Abstract
Reducing cell death during the secondary injury is a major priority in the development of a cure for traumatic spinal cord injury (SCI). One of the earliest processes that follow SCI is the excitotoxicity resulting from the massive release of excitotoxicity mediators, including ATP, which induce an excessive and/or prolonged activation of their receptors and a deregulation of the calcium homeostasis. Diadenosine tetraphosphate (Ap4A) is an endogenous purinergic agonist, present in both extracellular and intracellular fluids, with promising cytoprotective effects in different diseases including neurodegenerative processes. In a search for efficient neuroprotective strategies for SCI, we have tested the capability of Ap4A to reduce the excitotoxic death mediated by the ATP-induced deregulation of calcium homeostasis and its consequences on tissue preservation and functional recovery in a mouse model of moderate contusive SCI. Our analyses with the murine neural cell line Neuro2a demonstrate that treatment with Ap4A reduces ATP-dependent excitotoxic death by both lowering the intracellular calcium response and decreasing the expression of specific purinergic receptors. Follow-up analyses in a mouse model of contusive SCI showed that acute administration of Ap4A following SCI reduces tissue damage and improves motor function recovery. These results suggest that Ap4A cytoprotection results from a decrease of the purinergic tone preventing the effects of a massive release of ATP after SCI, probably together with a direct induction of anti-apoptotic and pro-survival pathways via activation of P2Y2 proposed in previous studies. In conclusion, Ap4A may be a good candidate for an SCI therapy, particularly to reduce excitotoxicity in combination with other modulators and/or inhibitors of the excitotoxic process that are being tested.
Keywords: Spinal cord injury, Neuroprotection, Diadenosine, Tissue damage, Apoptosis, Excitotoxicity, Intracellular calcium, Secondary injury, Neuro-2a, Mouse model
Introduction
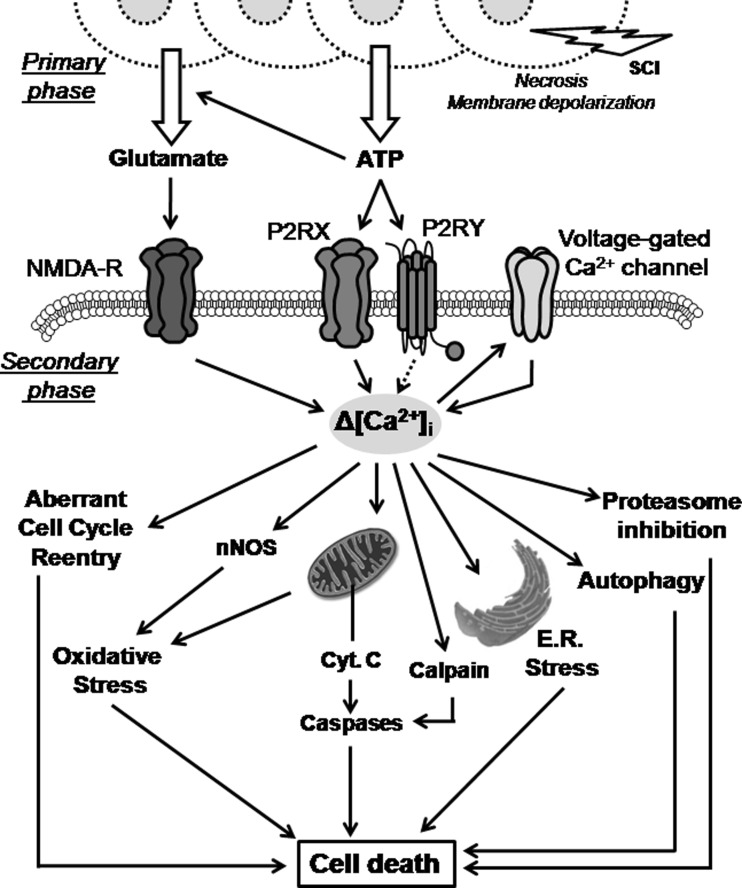
Cell death is one of the features in the causes of the disabilities induced by traumatic spinal cord injuries (SCI). SCI induces an immediate first wave of neural cell death, mainly necrotic, followed by delayed oligodendrocyte and neuronal death that extends the injury in time and space along the spinal cord [1].One of the earliest processes that follow SCI is the excitotoxicity that results from the massive release of excitatory mediators, which induce an excessive and/or prolonged activation of their receptors and the deregulation of the calcium homeostasis [2]. Excitotoxicity leads to mitochondrial dysfunction, oxidative stress, or endoplasmic reticulum stress (see in Fig. 1) that finally converge in the activation of both intrinsic and extrinsic pathways of apoptosis [3–7].
Fig. 1.
Excitotoxic-induced cell death pathways. Massive release of glutamate and ATP from necrotic and broken cells at trauma level activates NMDA and purinergic receptors expressed in neighbor cells. The subsequent increase in intracellular calcium concentration induces the activation of several cell death pathways implying endoplasmic reticulum and oxidative stress, autophagy, pro-apoptotic caspase cleavage, etc. All these mechanisms are part of the secondary wave of neural death activated minutes to few days after injury in the spinal cord
The major mediator in the excitotoxic processes is glutamate either released by breakage of the plasma membrane of necrotic neural cells or by massive synaptic release due to unbalance of the membrane potential and increase in intracellular sodium and calcium concentration [3, 8, 9]. However, following trauma to the central nervous system, including SCI, large amounts of ATP and other nucleotides are also released by the damaged tissue [10–12]. Released ATP acts as an excitotoxin inducing the activation of the purinergic system and leading to a direct calcium-dependent excitotoxic cell death of neurons [13–16] and oligodendrocytes [17], or indirectly, favoring glutamate release from neurons [18, 19] and glia [20].
Nucleotides and nucleosides are key signaling molecules that interact with two basic types of membrane purinergic receptors, the nucleoside P1 receptors and the nucleotide P2 receptors. The P2 receptors are additionally subclassified into ionotropic P2X receptors and metabotropic P2Y receptors [21–24]. This is a primitive system with a wide range of physiological and pathologic functions that include neurotransmitter and co-transmitter activity in the central, peripheral, and autonomous nervous systems, as well as activity in pain and mechanosensory transduction [25–28]. Several P2 receptors, including P2X4 and P2X7, are proposed to mediate various aspects of neural cell signaling in SCI and stroke, such as the remodeling and reparation of damaged tissue, induction of gliosis, formation of the glial scar, development of inflammatory and pain processes or excitotoxic cell death [29–34].
Recent therapeutic approaches are trying to attenuate excitotoxic neural death after SCI. As a result, riluzole—a sodium channel blocker that decreases the pre-synaptic glutamate release by reducing the membrane potential depolarization and the derived calcium influx—has entered clinical phase II-B/III for SCI treatment [35–39]. In addition, minocycline, an antibiotic, anti-apoptotic, and anti-inflammatory drug [40, 41], that also has anti-excitotoxic effects [42] is already in clinical phase III for SCI treatment [43]. Following these approaches, we hypothesize that modulation of the purinergic system may be beneficial against the excitotoxic damage induced by the massive release of ATP after SCI. Previous evidences suggest a beneficial role of blocking purinergic receptors in similar neuropathological conditions. Blockade of P2X7 with PPADS, oxo-ATP [10], or brilliant blue G [44] provides neuroprotection in rat models of traumatic SCI resulting in the reduction of neuronal damage, microglial activation, neutrophil infiltration, and inflammatory response and increasing tissue preservation, although other groups failed to reproduce brilliant blue G results [45].
Among the purines, diadenosine polyphosphates (ApnAs) emerge as interesting therapeutical molecules. They are partial agonists, and even antagonists in presence of the full agonist, of different P2 receptors [46–50] that may induce a decrease in the receptor response and a negative modulation of the calcium response. They comprise a group of compounds formed by the union of two adenosine moieties linked by their ribose 5′-ends by a 2 to 7 phosphate chain. ApnAs act on both P2X (1, 2, 3, and 4) [48, 51, 52] and P2Y (1, 2, 4, 12, and 13) [52–57] purinergic receptors and diadenosine-specific receptors or P4 receptors [58]. They are naturally present in the cytoplasm of eukaryotic and prokaryotic cells [59], co-stored with ATP in granules of platelets and chromaffin cells and in synaptic vesicles of the cholinergic synaptic terminals [60–63], and present in several types of fluids. ApnAs regulate the function and composition of tears [64], intraocular pressure [65], and increase wound healing capacities of the cornea [66]. Among ApnAs, the diadenosine with a 4-phosphate bridge or P1-P4-di (adenosine-5′), tetraphosphate (Ap4A) is neuroprotective against ocular sympathetic nerve degeneration induced by 6-hydroxidopamine (6-OHDA) [67] and protects brain against ischemia,6-OHDA [68] or methamphetamine [69] exposure. Though this general neuroprotective effect is known, its role in SCI has not been explored yet. In the present work, we have used in vitro analyses in neural cell lines to determine the ability of Ap4A to reduce neuronal cell death by reducing the excitotoxic rise in intracellular calcium concentration and in vivo studies in a mouse model of contusive spinal cord injury to evaluate its therapeutic potential to reduce tissue damage and to ameliorate locomotor impairment following SCI.
Material and methods
In vitro experiments
Cell culture
Neuro-2a mouse neuroblastoma cells (CCL-131; ATCC, Manassas, VA, USA) were grown in 100 mm2 dishes with Dulbecco’s modified Eagle’s medium (DMEM, Lonza, Basel, Switzerland) supplemented with 10 % fetal bovine serum (FBS; Lonza) and 1 % penicillin/streptomycin solution (Gibco, ThermoFisher Scientific, Waltham, MA, USA) at 37 °C and 5 % CO2.
