Abstract
Two different methods for single step transesterification from pig meat without fat extraction have been tested. Freeze-drying of the meat with and without anhydrous salt, followed by a base-catalyzed transmethylation (KOH/MeOH) was carried out. Both methods were compared with the standard Folch procedure of fat extraction followed by transmethylation. The methods were tested on a complete sample set of biceps femoris of pig thigh, used for the production of dry-cured ham. The set was divided in three subgroups according to total fat content. Both derivatization protocols on freeze-dried pork muscle were proven to be a valid alternative to the Folch procedure for FAME analysis. Freeze-drying method offered several advantages in comparison with the Folch procedure, including a lower solvent requirement, and process temperature, as well as considerable saving of time. In freeze-drying, the addition of an anhydrous salt (Na2SO4) gave more friable samples which resulted in higher yields for some fatty acids, particularly evident in the case of tissues with high lipid content.
Keywords: Fatty acids analysis, Meat lipids, Single step transesterification, Ultra Fast GC
Introduction
Fatty acid (FA) composition determines physical properties, stability, and nutritional value of lipids. Variations in plant and animal lipid FA composition help determine the origin of the lipids. The profile of total fatty acids is considered as a kind of “identity card”, particularly for phospholipids, which are less prone to changes due to their function (Grahl-Nielsen 1999; Joensen and Grahl-Nielsen 2001).
FA composition of the pig adipose tissue and lean cuts has a great importance for meat quality control and it provides information about animal feeding and the history of the product (Wood et al. 2008). Moreover, the composition of depot and intramuscular fat reflects the diet lipid composition in monogastric animals.
In pigs, linoleic and linolenic acids are derived entirely from the diet, and they are metabolized in the liver to produce two families of polyunsaturated fatty acids (PUFA), which are specific to animals, respectively, the n-6 and n-3 series (Nieto and Ros 2012). Changes in the FA composition of pig muscle fat induced by diet may increase the susceptibility of FAs to oxidation to a higher extent than it would be expected by the kind of alteration (Kolakowska et al. 1998).
High saturated FA (SFA) intake contributes to increase the LDL-cholesterol level, which was reported to be positively related to the occurrence of heart diseases (Nieto and Ros 2012). However, some monounsaturated FAs (MUFA) and PUFA, in particular long-chain n-3 PUFA reported to have favourable effects on human health (Christie 2003). A higher n-3:n-6 PUFA ratio is recommended (Wood et al. 2004).
In Italy, as well as in other European countries (such as Spain), one of the main destinations of pig thigh is cured ham. For this reason, fatty acids composition of thigh lipids was assessed in detail by different studies (Lo Fiego 1996; Lo Fiego et al. 2005, 2010; Rossi and Corino 2002). FAs are generally analyzed after derivatization into fatty acid methyl esters (FAMEs). Fat extraction, FAs fractioning followed by derivatization (FAME production), and finally GC analysis are the main steps of the procedure. Short narrow-bore columns in combination with fast GC systems are able to give perfect separations of complex FAME mixtures in a few minutes (Ficarra et al. 2010). However, the full exploitation of these systems requires a method that could bypass fat extraction, that is normally carried out by either Folch method (Folch et al. 1957) modified by Boselli et al. (2001) or by Association of Official Analytical Chemists recommended methods (AOAC 2012) based on the use of Soxhlet apparatus. Both methods are solvent and time consuming and the operator has to assist the whole extraction procedure.
The opportunity to bypass fat extraction was verified in several studies on different matrices, such as dairy products and other foodstuffs (Destaillats et al. 2007; Golay et al. 2006; Grob and Suter 2000; Suter et al. 1997a, b, 1999). In literature, different alternative methods for lipid extraction are reported. Supercritical fluid extraction was successfully tested for lipid extraction, yielding good recoveries of nonpolar lipids (List et al. 1989). The main advantages are: high quality and purity of the obtained product; quick extraction and separation phases; low consumption of hazardous organic solvents; extract free of residues; selective extraction of a specific compound; reduction in separation cost; less damage to the fatty acids due to low extraction temperature (Raventós et al. 2002; Sahena et al. 2009). The method was applied on different meat matrices (Berg et al. 1997; Merkle and Larick 1995; Taylor and Larick 1995). However, an expensive extraction equipment is required.
Accelerated solvent extraction (ASE) led to high extraction efficiency using common solvents at elevated temperatures and pressures. ASE technology was applied to fish (Dodds et al. 2004), plant and animal tissues (Schäfer 1998). Also in this case, an expensive and dedicated equipment is required.
On the contrary, Ficarra et al. (2013) have set up a fast procedure based on tools commonly available in many food laboratories. In fact, the method relies on meat freeze-drying process prior direct transmethylation with methanolic KOH. This procedure was successfully applied to meat samples with different amount of intramuscular fat. The method was reliable and performed comparably with Folch method. It showed several advantages, such as bypassing the fat extraction, the need of high amount of solvent and its disposal.
Base-catalyzed methylation requires anhydrous condition (Christie 1993). Anhydrous Na2SO4 is largely used for water removal from organic solutions. Therefore, the presence of a desiccant salt during transmethylation reduces the risk of hydrolysis due to traces of water from the environment that can reduce the reaction yield.
The aim of this study was to compare the method set up by Ficarra et al. (2013) on a complete samples set of biceps femoris (BF) from pig thighs, with or without a protocol modification based on the addition of anhydrous salt during freeze-drying process, with the standard Folch procedure modified by Boselli et al. (2001).
Materials and methods
Sampling and total fat determination (Soxhlet extraction)
Samples were taken from biceps femoris (BF) of the pig thigh (destined to the production of dry-cured ham) of pigs (Sus scrofa domesticus L.) with different genetic types [(Italian landrace × Large White), and two hybrids: Hypor and Goland], male and female, and with a different amount of BF fat concentration. To obtain a representative sampling, the meat (about 7.00 g of each sample) was selected from different parts of the pig BF. The samples were ground and then stocked in a freezer at −20 °C.
Twelve samples were selected on the basis of their fat content (Table 1). To determine the total fat amount in BF, each sample was subjected to acid digestion and then extracted with the Soxhlet apparatus (AOAC 1990a; AOAC 1990b), using 3.00 g of ground BF. The extraction was carried out with petroleum ether for 6 h.
Table 1.
Characteristics of biceps femoris samples
| Sample label | Subgroup | Mean value inside the subgroup (g/100 g) | Genetic type | Sex |
|---|---|---|---|---|
| 1 | Low | 1.26 (±0.04) | 1 | M |
| 2 | 3 | M | ||
| 3 | 3 | F | ||
| 4 | 1 | M | ||
| 5 | Medium | 2.64 (±0.03) | 3 | F |
| 6 | 2 | F | ||
| 7 | 3 | M | ||
| 8 | 1 | M | ||
| 9 | High | 5.79 (±0.60) | 1 | F |
| 10 | 2 | M | ||
| 11 | 2 | M | ||
| 12 | 1 | M |
Mean values of lipid content (expressed as g/100 g ± standard deviation) of the subgroups of biceps femoris muscles from pigs with different genetic type [1, Italian landrace × Large White 3 (L × LW); 2, Hypor; 3, Goland], and sex (F, female; M, male)
Each sample was analysed in triplicate (12 × 3 = 36 analysis for each method).
