Abstract
Controversy exists over the role of stress and depression in the pathophysiology of type 2 diabetes mellitus. Depression has been shown to increase the risk for progressive insulin resistance and incident type 2 diabetes mellitus in multiple studies, whereas the association of stress with diabetes is less clear, owing to differences in study designs and in forms and ascertainment of stress. The biological systems involved in adaptation that mediate the link between stress and physiological functions include the hypothalamic–pituitary–adrenal axis and the autonomic nervous and immune systems. The hypothalamic–pituitary–adrenal axis is a tightly regulated system that represents one of the body’s mechanisms for responding to acute and chronic stress. Depression is associated with cross-sectional and longitudinal alterations in the diurnal cortisol curve, including a blunted cortisol awakening response and flattening of the diurnal cortisol curve. Flattening of the diurnal cortisol curve is also associated with insulin resistance and type 2 diabetes mellitus. In this article, we review and summarize the evidence supporting hypothalamic–pituitary–adrenal axis dysregulation as an important biological link between stress, depression, and type 2 diabetes mellitus.
Keywords: cortisol, stress, depression, diabetes, hypothalamic–pituitary–adrenal axis
Introduction
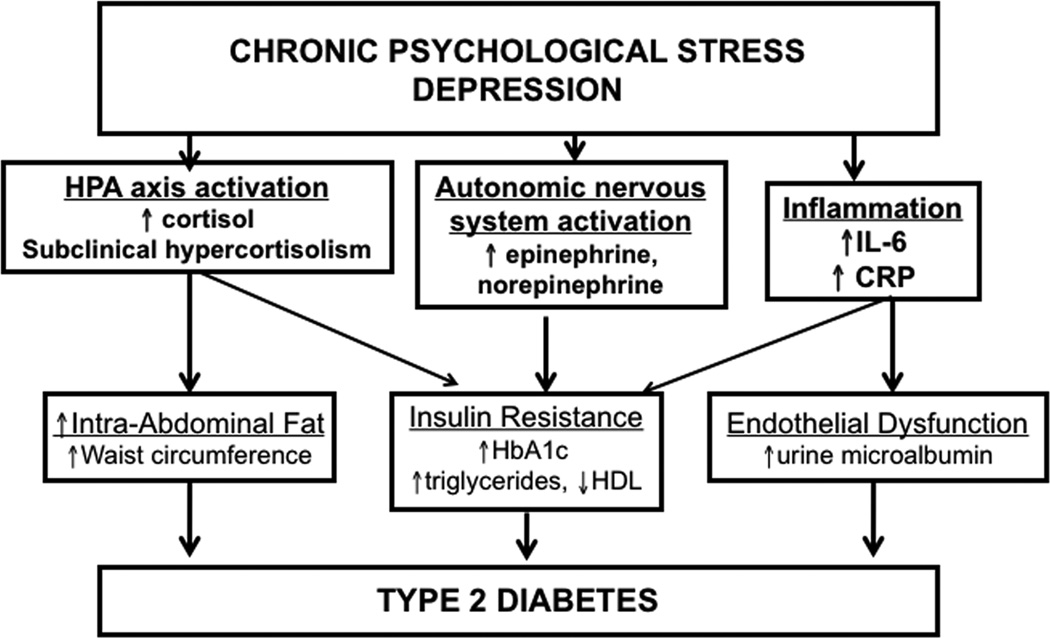
Allostasis describes the capacity of an organism to adapt to a changing environment or stressful challenge to support homeostatic systems essential to life.1,2 Stress can be measured as the interpretation and perception of stressors or the actual exposure to events assumed to be stressful. Allostatic load summarizes the cumulative impact of physiological wear and tear related to maladaptive stress patterns that predispose individuals to disease.1,3 The biological systems involved in adaptation that mediate the link between stress and physiological functions include the hypothalamic–pituitary–adrenal (HPA) axis and the autonomic nervous and immune systems.1,4 Various types of chronic psychological stress are associated with increased allostatic load.5–7 We hypothesize a graded positive association of stress and depression with type 2 diabetes mellitus (diabetes) risk (Fig. 1).
Figure 1.
A hypothesized relationship between stress, depression, cortisol, and diabetes.
Physiology of the HPA axis
As widely reviewed, the HPA axis is a tightly regulated system that represents one of the body’s mechanisms for responding to acute and chronic stress.8 In response to physiological or psychological stressors, the HPA axis is activated, resulting in secretion of corticotropin-releasing hormone (CRH) from the hypothalamus, which stimulates the anterior pituitary gland to release adrenocorticotropic hormone (ACTH). ACTH then stimulates release of cortisol from the adrenal gland, resulting in a cascade of physiological events. Once the stressor has resolved, the response is terminated through a negative feedback loop, in which cortisol suppresses further release of ACTH and CRH.8 Activation of the HPA axis is also accompanied by stimulation of the sympathetic nervous system, resulting in the release of catecholamines and interleukin-6, which activates a cytokine cascade.9 Chronic stress may impair the feedback mechanisms that return these hormonal systems to normal, resulting in chronic elevation in levels of cortisol, catecholamines, and inflammatory markers.
Depression and diabetes
Depression and depressive symptoms increase risk for progressive insulin resistance10 and incident diabetes.11–15 Rates of co-existing major depressive disorder (MDD) and diabetes are high, with at least 10–15% of individuals with diabetes suffering from depression.16,17 A meta-analysis found that the odds of depression in diabetic individuals were twice those of the nondiabetic comparison groups and that there was no difference by sex, type of diabetes, or assessment method.16 The prevalence of comorbid depression was significantly higher in diabetic women (28%) than in diabetic men (18%), in uncontrolled (30%) than in controlled (21%) studies, in clinical (32%) than in community (20%) samples, and when assessed by self-report questionnaires (31%) than by standardized diagnostic interviews (11%).16 Among individuals with diabetes, depression is associated with worse glycemic control18 and health-related outcomes in diabetes (i.e., weight gain, adherence to therapy,19 long-term diabetic macrovascular and microvascular complications)20 and is associated with higher costs to the healthcare system.19 This interaction predicts not only greater incidence, but also earlier incidence of complications from diabetes in older adults.18–22 Treatment of depression through pharmacotherapy and/or cognitive behavioral therapy has resulted in improved glycemic control in some,23–25 but not all,26 trials. The biological association between depression and diabetes is hypothesized to be due to a dysregulated and overactive HPA axis, a shift in sympathetic nervous system tone toward enhanced sympathetic activity, and a proinflammatory state.9
Chronic psychological stress and diabetes
Chronic psychological stress is a state of mental or emotional strain where an individual perceives that environmental demands tax or exceed his/her adaptive capacity.27 Stress can be measured as the interpretation and perception of stressors or the actual exposure to events assumed to be stressful. To this point, most of the work on stress and incident diabetes has been concentrated on work-related stress and the results have been mixed, with reports of both positive and negative associations, depending on sex, length of follow-up, diabetes ascertainment method, and stress measurement instrument.28 In 2008, a meta-analysis attempted to examine the association of general chronic psychological stress on diabetes risk but was unable to determine an etiological association because of a lack of published longitudinal cohorts.29 Since that time, six prospective longitudinal studies with follow-up durations ranging from 10–35 years have been published,30–32 using different methods to assess stress and revealing divergent findings.30–36 Two of these studies found a positive association in women only;30,33 three studies found a positive association in men but not in women;31,32 one study of only men showed a positive association at 35 years;34 and one study found a positive association in pre-diabetic men and women.35 Another study of men and women revealed a hazard ratio of 1.33 for incident diabetes at 18 years for those with chronic psychological stress versus none (adjusted for age, sex, education level, and household income), but when further adjusted for level of energy, health status, health problems, and activity level, the results were not significant.36 Among African Americans in the Jackson Heart Study, higher global perceived stress scores (GPSSs) were weakly associated with a higher prevalence of diabetes in women, and higher GPSSs and major life events scores were cross-sectionally associated with obesity in men and women.37 Collectively, these studies support a positive association between chronic psychological stress and incident diabetes, but the link is difficult to assess because of the aforementioned differences in study design and methods. Moreover, there remains a lack of published studies assessing the association of chronic psychological stress with incident diabetes specifically in U.S. racial/ethnic minorities, who have a higher prevalence of diabetes and associated complications.