Cell death assay
ATP-mediated citoplasmatic calcium rise and excitotoxicity was induced by incubation of the cells in a death-inducing concentration of ATP (Sigma-Aldrich, St Louis, MO, USA) for 24 h. ATP (300 μM) concentration was chosen to induce cell death following the results from dose-response experiments carried out in Neuro-2a cell line cultures. This concentration lies within the range of citoplasmic (5 mM) and vesicular (100 mM) concentrations and guarantees the activation of P2X7 receptor (EC50 = 100 μM). Ap4A (100 μM; Sigma-Aldrich) or vehicle were added to the culture medium at the same time and/or 24 h before ATP administration. 100 μM Ap4A is a cytoprotective concentration that has been commonly employed in previous studies [67, 69]. Cells were mechanically detached after 24 h exposure to ATP, washed with PBS, and fixed in 70 % ethanol at −20 °C overnight. For determining DNA content, cells were stained with propidium iodide and analyzed in a FACS Canto II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Dot plot showing pulse width versus area was used to distinguish between single cells and aggregates. A total of 30,000 gated single events were collected using a FACS Canto II flow cytometry (BD Biosciences) and the FACS Diva 6.1 software (BD Biosciences) and analyzed with Flow Jo Software (Celeza GmbH, Olten, Switzerland) to determine the percentage of population in sub-G0/G1 phase.
Measurement of intracellular calcium
Neuro-2a cells were plated in 96 well plates (90.000 cells/well) in DMEM medium with 10 % FBS for 1 day. Cells were washed twice with Krebs’ solution (in mM: 105 NaCl (Sigma-Aldrich), 5 KCl (Sigma-Aldrich), 10 Na-HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; Merck), 5 sodium bicarbonate (NaHCO3; Sigma-Aldrich), 60 mannitol (Sigma-Aldrich), 5 sucrose (Sigma-Aldrich), 0.5 MgCl2 (Sigma-Aldrich), and 1.3 CaCl2 (Sigma-Aldrich) pH 7.4). Then they were loaded with 50 μl/well of 10 μM fura-2 (ThermoFisher Scientific cat# F-1221) containing 0.2 % pluronic acid (Sigma-Aldrich) in Krebs’ solution. After 30 min at 37 °C, the wells were washed twice with Krebs’ solution, and 45 μl of Krebs’ solution was added to each well. Intracellular calcium concentration was estimated by alternatively exciting with 340 and 380 nm and measuring the fluorescence emission at 510 nm in fluorescence plate reader (Infinite M200, Tecan Group LTD. Mannendorf, Switzerland) at 37 °C. After 5 to 10 min reading, 5 μl of control (Krebs’ buffer) or a 10-fold concentrated ATP solution (final concentration of 300 μM) was added to each well by means of the injector system, and the fluorescence was measured for an additional 10 to 50 min. The ratio (R) of light excited at 340 nm to that at 380 nm was converted into Ca2+ concentration using the method of Grynkiewiczet al. [70]. An in situ Kd value for fura-2 of 350 nM was used [71]. Rmin was obtained by bathing cells in a Ca2+-free isotonic solution of pH 8.0 containing 10 mM EGTA (ethylene glycol tetraacetic acid; Sigma-Aldrich) and 5 μM ionomycin (Sigma-Aldrich). Rmax was obtained by bathing the cells in isotonic solution with 1.3 mM Ca2+ and 5 μM ionomycin. Calibration was performed separately for each experiment. Baseline levels from Neuro-2a cells in the absence of fura-2 were subtracted from records to control for auto-fluorescence. The increase in intracellular calcium concentration ([Ca2+]i) was expressed as a percentage of the peak level in comparison with the pre-injection baseline according to the following formula: Δ[Ca2+]i = [(peak − baseline) / baseline] × 100. Experiments testing free extracellular calcium conditions are done using isotonic buffer with 10 mM EGTA and pH 8.0.
Western blot
After treatment, cells were mechanically detached from the plate and lysed in buffer containing 25 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; Merck, Darmstadt, Germany), 150 mM sodium chloride (NaCl; Sigma-Aldrich), 1 % nonyl phenoxypolyethoxylethanol (Tergitol type NP40; Sigma-Aldrich), 1 % sodium deoxycholate (DOC; Sigma-Aldrich), 10 % glycerol (USB Corporation, Affymetrix, Santa Clara, CA, USA), 10 mM magnesium chloride (MgCl2; Sigma-Aldrich), 2 mM 3-[(3-cholamidopropyl)-dimethylammonium]-1-propane sulfonate (CHAPS; Sigma-Aldrich), 0.1 % sodium dodecyl sulfate (SDS; Sigma-Aldrich), 2 mM ethylenediaminetetraacetic acid (EDTA, USB Corpotation), and 1 tablet of Complete protease inhibitor cocktail (Roche, Basel, Switzerland) every 50 ml of buffer. Samples were sonicated and cleared by centrifugation (10,000×g for 15 min at 4 °C). The protein content was determined by the Bradford method.
Homogenates containing 50–100 mg of protein were separated using conventional SDS-polyacrylamide gel electrophoresis in reducing conditions (5 % β-mercaptoethanol; Sigma-Aldrich) and transferred to 0.45 μm pore size polyvinylidenedifluoride membrane (PVDF, Immobilon, Merck Millipore; Darmstadt, Germany). The membrane was blocked with a solution of 5 % nonfat milk in TBS-T (Tris buffer saline plus 0.05 % (v/v) Tween20) for 1 h at room temperature (RT) and then incubated with the following primary antibodies diluted in blocking solution: anti-P2X1 (1:500; Alomone Labs (Jerusalem, Israel) cat#APR-001, RRID:AB_2040052), anti-P2X2 (1:500; Alomone Labs cat#APR-003, RRID:AB_2040054), anti-P2X4 (1:500; Alomone Labs cat#APR-002, RRID:AB_2040058), anti-P2X7 (1:500; Alomone Labs cat#APR-008, RRID:AB_2040065), anti-P2Y1 (1:500; Alomone Labs cat#APR-009, RRID:AB_2040070), anti-P2Y2 (1:500; Alomone Labs cat#APR-010, RRID:AB_2040078), anti-P2Y6 (1:500; Alomone Labs cat#APR-011, RRID:AB_2040082), and β-tubulin (1:1000; Sigma-Aldrich Cat# T5293, RRID:AB_477580) as loading control. Primary antibodies were incubated overnight at 4 °C followed by incubation at RT for 1 h with the corresponding secondary antibody diluted in 5 % non-fat milk in TBS-T buffer: HRP-conjugated goat anti-rabbit (1:10.000; ThermoFisher Scientific cat#31,460; RRID:AB_228341) or HRP-conjugated goat anti-Mouse (1:10.000; ThermoFisher Scientific cat#31,430; RRID:AB_228307) and developed using the enhanced chemiluminescence detection system (ECL; Pierce, ThermoFisher Scientific). All employed antibodies recognized the specific band or bands of the expected molecular weight.
In vivo experiments
C57BL/6J mice (Harlan Laboratories, Indianapolis, IN, USA) were housed in plastic cages in a temperature and humidity controlled room maintained on a 12:12 h reverse light/dark cycle with free access to food and water. Animal experimental procedures were in accordance with the European Communities Council Directive (2010/63/EU) and were approved by the Hospital Nacional de Parapléjicos Animal Care and Use Committee (ref# 63/2010).
SCI procedure, treatment, and locomotor recovery assays
Female mice 20 g of weight (12–14 weeks of age) were anesthetized through isoflurane (Forane, Baxter Health Care Corporation, Deerfield, IL, USA) inhalation (2 % in oxygen for induction and 1.5 % during surgery). The spinal cord was exposed by laminectomy in the 9th thoracic vertebrae (T9) and subsequently received a 50 Kdyne contusion using an IH Spinal Cord Impactor device (Precision System & Instrumentation, Lexington, KY, USA). After surgery, animals were maintained by daily manual bladder emptying for 2 weeks and by administration of Buprenorfine as analgesic (0.03 mg/Kg Buprex; Reckitt Benckiser Pharmaceuticals Limited, Richmond, VA, USA) and enrofloxazine as antibiotic (0.4 mg/Kg Baytril; Bayer AG, Leverkusen, Germany) for 2 days. The Sham group of animals was laminectomized but not contusioned.
As excitotoxic process starts minutes after injury, a first dose of Ap4A was administered directly on the contused area of the spinal cord by placing a 1-mm2 piece of Spongostan (Ferrosan Medical Devices, Søborg, Denmark) soaked in 2 mM Ap4A. Spongostan is an absorbable and haemostatic gel-foam used for dural application of drugs to the spinal cord [72, 73]. Afterwards, animals received a dose of 20 mg/Kg Ap4A in 200 μl of saline buffer and intraperitoneally administered 1 h after injury and then once daily for 7 days. Vehicle-treated animals received Spongostan soaked in saline buffer and then intraperitoneal injections of 200 μl saline buffer 1 h after injury and then once daily for 7 days.
Motor function recovery
The rate of motor function recovery was assessed in an open field weekly for 3 to 7 weeks after injury following the Basso Mouse Scale (BMS) [74]. Evaluations of each individual were performed for 4 min by two observers blinded to the treatment. The correspondent subscore scales were also used analyzing the different parameters individually for a more extensive analysis of certain motor features.
Motor function was also evaluated according to the rotarod performance test [75]. Weekly, animals were placed in a mouse rotarod 47600 device (UgoBasile, Gemonio, Varese, Italy) with accelerated rotational movement (from 4 to 40 rpm in 5 min test). The latency time it takes the mouse to fall off the rod was measured in three trials separated by 15 min rest.