Chemicals
All solvents and reagents were of analytical grade. Chloroform, petroleum ether (40–60 °C), methanol, n-hexane, and potassium hydroxide were obtained from Sigma-Aldrich® (Milan, Italy). Anhydrous Na2SO4 and KCl were purchased from Carlo Erba Reagents S.p.A. (Rodano, Milan, Italy).
Single FAME standards were purchased from Larodan Fine Chemicals AB (Malmö, Sweden), methyl nonadecanoate (C19:0; ≥99%) used as internal standard (100 uL for each sample) was purchased from Fluka (Milan, Italy) and prepared at a concentration of 1.00% (w/v) in hexane.
Methods of sample preparation
Lipid extraction: modified Folch procedure
Total intramuscular lipids extraction was operated as previously described (Ficarra et al. 2013), following the Folch procedure (Folch et al. 1957) modified by Boselli et al. (2001). Briefly, total intramuscular lipids were extracted from 1.00 g of fresh muscle (3 replicates) by adding 60 mL of a solution of chloroform/methanol (2:1) and by homogenizing for 3 min. The sample was heated for 20 min at 60 °C, then 60 mL of a solution of chloroform/methanol (2:1) was added and a second homogenization was carried out. After filtering with suction on a Büchner funnel, a KCl aqueous solution (34 mL; 0.88% w/v) was added to the sample, which was left to stand at −20 °C overnight. The organic phase was recovered and dehydrated with anhydrous Na2SO4 for 2 h at the same temperature. Finally, the organic phase was filtered and evaporated to dryness on a rotary evaporator (VV 2000; Heidolph, Kelheim, Germany) at 40 °C.
Meat freeze-drying
An aliquot of ground meat (1.00 g) was transferred in a 100 mm × 16 mm (i.d.) test tube with Teflon-lined cap (3 replicates for each sample). Tubes were frozen at −20 °C and then placed in a tube rack inside a desiccator. After connecting the desiccator to the freeze-drier (FreeZone 1L Labconco, Kansas City, MO, U.S.A.), samples were left up to a complete drying (24 h).
Meat freeze-drying with anhydrous Na2SO4
The procedure described in “Meat freeze-drying” section was carried out by adding 1.00 g of anhydrous Na2SO4 together with 1.00 g of ground meat before freeze-drying (3 replicates for each sample).
Preparation of fatty acid methyl esters (FAMEs) and analytical conditions
Preparation and sample analyses were carried out as previously described (Ficarra et al. 2010). A transesterification method was applied to the samples obtained with the different protocols for subsequent gas chromatography analysis. Strictly in this order, the sample with the addition of hexane (4 mL) and 100 µL of internal standard was mixed with an Ultra-Turrax (T25 basic, IKA-Werke, Staufen, Germany; 1 min at speed max). Then, KOH/MeOH solution (2 N; 0.4 mL) was added and the sample was mixed again with the Ultra-Turrax (1 min at speed max). After centrifugation, FAMEs were recovered in the upper phase.
FAMEs were separated using a TRACE™ GC Ultra (Thermo Electron Corporation, Rodano, Milano, Italy) equipped with the Ultra Fast Module (UFM), a Fast Flame Ionization Detector (FFID) and a UFM-Carbowax column, 5 m long, 0.1 mm i.d., 0.2 μm film thickness.
The temperature program ranged from 150 °C (held 10 s) to 240 °C (90 s, 102 °C/min) and held at 240 °C for 2.30 min. The injection (PVT split mode, 1 μL) was operated with a split ratio 1:150 and constant flow operating mode at 0.5 mL/min (helium as carrier gas). The injector and detector temperature were both set at 240 °C.
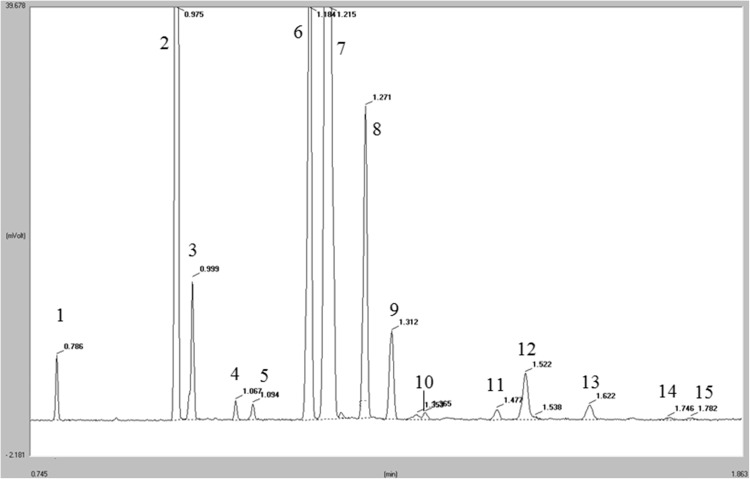
The identification of FAs was carried out by comparing the retention times with those of the reference pure standards, injected in the same conditions of the samples. GC trace of the standard mixture is showed in Fig. 1. The quantification was performed using the internal standard method. Chromatograms were acquired and processed by Chrom-Card software (ver. 2.3.3, Thermo Electron Corporation, Rodano, Italy).
Fig. 1.
GC trace of a standard mixture. 1: C14:0; 2: C16:0; 3: C16:1 (n-9); 4: C17:0; 5: C17:1 (n-9); 6: C18:0; 7: C18:1 (n-9); 8: C18:2 (n-6); 9: C19:0 (internal standard); 10: C18:3 (n-3); 11: C20:0; 12: C20:1 (n-9); 13: C20:2 (n-6); 14: C20:3 (n-6); 15: C20:4 (n-6)
Statistical analysis
Univariate and multivariate analyses were performed on the data set. Principal component analysis (PCA) of the autoscaled values was carried out to explore the whole data set. Differences between methods were assessed by analysis of variance (one-way ANOVA) based on three replicates for each sample. When a significant effect (at least p ≤ 0.05) was detected, comparative analyses were carried out using the post hoc Tukey’s multiple comparison test. All tests were performed using Statistica version 8.0 software (Stat 180 Soft Inc., Tulsa, OK, USA).
Results and discussion
To evaluate the effect of fat concentration and pork texture on the determination of FAs, samples were divided in three subgroups by means of their total lipid content: low (1.26% mean), medium (2.64% mean), and high (5.79% mean), as shown in Table 1. The division in subgroups also allows obtaining more homogeneous groups of samples in order to perform the data comparison.
Exploratory principal component analysis
The whole data set of autoscaled values was explored by Principal Component Analysis (PCA) to achieve preliminary but essential information about the homogeneity of the results across the different methods. Moreover, another aim was to evaluate if PCA could discriminate the different factors used for sampling. The first four principal components (PCs), all with eigenvalues >1.0, explained 86.53% of the total variance. All factors with eigenvalues <1.0 were discarded on the basis of Kaiser’s criterion (Kaiser 1958).