Salivary cortisol and the diurnal cortisol curve
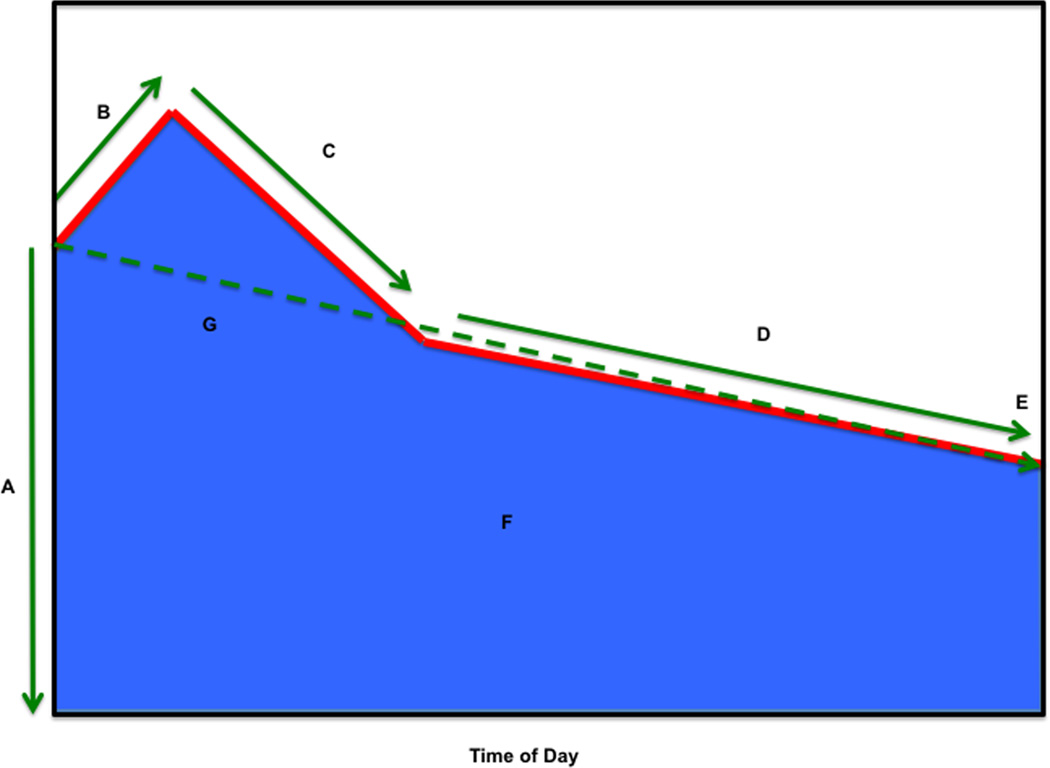
Epidemiological studies examining the contribution of HPA axis dysfunction to the pathophysiology of depression, psychological stress, and diabetes have been previously limited by imprecise measures of glucocorticoid exposure, owing to the cumbersome nature of the 24-h urinary free-cortisol measurement, the pulsatility and lability of hypothalamic and pituitary hormones, and the circadian variation in cortisol secretion. As noted above, production of cortisol from the adrenal gland has a classic circadian pattern, with cortisol rapidly rising after awakening, reaching a peak at 30–45 min, and then gradually declining over the course of the day. Salivary cortisol collection allows for noninvasive timed collection of free cortisol, which is stable for several days prior to processing, allowing for a valid assessment of the HPA axis in the free-living state. Timed salivary cortisol collections are used to construct a diurnal salivary cortisol curve (Fig. 2) with several cortisol features, including wake-up cortisol (salivary cortisol obtained at 0 min), cortisol awakening response (CAR; the cortisol rise from 0 to 30 min post-awakening), early decline in cortisol (the decline in cortisol from 30 min post-awakening to 2 h post-awakening), late decline in cortisol (decline in cortisol from 2 h post-awakening to bedtime), bedtime cortisol (cortisol prior to bedtime), and total area-under-the-curve (AUC) cortisol (the total AUC from 0 to 16 h).
Figure 2.
Summary of diurnal cortisol parameters. Shown is (A) wake-up cortisol (time 0), (B) cortisol awakening response (0–30 min), (C) early-decline slope cortisol (30 min to 2 h), (D) late-decline slope cortisol (2 hours to bedtime), (E) bedtime cortisol, (F) total AUC (0 min to bedtime) cortisol, and (G) overall-decline slope cortisol (0 min to bedtime, excluding 30-min cortisol). Reprinted, with permission, from Joseph et al.80
Our research focus has thus evolved to using salivary cortisol to assess the diurnal cortisol curve and HPA axis activity in population-based studies. Salivary cortisol has the advantage of allowing examination of more salient features of the diurnal cortisol curve in relation to depression among community-dwelling individuals. Given that cortisol levels rise with age and there may be differences in the cortisol curve over the life span, we will examine studies in children/adolescents < 18 years old, young and middle-aged adults 18–64 years old, and older adults ≥ 65 years old.
Diurnal cortisol curve and depression
The relationship between non-diurnal cortisol curve measures and depression was summarized in a recent meta-analysis.38 Elevated cortisol and ACTH are associated with depression, with a stronger effect in older inpatients with melancholic or psychotic depression.38 We previously reviewed the literature showing excessive cortisol exposure in individuals with depression, evidenced by elevated 24-h urinary free-cortisol levels, adrenal gland enlargement, and failure to suppress cortisol in response to the dexamethasone suppression test.39 The association of diurnal cortisol curve features and depression across the life span proceeding from childhood to older adulthood is discussed below.
Children, adolescents, and young adults
We previously reviewed two smaller studies (< 100 participants) examining the cross-sectional association of the diurnal cortisol rhythm with depression.9 These studies revealed a flatter diurnal cortisol curve with greater emotional distress and higher children’s depression inventory scores among post-pubertal adolescents.9 An analysis of the Tracking Adolescents’ Individual Lives Survey (TRAILS) of children in the general population aged 10–12 years (n = 1604) revealed higher cortisol (mainly CAR) in relation to depressive problems.40 In a study of 17- to 23-year-old women, cortisol secretion patterns were compared for 57 currently depressed, at-risk (based on trait-level positive and negative affect), and control women over 5 days and in response to a laboratory stressor. After controlling for potentially confounding biobehavioral variables, the depressed group displayed a larger CAR and higher average diurnal cortisol compared to control participants. Individuals at-risk for depression also had significantly higher waking cortisol levels than control participants.41 Longitudinal studies have reported discrepant findings; for example, in the Youth Emotion Project with 230 U.S. suburban high school students (mean = 17 years of age), a higher CAR was found to be predictive of MDD 1 year later.42 On the other hand, in a British study that randomly recruited participants from the Avon Longitudinal Study of Parents and Children (ALSPAC) and among 668 adolescents with 3 years follow-up, researchers found no association of morning cortisol, CAR, decline slope, bedtime cortisol, or AUC cortisol with the development of depression from ages 15 to 18 years.43
Young and middle-aged adults
In 1999, a Swedish study of 257 participants aged 30–64 years, who were randomly selected from the general population, examined the association of salivary cortisol and depressive symptoms using the Major Depression Inventory scale. The mean of samples collected at awakening, 30 min later, and bedtime over three consecutive workdays were used in the analyses. Depressive symptoms were negatively correlated to wake-up and 30-min cortisol (P < 0.05) but not to the CAR or evening cortisol. A flatter diurnal rhythm of cortisol was related to higher levels of depression (P < 0.05).44 We previously reviewed two smaller studies (< 100 participants) of the association of the diurnal cortisol rhythm with depression in hospitalized patients suspected of having, or being at risk for, cardiovascular disease9 and found that rhe participants with concurrent depression had a flatter diurnal cortisol curve. Contrary to this, Conrad et al. did not note any significant differences in salivary cortisol diurnal variation among 46 depressed subjects at risk for cardiovascular disease compared to 19 subjects without depression.9 In the Netherlands Study of Depression and Anxiety (NESDA), which recruited 1588 participants from the community, general practice care, and specialized mental health care (308 control (mean age = 48 years), 579 with remitted depression (mean age = 45 years), and 701 with current depression (mean age = 42 years), both the remitted and current MDD groups showed a significantly higher CAR compared with control subjects (effect size (Cohen’s d) range: 0.15–0.25), with higher morning AUC cortisol. Evening cortisol levels were higher among the current MDD group at 10 PM but not at 11 PM. Participants with comorbid anxiety disorders, but not with other depression characteristics, had a higher CAR.45 In 2010, a systematic review and meta-analysis of mostly small studies (except for the aforementioned NESDA study45) concluded that there is no firm evidence of a difference of salivary cortisol in middle-aged (mean ages ranged from 18–65 years) depressed compared to control persons.46
Following publication of the abovementioned meta-analysis,46 further related analyses were reported in the literature, including a cross-sectional study of 26 premenopausal depressed women and 23 never-depressed women who were matched for age and body mass index. This study found that depression and greater depression severity were associated with flatter diurnal cortisol curve slopes over the course of the day.47 However, mixed findings have been reported in recent longitudinal studies, including in the Danish PRISME (Psychological risk factors in the work environment and biological mechanism in the development of stress, burnout, and depression) project—a longitudinal Danish study that collected morning and evening salivary cortisol and assessed depressive symptoms of 4467 public employees, with follow-up interviews for participants with elevated scores to diagnose depression. Over 2 years, each 1.0 nmol/L increase in daily mean cortisol concentration was associated with a 47% (95% CI: 0.32, 0.90) reduction in diabetes risk, while each 1.0 nmol/L difference in morning and evening salivary cortisol concentration was associated with a 36% lower risk of depression (95% CI: 0.45, 0.90). These data suggested that a steeper cortisol slope over the day was protective for incident depression.48 To confirm these findings, the same research group conducted a population-based study of the association of depressive symptoms or diagnosed depression and the diurnal cortisol curve among 3536 public-sector employees aged 19–66 years. They found no association of depressive symptoms or depression with morning cortisol, CAR, cortisol decline slope, evening cortisol, or total AUC cortisol in two cross-sectional analyses (2007 and 2009) or in their longitudinal analysis from 2007 to 2009.49