Histology
Twenty-one days after surgery, animals were euthanized with a lethal dosage of sodium pentobarbital and transcardially perfused with saline buffer with sodium heparin (1 unit/ml; ChiesiEspaña, Barcelona, Spain) followed by 50 ml of 4 % (w/v) paraformaldehyde in 0.1 M phosphate buffer pH 7.4. A segment of 1 cm of spinal cord around the injured area was removed, post-fixed in 4 % paraformaldehyde solution (overnight at 4 °C), and cryoprotected in 30 % (w/v) sucrose (1–2 days at 4 °C). Finally, tissue was embedded in optimum cutting temperature compound (OCT; Tissue-Tek, Sakura Finetek Europe B.V. Alphen aan den Rijn, the Netherlands) and frozen (−20 °C). Embedded tissue was cut in 20 μm sections using a cryostat (HM560, Microm GmbH, Walldorf, Germany) and mounted in microscope glass slides (Superfrost Plus, ThermoFisher Scientific, Waltham, MA, USA).
Eriochrome cyanine stain of myelin
To determine the area and volume of spared tissue, we followed a modified method of Eriochrome Cyanine staining from Rabchevsky and colleagues [76]. Briefly, spinal cord sections were sequentially stained with Eriochrome Cyanine followed by counter-staining with 5 % iron Alum and Borax-Ferricyanide solutions. After dehydration with increasing concentrations of ethanol and Histochoice Cleaning Agent (Sigma-Aldrich), the samples were mounted using DPX Mountant (Sigma-Aldrich). This protocol stained white matter myelin and allowed us to differentiate the spared white matter from the grey matter or the damaged tissue. The area and volume of spared white matter were estimated through the stereological analysis of sections comprising 1 cm around the epicenter (100 μm between sections), using the Cavalliery method in a Stereology Microscope (Olympus BX61, Center Valley, PA, USA).
Data analysis
All data are expressed as mean ± SEM unless specifically stated. Statistical significance of the treatment effects was tested using unpaired Student’s t test, one-way ANOVA with Tukey post hoc test, or chi-square test depending to the characteristics of the data. The correlation between locomotor improvements and tissue conservation was calculated using the Pearson’s Correlation Coefficient. All analyses were conducted in Prism Software 5 (GraphPad Software Inc., La Jolla, Ca, USA). Differences were considered statistically significant when P < 0.05.
Results
Ap4A treatment reduces the ATP-induced death of Neuro-2a cells
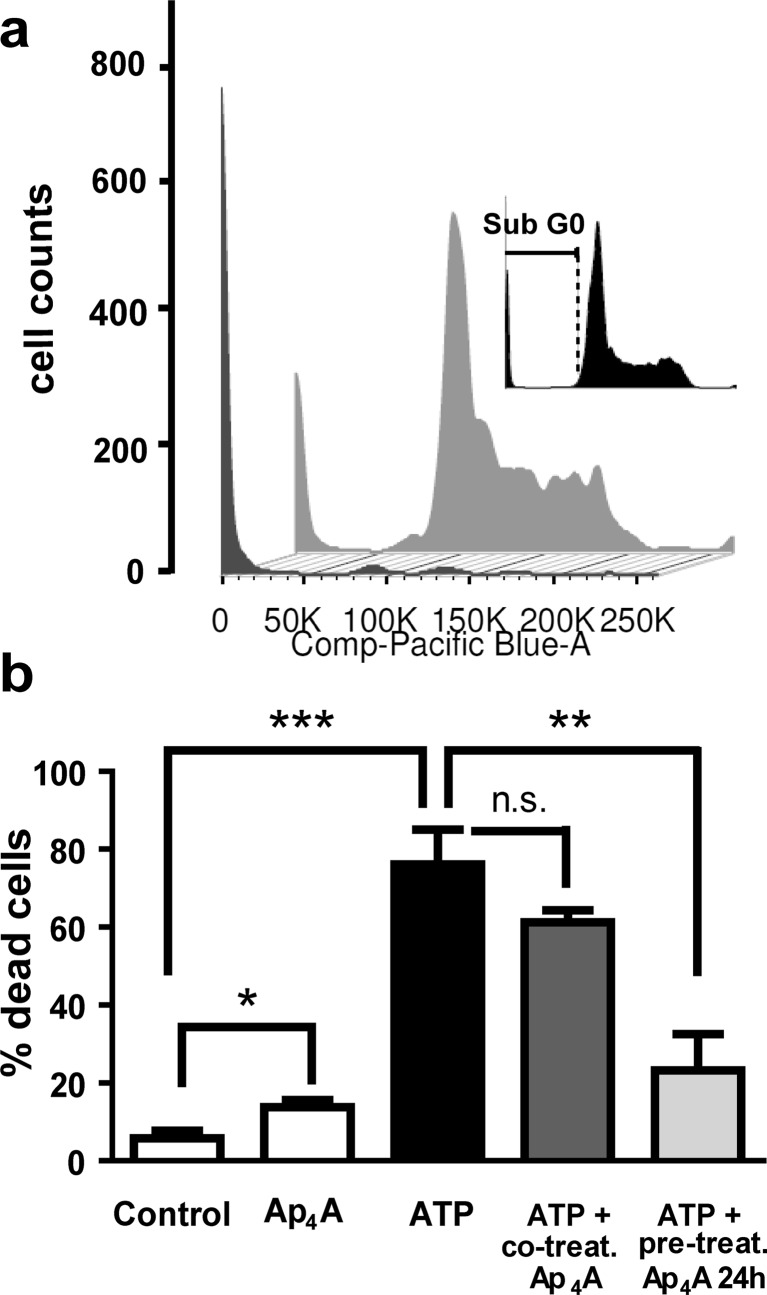
We analyzed the neuroprotective effects of Ap4A treatment using cultures of the murine neuroblastoma cell line Neuro-2a under excytotoxic conditions. We evaluated the percentage of death by flow cytometry of propidium iodide-stained cells. In Fig. 2a, we show representative cytometric cell cycle histograms of data obtained after ATP administration. The presence of ATP (300 μM) for 24 h induced apoptotic death as indicated by a significant increase in the percentage of Neuro-2a cells with condensed nuclei (sub-G0/G1 stage) from 5.80 ± 1.87 % in non-stimulated cells to 76.10 ± 8.92 % in ATP-stimulated ones (T4 = 7.71; p = 0.0008). Treatment with Ap4A (100 μM) also induced cell death but to a much lesser extent (13.77 ± 1.88 %; T4 = 3.0, p = 0.02, versus control). Co-treatment of ATP with Ap4A during 24 h induced a non-significant attenuation of the ATP-induced death (61.33 ± 2.96 %, corresponding to a 19.41 % reduction; T4 = 1.57, p = 0.096). Interestingly, adding a pre-incubation with Ap4A for 24 h markedly reduced the pro-apoptotic effect of ATP (23.20 ± 9.33 %, a reduction of 69.51 % versus ATP-treated; T4 = 4.10, p = 0.0074; Fig. 2b).
Fig. 2.
Reduction of ATP-induced apoptotic cell death by Ap4A in Neuro-2a cells. Neuroprotective effect of Ap4A was analyzed by flow cytometry in Neuro-2a cultures stimulated with ATP (300 μM, 24 h). Panel a shows representative flow cytometry histogram of cell cycle of propidium iodide-stained Neuro-2a under ATP treatment, with (gray trace) or without (black trace) Ap4A pre-treatment (100 μM, 24 h previous to ATP treatment). Insert in a shows the sub-G0/G1 region (apoptotic cells with condensed nuclei) of the cell cycle. The bar graph in b represents the percentages of apoptotic cells in each condition, showing an increase in cell death due to ATP treatment that was significantly reduced by Ap4A pre-treatment (mean ± standard deviation, n = 3). ***p < 0.001; **p < 0.01; *p < 0.05; n.s = non significant (Student’s t test)
Ap4A treatment reduces the ATP-induced rise in intracellular calcium concentration
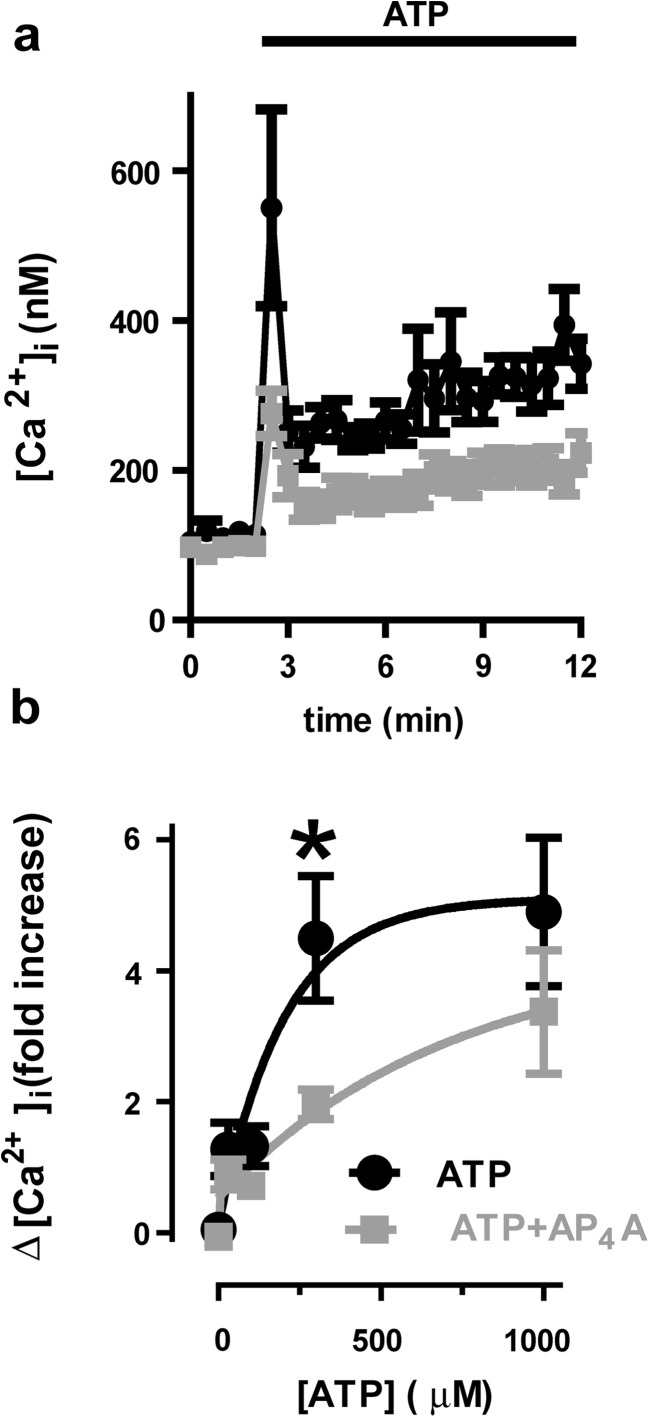
To explore the processes involved in Ap4A cytoprotection, we evaluated the effects Ap4A on ATP-induced calcium rise in Neuro-2a cells loaded with the ratiometric calcium sensitive dye fura-2. The addition of ATP (300 μM) increased intracellular calcium from a baseline of 96.39 ± 6.11 nM to a fast peak of 538.46 ± 110.94 nM (a 4.58-fold increase; n = 9; Fig. 3a, b) due to P2X receptor activation and a posterior slow rate increase due to P2Y receptor activation. Pre-incubation with Ap4A (100 μM) for 24 h did not change the intracellular calcium baseline concentration (99.92 ± 10.84 nM) but significantly reduced both the fast peak (290.85 ± 25.88 nM; a 1.91-fold increase; T15 = 2.32; p = 0.017 vs. ATP alone, n = 9) and the slow increase induced by ATP.