All FAs (except for PUFA) were strictly grouped and weighed on PC1 with negative sign (Table 2). PUFA (C20:3 (n-6) and C20:4 (n-6)) weighed on PC2 with negative sign, while sex mainly weighed on PC3. Finally, PC4 was mainly characterized by genetic type.
Table 2.
Loading values for the first four Principal Components
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| Genetic type | 0.30 | 0.15 | 0.57 | 0.70 |
| Sex | −0.18 | 0.18 | 0.89 | −0.18 |
| C14:0 | −0.96 | 0.06 | −0.02 | 0.14 |
| C16:0 | −0.98 | 0.06 | −0.04 | 0.11 |
| C16:1 (n-9) | −0.76 | 0.22 | −0.19 | 0.34 |
| C17:0 | −0.85 | −0.19 | 0.17 | −0.30 |
| C17:1 (n-9) | −0.91 | −0.07 | 0.13 | −0.24 |
| C18:0 | −0.98 | 0.03 | −0.11 | 0.06 |
| C18:1 (n-9) | −0.96 | 0.12 | −0.10 | 0.18 |
| C18:2 (n-6) | −0.92 | −0.07 | 0.02 | −0.08 |
| C18:3 (n-3) | −0.85 | 0.20 | −0.02 | −0.04 |
| C20:0 | −0.91 | −0.11 | 0.14 | 0.06 |
| C20:1 (n-9) | −0.94 | 0.12 | −0.12 | 0.20 |
| C20:2 (n-6) | −0.83 | −0.11 | 0.11 | −0.22 |
| C20:3 (n-6) | −0.22 | −0.77 | 0.23 | 0.09 |
| C20:4 (n-6) | −0.07 | −0.75 | −0.11 | 0.31 |
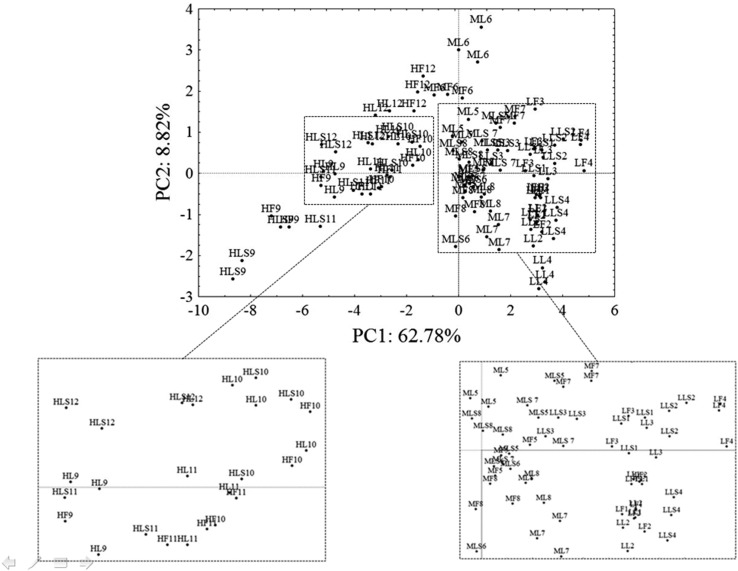
The different distribution of the samples on PC1 (62.78% of the total variance) was clearly identified as a consequence of the different fat content (the higher the fat, the lower the scores). However, the different protocols did not affect the distribution of the samples neither on the PC1 nor on the PC2 (Fig. 2).
Fig. 2.
PC1 versus PC2 score plot; samples extracted by Folch procedure, lyophylized samples, and lyophylized with anhydrous Na2SO4. LF low (lipid content) Folch; MF medium Folch; HF high Folch; LL low lyophylized; ML medium lyophylized; HL high lyophylized; LLS low lyophylized plus salt (anhydrous Na2SO4); MLS medium lyophylized plus salt (anhydrous Na2SO4); HLS high lyophylized plus salt (anhydrous Na2SO4)
On PC2 (8.82% of the total variance) the influence of PUFA was of crucial importance (Table 2). On the other hand, it is likely that the high susceptibility to degradation of PUFA may have significantly affected their yield in the different tested methods.
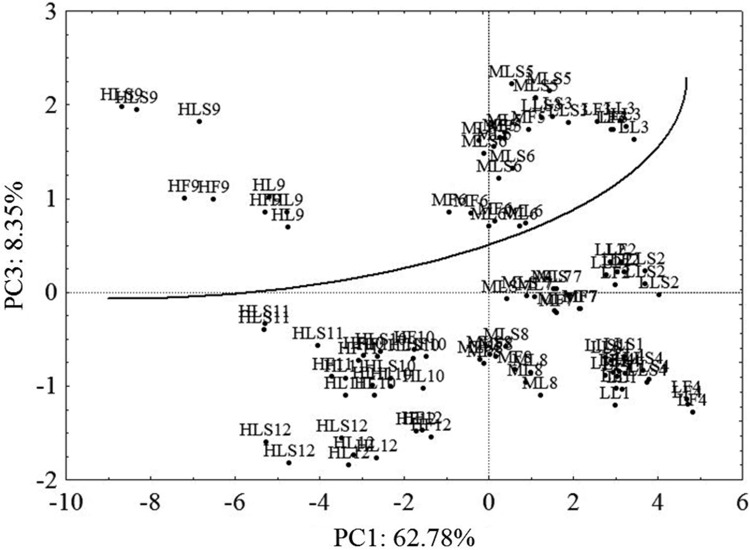
The plot of PC1 versus PC3 (Fig. 3) allowed to easily discriminate the samples on the basis of sex of the animal, whereas the female group was at the top and the male one was at the bottom. However, also in this case samples from different methods were not separated in clusters.
Fig. 3.
PC1 versus PC3 score plot; samples extracted by Folch procedure, lyophylized samples, and lyophylized with anhydrous Na2SO4. LF low (lipid content) Folch; MF medium Folch; HF high Folch; LL low lyophylized; ML medium lyophylized; HL high lyophylized; LLS low lyophylized plus salt (anhydrous Na2SO4); MLS medium lyophylized plus salt (anhydrous Na2SO4); HLS high lyophylized plus salt (anhydrous Na2SO4)
PC4 was characterized by genetic type, but also by quite high loading values for C16:1, C17:0, and C20:4. However, a complete segregation in different clusters of the different genetic types was not possible.
At first glance, the different methods were hence comparable as there was not segregation at all in different clusters of samples.
Quantification of FAMEs obtained with the three protocols and comparisons by one-way ANOVA
The quantification of FAMEs (mg of each FA/100 g of BF) obtained with the three protocols is reported in Table 3.
Table 3.