Studies specifically with participants with known depression or a history of depression reveal a mix of findings consistent with cortisol dysregulation. Longitudinal data obtained from 837 NESDA participants revealed that a lower CAR was associated with an unfavorable course, namely, depression remission lasting no longer than 3 months (relative risk (RR) = 0.83; P = 0.03).50 In a study of 187 remitted, highly recurrent MDD patients, cortisol concentrations were found to be lower in patients with more previous episodes (P = 0.047) but were not associated with recurrence(s) during follow-up.51 In contrast, a study of 55 recurrently depressed patients reported that lower mean 8 AM cortisol levels predicted earlier time to recurrence over 5.5 years, after correction for residual symptoms (P = 0.015).52 NESDA subjects (n = 549) with a lifetime diagnosis of MDD and in remission for at least 6 months preceding the baseline assessment were followed over 4 years to assess recurrence.53 It was found that a higher CAR, but not evening cortisol, was associated with earlier time to recurrence of MDD (hazard ratio (HR) = 1.03l; 95% CI: 1.003–1.060; P = 0.03).53
Older adults
Among 61 older adults (age > 60 years) with depression and 40 older adults without depression, 8 AM, noon, 4 PM, 8 PM, and total AUC cortisol levels were higher among depressed compared to non-depressed participants.54,55 In a meta-analysis of 20 studies of the association of depression with cortisol dysregulation among older adults (> 60 years), those with depression displayed higher levels of basal cortisol during all phases of the diurnal cycle, but particularly during the evening and night hours, and higher levels of post-dexamethasone cortisol.56 Furthermore, in the Netherlands Study of Depression in Older Persons, older adults (mean age = 70 years) with MDD (n = 311) showed significantly higher awakening cortisol, a blunted CAR, and non-significantly higher cortisol levels throughout the day compared to non-depressed older persons (n = 109).57
Diurnal cortisol curve and depression: conclusions
In summary, among children and adolescents there is a consistent cross-sectional finding of a higher CAR in those with depression or greater depressive symptoms. However, there is a need for further research on the temporality of this association since longitudinal studies revealed divergent findings, with the largest study showing no prospective association of cortisol curve features with incident depression. The data for individuals aged 18–64 years of age are even less clear. Some studies report cross-sectional positive associations of CAR with prevalent depression,45 while other studies do not.44,49 Some studies reveal a cross-sectional and longitudinal association of a flatter cortisol curve with incident depression,9,48 while other studies have not found this association.9,47,49 The small sample of studies in older adults has provided evidence for a higher level of cortisol throughout the day and possibly at bedtime. Studies of individuals with previous or current depression alone reveal that a higher CAR and lower 8 AM cortisol are associated with time to recurrence and a lower CAR is associated with an unfavorable course among those with depression.50, 52, 53 These results reveal that a lower wake-up cortisol or a higher CAR may be associated with a decreased time to recurrence among those with a history of depression, but further studies are required to verify these findings. It is important to understand why there may be differences in the findings, most of which relate to the CAR. In healthy populations, an increased CAR is important for meeting specific demands of the upcoming day (i.e., workday versus weekend) and decreasing stress on that specific day, and has therefore been linked to coping.58 It has also been speculated that this link may become dysregulated once the coping mechanisms employed are inefficient in eliminating feelings of stress over time. In that case, a heightened CAR (and possibly inflexible or stiff CAR) may switch from signaling coping to signaling anticipation of stress of the next day. After a certain threshold, a persistent increased CAR may be downregulated and become blunted. This could explain why some studies have observed a heightened CAR and others a blunted CAR in relation to depression.58 It is important to consider that treatment with selective serotonin reuptake inhibitors (SSRIs) and cognitive therapy is associated with a reduction in total AUC cortisol, and, in turn, reduction of cortisol significantly correlates with improvement in depressive symptoms and mental cognition.51,59,60 The treatment results support a relationship between depression and dysregulation of cortisol. Studies of neuroendocrine blockade with CRH-1 receptor antagonist revealed significant reductions in depressive and anxiety symptoms among 20 patients with MDD, but this antagonist did not impair the CRH and cortisol secretory activity either at baseline or following an exogenous CRH challenge.61 Notably, the assessment of cortisol did not include a diurnal assessment to examine cortisol features throughout the day, but instead focused on 24-h urinary cortisol collection, which may not have been sensitive enough to detect differences with CRH-1 receptor blockade. For a more detailed review on the role of cortisol and the glucocorticoid receptor in the neurobiology of depression, the reader is referred to Ref. 62.
Diurnal cortisol curve and stress
In addition to depression, various forms of stress, whether inflammatory, traumatic, or psychological, activate the HPA axis.63,64 Changes in the diurnal cortisol slope represent an important indicator of a stress-related alteration of the diurnal cortisol rhythm,65 and acute and chronic stress exposures have been linked to flatter cortisol decline.66–68 The experience of life stress has also consistently been related to a higher CAR.69 In the following discussion, we focus on psychological stress, including work-related stress and discrimination, which may have different effects on activation of the HPA axis.
Work-related stress
The literature to date on measures of work-related stress (i.e., job strain—high job demand and low job control) and salivary cortisol levels reveals inconsistent evidence of an association. As we have previously discussed,70 job strain has been associated with higher morning cortisol levels, lower average cortisol levels, a steeper CAR, and increased total AUC cortisol, and for each of these observations there are other studies that report no significant association. One of the larger analyses was from the Whitehall II study, in which researchers assessed the cross-sectional association of work stress, using job demand–control (JDC) and effort–reward imbalance (ERI) models, with the diurnal cortisol curve (six salivary cortisol collections over a typical work day) among 2126 occupational workers (mean age = 57.1 years).71 The JDC model postulates that a combination of lower control (less skill utilization and lower decision authority) and higher work demand (more quantitative work load and conflicting demands) will trigger job strain; whereas, the ERI model emphasizes social reciprocity, such that a sustained unfair trade-off between effort (cost) and reward (gain) will elicit negative emotions and further lead to adverse long-term health consequences.71 Modest differences in cortisol patterns were found for ERI models only, showing that lower reward (β = −0.001; P = 0.04) and higher ERI (β = 0.002; P = 0.05) were related to a flatter slope in cortisol across the day.71 Given the previous inconsistent findings, Rudolph et al. performed an analysis on data from the Multi-Ethnic Study of Atherosclerosis (MESA) and found that, among employed participants, job strain was associated with lower salivary cortisol levels and total AUC cortisol, and was not associated with CAR, using propensity score matching on an extensive set of variables to control for sources of confounding.70 The authors suggested that the differences between their results and those from the prior literature could be attributed to the middle- and older-aged racially/ethnically diverse sample in MESA, compared to the previous research in younger, mostly white cohorts. In addition, previous findings were influenced by residual confounding and multiple sources of variability using cortisol measures from a single day.70 Further studies are required with similar methodologies and assessment of work stress to allow for an accurate comparison across studies.