Fig. 3.
Reduction of intracellular calcium response to ATP by Ap4A treatment in Neuro-2a cells. Representative traces of ATP-induced calcium rise determinations (black) a due to P2 receptor activity and the inhibitory effect of a 24-h pre-treatment with Ap4A (100 μM, 24 h, gray). In b, we summarize the dose response in intracellular calcium by stimulation with ATP with (gray) or without (black) a 24-h pre-treatment with Ap4A
Ap4A reduces calcium response by a partial agonism effect and the reduction in the expression of P2 receptors
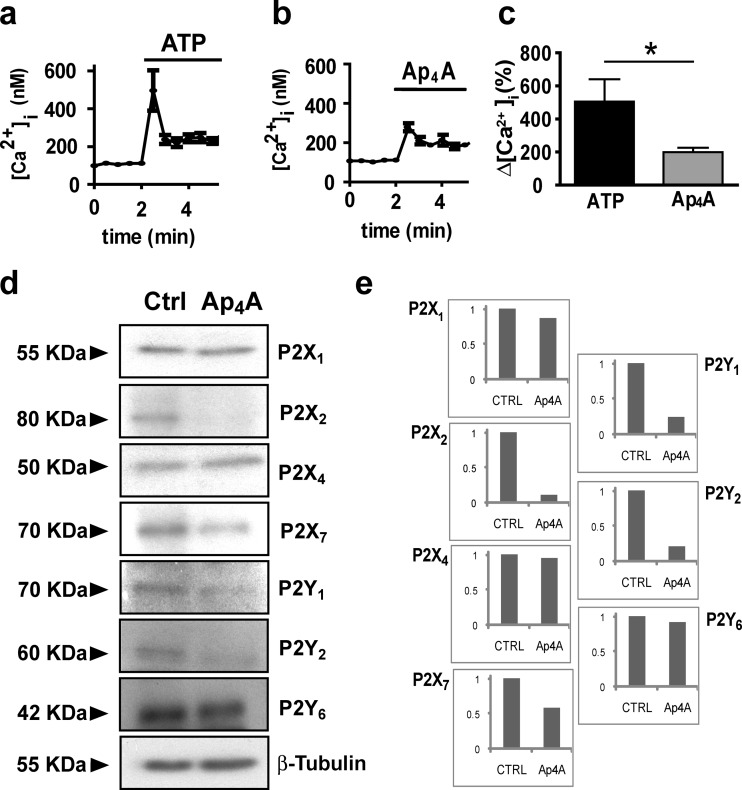
Two different mechanisms may underlie the effects of Ap4A on ATP-dependent intracellular calcium increase. On the one hand, Ap4A may function as a competitive partial agonist, binding to ATP receptors but with lower effects on calcium entrance. On the other, Ap4A may reduce the amount of purinergic receptors in cell membrane by inducing their internalization and degradation. In agreement with partial agonism competition, addition of Ap4A (100 μM) to Neuro-2a cultures had a lower effect on intracellular calcium than ATP (from a base line of 111.46 ± 8.56 nM to a peak of 278.76 ± 20.38 nM; a 1.5-fold increase; T15 = 2.44; p = 0.014 vs. ATP, n = 9) (Fig. 4a–c). Higher Ap4A concentrations (300 and 1000 μM) did not elicit any further increase in intracellular calcium concentrations (data not shown). However, in agreement with the degradation of purinergic receptors associated to the second mechanism, Western blot evaluation of protein samples from Neuro-2a cultures revealed that Ap4A treatment (100 μM; 24 h) reduces the expression of P2X2 (−88.32 %), P2X7 (−41.02 %), P2Y1 (−75.92 %), and P2Y2 (−78.09 %), whereas P2X1 (−13.41 %), P2Y6 (−7.39 %), and P2X4 (−4.53 %) expression remained unchanged (Fig. 4d, e). Therefore, Ap4A seems to reduce ATP-induced cell death and calcium entrance through both proposed mechanisms, partial agonism and receptor repression.
Fig. 4.
Effect of Ap4A on P2 responses and expression. The effect of Ap4A on ATP-induced calcium rise can be due to a partial agonism effect and/or reduction in receptor expression. Representative traces of calcium determinations after addition of ATP (300 μM) and Ap4A (100 μM) (a, b, and c) show a lower effect of Ap4A, maybe due to a partial agonism on P2X receptors. Western blot analysis of P2X and P2Y receptor expression d showed that Ap4A treatment (100 μM, 24 h) reduces the expression of P2X2, P2X7, P2Y1, and P2Y2 receptors in Neuro-2a cells, while the expression of P2X1, P2X4, and P2Y6 receptors remains unchanged. Panel d shows normalized expression versus the housekeeping protein β-tubulin
Ap4A treatment improves motor function recovery after SCI in mice
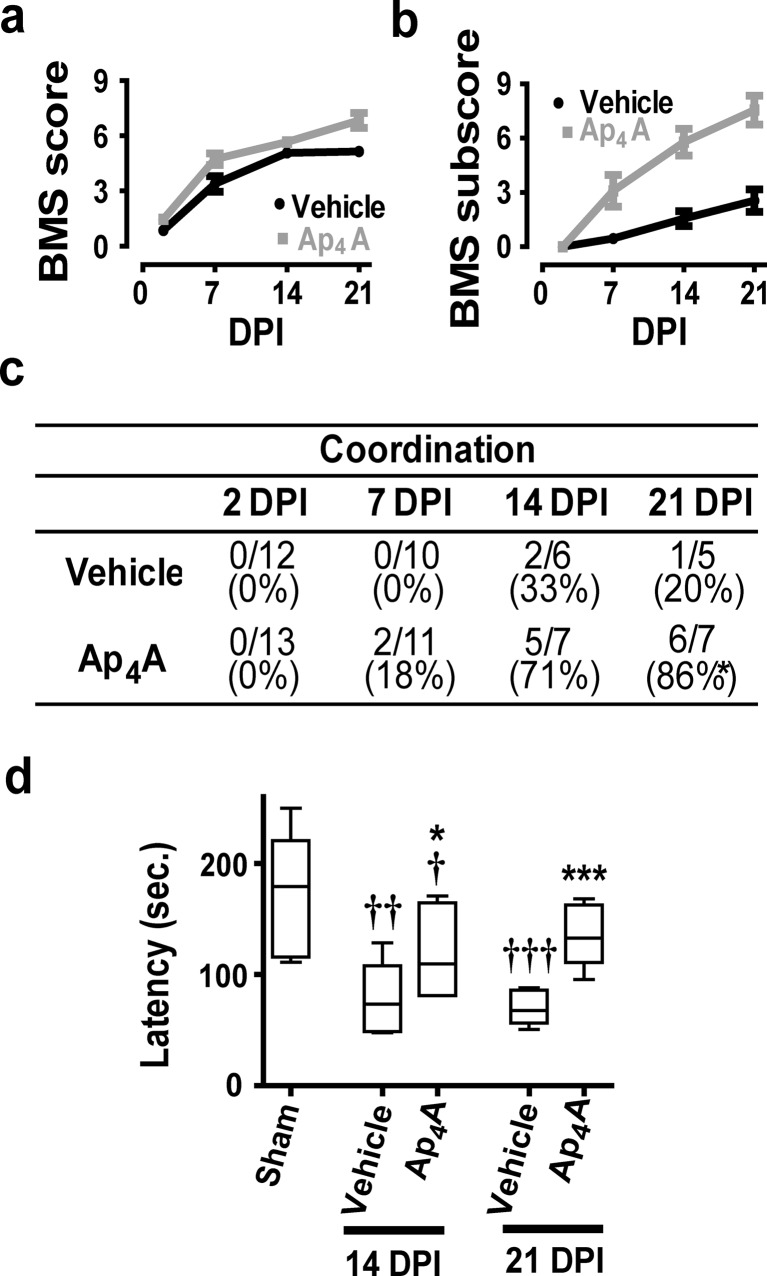
To explore the therapeutic potential of Ap4A, we evaluated the locomotor recovery in injured mice treated with Ap4A or vehicle. According to the Basso Mouse Scale (BMS), before injury, all mice reached the maximum score of 9 points in the scale. SCI caused a total loss of hind limb locomotor ability during the first 2 days post-injury (DPI), with a BMS score of 0 or 1 (Fig. 5a). Locomotion was partially recovered spontaneously at 21 DPI in vehicle-treated animals, reaching a final BMS score of 5.14 ± 0.23 (n = 7). Intraperitoneal injection of 20 mg/Kg Ap4A per day during 7 days induced a faster and larger locomotor recovery, up to 6.83 ± 0.41 points in the BMS score at 21 DPI (n = 9). A lower dose (10 mg/Kg) led to much lesser improvements in motor function recovery (data not shown). Comparison of the BMS subscores emphasize the differences between the vehicle-treated animals (2.56 ± 0.63 at 21 DPI; n = 7) and the 20 mg/Kg Ap4A-treated group (7.55 ± 0.80; n = 9) (Fig. 5b). Differences were particularly large when considering the capacity of injured mice to perform coordinative walk between forelimbs and hindlimbs. An individualized analysis revealed that a significantly higher number of Ap4A-treated mice recovered a mostly coordinated locomotion than vehicle-treated mice (86 vs. 20 % at 21 DPI, respectively; Fisher exact test p = 0.046) (Fig. 5c).