FA profile of the sample set. Data (mg/100 g of BF) are the mean of 3 repetitions ± the standard deviation
| Sample | Method | C14:0 | C16:0 | C16:1 (n-9) | C17:0 | C17:1 (n-9) | C18:0 | C18:1 (n-9) | C18:2 (n-6) | C18:3 (n-3) | C20:0 | C20:1 (n-9) | C20:2 (n-6) | C20:3 (n-6) | C20:4 (n-6) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA (F values) | 0.48n.s. | 0.48n.s. | 0.85n.s. | 0.55n.s. | 1.14n.s. | 0.35n.s. | 0.28n.s. | 0.41n.s. | 2.04n.s. | 1.18n.s. | 0.19n.s. | 1.87n.s. | 11.68*** | 15.98*** | |
| La | Lb | ||||||||||||||
| LSb | LSa | ||||||||||||||
| FOLa | FOLa | ||||||||||||||
| 1 | La | 12.45 ± 1.49 | 272.68 ± 29.93 | 24.44 ± 2.33 | 5.41 ± 0.74 | 4.11 ± 0.42 | 203.33 ± 24.44 | 483.30 ± 62.35 | 160.63 ± 14.73 | 3.42 ± 0.30 | 3.45 ± 0.34 | 7.28 ± 0.79 | 3.70 ± 0.25 | 4.79 ± 0.21 | 47.95 ± 2.56 |
| LSb | 14.79 ± 1.70 | 288.95 ± 28.61 | 29.76 ± 2.89 | 4.44 ± 0.48 | 3.72 ± 0.11 | 198.21 ± 20.28 | 490.00 ± 49.69 | 131.37 ± 10.87 | 3.28 ± 0.36 | 3.03 ± 0.09 | 9.20 ± 0.92 | 6.80 ± 0.28 | 6.08 ± 0.19 | 30.95 ± 2.85 | |
| FOLc | 13.57 ± 0.59 | 272.52 ± 22.02 | 26.79 ± 1.89 | 4.40 ± 0.23 | 4.20 ± 0.19 | 194.82 ± 13.27 | 458.33 ± 39.88 | 164.93 ± 4.36 | 2.67 ± 0.22 | 2.59 ± 0.21 | 9.21 ± 0.92 | 6.71 ± 0.26 | 5.96 ± 0.29 | 43.97 ± 1.91 | |
| 2 | L | 12.72 ± 0.75 | 258.03 ± 15.85 | 44.52 ± 4.12 | 3.48 ± 0.37 | 2.74 ± 0.25 | 138.86 ± 3.91 | 484.18 ± 55.58 | 138.41 ± 11.02 | 4.72 ± 0.34 | 5.60 ± 0.73 | 11.32 ± 1.37 | 6.39 ± 0.78 | 6.71 ± 0.69 | 50.32 ± 1.86 |
| LS | 12.81 ± 1.17 | 246.59 ± 15.98 | 41.05 ± 2.94 | 2.88 ± 0.26 | 2.86 ± 0.37 | 132.59 ± 10.40 | 466.59 ± 23.96 | 117.61 ± 14.90 | 2.73 ± 0.20 | 2.79 ± 0.28 | 8.01 ± 0.88 | 5.42 ± 0.39 | 5.21 ± 0.70 | 36.69 ± 3.01 | |
| FOL | 14.11 ± 1.59 | 291.65 ± 14.46 | 45.47 ± 4.18 | 3.05 ± 0.28 | 3.43 ± 0.42 | 159.14 ± 6.46 | 547.54 ± 21.85 | 156.73 ± 6.52 | 2.66 ± 0.32 | 2.61 ± 0.40 | 10.91 ± 0.68 | 7.21 ± 0.83 | 6.90 ± 0.40 | 45.90 ± 5.02 | |
| 3 | L | 12.34 ± 1.76 | 351.16 ± 15.37 | 44.62 ± 1.54 | 3.86 ± 0.23 | 4.02 ± 0.26 | 159.09 ± 11.67 | 595.88 ± 66.17 | 160.12 ± 10.71 | 2.63 ± 0.19 | 3.65 ± 0.22 | 7.78 ± 0.34 | 2.13 ± 0.24 | 6.14 ± 0.78 | 43.12 ± 3.66 |
| LS | 24.69 ± 2.05 | 370.28 ± 48.64 | 139.52 ± 12.53 | 5.32 ± 0.28 | 6.82 ± 0.44 | 205.29 ± 13.53 | 803.28 ± 39.69 | 152.76 ± 10.25 | 3.93 ± 0.52 | 3.94 ± 0.33 | 11.94 ± 1.04 | 5.66 ± 0.58 | 7.10 ± 0.24 | 35.94 ± 1.52 | |
| FOL | 18.59 ± 0.33 | 342.81 ± 5.68 | 51.93 ± 3.40 | 4.36 ± 0.38 | 4.70 ± 0.26 | 171.12 ± 10.83 | 658.18 ± 49.67 | 166.02 ± 11.72 | 2.47 ± 0.33 | 2.46 ± 0.34 | 7.49 ± 0.24 | 6.64 ± 0.79 | 5.05 ± 0.52 | 38.23 ± 5.38 | |
| 4 | L | 10.03 ± 1.15 | 218.36 ± 5.80 | 28.99 ± 3.03 | 4.05 ± 0.23 | 3.70 ± 0.18 | 125.86 ± 3.07 | 371.68 ± 21.77 | 145.27 ± 6.35 | 4.80 ± 0.46 | 2.63 ± 0.37 | 5.02 ± 0.35 | 5.95 ± 0.40 | 7.43 ± 0.35 | 55.82 ± 1.59 |
| LS | 8.26 ± 0.66 | 188.69 ± 18.96 | 57.60 ± 6.10 | 2.71 ± 0.25 | 3.37 ± 0.33 | 99.90 ± 9.83 | 357.11 ± 40.11 | 125.48 ± 4.93 | 3.14 ± 0.36 | 2.53 ± 0.17 | 5.51 ± 0.19 | 4.19 ± 0.46 | 7.19 ± 0.66 | 43.81 ± 1.83 | |
| FOL | 6.28 ± 0.72 | 160.69 ± 5.09 | 24.95 ± 2.16 | 2.41 ± 0.19 | 2.24 ± 0.28 | 91.39 ± 0.90 | 277.89 ± 1.21 | 116.91 ± 8.65 | 1.98 ± 0.17 | 1.02 ± 0.15 | 3.83 ± 0.10 | 2.46 ± 0.12 | 4.52 ± 0.20 | 37.28 ± 3.79 | |
| 5 | L | 32.98 ± 2.70 | 525.52 ± 75.68 | 86.98 ± 13.53 | 4.70 ± 0.55 | 5.95 ± 0.63 | 236.84 ± 25.84 | 991.95 ± 114.27 | 141.89 ± 18.41 | 11.37 ± 1.11 | 6.91 ± 0.86 | 20.26 ± 2.45 | 10.19 ± 0.55 | 4.62 ± 0.34 | 42.85 ± 2.38 |
| LS | 31.92 ± 2.77 | 603.76 ± 66.06 | 24.85 ± 1.17 | 5.54 ± 0.80 | 5.91 ± 0.36 | 310.68 ± 40.55 | 1141.77 ± 99.90 | 147.85 ± 9.87 | 3.86 ± 0.29 | 4.92 ± 0.52 | 19.90 ± 2.10 | 7.78 ± 0.83 | 6.99 ± 0.84 | 31.88 ± 3.95 | |
| FOL | 35.61 ± 1.12 | 608.56 ± 69.77 | 100.86 ± 12.03 | 5.66 ± 0.55 | 5.95 ± 0.53 | 310.10 ± 25.61 | 1135.99 ± 97.77 | 179.01 ± 17.77 | 3.87 ± 0.43 | 4.68 ± 0.18 | 19.80 ± 2.06 | 7.32 ± 0.44 | 6.61 ± 0.35 | 44.07 ± 2.07 | |
| 6 | L | 29.26 ± 1.74 | 487.98 ± 17.51 | 78.59 ± 3.42 | 5.31 ± 0.68 | 6.96 ± 0.08 | 278.47 ± 25.49 | 906.85 ± 118.76 | 195.15 ± 10.19 | 13.23 ± 2.01 | 3.44 ± 0.29 | 19.36 ± 2.28 | 7.39 ± 0.88 | 1.89 ± 0.13 | 33.06 ± 4.60 |
| LS | 27.87 ± 1.96± | 557.13 ± 32.55 | 101.39 ± 7.45 | 4.61 ± 0.46 | 6.44 ± 0.49 | 284.35 ± 14.51 | 1096.59 ± 79.10 | 219.07 ± 13.13 | 6.10 ± 0.68 | 3.73 ± 0.45 | 17.04 ± 1.89 | 10.41 ± 0.32 | 7.82 ± 0.93 | 43.82 ± 4.69 | |