Discrimination
In a study of White and African American young adults, Skinner et al.72 examined the relation between discrimination and diurnal cortisol and found that perceived discrimination was associated with a flatter diurnal cortisol slope among all groups. In contrast, a study examining White and African American adults found that discrimination was associated with flatter diurnal cortisol slopes in White adults, but steeper diurnal cortisol slopes in African American adults.73 In a study that assessed the association of self-reported discrimination and diurnal cortisol rhythm (six salivary cortisol samples collected over 3 days) among 140 young adults (mean age 22.8 years), self- reported discrimination predicted flatter diurnal cortisol slopes only for racial/ethnic minority individuals (African Americans, Hispanic Americans, and Asian Americans),74 suggesting that Whites may have a more robust HPA axis response to perceived discrimination than minorities. Given the discrepant findings in the current literature, additional studies are needed.
In conclusion, heterogeneity in study design, age, and population make it difficult to draw firm conclusions from the current literature. Keeping the aforementioned limitations in mind, there is a suggestion of a flatter diurnal cortisol curve with higher levels of work stress and discrimination that may vary by age and race/ethnicity. Thus, further exploration of the role of stress in cross-sectional and longitudinal changes in the diurnal cortisol curve is of critical importance.
Diurnal cortisol curve and diabetes
Cross-sectional studies of the association of diurnal cortisol and diabetes suggest a flattening of the diurnal cortisol curve in individuals with diabetes compared to those without diabetes. In a small study of 30 subjects, diabetic individuals showed a blunted CAR compared to non-diabetic individuals.75 This finding was confirmed by the Cooperative Research in the Region of Augsburg (KORA)-F3 study, which also found higher bedtime cortisol levels in participants with diabetes (n = 63) compared to those without diabetes (n = 916; P < 0.01), as well as a trend toward lower wake-up cortisol levels (P = 0.07).76 In the Whitehall II study of 508 White men and women, including 238 participants with diabetes, those with diabetes had a flatter slope across the day and higher bedtime cortisol values, but no difference in wake-up cortisol levels or CAR, suggesting that raised evening cortisol levels promoted a flattening of the slope throughout the day.77
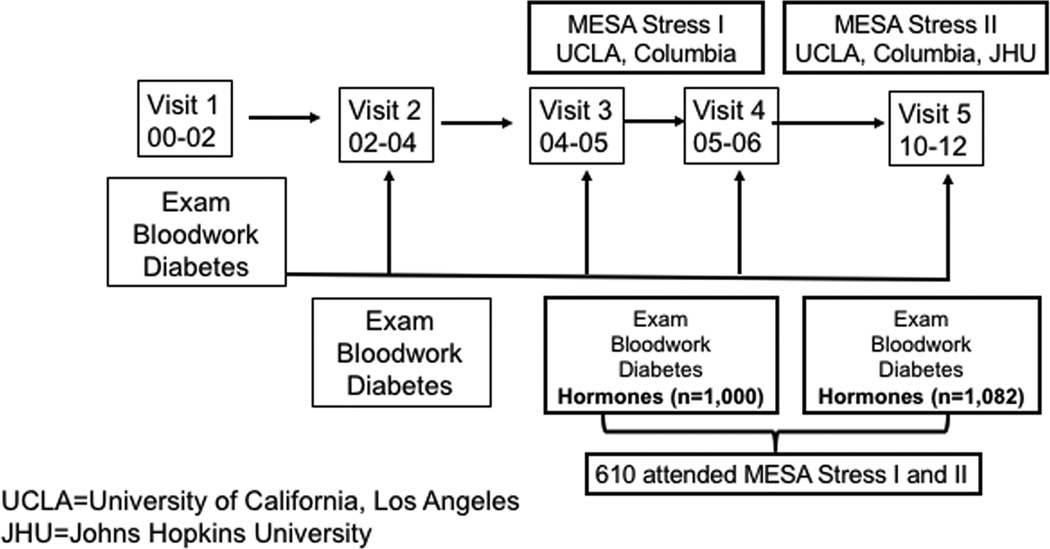
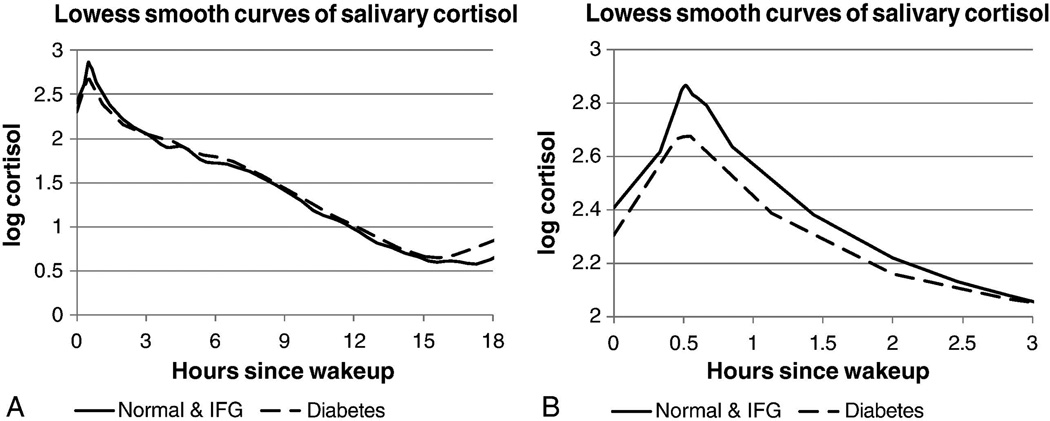
Our research group has studied participants in MESA Stress, an ancillary study to the main multicenter longitudinal cohort study of the prevalence and correlates of subclinical cardiovascular disease and the factors that influence its progression.78 MESA Stress I collected detailed measures of stress hormones, including salivary cortisol measures, between 2004 and 2006 (during the period of MESA Exam 3 and Exam 4) at the New York and Los Angeles MESA sites. MESA Stress II collected similar data, on a subsample of 1082 participants at the New York, Los Angeles, and Baltimore MESA sites between 2010 and 2012 during MESA Exam 5 (Fig. 3). The MESA Stress populations included non-Hispanic Whites (NHWs), African Americans, and Hispanic Americans. Among multiethnic participants, those with diabetes (n = 177) had a significantly blunted CAR, flatter early-decline cortisol, and sex differences with total AUC cortisol—men with diabetes had a lower total AUC cortisol compared to men without diabetes and women with diabetes had a higher total AUC cortisol compared to women without diabetes (Fig. 4).79 Wake-up cortisol was non-significantly lower among those with diabetes compared to those without diabetes.79 These results in a multiethnic population were consistent with prior data among NHW cohorts showing an association of a blunted CAR and flattening of cortisol decline with diabetes mellitus.
Figure 3.
MESA Stress Ancillary Studies.
Figure 4.
