 17b-Oestradiol anti-inflammatory effects in primary astrocytes require oestrogen receptor β-mediated neuroglobin up-regulation. J Neuroendocrinol. 2013;25:260–270. doi: 10.1111/jne.12007. [DOI] [PubMed] [Google Scholar]
- 12.De Marinis E, Acaz-Fonseca E, Arevalo MA, Ascenzi P, Fiocchetti M, Marino M, Garcia-Segura LM. Estradiol receptors agonists induced effects in rat intestinal microcirculation during sepsis. Microvasc Res. 2013;85:118–127. doi: 10.1016/j.mvr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Fan X, Warner M, Xu XJ, Gustafsson JA, Wiesenfeld-Hallin Z. Ablation of estrogen receptor a or b eliminates sex differences in mechanical pain threshold in normal and inflamed mice. Pain. 2009;143:37–40. doi: 10.1016/j.pain.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Spooner MF, Robichaud P, Carrier JC, Marchand S. Endogenous pain modulation during the formalin test in estrogen receptor beta knockout mice. Neuroscience. 2007;150:675–680. doi: 10.1016/j.neuroscience.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 15.Edvardsson K, Ström A, Jonsson P, Gustafsson JÅ, Williams C. Estrogen receptor induces antiinflammatory and antitumorigenic networks in colon cancer cells. Mol Endocrinol. 2011;25:969–979. doi: 10.1210/me.2010-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao DY, Ji Y, Tang B, Traub RJ. Estrogen receptor β activation is antinociceptive in a model of visceral pain in the rat. J Pain. 2012;13:685–694. doi: 10.1016/j.jpain.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu S, Cheng Y, Keast JR, Osborne PB. 17β-estradiol activates estrogen receptor βsignalling and inhibits transient receptor potential vanilloid receptor 1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology. 2008;149:540–5548. doi: 10.1210/en.2008-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardell LR, Hyldtoft L, Del Tredici AL, Andersen CB, Fairbairn LC, Lund BW, Gustafsson M, Brann MR, Olsson R, Piu F. Differential modulation of inflammatory pain by a selective estrogen receptor β agonist. Eur J Pharmacol. 2008;592:158–159. doi: 10.1016/j.ejphar.2008.06.107. [DOI] [PubMed] [Google Scholar]
- 19.Sapsed-Byrnea S, Ma D, Ridoutb D, Holdcrofta A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742(1–2):10–16. doi: 10.1016/S0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- 20.Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, Frail DE, Henderson RA, Zhu Y, Keith JC., Jr Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144(10):4241–4249. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 21.Cattaneo M. The platelet P2 receptors in inflammation. Hamostaseologie. 2015;35(3):262–266. doi: 10.5482/HAMO-14-09-0044. [DOI] [PubMed] [Google Scholar]
- 22.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 23.Wynn G, Ma B, Ruan HZ, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G647–G657. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- 24.Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X3 receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. 2009;137:2096–2104. doi: 10.1053/j.gastro.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang Z, Xiong Y, Yan N, Li X, Mao Y, Ni X, He C, LaMotte RH, Burnstock G, Sun J. Functional up-regulation of P2X3 receptors in the chronically compressed dorsal root ganglion. Pain. 2008;140:23–34. doi: 10.1016/j.pain.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira MC, Pelegrini-da-Silva A, Tambeli CH, Parada CA. Peripheral mechanisms underlying the essential role of P2X3, 2/3 receptors in the development of inflammatory hyperalgesia. Pain. 2009;141(1–2):127–134. doi: 10.1016/j.pain.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh YL, Chiang H, Lue JH, Hsieh ST. P2X3-mediated peripheral sensitization of neuropathic pain in resiniferatoxin-induced neuropathy. Exp Neurol. 2012;235:316–325. doi: 10.1016/j.expneurol.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Fan J, Yu LH, Zhang YM, Ni X, Ma B, Burnstock G. Estrogen altered visceromotor reflex and P2X3 mRNA expression in a rat model of colitis. Steroids. 2009;74:956–962. doi: 10.1016/j.steroids.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y, Jiang Q, Yu L, Lu ZY, Meng SP, Su D, Burnstock G, Ma B. 17β-estradiol rapidly attenuates P2X3 receptor-mediated peripheral pain signal transduction via ERα and GPR30. Endocrinology. 2013;154(7):2421–2433. doi: 10.1210/en.2012-2119. [DOI] [PubMed] [Google Scholar]
- 30.Ma B, Yu LH, Fan J, Cong BH, He P, Ni X, Burnstock G. Estrogen modulation of peripheral pain signal transduction: involvement of P2X3 receptors. Purinergic Signal. 2011;7:73–83. doi: 10.1007/s11302-010-9212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Y, Li Z, Li HJ, Du D, Wang LP, Yu LH, Geoffrey B, Chen AM, Ma B. A comparative study of 17β-estradiol and estriol on peripheral pain behavior in rats. Steroids. 2012;77:241–249. doi: 10.1016/j.steroids.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Rovenský J, Stančíkova M, Svík K, Bauerová K, Jurčovičová J. The effects of β-glucan isolated from Pleurotusostreatus on methotrexate treatment in rats with adjuvant arthritis. Rheumatol Int. 2011;31(4):507–511. doi: 10.1007/s00296-009-1258-z. [DOI] [PubMed] [Google Scholar]