| FOL | 32.75 ± 3.57 | 743.86 ± 28.49 | 65.15 ± 3.10 | 5.55 ± 0.75 | 7.31 ± 0.81 | 352.07 ± 35.47 | 1380.26 ± 121.34 | 308.49 ± 47.48 | 7.95 ± 0.76 | 4.23 ± 0.44 | 22.62 ± 0.85 | 7.01 ± 0.81 | 3.32 ± 0.11 | 36.58 ± 0.57 | |
| 7 | L | 28.05 ± 1.84 | 463.48 ± 58.07 | 77.75 ± 4.77 | 6.04 ± 0.55 | 6.81 ± 0.66 | 269.30 ± 9.00 | 1011.79 ± 93.20 | 183.92 ± 8.30 | 3.16 ± 0.29 | 2.95 ± 0.18 | 18.26 ± 1.26 | 5.50 ± 0.51 | 6.48 ± 0.46 | 54.91 ± 5.30 |
| LS | 30.33 ± 3.87 | 527.62 ± 61.35 | 90.84 ± 6.71 | 7.05 ± 1.15 | 6.24 ± 0.82 | 299.13 ± 32.48 | 1041.46 ± 135.50 | 200.13 ± 26.36 | 4.55 ± 0.24 | 3.48 ± 0.22 | 16.33 ± 1.98 | 6.90 ± 0.41 | 5.17 ± 0.40 | 42.41 ± 4.29 | |
| FOL | 21.94 ± 2.46 | 454.65 ± 55.45 | 75.80 ± 8.17 | 5.57 ± 0.51 | 5.71 ± 0.53 | 250.96 ± 23.94 | 935.30 ± 95.28 | 159.78 ± 13.29 | 3.60 ± 0.27 | 2.93 ± 0.07 | 16.55 ± 1.20 | 5.95 ± 0.17 | 3.80 ± 0.10 | 37.84 ± 1.19 | |
| 8 | L | 23.60 ± 1.88 | 439.19 ± 41.32 | 56.22 ± 3.45 | 5.44 ± 0.10 | 7.99 ± 0.67 | 269.37 ± 37.05 | 883.66 ± 23.22 | 184.20 ± 12.94 | 4.09 ± 0.16 | 2.53 ± 0.12 | 18.11 ± 1.75 | 10.47 ± 1.24 | 5.92 ± 0.59 | 45.33 ± 4.00 |
| LS | 31.45 ± 1.42 | 536.93 ± 42.79 | 89.59 ± 6.16 | 8.23 ± 0.59 | 8.25 ± 0.50 | 285.53 ± 15.12 | 979.01 ± 77.44 | 193.95 ± 14.43 | 7.36 ± 0.68 | 3.82 ± 0.39 | 14.95 ± 0.90 | 9.61 ± 0.68 | 6.11 ± 0.64 | 32.29 ± 1.15 | |
| FOL | 28.46 ± 0.70 | 524.61 ± 48.34 | 63.89 ± 3.90 | 7.77 ± 0.82 | 8.73 ± 0.97 | 307.24 ± 11.44 | 1010.74 ± 37.00 | 227.74 ± 9.54 | 5.12 ± 0.42 | 3.16 ± 0.31 | 17.94 ± 1.49 | 9.28 ± 0.92 | 6.30 ± 0.36 | 42.87 ± 1.86 | |
| 9 | L | 61.82 ± 1.98 | 1037.69 ± 12.46 | 135.35 ± 2.37 | 12.70 ± 0.30 | 13.47 ± 0.67 | 598.50 ± 4.20 | 1888.35 ± 20.06 | 337.46 ± 3.20 | 10.69 ± 0.57 | 8.45 ± 0.94 | 31.00 ± 1.63 | 12.07 ± 1.21 | 5.37 ± 0.33 | 43.98 ± 3.52 |
| LS | 77.97 ± 3.72 | 1314.51 ± 130.93 | 76.22 ± 4.13 | 17.87 ± 0.38 | 17.88 ± 1.45 | 770.96 ± 63.86 | 2155.39 ± 233.74 | 375.35 ± 39.12 | 12.74 ± 1.38 | 12.50 ± 0.96 | 39.88 ± 2.05 | 17.60 ± 1.31 | 8.69 ± 0.58 | 44.68 ± 3.73 | |
| FOL | 55.44 ± 6.06 | 1284.77 ± 108.60 | 175.04 ± 6.73 | 16.05 ± 0.41 | 11.28 ± 1.24 | 710.95 ± 75.95 | 2194.62 ± 224.20 | 425.80 ± 21.20 | 11.43 ± 0.96 | 10.55 ± 0.86 | 33.19 ± 2.83 | 11.57 ± 1.55 | 7.43 ± 0.83 | 44.87 ± 2.52 | |
| 10 | L | 55.93 ± 0.82 | 945.02 ± 97.00 | 139.08 ± 14.33 | 6.20 ± 0.63 | 7.28 ± 0.59 | 549.15 ± 53.30 | 1888.74 ± 182.36 | 243.10 ± 12.05 | 7.32 ± 0.78 | 6.10 ± 0.50 | 32.70 ± 0.94 | 8.61 ± 0.84 | 5.06 ± 0.41 | 42.97 ± 2.22 |
| LS | 56.17 ± 1.95 | 958.45 ± 64.89 | 154.18 ± 12.77 | 6.54 ± 0.42 | 7.81 ± 0.67 | 518.68 ± 46.27 | 1800.25 ± 142.14 | 233.10 ± 18.25 | 7.57 ± 0.72 | 5.72 ± 0.14 | 34.99 ± 0.83 | 7.39 ± 0.68 | 7.02 ± 0.71 | 35.61 ± 2.74 | |
| FOL | 51.69 ± 4.45 | 906.11 ± 87.44 | 133.35 ± 14.50 | 6.39 ± 0.58 | 7.89 ± 0.45 | 510.00 ± 50.10 | 1742.32 ± 205.10 | 240.01 ± 28.33 | 6.60 ± 0.14 | 5.55 ± 0.32 | 31.44 ± 3.47 | 10.65 ± 0.82 | 6.48 ± 0.67 | 39.62 ± 2.63 | |
| 11 | L | 57.98 ± 2.27 | 981.80 ± 33.93 | 148.55 ± 1.81 | 7.30 ± 0.29 | 7.50 ± 0.68 | 555.12 ± 19.14 | 1878.10 ± 29.16 | 291.07 ± 24.30 | 11.59 ± 0.73 | 7.39 ± 0.16 | 34.90 ± 0.52 | 8.69 ± 0.73 | 5.66 ± 0.54 | 49.87 ± 1.44 |
| LS | 67.49 ± 5.99 | 1140.40 ± 79.15 | 144.23 ± 9.87 | 9.02 ± 0.50 | 9.54 ± 1.11 | 633.29 ± 36.82 | 2210.70 ± 135.73 | 300.61 ± 13.45 | 12.20 ± 0.55 | 8.67 ± 0.90 | 41.19 ± 3.54 | 12.91 ± 0.86 | 7.82 ± 0.73 | 43.53 ± 4.22 | |
| FOL | 63.67 ± 4.80 | 1022.21 ± 101.10 | 149.49 ± 7.99 | 7.06 ± 0.48 | 7.96 ± 0.79 | 521.57 ± 18.74 | 1868.91 ± 67.46 | 290.52 ± 21.38 | 10.17 ± 0.94 | 7.90 ± 0.59 | 33.39 ± 3.03 | 7.85 ± 0.72 | 6.79 ± 0.39 | 45.98 ± 4.05 | |
| 12 | L | 49.66 ± 1.30 | 997.17 ± 22.55 | 124.42 ± 0.22 | 7.14 ± 0.21 | 8.71 ± 0.51 | 597.50 ± 24.20 | 2137.77 ± 53.51 | 275.31 ± 16.15 | 10.20 ± 0.59 | 7.33 ± 0.06 | 35.72 ± 1.69 | 8.55 ± 0.93 | 3.10 ± 0.15 | 44.39 ± 4.17 |
| LS | 50.74 ± 4.81 | 1194.68 ± 89.56 | 233.05 ± 13.04 | 7.06 ± 0.45 | 10.31 ± 1.12 | 678.48 ± 68.85 | 2277.38 ± 246.18 | 278.46 ± 38.54 | 11.24 ± 0.81 | 7.67 ± 0.93 | 35.69 ± 3.52 | 10.48 ± 1.03 | 5.98 ± 0.63 | 41.52 ± 4.19 | |
| FOL | 45.82 ± 3.88 | 738.16 ± 74.84 | 165.39 ± 12.33 | 6.77 ± 0.59 | 6.84 ± 0.65 | 483.21 ± 22.21 | 1630.01 ± 42.68 | 200.25 ± 7.56 | 8.43 ± 0.93 | 4.75 ± 0.30 | 29.06 ± 1.60 | 9.63 ± 1.04 | 4.53 ± 0.47 | 30.38 ± 3.02 |