Fig. 5.
Improvement of mouse locomotor capacities by Ap4A treatment after SCI. We used the open field Basso Mouse Scale (BMS) to determine the locomotor skills of spinal cord injured mice after Ap4A treatment. BMS score (a) revealed that intraperitoneal treatment with Ap4A (20 mg/Kg) ameliorated the locomotor impairment derived from SCI. Improvements become more evident when locomotion parameters are coded according to the BMS subscore (b) (symbols represent mean ± SEM). The specific analysis of interlimb coordination (c) show that Ap4A treatment significantly increases the percentage of animals with coordinative capacities 21 days post-injury (DPI; *p < 0.05 in a chi-square test). In agreement, rotarod analysis of coordination and balance of mice at 14 and 21 DPI (d) reveal an increase of the latency time in Ap4A-treated mice versus vehicle-treated (bars represent the median and dispersion of the sample. *p < 0.05 and ***p < 0.001 vs. vehicle; † p < 0.05, †† p < 0.01, and ††† p < 0.001 vs. sham in Student’s t test; n = 6–12)
Coordinative skills together with balance capacities were also evaluated using the rotarod performance test [75], which determines the time that mice remain in an accelerating rod before falling (latency time) (Fig. 5d). In these setting, sham animals were able to remain in the device 173.60 ± 18.35 s (n = 9), whereas vehicle-treated group significantly worsen their performance, remaining 77.28 ± 14.83 (n = 5) and 70.5 ± 6.93 s (n = 6) at 14 and 21 DPI, respectively (T12 = 3.54, p = 0.002 vs. Sham). Treatment with Ap4A increased significantly the latency time relative to vehicle-treated group up to 119.0 ± 15.97 s at 14 DPI (T9 = 1.88; p = 0.046 vs. vehicle 14 DPI; n = 5) and 134.4 ± 11.09 s at 21 DPI (T9 = 4.64; p = 0.0006 vs. vehicle 21 DPI; n = 6), although they did not recover the values of the sham group.
Motor function improvements are associated to reduced tissue damage
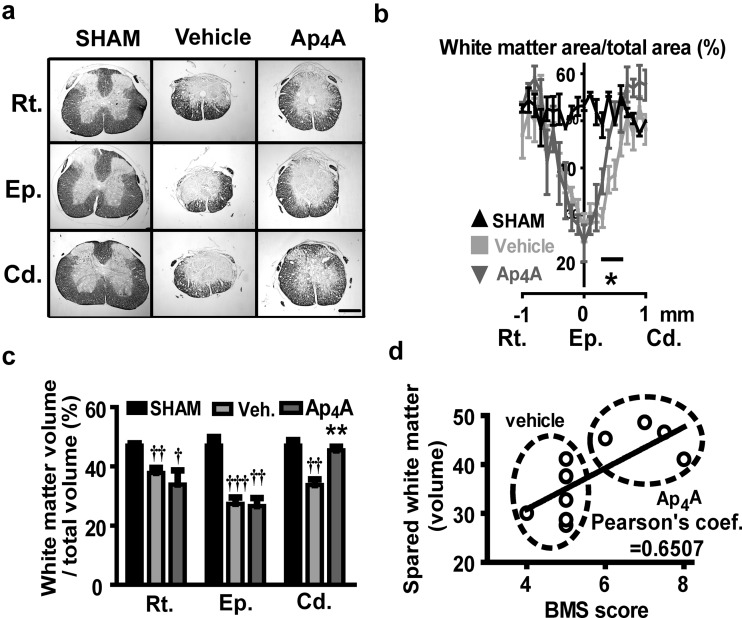
The observed improvement in locomotor recovery induced by Ap4A treatment led us to evaluate the underlying tissue preservation in the damaged spinal cord. To analyze the effect of Ap4A on tissue preservation, we stained mice spinal cord slices with eriochrome cyanine histological staining and quantified the spared white matter area and volume around the injury epicenter using Cavalieri’s stereological method combined with point-counting. White matter in sham group represented approximately the 50 % of the transversal section area of the spinal cord. At 21 DPI, tissue damage reduced the spared white matter to approximately 25 % of the section at the epicenter. Tissue preservation increased gradually, showing sham values at 1 mm caudal and rostral to the injury site (Fig. 6a, b). Ap4A treatment significantly increased white matter preservation in sections caudal to the injury site (45.41 ± 1.60 % white matter volume from 0.2 to 0.6 mm vs. 33.66 ± 1.96 % in vehicle-treated group; T9 = 4.06, P = 0.0014, n = 4–7; Fig. 6c). Moreover, while the relative area of white matter in Ap4A-treated mice reached extensions similar to sham individuals (around 50 %) in sections separated 0.4–0.5 mm caudal to the epicenter, vehicle-treated animals did not reached a similar preservation until 0.6–0.7 mm away from the epicenter (Fig. 6b). Conversely, vehicle and Ap4A-treated groups did not show differences in tissue preservation at the epicenter or the adjacent rostral sections.
Fig. 6.
Reduction of histological damage after SCI by Ap4A treatment. a Representative slices of the spinal cord stained with eriochrome cianine used to measure the spared white matter tissue at 21 DPI. Note that caudal sections in Ap4A-treated animals, but not in vehicle-treated ones, partially preserve the tissue structure of the naïve spinal cord. Scale bar for all images = 0.5 mm. Codes in the y-axis correspond to Cd = caudal; Ep = epicenter, and Rt = rostral. Estimations of the percentage of preserved white matter in transverse sections comprising 3 mm surrounding the injury epicenter of the spinal cord b show that Ap4A causes a significant increase of spared tissue area in caudal segments of the injury zone (symbols represent mean ± SEM; *p < 0.05 in Student’s t test vs. vehicle; n = 4–7). The estimated volume of spared white matter c at the injury epicenter, and the adjacent caudal and rostral regions (300 μm long in the three cases) also reveals the protective effect of Ap4A in the region caudal to the injury zone relative to the vehicle. d This greater tissue conservation in caudal areas significantly correlates with the locomotor improvements observed in BMS scale evaluations (see Fig. 5) (bars represent means ± SEM; † p < 0.05, †† p < 0.01, and ††† p < 0.01 vs. sham; **p < 0.01 in Student’s t test vs. vehicle; n = 4–7)
Accordingly, motor function recovery at 21 DPI (BMS score) was significantly correlated with tissue preservation in sections caudal to the injury (Pearson’s correlation coefficient = 0.6507, p = 0.03) but not with preservation at the epicenter or in sections rostral to the injury (Pearson’s correlation coefficients −0.0627 and 0.0612 respectively, n = 4–7 mice; Fig. 6d).
Discussion
Neuroprotection is a major priority in the development of an effective therapy for traumatic spinal cord injury [77]. Several approaches are being tested [78, 79], but only high-dose intravenous administration of methylprednisolone has reached the clinical practice with controversial benefits [80]. In search for efficient neuroprotective strategies, we have evaluated the ability of diadenosine tetraphosphate (Ap4A) to reduce the excitotoxic death mediated by the ATP-induced deregulation of calcium homeostasis and its consequences on tissue preservation and functional recovery in a mouse model of moderate contusive SCI.
Our studies with the mice-derived neural cell line Neuro2a indicate that Ap4A treatment protects neural cells from death induced by administration of excitotoxic concentrations of ATP, as reported in other cellular models of neuronal death induced by methamphetamine [69], ischemia or 6-hydroxydopamine [68]. Neuroprotection was highest when cultures were pre-incubated with Ap4A for 24 h before ATP stimulation. Pre-treatment with Ap4A reduced the rise of intracellular levels of calcium induced by ATP. As shown in Fig. 4d and in the literature, Neuro2a cells express several types of P2X ligand-gated ion channel receptors [81] and G-protein-coupled P2Y receptors [82]. Both the fast peak, under control of P2X receptors, and the slow rate increase, under control of P2Y receptors, were reduced by Ap4A. This result agreed with a reduction in the levels of Ap4A purinergic receptors P2X2, P2Y1, and P2Y2, suggesting that regulation of calcium levels may result from a ligand-induced internalization and degradation of ATP purinergic receptors, a well-known regulatory mechanism of the function of several P2X [83–88] and P2Y receptors [89, 90]. However, Ap4A failed to reduce the expression of P2X1, P2X4, both ionotropic receptors for which Ap4A is a partial agonist. Interestingly, Ap4A treatment also reduced the expression of P2X7, which is not receptor for Ap4A [91], suggesting that Ap4A can activate alternative regulatory mechanisms for non-target purinergic receptor internalization and degradation. Further studies are required to clarify the different response of these receptors. Ap4A can also negatively modulate the calcium response to ATP and the consequent excitotoxic cell death by competing for receptors shared by both purines [92–94]. As we mentioned before, Ap4A is partial agonist for P2X1 and P2X4, full agonist but less potent than ATP for P2X2 and P2X3 [48] and partial agonist on P2Y1, P2Y2, and P2Y12, antagonizing the effects of ATP [49, 50]. According to our experiments, Ap4A increases intracellular calcium, without reaching the cytotoxic levels elicited by ATP, and when co-administered with ATP, Ap4A protects Neuro2a cells from ATP excitotoxicity, although to a much lesser extent than when Ap4A is administered 24 h before (see Fig. 2).