Salivary cortisol curve from 0 to 3 h in MESA Stress I. Adapted, with permission, from Champaneri et al.79
We next examined the association of diurnal cortisol curve features with measures of glycemia and insulin resistance among 850 participants with and without diabetes, using data from MESA Stress Exam II.80 Among those with diabetes mellitus, early-decline slope, overall-decline slope, bedtime cortisol, and AUC cortisol were significantly and positively associated with a 5.4% (95% CI: 1.3, 9.7), 54.7% (95% CI: 12.4, 112.9), 4% (95% CI: 1.6, 6.4), and 6.8% (95% CI: 3.3,10.4) higher hemoglobin A1c (HbA1c) per 1-unit increase in log cortisol feature, respectively. These findings indicate that an overall flattening of the cortisol curve and increased total daily cortisol exposure are associated with worsened glycemia in the presence of diabetes. We hypothesized a cyclical relationship between subclinical hypercortisolism and glycemia, with hyperglycemia promoting HPA axis dysfunction and hypercortisolism promoting hyperglycemia (Fig. 5). There was no association of HbA1c with cortisol curve features in participants without diabetes; however, in this population there was a significant inverse association of cortisol curve features with insulin resistance, estimated using homeostatic model assessment of insulin resistance (HOMA-IR). Specifically, higher wake-up cortisol and AUC cortisol were associated with an 8.2% lower (95% CI: –13.3, –2.7) and 7.9% lower (95% CI: –14.6, –0.6) log HOMA-IR, respectively. We postulate that a higher wake-up cortisol contributing to a higher total AUC cortisol represents normal HPA axis plasticity in normoglycemic individuals without central adiposity. Previous studies have shown that a higher wake-up cortisol is indicative of improved HPA reactivity and lower odds of metabolic disturbances, including central obesity,81–83 generalized obesity,82,83 and metabolic syndrome.84 The association of lower HOMA-IR with higher wake-up cortisol and total AUC cortisol was fully blunted in a model including waist circumference, a correlate for visceral adiposity and a known mediator of insulin85 and leptin resistance.86 This suggests that, as individuals gain visceral adiposity, they may lose the beneficial effect of the interaction between cortisol and the adipoinsular axis and succumb to the detrimental effects of cortisol, including insulin resistance, lipolysis, and glycogenolysis, leading to worsening hyperglycemia, as seen in participants with diabetes mellitus in this study.80
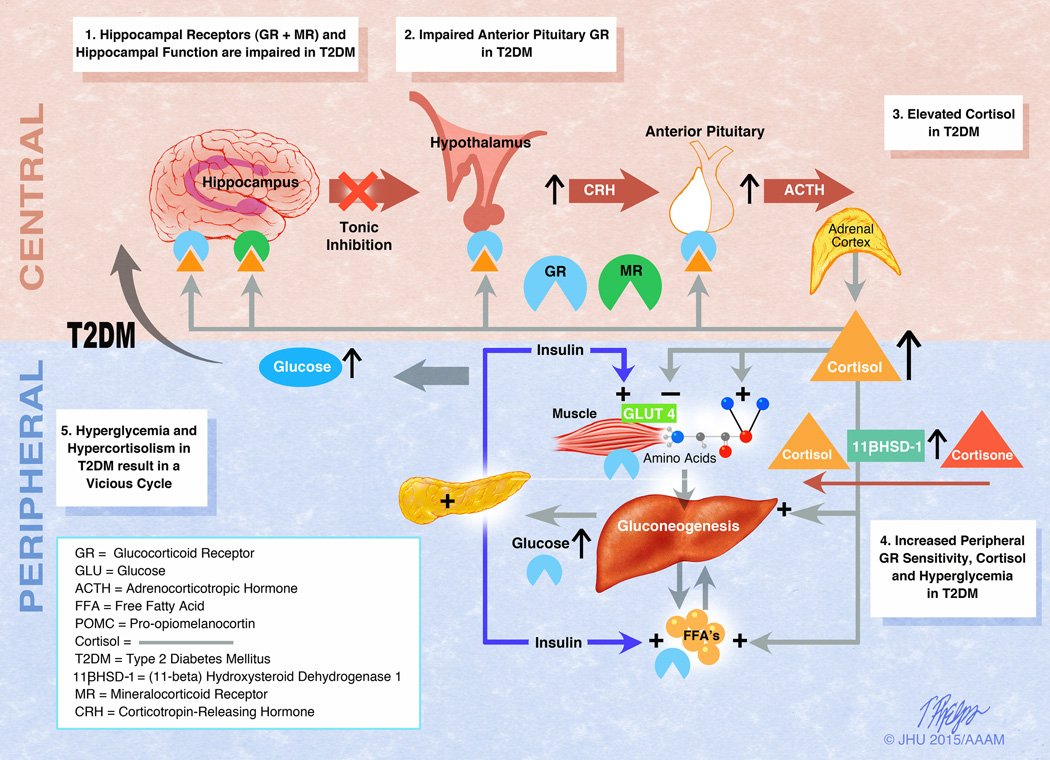
Figure 5.
The hypothetical relationship between subclinical hypercortisolism and glycemia in diabetes. (1) Hyperglycemia in diabetic mice leads to decreased hippocampal function owing to astrogliosis and neuronal apoptosis.93 There is also evidence of decreased mineralocorticoid receptor–mediated stress responsivity in diabetic mice94 and decreased glucocorticoid receptor levels in sedentary mice.95 These processes lead to hippocampal atrophy, which has been seen in humans75 and a reduction in inhibition of hypothalamic activity by the hippocampus. (2) Human studies have shown decreased negative feedback via the dexamethasone suppression test in diabetes, suggesting abnormalities at the level of the anterior pituitary.75,96 Glucocorticoid receptor dysfunction at the level of the anterior pituitary results in increased production of ACTH, which acts on the zona fasciculata of the adrenal gland. (3) Findings of elevated cortisol throughout the day in diabetes in women have been reported in a multi-ethnic study97 and in both sexes in a study of Caucasians.77 (4) In the periphery, there is increased basal glucocorticoid receptor sensitivity in diabetes, which is associated with poorer glucose control92 and a lack of stress-induced modulation of glucocorticoid receptor sensitivity in diabetes mellitus.98 Peripheral cortisol synthesis from conversion of cortisone to cortisol is increased due to whole-body and liver cortisol regeneration by 11 β-hydroxysteroid dehydrogenase type 1 in diabetes with obesity.99 Blocking the glucocorticoid receptor with mifepristone, or selective glucocorticoid receptor II antagonists specific to liver or adipose tissue, decrease glucose levels in diabetes.100,101 In addition, glucose transporter type 4 (GLUT4) translocation is decreased.90 These data indicate that cortisol does have an effect of peripheral glycemia in diabetes. (5) Individually and collectively, these processes may lead to worsening hyperglycemia and further blunting of the HPA axis, creating a vicious cycle. Reprinted, with permission, from Joseph et al.80
Given that cross-sectional associations do not provide evidence on the temporality of the association, we studied the effect of baseline diabetes status on cortisol curves over 6 years, using data from MESA Stress I and MESA Stress II in 90 participants with diabetes and 490 participants without diabetes who attended both exams.87 In this novel longitudinal study examining the association of diabetes status with long-term changes in daily cortisol curve features, we did not identify any statistically significant differences in change in cortisol curve features by diabetes status, suggesting that either larger studies with longer follow-up are needed or that the direction of the association is toward HPA axis dysfunction leading to the development of diabetes. The latter hypothesis was tested recently in the Whitehall II study.88 Among 3270 participants, 210 developed diabetes over 9 years, with higher evening cortisol levels predicting new-onset diabetes mellitus (odds ratio (OR) = 1.18; 95% CI: 1.01–1.37) and a trend for a flatter slope at baseline in participants with incident diabetes (OR = 1.15; 95% CI, 0.99–1.33).88 The cortisol curve features related to incident diabetes are similar to the features related to prevalent diabetes in the Whitehall II study and MESA Stress and provide evidence that a blunted HPA axis profile may lead to the development of diabetes. However, the longitudinal relationship between baseline cortisol and the development of diabetes has not been investigated in a multiethnic cohort, and additional studies are needed to confirm the findings from the Whitehall II study.
In conclusion, diabetes and worsened glycemia in diabetes is associated with a flatter diurnal cortisol curve, with evidence of a blunted CAR and flatter slope across the day. Similarly, among those without diabetes, higher morning cortisol and total AUC cortisol are associated with lower insulin resistance, which was blunted with the inclusion of waist circumference, and the development of diabetes is associated with a flatter diurnal cortisol curve and higher bedtime cortisol. These results suggest that a more dynamic diurnal cortisol profile with higher morning cortisol, a steeper slope throughout the day, and a low bedtime cortisol may be protective for the development of diabetes.