Results of the one-way ANOVA and the Tukey’s test for total lipid content are reported as F values and superscript letters, respectively. Different letters identify samples significantly different (p ≤ 0.05)
aLyophilized samples without Na2SO4, b Lyophilized samples with Na2SO4, c Folch samples
n.s. not significant
*** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05
ANOVA and related Tukey’s test were carried out on the whole data set. No significant difference among the treatments was evidenced except for the omega-6 PUFA, C20:3 (n-6) and C20:4 (n-6). For both PUFA Folch method gave the poorest results, while freeze-dried samples without Na2SO4 and freeze-dried samples with Na2SO4 gave higher results for C20:4 (n-6) and C20:3 (n-6), respectively.
One-way ANOVA and Tukey’s test were carried out for each subgroup (low, medium, and high total lipid content) to evaluate the effect of the different protocols in relationship to the fat content. Results are reported in Table 4.
Table 4.
FA mean values inside each subgroup. Data are expressed as mg/100 g of BF ± the standard deviation
| Method of sample preparation | C14:0 | C16:0 | C16:1 (n-9) | C17:0 | C17:1 (n-9) | C18:0 | C18:1 (n-9) | C18:2 (n-6) | C18:3 (n-3) | C20:0 | C20:1 (n-9) | C20:2 (n-6) | C20:3 (n-6) | C20:4 (n-6) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low content (F values) | 1.48n.s. | 0.05n.s. | 4.85* | 1.28n.s. | 0.89n.s. | 0.04n.s. | 0.38n.s. | 4.71* | 13.22*** | 10.62*** | 0.39n.s. | 1.70n.s. | 2.04n.s. | 17.36*** |
| La | 11.88 | 275.06 | 35.64a | 4.20 | 3.64 | 159.00 | 483.76 | 151.11b | 3.89b | 3.83b | 7.85 | 4.54 | 6.27 | 49.30b |
| LSb | 15.14 | 273.63 | 66.98b | 3.84 | 4.19 | 156.79 | 529.25 | 131.80a | 3.27b | 3.07b | 8.66 | 5.52 | 6.40 | 36.85a |
| FOLc | 13.14 | 266.92 | 37.29a | 3.55 | 3.64 | 154.12 | 485.49 | 151.15b | 2.45a | 2.17a | 7.86 | 5.76 | 5.61 | 41.34a |
| Medium content (F values) | 0.61n.s. | 5.05* | 0.02n.s. | 2.23n.s. | 0.15n.s. | 5.04* | 4.50* | 2.98n.s. | 3.11n.s. | 0.13n.s. | 3.06n.s. | 1.74n.s. | 5.27** | 2.77n.s. |
| La | 28.47 | 479.04a | 74.88 | 5.37 | 6.93 | 263.49a | 948.56a | 176.29 | 7.96 | 3.96 | 19.00 | 8.39 | 4.59a | 44.04 |
| LSb | 30.39 | 556.36ab | 76.67 | 6.36 | 6.71 | 294.92ab | 1064.71ab | 190.25 | 5.47 | 3.99 | 17.05 | 8.68 | 6.52b | 37.60 |
| FOLc | 29.69 | 582.92b | 76.42 | 6.14 | 6.93 | 305.10b | 1115.57b | 218.75 | 5.13 | 3.75 | 19.23 | 7.39 | 5.01ab | 40.34 |
| High content (F values) | 3.55* | 4.12* | 0.92n.s. | 0.60n.s. | 2.92n.s. | 3.86* | 3.87* | 0.08n.s. | 2.33n.s. | 1.68n.s. | 13.34*** | 3.22n.s. | 12.12*** | 2.97n.s. |
| La | 56.35ab | 990.42a | 136.85 | 8.33 | 9.24 | 575.07ab | 1948.24ab | 286.73 | 9.95 | 7.31 | 33.58a | 9.48 | 4.94a | 45.31 |
| LSb | 63.09b | 1152.01b | 151.92 | 10.12 | 11.39 | 650.35b | 2110.93b | 296.88 | 10.94 | 8.64 | 37.94b | 12.10 | 7.38b | 41.3 |
| FOLc | 54.15a | 987.81a | 155.82 | 9.07 | 8.49 | 556.43a | 1858.96a | 289.15 | 9.16 | 7.19 | 31.77a | 9.93 | 6.31b | 40.21 |
Results of the one-way ANOVA and the Tukey’s test for low, medium, and high total lipid content are reported as F values and superscript letters, respectively. Different letters identify samples significantly different (p ≤ 0.05)
aLyophilized samples without Na2SO4, b Lyophilized samples with Na2SO4, c Folch samples
n.s. not significant
*** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05
For low lipid content, there were no statistically significant differences for the most abundant fatty acids (C16:0, and C18:1 (n-9)), as well as for C14:0, C17:0, C17:1 (n-9), C18:0, C20:1 (n-9), C20:2 (n-6), and C20:3 (n-6). Significant differences were found for C16:1 (n-9), C18:2 (n-6), C18:3 (n-3), C20:0, and C20:4 (n-6). Data obtained with Folch method showed the highest values along with the ones obtained with freeze-drying method without Na2SO4 for C18:2 (n-6) (p ≤ 0.05), only. For all other FAs, according to Tukey’s test, freeze-dried samples showed significant higher yields of FAME in comparison with those obtained with Folch method. The effect of freeze-drying without Na2SO4 was significant for C20:4 (n-6) (p ≤ 0.001), while the addition of Na2SO4 was significant for C16:1 (n-9) (p ≤ 0.05). For C18:3 (n-3) (p ≤ 0.001) and C20:0 (p ≤ 0.001), both freeze-drying methods figures were significantly higher than in Folch method.