Together, these results demonstrate the cytoprotective effects of Ap4A on ATP-induced excitotoxicity, due to the internalization and degradation of P2X receptors reducing the entrance of extracellular calcium and likely of P2Y receptors decreasing the long-term mobilization of calcium from intracellular reservoirs. However, we cannot exclude other effects on pathways associated to the activation of the P2Y and P2X receptors. However, we cannot exclude other effects on pathways associated to the activation of the P2Y and P2X receptors. The mobilization of calcium can be also due to the activation of voltage-dependent calcium channels by different types of diadenosine polyphosphates [58, 95], which expression or function could be affected by the 24 h pre-incubation with Ap4A. In addition, Ap4A has been described as alarmone–low molecular weight intracellular compounds that are synthesized in response to a specific metabolic stress and that act homeostatically to increase the probability of cell survival- regulating cellular metabolic state and/or gene expression [96]. Ap4A cytoprotection can be also a consequence of the effects of its hydrolysis to adenosine, as previously described [97], although preliminary data from an ongoing study using adenosine deaminase to transform adenosine in inosine indicates that adenosine does not participate in Ap4A cytoprotection.
The cytoprotective effects of Ap4A in vitro demonstrated in the present work, together with results from previous studies in different neurodegenerative diseases [67–69], suggested that Ap4A administration can be a valid neuroprotective treatment for nervous system injuries involving excitotoxic processes induced by massive ATP release, including SCI [10–12]. In agreement, we observed that intraperitoneal administration of Ap4A after contusion in a mouse model of SCI induces improved inter-limb coordination earlier and to a larger extent than vehicle-treated mice. Coordination is a major milestone rarely reached by untreated C57BL/6J after a moderate SCI [74, 98] that precedes a more extensive recovery [99], depending on the conservation of local networks or propriospinal interneurons (PNs) that build up the central patterns generators (CPGs) and rhythmic locomotive movements [100, 101]. The conservation of these circuits is reflected in the increase of spared white matter in Ap4A-treated mice, especially in sections caudal to the injury place.
The beneficial effects of Ap4A treatment on a mouse model of contusive SCI have important implications. On the one hand, they confirm the contribution of ATP activation of the purinergic signaling in the development of SCI impairments, as previously shown in studies of Dr. Nedergaard’s laboratory [10, 44]. According to Wang and colleagues [10], ATP release is strongly increased in peritraumatic areas during the first 6 h following the trauma, activating purinergic receptors in some cases overexpressed after SCI (vg. P2X4 and P2X7 [102, 103]). Signaling through purinergic receptors increases the concentration of intracellular calcium and induces excitotoxic death of neurons and oligodendrocytes [10], as well as modulates the acute and chronic inflammation and neuropathic pain [104, 105]. The ATP-mediated rise of intracellular calcium also contributes to exacerbate excitotoxicity indirectly, inducing the release of glutamate [106, 107]. On the other hand, the beneficial effect of Ap4A treatment supports the modulation of the purinergic system as a promising neuroprotective target for SCI, as proposed by previous authors [10, 44]. However, unlike previous analyses that searched for antagonists to P2X7 as their approach for these therapies [10], our results indicate that other purinergic receptors, particularly the ionotropic P2Y receptors, may also be targeted to modulate ATP-induced cell death and improve functional recovery. Moreover, according to our in vitro analyses, the use of an agonist such as Ap4A may elicit a broader cytoprotective response based on (i) a long-term regulation of intracellular calcium concentration, (ii) internalization of purinergic receptors including P2X7 which is overexpressed after SCI [103], and (iii) possibly through anti-apoptotic mechanisms not explored here such as the reduction of caspase cleavage, DNA fragmentation, and activation of the Erk1/2 pro-survival pathway induced by the activation of P2Y2 receptor [108], one of the purinergic receptors with highest affinity for Ap4A [51, 109].
Therefore, we suggest that the acute administration of an endogenous purine-signaling molecule as Ap4A can be a promising therapy to reduce ATP-induced excitotoxicity and other cell death pathways triggered by SCI, as already proved in the amelioration of multiple types of disease models and neurodegenerative processes [51, 67, 68]. In the present study, we have combined the systemic delivery of Ap4A with its topic administration taking advantage of the spinal cord exposure during the surgical procedures in order to maximize the chances of the drug to reach the damaged tissue. As a consequence, uncertainties may be risen on the contribution of each administration strategy to the observed improvements. Previous studies have already demonstrated the therapeutic potential of both topic and systemic administration of Ap4A in different CNS pathologies [67, 68, 110], but information on the effects of their combination is still lacking. Therefore, additional studies will be necessary to optimize the dosage and to establish the therapeutic efficiency of each delivery system, as well as to determine the precise—direct and indirect—mechanisms underlying functional recovery, tissue sparing, and neuroprotection.
We also suggest that Ap4A acts in a biphasic way, with a early fast phase, which reduces calcium excitotoxicity by competing with ATP for purinergic receptors and probably reduces apoptosis by signaling through the P2Y2 receptor to activate Erk1/2 pathway [108], and a secondary phase in which Ap4A reduces the purinergic tone and calcium response promoting the internalization of purinergic receptors. Other studies have already targeted the purinergic system after SCI using P2X7 antagonists reaching promising cytoprotective effects [10, 44]. However, contrary to the drugs employed in these approaches, Ap4A is an endogenous purine that does not fully antagonize ATP but functions as a partial agonist restricting intracellular calcium concentrations to non-toxic levels while activating different pro-survival pathways. Finally, we suggest that combination of Ap4A administration with therapies based on P2X7 antagonists [10, 44] as well as inhibitors of glutamate derived excitotoxicity such as Riluzole [35, 39], may lead to more efficient cytoprotective therapies for excitotoxic death in SCI.
Acknowledgments
We would like to thank Dr. Claire H. Mitchell and Dr. Jesús Pintor for their helpful comments and their kind criticism in the preparation of this manuscript. This work was supported by Fundación para la Investigación Sanitaria de Castilla la Mancha (FISCAM). PI-2010/19. We thank the technical and logistic support to the Fundación del Hospital Nacional de Parapléjicos para la Investigación y la Integración (FUHNPAIIN) and the microscopy and cytometry facilities of the Experimental Neurology Unit, Hospital Nacional de Parapléjicos, Toledo, Spain.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
David Reigada, Phone: 34-925396834, Email: dreigada@sescam.jccm.es.
Rosa María Navarro-Ruiz, Phone: 34-925396834, Email: rosanavarro8@gmail.com.
Marcos Javier Caballero-López, Phone: 34-925396834, Email: marcosinvestigacion@gmail.com.
Ángela Del Águila, Phone: 34-925396834, Email: mdeda@sescam.jccm.es.
Teresa Muñoz-Galdeano, Phone: 34-925396834, Email: tmunozd@sescam.jccm.es.
Rodrigo M. Maza, Phone: 34-925396834, Email: rodrigom@sescam.jccm.es
Manuel Nieto-Díaz, Phone: 34-925396834, Email: mnietod@sescam.jccm.es.