Mechanistic links: perturbation of the diurnal cortisol curve, depression, and diabetes
Clinical hypercortisolism (Cushing’s disease or syndrome) leads to the development of type 2 diabetes mellitus in one-third of affected individuals by inducing visceral adiposity, activating lipolysis with free fatty acid release, skeletal muscle insulin resistance, decreasing insulin secretion, and increasing hepatic glucose production.89,90 There are changes in the HPA axis with neuropsychiatric conditions, including depression—a relationship that is bidirectional, as hypercortisolism can also lead to neuropsychiatric disease, evidenced by the high prevalence of MDD (50–81%), anxiety (66%), and bipolar disorders (30%) in Cushing’s syndrome.90 Chronic exposure to high cortisol levels leads to structural and functional changes in various glucocorticoid receptor–rich brain regions fundamental for emotional and cognitive function, including the hippocampus, amygdala, and prefrontal cortex.90
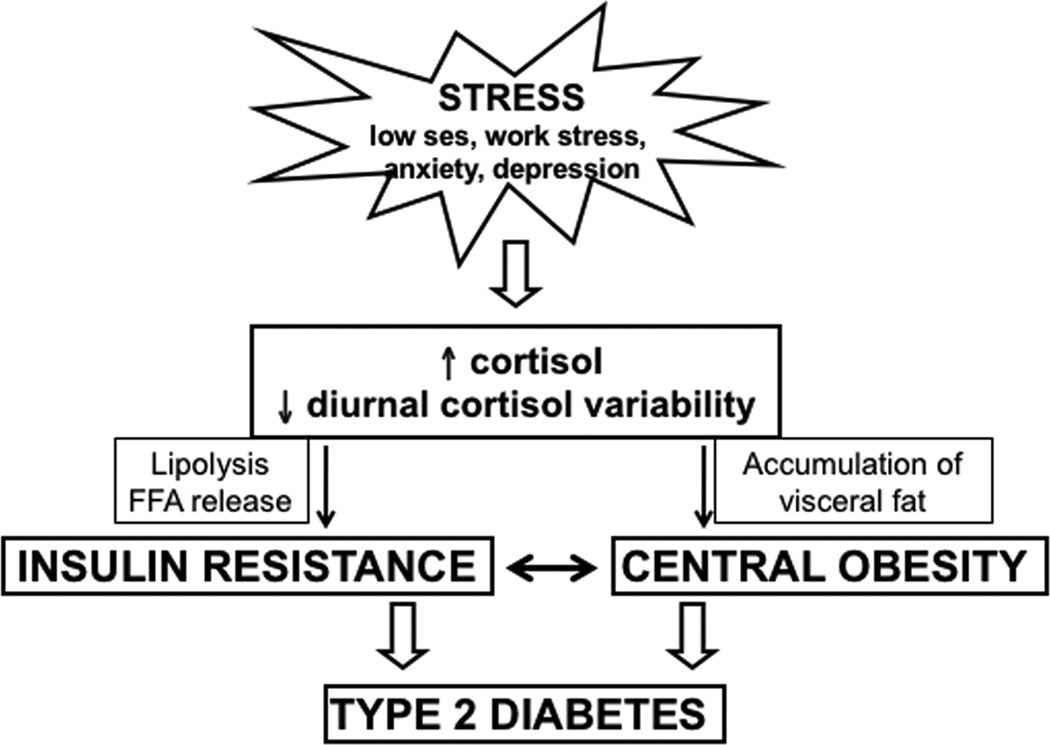
The larger question is how smaller perturbations in cortisol, specifically subclinical hypercortisolism that occurs more commonly in states of stress and depression, lead to type 2 diabetes mellitus. In states of subclinical hypercortisolism, similar pathophysiology occurs with accumulation of visceral fat through promotion of differentiation and proliferation of adipocytes, fat redistribution from peripheral to central depots, increasing the size and number of adipocytes, lipolysis and release of free fatty acids, and glucometabolic disturbances, including insulin resistance.89 These effects are likely mediated through glucocorticoid receptors that are more abundant on visceral than subcutaneous adipose tissue.89 Thus, because cortisol leads to visceral adiposity and insulin resistance (metabolic precursors to diabetes), subclinical hypercortisolism may provide an additional biological explanatory link between depression and type 2 diabetes mellitus (Fig. 6).39
Figure 6.
Mechanistic links between perturbation of the diurnal cortisol curve and diabetes.
Conclusions
Depression has a clear association with both prevalent and incident diabetes. The link between measures of stress and diabetes is less clear, possibly because of differences in measurements of stress and varying effects of different forms of stress on the HPA axis. We hypothesize that differences in allostatic load between chronic stress and depression may exist, with depression representing a state of greater mental wear and tear and, thus, HPA axis dysfunction. One could also consider a gradation of risk from small levels of consistent acute stress, to generalized chronic stress, to depression with greater burden, as an individual’s psychological load progresses. We hypothesize that HPA axis dysregulation is an important biological link between stress, depression, and diabetes. Depression is associated with a flatter or blunted diurnal cortisol curve, which is relatively preserved across the life span. Blunting of the diurnal cortisol curve is an important feature for cardiometabolic risk and has specifically predicted incident diabetes88 and higher HbA1c in individuals with diabetes.87 A blunted cortisol curve can be mediated by a lower wake-up cortisol, a decreased CAR, or higher bedtime cortisol, as revealed by studies of the HPA axis and diabetes.79,87,88 Thus, we consider HPA axis dysregulation to be a critical link in the high prevalence of co-existent depression and diabetes. Establishing the temporality of the association will require further study and is potentially bidirectional. Recently, two studies have made initial inroads in understanding the association of stress, allostatic load, and diabetes. First, a study of 140 diabetic men and women and 280 nondiabetic matched controls measured markers of allostatic load and cortisol in response to a standardized mental stressor.91 Participants with diabetes showed impaired post-stress recovery in systolic and diastolic blood pressure, heart rate, and cholesterol, and blunted stress reactivity in systolic blood pressure, cortisol, cholesterol, and interleukin-6. Salivary cortisol output was 36% higher in the diabetic compared to control groups, with significant differences in cortisol samples on waking, at 1600–1630, and at 2000–2030, consistent with a flattening of the diurnal cortisol curve. Diabetic persons reported greater depressive symptoms and higher stress than did healthy controls in this study.91 A second study of psychophysiological stress in 37 diabetic patients and 37 healthy controls revealed that both glucocorticoid and mineralocorticoid sensitivity decreased in healthy controls, but did not change in people with diabetes.92 Participants with diabetes showed blunted stress responsivity in systolic blood pressure, diastolic blood pressure, heart rate, interleukin-6, monocyte chemoattractant protein-1, and impaired post-stress recovery in heart rate. Participants with diabetes had higher cortisol levels, as measured by the total amount excreted over the day and increased glucocorticoid sensitivity at baseline. This study suggests that impaired stress responsivity in type 2 diabetes is, in part, due to a lack of stress-induced changes in mineralocorticoid and glucocorticoid sensitivity.92 The authors surmised that diabetes is characterized by disruption of stress-related processes across multiple biological systems and increased exposure to life stress, with chronic allostatic load providing a unifying theory.91 These two studies represent important steps in the journey toward fully understanding the relationships between stress, depression, allostatic load, and diabetes, which will be further elucidated in the coming years in longitudinal studies of multiethnic individuals. Once the appropriate biological targets are identified, interventions directed at the HPA axis may improve outcomes in both depression and diabetes.