The ANOVA for medium total lipid content (Table 4) showed statistically significant differences for the most abundant FAs, C16:0, C18:0, C18:1 (n-9). Folch samples showed the highest concentrations in comparison with freeze-dried ones, although p was lower than 0.05 in all cases. All other FAs did not show significant differences, but C20:3 (n-6) (p ≤ 0.01).
Finally, the ANOVA for high total lipid content was reported in Table 4. As for medium fat content, the ANOVA showed statistically significant differences for C16:0, C18:0, C18:1 (n-9) (all with p ≤ 0.05). For these FAs, samples obtained with freeze-dried method with Na2SO4 had the highest yields, while Folch samples showed the lowest concentrations. The effect of freeze-drying with the addition of salt was significant also for C14:0 (p ≤ 0.05) and C20:1 (n-9) (p ≤ 0.001), while for C20:3 (n-6) (p ≤ 0.001) freeze-drying without Na2SO4 was significantly lower.
Even if some differences were observed for the yields of FAs obtained with the different methods, they were not univocal, in particular for the most abundant FAs. However, p values were lower than 0.05 in all these cases.
Generally speaking, the ANOVA as well as PCA showed a large comparability among the methods, confirming on a complete set of samples the encouraging results achieved in a preliminary study (Ficarra et al. 2013). A freeze-drying method prior derivatization was already successfully tested on different kinds of meat (beef, chicken, and lamb) in comparison with a saponification procedure showing no differences between the methods for all major fatty acids (Lee et al. 2012).
The advantages of freeze-drying method in comparison with Folch procedure are evident in terms of lower solvent disposal, lower temperature to subject the samples, and considerable saving of time. Folch method, in fact, requires the continuous and careful presence of an operator during all the procedure, while freeze drying process can work up a large number of samples without operators at the same time.
The differences found for PUFA can be explained due to the high susceptibility to oxidation of these compounds. The susceptibility was associated with the sample storage, while freeze-drying of samples is considered the safest drying process to preserve PUFA, because they are kept trapped within intact cellular matrices (Calvo et al. 2011; Lee et al. 2012). Moreover, the higher performance of the freeze-dried method for C20:4 (n-6) does not confirm what reported by Ficarra et al. (2013) about a better reliability of Folch method for PUFA determination. However, they did not determine C20:3 (n-6) and the values for C20:4 (n-6) were considerably lower.
The use of anhydrous Na2SO4 during the freeze drying step showed a clear improvement of FA yields, in particular for samples with high fat content. The composition of the samples obtained by freeze-drying procedure mainly includes protein structures. The addition of anhydrous Na2SO4 to sample is conceivable and allows obtaining a more friable freeze-dried product that thereby makes the derivatization more effective.
Conclusion
Direct derivatization method on freeze-dried pork muscle has proven to be a valid alternative method to the Folch procedure for FAME analysis. Freeze-drying process offers advantages in terms of FA yields, as for C20:3 (n-6) (with Na2SO4) and C20:4 (n-6) (without Na2SO4). The addition of anhydrous Na2SO4 is feasible to achieve a more friable and anhydrous product for base-catalyzed methylation, as showed in particular for samples with high fat content.
Acknowledgements
The research was supported by a financial contribution from MIUR-PRIN 2007 (Prof. Domenico Pietro Lo Fiego).