References
- 1.Liu XZ, Xu XM, Hu R, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olney JW. Glutaate-induced retinal degeneration in neonatal mice. Electron microscopy of the acutely evolving lesion J Neuropathol Exp Neurol. 1969;28:455–474. doi: 10.1097/00005072-196907000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 4.Ray SK, Hogan EL, Banik NL. Calpain in the pathophysiology of spinal cord injury: neuroprotection with calpain inhibitors. Brain Res Rev. 2003;42:169–185. doi: 10.1016/S0165-0173(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 5.Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103:203–218. doi: 10.1016/S0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Ashwell KW, Waite P. Advances in secondary spinal cord injury: role of apoptosis. Spine (Phila Pa 1976) 2000;25:1859–1866. doi: 10.1097/00007632-200007150-00022. [DOI] [PubMed] [Google Scholar]
- 7.Demjen D, Klussmann S, Kleber S, et al. Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat Med. 2004;10:389–395. doi: 10.1038/nm1007. [DOI] [PubMed] [Google Scholar]
- 8.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 9.Choi D. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Arcuino G, Takano T, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 11.Neary JT, Rathbone MP, Cattabeni F, et al. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- 12.Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Zhang M, Laties AM, Mitchell CH. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthalmol Vis Sci. 2005;46:2183–2191. doi: 10.1167/iovs.05-0052. [DOI] [PubMed] [Google Scholar]
- 14.Hu H, Lu W, Zhang M, et al. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp Eye Res. 2010;91:425–432. doi: 10.1016/j.exer.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell CH, Lu W, Hu H, et al. The P2X7 receptor in retinal ganglion cells: a neuronal model of pressure-induced damage and protection by a shifting purinergic balance. Purinergic Signal. 2009;5:241–249. doi: 10.1007/s11302-009-9142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cisneros-Mejorado A, Pérez-Samartín A, Gottlieb M, Matute C. ATP signaling in brain: release, excitotoxicity and potential therapeutic targets. Cell Mol Neurobiol. 2015;35:1–6. doi: 10.1007/s10571-014-0092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domercq M, Perez-Samartin A, Aparicio D, et al. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia. 2010;58:730–740. doi: 10.1002/glia.20958. [DOI] [PubMed] [Google Scholar]
- 18.Cho J-H, Choi I-S, Jang I-S. P2X7 receptors enhance glutamate release in hippocampal hilar neurons. Neuroreport. 2010;21:865–870. doi: 10.1097/WNR.0b013e32833d9142. [DOI] [PubMed] [Google Scholar]
- 19.Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- 20.Duan S, Anderson CM, Keung EC, et al. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Straub R, Bolis L, editors. Cell Membr. Recept. Drugs Horm. A Multidiscip. Approach. New York: Raven Press; 1978. pp. 107–118. [Google Scholar]
- 22.Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 23.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 24.Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 25.Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Pankratov Y, Lalo U, Krishtal O, Verkhratsky A. Ionotropic P2X purinoreceptors mediate synaptic transmission in rat pyramidal neurones of layer II/III of somato-sensory cortex. J Physiol. 2002;542:529–536. doi: 10.1113/jphysiol.2002.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol. 2014;99:16–34. doi: 10.1113/expphysiol.2013.071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnstock G. Purinergic signalling. Br J Pharmacol. 2006;147(Suppl):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue K. P2 receptors and chronic pain. Purinergic Signal. 2007;3:135–144. doi: 10.1007/s11302-006-9045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue K. ATP receptors of microglia involved in pain. Novartis Found Symp. 2006;276:263–272. doi: 10.1002/9780470032244.ch21. [DOI] [PubMed] [Google Scholar]
- 31.Burnstock G, Krügel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Zayas AE, Torrado AI, Rosas OR, et al. Blockade of P2 nucleotide receptors after spinal cord injury reduced the gliotic response and spared tissue. J Mol Neurosci. 2011;46:167–176. doi: 10.1007/s12031-011-9567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franke H, Krügel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622–644. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 34.Majumder P, Trujillo CA, Lopes CG, et al. New insights into purinergic receptor signaling in neuronal differentiation, neuroprotection, and brain disorders. Purinergic Signal. 2007;3:317–331. doi: 10.1007/s11302-007-9074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagoshi N, Nakashima H, Fehlings MG. Riluzole as a neuroprotective drug for spinal cord injury: from bench to bedside. Molecules. 2015;20:7775–7789. doi: 10.3390/molecules20057775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fehlings MG, Wilson JR, Frankowski RF, et al. Riluzole for the treatment of acute traumatic spinal cord injury: rationale for and design of the NACTN phase I clinical trial. J Neurosurg Spine. 2012;17:151–156. doi: 10.3171/2012.4.AOSPINE1259. [DOI] [PubMed] [Google Scholar]
- 37.Wilson JR, Fehlings MG. Riluzole for acute traumatic spinal cord injury: a promising neuroprotective treatment strategy. World Neurosurg. 2014;81:825–829. doi: 10.1016/j.wneu.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Chow DSL, Teng Y, Toups EG, et al. Pharmacology of riluzole in acute spinal cord injury. J Neurosurg Spine. 2012;17:129–140. doi: 10.3171/2012.5.AOSPINE12112. [DOI] [PubMed] [Google Scholar]
- 39.Grossman RG, Fehlings MG, Frankowski RF, et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31:239–255. doi: 10.1089/neu.2013.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells JEA, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 41.Garrido-Mesa N, Zarzuelo A, Gálvez J. Minocycline: far beyond an antibiotic. Br J Pharmacol. 2013;169:337–352. doi: 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol. 2001;166:7527–7533. doi: 10.4049/jimmunol.166.12.7527. [DOI] [PubMed] [Google Scholar]
- 43.Nagoshi N, Fehlings MG. Investigational drugs for the treatment of spinal cord injury: review of preclinical studies and evaluation of clinical trials from phase I to II. Expert Opin Investig Drugs. 2015;24:645–658. doi: 10.1517/13543784.2015.1009629. [DOI] [PubMed] [Google Scholar]
- 44.Peng W, Cotrina ML, Han X, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcillo A, Frydel B, Bramlett HM, Dietrich WD. A reassessment of P2X7 receptor inhibition as a neuroprotective strategy in rat models of contusion injury. Exp Neurol. 2012;233:687–692. doi: 10.1016/j.expneurol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pojoga LH, Haghiac ML, Moose JE, Hilderman RH. Determination of ATP impurity in adenine dinucleotides. Nucleosides Nucleotides Nucleic Acids. 2004;23:581–598. doi: 10.1081/NCN-120030716. [DOI] [PubMed] [Google Scholar]
- 47.Pojoga LH, Haghiac M, Hilderman RH. Inhibition by adenine dinucleotides of ATP-induced prostacyclin release by bovine aortic endothelial cells. Biochem Pharmacol. 2002;64:405–412. doi: 10.1016/S0006-2952(02)01217-0. [DOI] [PubMed] [Google Scholar]
- 48.Wildman SS, Brown SG, King BF, Burnstock G. Selectivity of diadenosine polyphosphates for rat P2X receptor subunits. Eur J Pharmacol. 1999;367:119–123. doi: 10.1016/S0014-2999(98)00976-5. [DOI] [PubMed] [Google Scholar]
- 49.Chang H, Yanachkov IB, Michelson AD, et al. Agonist and antagonist effects of diadenosine tetraphosphate, a platelet dense granule constituent, on platelet P2Y1, P2Y12 and P2X1 receptors. Thromb Res. 2010;125:159–165. doi: 10.1016/j.thromres.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conant AR, Fisher MJ, McLennan AG, Simpson AW. Diadenosine polyphosphates are largely ineffective as agonists at natively expressed P2Y(1) and P2Y(2) receptors on cultured human saphenous vein endothelial cells. J Vasc Res. 2000;37:548–555. doi: 10.1159/000054088. [DOI] [PubMed] [Google Scholar]
- 51.Crooke A, Mediero A, Guzmán-Aránguez A, Pintor J. Silencing of P2Y2 receptor delays Ap4A-corneal re-epithelialization process. Mol Vis. 2009;15:1169–1178. [PMC free article] [PubMed] [Google Scholar]
- 52.Pintor J, King BF, Miras-Portugal MT, Burnstock G. Selectivity and activity of adenine dinucleotides at recombinant P2X2 and P2Y1 purinoceptors. Br J Pharmacol. 1996;119:1006–1012. doi: 10.1111/j.1476-5381.1996.tb15771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lazarowski ER, Watt WC, Stutts MJ, et al. Pharmacological selectivity of the cloned human P2U-purinoceptor: potent activation by diadenosine tetraphosphate. Br J Pharmacol. 1995;116:1619–1627. doi: 10.1111/j.1476-5381.1995.tb16382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schachter JB, Li Q, Boyer JL, et al. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinoceptor. Br J Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Communi D, Motte S, Boeynaems JM, Pirotton S. Pharmacological characterization of the human P2Y4 receptor. Eur J Pharmacol. 1996;317:383–389. doi: 10.1016/S0014-2999(96)00740-6. [DOI] [PubMed] [Google Scholar]
- 56.Marteau F, Le Poul E, Communi D, et al. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- 57.Zhang H (2008) P2,P3-[18F] Monofluoromethylene diadenosine-5′,5″’-P1,P4-tetraphosphate. In: Mol. Imaging Contrast Agent Database (MICAD). Natl. Cent. Biotechnol. Information, NLM, NIH, Bethesda, MD. http://www.ncbi.nlm.nih.gov/books/NBK22992/pdf/Bookshelf_NBK22992.pdf. [PubMed]
- 58.Pintor J, Miras-Portugal MT. A novel receptor for diadenosine polyphosphates coupled to calcium increase in rat midbrain synaptosomes. Br J Pharmacol. 1995;115:895–902. doi: 10.1111/j.1476-5381.1995.tb15894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zamecnik PC, Stephenson ML, Janeway CM, Randerath K. Enzymatic synthesis of diadenosine tetraphosphate and diadenosine triphosphate with a purified lysyl-sRNA synthetase. Biochem Biophys Res Commun. 1966;24:91–97. doi: 10.1016/0006-291X(66)90415-3. [DOI] [PubMed] [Google Scholar]