Acknowledgments
The authors thank the other investigators, staff, and participants of MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. MESA was supported by contracts NO1-HC-95159 through NO1-HC-95165 and NO1-HC-95169 from the National Heart, Lung, and Blood Institute. MESA Stress was supported by RO1 HL10161-01A1 and R21 DA024273 (Principal Investigator: Ana Diez Roux). Joshua J. Joseph was supported by an institutional training grant from the National Institute of Diabetes, Digestive, and Kidney Diseases (T32 DK062707).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Carlson ED, Chamberlain RM. Allostatic load and health disparities: A theoretical orientation. Res. Nurs. Health. 2005;28:306–315. doi: 10.1002/nur.20084. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol. Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 4.Chrousos GP. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 5.Flier JS, Underhill LH, McEwen BS. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 6.Seeman TE, Singer BH, Ryff CD, Love GD, Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosom. Med. 2002;64:395–406. doi: 10.1097/00006842-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Mair CA, Cutchin MP, Kristen Peek M. Allostatic load in an environmental riskscape: The role of stressors and gender. Health Place. 2011;17:978–987. doi: 10.1016/j.healthplace.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golden SH, Wand GS, Malhotra S, Kamel I, Horton K. Reliability of hypothalamic–pituitary–adrenal axis assessment methods for use in population-based studies. Eur. J. Epidemiol. 2011;26:511–525. doi: 10.1007/s10654-011-9585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Champaneri S, Wand GS, Malhotra SS, Casagrande SS, Golden SH. Biological Basis of Depression in Adults with Diabetes. Curr. Diab. Rep. 2010;10:396–405. doi: 10.1007/s11892-010-0148-9. [DOI] [PubMed] [Google Scholar]
- 10.Shomaker LB, et al. Longitudinal Study of Depressive Symptoms and Progression of Insulin Resistance in Youth at Risk for Adult Obesity. Diabetes Care. 2011;34:2458–2463. doi: 10.2337/dc11-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan SS, Clavarino AM, Mamun AA, Kairuz T. Incidence and risk of diabetes mellitus associated with depressive symptoms in adults: Evidence from longitudinal studies. Diabetes Metab. Syndr. Clin. Res. Rev. 2014;8:82–87. doi: 10.1016/j.dsx.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and Risk for Onset of Type II Diabetes: A Prospective Population-Based Study. Diabetes Care. 1996;19:1097–1102. doi: 10.2337/diacare.19.10.1097. [DOI] [PubMed] [Google Scholar]
- 13.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and Type 2 Diabetes Over the Lifespan: A meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carnethon MR, Kinder LS, Fair JM, Stafford RS, Fortmann SP. Symptoms of Depression as a Risk Factor for Incident Diabetes: Findings from the National Health and Nutrition Examination Epidemiologic Follow-up Study, 1971–1992. Am. J. Epidemiol. 2003;158:416–423. doi: 10.1093/aje/kwg172. [DOI] [PubMed] [Google Scholar]
- 15.Golden SH, et al. Examining a bidirectional association between depressive symptoms and diabetes. Jama. 2008;299:2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. 2001;24:1069. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 17.Katon W, et al. Behavioral and Clinical Factors Associated With Depression Among Individuals With Diabetes. Diabetes Care. 2004;27:914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 18.Lustman PJ, et al. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23:934. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 19.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch. Intern. Med. 2000;160:3278. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 20.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26:2822. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 21.De Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom. Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen. Hosp. Psychiatry. 2003;25:246–252. doi: 10.1016/s0163-8343(03)00055-0. [DOI] [PubMed] [Google Scholar]
- 23.Lustman PJ, et al. Effects of Nortriptyline on Depression and Glycemic Control in Diabetes: Results of a Double-Blind, Placebo-Controlled Trial. Psychosom. Med. 1997;59:241–250. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus. Ann. Intern. Med. 1998;129:613. doi: 10.7326/0003-4819-129-8-199810150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Fluoxetine for Depression in Diabetes: A Randomized Double-Blind Placebo-Controlled Trial. Diabetes Care. 2000;23:618–623. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- 26.Katon WJ, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch. Gen. Psychiatry. 2004;61:1042. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Jama. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 28.Cosgrove MP, Sargeant LA, Caleyachetty R, Griffin SJ. Work-related stress and Type 2 diabetes: systematic review and meta-analysis. Occup. Med. 2012;62:167–173. doi: 10.1093/occmed/kqs002. [DOI] [PubMed] [Google Scholar]
- 29.Chida Y, Hamer M. An association of adverse psychosocial factors with diabetes mellitus: a meta-analytic review of longitudinal cohort studies. Diabetologia. 2008;51:2168–2178. doi: 10.1007/s00125-008-1154-1. [DOI] [PubMed] [Google Scholar]
- 30.Heraclides A, Chandola T, Witte DR, Brunner EJ. Psychosocial Stress at Work Doubles the Risk of Type 2 Diabetes in Middle-Aged Women: Evidence from the Whitehall II Study. Diabetes Care. 2009;32:2230–2235. doi: 10.2337/dc09-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato M, et al. Psychological factors, coffee and risk of diabetes mellitus among middle-aged Japanese: a population-based prospective study in the JPHC study cohort. Endocr. J. 2009;56:459–468. doi: 10.1507/endocrj.k09e-003. [DOI] [PubMed] [Google Scholar]
- 32.Rod NH, Grønbaek M, Schnohr P, Prescott E, Kristensen TS. Perceived stress as a risk factor for changes in health behaviour and cardiac risk profile: a longitudinal study. J. Intern. Med. 2009;266:467–475. doi: 10.1111/j.1365-2796.2009.02124.x. [DOI] [PubMed] [Google Scholar]
- 33.Williams ED, Magliano DJ, Tapp RJ, Oldenburg BF, Shaw JE. Psychosocial Stress Predicts Abnormal Glucose Metabolism: The Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Ann. Behav. Med. 2013;46:62–72. doi: 10.1007/s12160-013-9473-y. [DOI] [PubMed] [Google Scholar]
- 34.Novak M, et al. Perceived stress and incidence of Type 2 diabetes: a 35-year follow-up study of middle-aged Swedish men. Diabet. Med. 2013;30:e8–e16. doi: 10.1111/dme.12037. [DOI] [PubMed] [Google Scholar]
- 35.Virtanen M, et al. Psychological Distress and Incidence of Type 2 Diabetes in High-Risk and Low-Risk Populations: The Whitehall II Cohort Study. Diabetes Care. 2014;37:2091–2097. doi: 10.2337/dc13-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mommersteeg PM, Herr R, Zijlstra WP, Schneider S, Pouwer F. Higher levels of psychological distress are associated with a higher risk of incident diabetes during 18 year follow-up: results from the British household panel survey. BMC Public Health. 2012;12:1109. doi: 10.1186/1471-2458-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebreab SY, et al. The contribution of stress to the social patterning of clinical and subclinical CVD risk factors in African Americans: The Jackson Heart Study. Soc. Sci. Med. 2012;75:1697–1707. doi: 10.1016/j.socscimed.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stetler C, Miller GE. Depression and Hypothalamic-Pituitary-Adrenal Activation: A Quantitative Summary of Four Decades of Research: Psychosom. Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- 39.Golden SH. A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr. Diabetes Rev. 2007;3:252–259. doi: 10.2174/157339907782330021. [DOI] [PubMed] [Google Scholar]
- 40.Dietrich A, et al. Cortisol in the morning and dimensions of anxiety, depression, and aggression in children from a general population and clinic-referred cohort: An integrated analysis. The TRAILS study. Psychoneuroendocrinology. 2013;38:1281–1298. doi: 10.1016/j.psyneuen.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Dienes KA, Hazel NA, Hammen CL. Cortisol secretion in depressed, and at-risk adults. Psychoneuroendocrinology. 2013;38:927–940. doi: 10.1016/j.psyneuen.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adam EK, et al. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carnegie R, et al. Cortisol awakening response and subsequent depression: prospective longitudinal study. Br. J. Psychiatry. 2014;204:137–143. doi: 10.1192/bjp.bp.113.126250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sjögren E, Leanderson P, Kristenson M. Diurnal saliva cortisol levels and relations to psychosocial factors in a population sample of middle-aged Swedish men and women. Int. J. Behav. Med. 2006;13:193–200. doi: 10.1207/s15327558ijbm1303_2. [DOI] [PubMed] [Google Scholar]