References
- AOAC . Association of Official Analytical Chemist. Official methods of analysis of the association of official analytical chemists. 19. Arlington: AOAC; 2012. [Google Scholar]
- AOAC Method 24.005 (1990a). Standard method for crude lipids determination or ethereal extract. Official methods of analysis, Association of Official Analytical Chemists, 15th ed. AOAC, Arlington, VA, USA
- AOAC 960.39 (1990b). Standard method for crude lipids determination or ethereal extract. Official methods of analysis,15th ed. AOAC, Arlington, VA, USA
- Berg H, Magard M, Johansson G, Mathiasson L. Development of an SFE method for determination of lipid classes and total fat in meats and its comparison with conventional methods. J Chromatogr A. 1997;785:345–352. doi: 10.1016/S0021-9673(97)00686-9. [DOI] [PubMed] [Google Scholar]
- Boselli E, Velazco V, Caboni MF, Lercker G. Pressurized liquid extraction of lipids for the determination of oxysterols in egg-containing food. J Chromatogr A. 2001;917:239–244. doi: 10.1016/S0021-9673(01)00688-4. [DOI] [PubMed] [Google Scholar]
- Calvo P, Castano AL, Hernandez MT, Gonzalez-Gomez D. Effects of microcapsule constitution on the quality of microencapsulated walnut oil. Eur J Lipid Sci Technol. 2011;113:1273–1280. doi: 10.1002/ejlt.201100039. [DOI] [Google Scholar]
- Christie W. Advances in lipid methodology—two. Dundee: The Oily Press Ltd; 1993. [Google Scholar]
- Christie W. Lipid analysis: isolation, separation, identification and structural analysis of lipids. 3. Urbana: American Oil Chemists’ Society; 2003. [Google Scholar]
- Destaillats F, Golay PA, Joffre F, De Wispelaere M, Hug B, Giuffrida F, Fauconnot L, Dionisi F. Comparison of available analytical methods to measure trans-octadecenoic acid isomeric profile and content by gas-liquid chromatography in milk fat. J Chromatogr A. 2007;1145:222–228. doi: 10.1016/j.chroma.2007.01.062. [DOI] [PubMed] [Google Scholar]
- Dodds ED, McCoy MR, Geldenhuys A, Rea LD, Kennish JM. Microscale recovery of total lipids from fish tissue by accelerated solvent extraction. J Am Oil Chem Soc. 2004;81:835–840. doi: 10.1007/s11746-004-0988-2. [DOI] [Google Scholar]
- Ficarra A, Lo Fiego DP, Minelli G, Antonelli A. Ultra fast analysis of subcutaneous pork fat. Food Chem. 2010;121:809–814. doi: 10.1016/j.foodchem.2010.01.003. [DOI] [Google Scholar]
- Ficarra A, De Paola EL, Lo Fiego DP, Minelli G, Antonelli A. Single step extraction and derivatization of meat lipids for fatty acid Ultra Fast GC analysis. Riv Ital Sostanze Grasse. 2013;90:81–86. [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Golay PA, Dionisi F, Hug B, Giuffrida F, Destaillats F. Direct quantification of fatty acids in dairy powders with special emphasis on trans fatty acid content. Food Chem. 2006;101:1115–1120. doi: 10.1016/j.foodchem.2006.03.011. [DOI] [Google Scholar]
- Grahl-Nielsen O. Comment: fatty acid signatures and classification trees: new tools for investigating the foraging ecology of seals. Can J Fish Aquat Sci. 1999;56:2219–2223. doi: 10.1139/f99-191. [DOI] [Google Scholar]
- Grob K, Suter B. Determination of total fat, milk fat and fatty acid composition through 1-min transesterification directly in the foods: collaborative studies. Mitt Geb Lebensmittelunters Hyg. 2000;91:224–233. [Google Scholar]
- Joensen H, Grahl-Nielsen O. The redfish species Sebastes viviparus, Sebastes marinus and Sebastes mentella have different composition of their tissue fatty acids. Comp Biochem Phys B. 2001;129:73–85. doi: 10.1016/S1096-4959(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Kaiser HF. The varimax criterion for analytic rotation in factor analysis. Psychometrika. 1958;23:187–200. doi: 10.1007/BF02289233. [DOI] [Google Scholar]
- Kolakowska A, Zygadlik B, Czarnecki H, Szczygielski M. Susceptibility of muscle lipids in pigs to oxidation depending on breed soybean or rapeseed diet. Pol J Food Nutr Sci. 1998;7:655–662. [Google Scholar]
- Lee MRF, Tweed JKS, Kim EJ, Scollan ND. Beef, chicken and lamb fatty acid analysis—a simplified direct bimethylation procedure using freeze-dried material. Meat Sci. 2012;92:863–866. doi: 10.1016/j.meatsci.2012.06.013. [DOI] [PubMed] [Google Scholar]
- List GR, Friedrich JP, King JW. Supercritical CO2 extraction and processing of oilseeds. Oil Mill Gaz. 1989;95:28–34. [Google Scholar]
- Lo Fiego DP. Carcass fatness and lipid quality in the heavy pig. Meat Focus Int. 1996;5:261–263. [Google Scholar]
- Lo Fiego DP, Santoro P, Macchioni P, De Leonibus E. Influence of genetic type, live weight at slaughter and carcass fatness on fatty acid composition of subcutaneous adipose tissue of raw ham in the heavy pig. Meat Sci. 2005;69:107–114. doi: 10.1016/j.meatsci.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Lo Fiego DP, Macchioni P, Minelli G, Santoro P. Lipid composition of covering and intramuscular fat in pigs at different slaughter age. Ital J Anim Sci. 2010;9:200–205. doi: 10.4081/ijas.2010.e39. [DOI] [Google Scholar]
- Merkle JA, Larick DK. Fatty acid content of supercritical carbon dioxide-extracted fractions of beef fat. J Food Sci. 1995;60:959–962. doi: 10.1111/j.1365-2621.1995.tb06270.x. [DOI] [Google Scholar]
- Nieto G, Ros G (2012) Modification of fatty acid composition in meat through diet: effect on lipid peroxidation and relationship to nutritional quality—a review. In: Catala A (ed) Lipid Peroxidation, chapter 12, In Tech Open Access Publisher ,Rijeka, pp 239–258. http://www.intechopen.com/books/lipid-peroxidation/modification-of-fatty-acid-composition-in-meat-through-diet-effect-on-lipid-peroxidation-and-relatio
- Raventós M, Duarte S, Alarcón R. Application and possibilities of supercritical CO2 extraction in food processing industry: an overview. Food Sci Technol Int. 2002;8:269–284. doi: 10.1106/108201302029451. [DOI] [Google Scholar]
- Rossi R, Corino C. Influence of long-term nutrition with different dietary fats on fatty acid composition of heavy pigs backfat. Ital J Anim Sci. 2002;1:7–16. doi: 10.4081/ijas.2002.7. [DOI] [Google Scholar]
- Sahena F, Zaidul ISM, Jinap S, Karim AA, Abbas KA, Norulaini NAN, Omar AKM. Application of supercritical CO2 in lipid extraction—a review. J Food Eng. 2009;95:240–253. doi: 10.1016/j.jfoodeng.2009.06.026. [DOI] [Google Scholar]
- Schäfer K. Accelerated solvent extraction of lipids for determining the fatty acid composition of biological material. Anal Chim Acta. 1998;358:69–77. doi: 10.1016/S0003-2670(97)00587-4. [DOI] [Google Scholar]
- Suter B, Grob K, Paciarelli B. Determination of fat content and fatty acid composition through 1-min transesterification in the food sample; principle. Z Lebensm Unters F A. 1997;204:252–258. doi: 10.1007/s002170050073. [DOI] [Google Scholar]
- Suter B, Grob K, Paciarelli B, Novoselac A. Determination of fat content and fatty acid composition through 1-min transesterification in the food sample. II. Solubilization of the fat, results. Mitt Geb Lebensmittelunters Hyg. 1997;88:259–276. [Google Scholar]
- Suter B, Grob K, Paciarelli B. Simultaneous determination of milk fat (butiryc acid) and total fat by 1-min transesterification directly in the food. Mitt Geb Lebensmittelunters Hyg. 1999;90:149–166. [Google Scholar]
- Taylor DL, Larick DK. Investigations into the effect of supercritical carbon dioxide extraction on the fatty acid and volatile profiles of cooked chicken. J Agric Food Chem. 1995;43:2369–2374. doi: 10.1021/jf00057a010. [DOI] [Google Scholar]
- Wood JD, Richardson RI, Nute GR, Fisher AV, Campo MM, Kasapidou E, Sheard PR, Enser M. Effects of fatty acids on meat quality: a review. Meat Sci. 2004;66:21–32. doi: 10.1016/S0309-1740(03)00022-6. [DOI] [PubMed] [Google Scholar]
- Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, Hughes SI, Whittington FM. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 2008;78:343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]