- 60.Miras-Portugal MT, Gualix J, Pintor J. The neurotransmitter role of diadenosine polyphosphates. FEBS Lett. 1998;430:78–82. doi: 10.1016/S0014-5793(98)00560-2. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez del Castillo A, Torres M, Delicado EG, Miras-Portugal MT. Subcellular distribution studies of diadenosine polyphosphates—Ap4A and Ap5A—in bovine adrenal medulla: presence in chromaffin granules. J Neurochem. 1988;51:1696–1703. doi: 10.1111/j.1471-4159.1988.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 62.Pintor J, Díaz-Rey MA, Torres M, Miras-Portugal MT. Presence of diadenosine polyphosphates—Ap4A and Ap5A—in rat brain synaptic terminals. Ca2+ dependent release evoked by 4-aminopyridine and veratridine. Neurosci Lett. 1992;136:141–144. doi: 10.1016/0304-3940(92)90034-5. [DOI] [PubMed] [Google Scholar]
- 63.Pintor J, Rotllán P, Torres M, Miras-Portugal MT. Characterization and quantification of diadenosine hexaphosphate in chromaffin cells: granular storage and secretagogue-induced release. Anal Biochem. 1992;200:296–300. doi: 10.1016/0003-2697(92)90469-N. [DOI] [PubMed] [Google Scholar]
- 64.Carracedo G, Peral A, Pintor J. Diadenosine polyphosphates in tears of Sjogren syndrome patients. Invest Ophthalmol Vis Sci. 2010;51:5452–5459. doi: 10.1167/iovs.09-5088. [DOI] [PubMed] [Google Scholar]
- 65.Pintor J. Presence of diadenosine polyphosphates in the aqueous humor: their effect on intraocular pressure. J Pharmacol Exp Ther. 2003;304:342–348. doi: 10.1124/jpet.102.041368. [DOI] [PubMed] [Google Scholar]
- 66.Pintor J, Bautista A, Carracedo G, Peral A. UTP and diadenosine tetraphosphate accelerate wound healing in the rabbit cornea. Ophthalmic Physiol Opt. 2004;24:186–193. doi: 10.1111/j.1475-1313.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 67.Hoyle CHV, Pintor JJ. Diadenosine tetraphosphate protects sympathetic terminals from 6-hydroxydopamine-induced degeneration in the eye. Acta Physiol. 2010;199:205–210. doi: 10.1111/j.1748-1716.2010.02089.x. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Chang C-F, Morales M, et al. Diadenosine tetraphosphate protects against injuries induced by ischemia and 6-hydroxydopamine in rat brain. J Neurosci. 2003;23:7958–7965. doi: 10.1523/JNEUROSCI.23-21-07958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harvey BK, Chou J, Shen H, et al. Diadenosine tetraphosphate reduces toxicity caused by high-dose methamphetamine administration. Neurotoxicology. 2009;30:436–444. doi: 10.1016/j.neuro.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 71.Negulescu PA, Machen TE. Intracellular ion activities and membrane transport in parietal cells measured with fluorescent dyes. Methods Enzymol. 1990;192:38–81. doi: 10.1016/0076-6879(90)92062-I. [DOI] [PubMed] [Google Scholar]
- 72.Gürer B, Kahveci R, Gökçe EC, et al. Evaluation of topical application and systemic administration of rosuvastatin in preventing epidural fibrosis in rats. Spine J. 2015;15:522–529. doi: 10.1016/j.spinee.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 73.Louw AM, Kolar MK, Novikova LN, et al. Chitosan polyplex mediated delivery of miRNA-124 reduces activation of microglial cells in vitro and in rat models of spinal cord injury. Nanomedicine. 2016;12:643–653. doi: 10.1016/j.nano.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 74.Basso DM, Fisher LC, Anderson AJ, et al. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 75.Hamm RJ, Pike BR, O’Dell DM, et al. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 76.Rabchevsky AG, Fugaccia I, Sullivan PG, Scheff SW. Cyclosporin a treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J Neurotrauma. 2001;18:513–522. doi: 10.1089/089771501300227314. [DOI] [PubMed] [Google Scholar]
- 77.Liverman BM, Altevogt CT, Joy JE, Johnson RT. Spinal cord injury: progress, promise, and priorities. Washington D.C: The National Academies Press; 2005. [Google Scholar]
- 78.Onose G, Anghelescu A, Muresanu DF, et al. A review of published reports on neuroprotection in spinal cord injury. Spinal cord Off J Int Med Soc Paraplegia. 2009;47:716–726. doi: 10.1038/sc.2009.52. [DOI] [PubMed] [Google Scholar]
- 79.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 80.Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gómez-Villafuertes R, del Puerto A, Díaz-Hernández M, et al. Ca2+/calmodulin-dependent kinase II signalling cascade mediates P2X7 receptor-dependent inhibition of neuritogenesis in neuroblastoma cells. FEBS J. 2009;276:5307–5325. doi: 10.1111/j.1742-4658.2009.07228.x. [DOI] [PubMed] [Google Scholar]
- 82.León-Otegui M, Gómez-Villafuertes R, Díaz-Hernández JI, et al. Opposite effects of P2X7 and P2Y2 nucleotide receptors on α-secretase-dependent APP processing in neuro-2a cells. FEBS Lett. 2011;585:2255–2262. doi: 10.1016/j.febslet.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 83.Ennion SJ, Evans RJ. Agonist-stimulated internalisation of the ligand-gated ion channel P2X(1) in rat vas deferens. FEBS Lett. 2001;489:154–158. doi: 10.1016/S0014-5793(01)02102-0. [DOI] [PubMed] [Google Scholar]
- 84.Lalo U, Allsopp RC, Mahaut-Smith MP, Evans RJ. P2X1 receptor mobility and trafficking; regulation by receptor insertion and activation. J Neurochem. 2010;113:1177–1187. doi: 10.1111/j.1471-4159.2010.06730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dutton JL, Poronnik P, Li GH, et al. P2X(1) receptor membrane redistribution and down-regulation visualized by using receptor-coupled green fluorescent protein chimeras. Neuropharmacology. 2000;39:2054–2066. doi: 10.1016/S0028-3908(00)00058-7. [DOI] [PubMed] [Google Scholar]
- 86.Li GH, Lee EM, Blair D, et al. The distribution of P2X receptor clusters on individual neurons in sympathetic ganglia and their redistribution on agonist activation. J Biol Chem. 2000;275:29107–29112. doi: 10.1074/jbc.M910277199. [DOI] [PubMed] [Google Scholar]
- 87.Bobanovic LK, Royle SJ, Murrell-Lagnado RD. P2X receptor trafficking in neurons is subunit specific. J Neurosci. 2002;22:4814–4824. doi: 10.1523/JNEUROSCI.22-12-04814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Royle SJ, Bobanović LK, Murrell-Lagnado RD. Identification of a non-canonical tyrosine-based endocytic motif in an ionotropic receptor. J Biol Chem. 2002;277:35378–35385. doi: 10.1074/jbc.M204844200. [DOI] [PubMed] [Google Scholar]
- 89.Koenig JA, Edwardson JM. Endocytosis and recycling of G protein-coupled receptors. Trends Pharmacol Sci. 1997;18:276–287. doi: 10.1016/S0165-6147(97)90643-X. [DOI] [PubMed] [Google Scholar]
- 90.Koenig JA. Assessment of receptor internalization and recycling. Methods Mol Biol. 2004;259:249–273. doi: 10.1385/1-59259-754-8:249. [DOI] [PubMed] [Google Scholar]
- 91.Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br J Pharmacol. 2009;157:1203–1214. doi: 10.1111/j.1476-5381.2009.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haghiac M, Pojoga LH, Hilderman RH. Studies on the effect of diadenlyated nucleotides on calcium mobilization and prostacyclin synthesis in bovine aortic endothelial cells. Cell Signal. 2001;13:145–150. doi: 10.1016/S0898-6568(00)00147-9. [DOI] [PubMed] [Google Scholar]
- 93.Gómez-Villafuertes R, Gualix J, Miras-Portugal MT, Pintor J. Adenosine 5′-tetraphosphate (Ap(4)), a new agonist on rat midbrain synaptic terminal P2 receptors. Neuropharmacology. 2000;39:2381–2390. doi: 10.1016/S0028-3908(00)00070-8. [DOI] [PubMed] [Google Scholar]
- 94.Miras-Portugal MT, Gualix J, Mateo J, et al. Diadenosine polyphosphates, extracellular function and catabolism. Prog Brain Res. 1999;120:397–409. doi: 10.1016/S0079-6123(08)63572-4. [DOI] [PubMed] [Google Scholar]
- 95.Miras-Portugal MT, Pintor J, Gualix J. Ca2+ signalling in brain synaptosomes activated by dinucleotides. J Membr Biol. 2003;194:1–10. doi: 10.1007/s00232-003-2024-x. [DOI] [PubMed] [Google Scholar]
- 96.Varshavsky A. Diadenosine 5′, 5′′′-P1, P4-tetraphosphate: a pleiotropically acting alarmone? Cell. 1983;34:711–712. doi: 10.1016/0092-8674(83)90526-3. [DOI] [PubMed] [Google Scholar]
- 97.Ribeiro FF, Xapelli S, Miranda-Lourenço C, et al. Purine nucleosides in neuroregeneration and neuroprotection. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 98.Reigada D, Nieto-Díaz M, Navarro-Ruiz R, et al. Acute administration of ucf-101 ameliorates the locomotor impairments induced by a traumatic spinal cord injury. Neuroscience. 2015;300:404–417. doi: 10.1016/j.neuroscience.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 99.Basso DM. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J Neurotrauma. 2004;21:395–404. doi: 10.1089/089771504323004548. [DOI] [PubMed] [Google Scholar]
- 100.Flynn JR, Graham BA, Galea MP, Callister RJ. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011;60:809–822. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 101.Bareyre FM, Kerschensteiner M, Raineteau O, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 102.Miras-Portugal MT, Gomez-Villafuertes R, Gualix J, et al. Nucleotides in neuroregeneration and neuroprotection. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 103.Gómez-Villafuertes R, Rodríguez-Jiménez FJ, Alastrue-Agudo A, et al. Purinergic receptors in spinal cord-derived ependymal stem/progenitor cells and their potential role in cell-based therapy for spinal cord injury. Cell Transplant. 2015;24:1493–1509. doi: 10.3727/096368914X682828. [DOI] [PubMed] [Google Scholar]
- 104.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 105.Gourine AV, Dale N, Llaudet E, et al. Release of ATP in the central nervous system during systemic inflammation: real-time measurement in the hypothalamus of conscious rabbits. J Physiol. 2007;585:305–316. doi: 10.1113/jphysiol.2007.143933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khakh BS, Henderson G. ATP receptor-mediated enhancement of fast excitatory neurotransmitter release in the brain. Mol Pharmacol. 1998;54:372–378. doi: 10.1124/mol.54.2.372. [DOI] [PubMed] [Google Scholar]
- 107.Jeremic A, Jeftinija K, Stevanovic J, et al. ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J Neurochem. 2001;77:664–675. doi: 10.1046/j.1471-4159.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- 108.Arthur DB, Georgi S, Akassoglou K, Insel PA. Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival. J Neurosci. 2006;26:3798–3804. doi: 10.1523/JNEUROSCI.5338-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol. 2003;140:1177–1186. doi: 10.1038/sj.bjp.0705544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Loma P, Guzman-Aranguez A, Pérez de Lara MJ, Pintor J. Diadenosine tetraphosphate induces tight junction disassembly thus increasing corneal epithelial permeability. Br J Pharmacol. 2015;172:1045–1058. doi: 10.1111/bph.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]