- 45.Vreeburg SA, et al. Major Depressive Disorder and Hypothalamic-Pituitary-Adrenal Axis Activity: Results From a Large Cohort Study. Arch. Gen. Psychiatry. 2009;66:617. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 46.Knorr U, Vinberg M, Kessing LV, Wetterslev J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology. 2010;35:1275–1286. doi: 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, Burke HM. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol. Psychol. 2013;93:150–158. doi: 10.1016/j.biopsycho.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grynderup MB, et al. A two-year follow-up study of salivary cortisol concentration and the risk of depression. Psychoneuroendocrinology. 2013;38:2042–2050. doi: 10.1016/j.psyneuen.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Vammen MA, et al. Salivary cortisol and depression in public sector employees: Cross-sectional and short term follow-up findings. Psychoneuroendocrinology. 2014;41:63–74. doi: 10.1016/j.psyneuen.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Vreeburg SA, et al. Salivary cortisol levels and the 2-year course of depressive and anxiety disorders. Psychoneuroendocrinology. 2013;38:1494–1502. doi: 10.1016/j.psyneuen.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 51.Lok A, et al. Longitudinal hypothalamic–pituitary–adrenal axis trait and state effects in recurrent depression. Psychoneuroendocrinology. 2012;37:892–902. doi: 10.1016/j.psyneuen.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Bockting CLH, et al. Lower cortisol levels predict recurrence in remitted patients with recurrent depression: A 5.5 year prospective study. Psychiatry Res. 2012;200:281–287. doi: 10.1016/j.psychres.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 53.Hardeveld F, et al. Increased cortisol awakening response was associated with time to recurrence of major depressive disorder. Psychoneuroendocrinology. 2014;50:62–71. doi: 10.1016/j.psyneuen.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 54.O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am. J. Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 55.Kohler S, et al. White matter hyperintensities, cortisol levels, brain atrophy and continuing cognitive deficits in late-life depression. Br. J. Psychiatry. 2010;196:143–149. doi: 10.1192/bjp.bp.109.071399. [DOI] [PubMed] [Google Scholar]
- 56.Belvederi Murri M, et al. HPA axis and aging in depression: Systematic review and meta-analysis. Psychoneuroendocrinology. 2014;41:46–62. doi: 10.1016/j.psyneuen.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Rhebergen D, et al. Hypothalamic–pituitary–adrenal axis activity in older persons with and without a depressive disorder. Psychoneuroendocrinology. 2015;51:341–350. doi: 10.1016/j.psyneuen.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Dedovic K, Ngiam J. The cortisol awakening response and major depression: examining the evidence. Neuropsychiatr. Dis. Treat. 2015:1181. doi: 10.2147/NDT.S62289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hinkelmann K, et al. Changes in cortisol secretion during antidepressive treatment and cognitive improvement in patients with major depression: A longitudinal study. Psychoneuroendocrinology. 2012;37:685–692. doi: 10.1016/j.psyneuen.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 60.McKay MS, Zakzanis KK. The impact of treatment on HPA axis activity in unipolar major depression. J. Psychiatr. Res. 2010;44:183–192. doi: 10.1016/j.jpsychires.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 61.Zobel AW, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J. Psychiatr. Res. 2000;34:171–181. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 62.Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: Pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 64.Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 65.Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Steffen PR, McNeilly M, Anderson N, Sherwood A. Effects of Perceived Racism and Anger Inhibition on Ambulatory Blood Pressure in African Americans: Psychosom. Med. 2003;65:746–750. doi: 10.1097/01.psy.0000079380.95903.78. [DOI] [PubMed] [Google Scholar]
- 67.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 68.Suglia SF, et al. Cumulative stress and cortisol disruption among Black and Hispanic pregnant women in an urban cohort. Psychol. Trauma Theory Res. Pract. Policy. 2010;2:326–334. doi: 10.1037/a0018953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol. Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 70.Rudolph KE, et al. Job Strain and the Cortisol Diurnal Cycle in MESA: Accounting for Between- and Within-Day Variability. Am. J. Epidemiol. 2016;183:497–506. doi: 10.1093/aje/kwv280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao J, Brunner EJ, Kumari M. Is There an Association between Work Stress and Diurnal Cortisol Patterns? Findings from the Whitehall II Study. PLoS ONE. 2013;8:e81020. doi: 10.1371/journal.pone.0081020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Dev. Psychopathol. 2011;23:1167–1186. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuller-Rowell TE, Doan SN, Eccles JS. Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology. 2012;37:107–118. doi: 10.1016/j.psyneuen.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeiders KH, Hoyt LT, Adam EK. Associations between self-reported discrimination and diurnal cortisol rhythms among young adults: The moderating role of racial–ethnic minority status. Psychoneuroendocrinology. 2014;50:280–288. doi: 10.1016/j.psyneuen.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruehl H, Wolf OT, Convit A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology. 2009;34:815–821. doi: 10.1016/j.psyneuen.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lederbogen F, et al. Flattened Circadian Cortisol Rhythm in Type 2 Diabetes. Exp. Clin. Endocrinol. Amp Diabetes. 2011;119:573–575. doi: 10.1055/s-0031-1275288. [DOI] [PubMed] [Google Scholar]
- 77.Hackett RA, Steptoe A, Kumari M. Association of diurnal patterns in salivary cortisol with type 2 diabetes in the Whitehall II study. J. Clin. Endocrinol. Metab. 2014 doi: 10.1210/jc.2014-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bild DE. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am. J. Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 79.Champaneri S, et al. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2012;61:986–995. doi: 10.1016/j.metabol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joseph JJ, et al. Diurnal salivary cortisol, glycemia and insulin resistance: The multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2015;62:327–335. doi: 10.1016/j.psyneuen.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duclos M, Pereira PM, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes. Res. 2005;13:1157–1166. doi: 10.1038/oby.2005.137. [DOI] [PubMed] [Google Scholar]
- 82.Champaneri S, et al. Diurnal salivary cortisol is associated with body mass index and waist circumference: The multiethnic study of atherosclerosis. Obesity. 2013;21:E56–E63. doi: 10.1002/oby.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumari M, Chandola T, Brunner E, Kivimaki M. A Nonlinear Relationship of Generalized and Central Obesity with Diurnal Cortisol Secretion in the Whitehall II Study. J. Clin. Endocrinol. Metab. 2010;95:4415–4423. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeSantis AS, et al. Associations of Salivary Cortisol Levels with Metabolic Syndrome and Its Components: The Multi-Ethnic Study of Atherosclerosis. J. Clin. Endocrinol. Metab. 2011;96:3483–3492. doi: 10.1210/jc.2011-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 86.Sáinz N, Barrenetxe J, Moreno-Aliaga MJ, Martínez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64:35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 87.Spanakis EK, et al. Lack of significant association between type 2 diabetes mellitus with longitudinal change in diurnal salivary cortisol: the multiethnic study of atherosclerosis. Endocrine. 2016 doi: 10.1007/s12020-016-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hackett RA, Kivimäki M, Kumari M, Steptoe A. Diurnal Cortisol Patterns, Future Diabetes, and Impaired Glucose Metabolism in the Whitehall II Cohort Study. J. Clin. Endocrinol. Metab. 2016;101:619–625. doi: 10.1210/jc.2015-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. The Pathogenetic Role of Cortisol in the Metabolic Syndrome: A Hypothesis. J. Clin. Endocrinol. Metab. 2009;94:2692–2701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 90.Pivonello R, et al. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 2016 doi: 10.1016/S2213-8587(16)00086-3. [DOI] [PubMed] [Google Scholar]
- 91.Steptoe A, et al. Disruption of multisystem responses to stress in type 2 diabetes: Investigating the dynamics of allostatic load. Proc. Natl. Acad. Sci. 2014;111:15693–15698. doi: 10.1073/pnas.1410401111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carvalho LA, et al. Blunted glucocorticoid and mineralocorticoid sensitivity to stress in people with diabetes. Psychoneuroendocrinology. 2015;51:209–218. doi: 10.1016/j.psyneuen.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stranahan AM, et al. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chan O. Hyperglycemia does not increase basal hypothalamo-pituitary-adrenal activity in diabetes but it does impair the HPA response to insulin-induced hypoglycemia. AJP Regul. Integr. Comp. Physiol. 2005;289:R235–R246. doi: 10.1152/ajpregu.00674.2004. [DOI] [PubMed] [Google Scholar]
- 95.Campbell JE, et al. Regular exercise prevents the development of hyperglucocorticoidemia via adaptations in the brain and adrenal glands in male Zucker diabetic fatty rats. AJP Regul. Integr. Comp. Physiol. 2010;299:R168–R176. doi: 10.1152/ajpregu.00155.2010. [DOI] [PubMed] [Google Scholar]
- 96.Hudson JI, et al. Abnormal results of dexamethasone suppression tests in nondepressed patients with diabetes mellitus. Arch. Gen. Psychiatry. 1984;41:1086–1089. doi: 10.1001/archpsyc.1983.01790220076012. [DOI] [PubMed] [Google Scholar]
- 97.Champaneri S, et al. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2012;61:986–995. doi: 10.1016/j.metabol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rohleder N, Wolf JM, Kirschbaum C. Glucocorticoid Sensitivity in Humans-Interindividual Differences and Acute Stress Effects. Stress Int. J. Biol. Stress. 2003;6:207–222. doi: 10.1080/1025389031000153658. [DOI] [PubMed] [Google Scholar]
- 99.Stimson RH, et al. Increased Whole-Body and Sustained Liver Cortisol Regeneration by 11 -Hydroxysteroid Dehydrogenase Type 1 in Obese Men With Type 2 Diabetes Provides a Target for Enzyme Inhibition. Diabetes. 2011;60:720–725. doi: 10.2337/db10-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Watts LM, et al. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes. 2005;54:1846–1853. doi: 10.2337/diabetes.54.6.1846. [DOI] [PubMed] [Google Scholar]
- 101.Beaudry JL, et al. Effects of Selective and Non-Selective Glucocorticoid Receptor II Antagonists on Rapid-Onset Diabetes in Young Rats. PLoS ONE. 2014;9:e91248. doi: 10.1371/journal.pone.0091248. [DOI] [PMC free article] [PubMed] [Google Scholar]