- 33.Tsubota M, Kawabata A. Role of hydrogen sulfide, a gasotransmitter, in colonic pain and inflammation. Yakugaku Zasshi. 2014;134(12):1245–1252. doi: 10.1248/yakushi.14-00209-2. [DOI] [PubMed] [Google Scholar]
- 34.Liu CY, Lu ZY, Li N, Yu LH, Zhao YF, Ma B. The role of large-conductance, calcium-activated potassium channels in a rat model of trigeminal neuropathic pain. Cephalalgia. 2015;35(1):16–35. doi: 10.1177/0333102414534083. [DOI] [PubMed] [Google Scholar]
- 35.Ji RR, Befort K, Brenner GJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain. 2007;8(4):334–342. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Mannino CA, South SM, Inturrisi CE, Quinones-Jenab V. Pharmacokinetics and effects of 17β-estradiol and progesterone implants in ovariectomized rats. J Pain. 2005;6:809–816. doi: 10.1016/j.jpain.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM. Differential expression of estrogen receptor α and β in rat dorsal root ganglion neurons. J Neurosci Res. 1999;57:603–615. doi: 10.1002/(SICI)1097-4547(19990901)57:5<603::AID-JNR3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 39.Papka RE, Storey-Workley M, Shughrue PJ, Merchentha-ler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Estrogenreceptor-α and β- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304:193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson S, Gustafsson JÅ. Estrogen receptors: therapies targeted to receptor subtypes. Clin Pharmacol Ther. 2011;89:44–55. doi: 10.1038/clpt.2010.226. [DOI] [PubMed] [Google Scholar]
- 41.Harris HA, Bruner-Tran KL, Zhang X, Osteen KG, Lyttle CR. A selective estrogen receptor-βagonist causes lesion regression in an experimentally induced model of endometriosis. Hum Reprod. 2005;20:936–941. doi: 10.1093/humrep/deh711. [DOI] [PubMed] [Google Scholar]
- 42.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 43.Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naïve rats and following neuropathic injury. Pain. 1999;80:273–282. doi: 10.1016/S0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- 44.Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci. 2002;22(1):93–102. doi: 10.1523/JNEUROSCI.22-01-00093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamura T, Katsu Y, Watanabe H, Iguchi T. Estrogen receptor subtypes selectively mediate female mouse reproductive abnormalities induced by neonatal exposure to estrogenic chemicals. Toxicology. 2008;253:117–124. doi: 10.1016/j.tox.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factorkappa B in osteoclasts. Biochem Biophys Res Commun. 2003;305:211–214. doi: 10.1016/S0006-291X(03)00695-8. [DOI] [PubMed] [Google Scholar]
- 47.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 48.Khare V, Paul G, Movadat O, Frick A, Jambrich M, Krnjic A, Marian B, Wrba F, Gasche C. IL-10R2 overexpression promotes IL-22/STAT3 signaling in colorectal carcinogenesis. Cancer Immunol Res. 2015;3(11):1227–1135. doi: 10.1158/2326-6066.CIR-15-0031. [DOI] [PubMed] [Google Scholar]
- 49.Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during ageing: from periphery to brain. Trends Mol Med. 2013;19(3):197–209. doi: 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adachi T, Kar S, Wang M, Carr BI. Transient and sustained ERK phosphorylation and nuclear translocation in growth control. Cell Physiol. 2002;192(2):151–159. doi: 10.1002/jcp.10124. [DOI] [PubMed] [Google Scholar]
- 51.Chen XQ, Wang B, Wu C, Pan J, Yuan B, Su YY, Jiang XY, Zhang X, Bao L. Endosome-mediated retrograde axonal transport of P2X3 receptor signals in primary sensory neurons. Cell Res. 2012;22:677–696. doi: 10.1038/cr.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimizu I, Iida T, Guan Y, Zhao C, Raja SN, Jarvis MF, Cockayne DA, Caterina MJ. Enhanced thermal avoidance in mice lacking the ATP receptor P2X3. Pain. 2005;116(1–2):96–108. doi: 10.1016/j.pain.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 53.Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50(2–3):83–107. doi: 10.1016/S0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 54.Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45(1):1–8. doi: 10.1016/S0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 55.Wagstaff SC, Bowler WB, Gallagher JA, Hipskind RA. Extracellular ATP activates multiple signalling pathways and potentiates growth factor-induced c-fos gene expression in MCF-7 breast cancer cells. Carcinogenesis. 2000;21(12):2175–2181. doi: 10.1093/carcin/21.12.2175. [DOI] [PubMed] [Google Scholar]