Abstract
The extraction of litchi (Litchi chinensis Sonn.) and oat (Avena sativa L.) seeds were investigated using n-butane as pressurized solvent by evaluating the effect of pressure in the range of 7–100 bar and temperature from 25 to 70 °C on the extract yield and chemical composition together with the antioxidant activity of the extracts obtained. It was experimentally observed extraction yields for both seeds up to ~3.5 wt%, with a total phenolic content around 126.4 mg GAE/100 g of extract, and an antioxidant activity up to 78.36%. Oat seeds extract presented higher values of these parameters evaluated compared to litchi extract. Based on the results found, it seems that n-butane may be a promising solvent to conventional extraction methods, as mild operating conditions and eco-friendly solvent can be used to provide good results without any residues in the final product.
Keywords: Litchi chinensis Sonn., Avena sativa L., Extraction, n-Butane, Chemical characterization, Antioxidant activity
Introduction
Litchi (Litchi chinensis Sonn.) is a fruit tree belonging to the Sapindaceae family, native from southern China, with adapted cultivation in countries with tropical and subtropical climates. The fruits of this plant have specific characteristics, with a thin, hard, indehiscent pericarp red color, guarding a white, juicy and edible aril. The aril contains a dark brown and relatively large seeds the ripe fruit can be consumed in natura or for juice production, vinegar, wine and jam (Lima et al. 2010).
The fruit of Litchi is rich in minerals and vitamins, containing by l00 g pulp approximately 50 mg of vitamin C, 0.6 mg of vitamin B2 and B3, and 0.5 mg of vitamin B1, in addition to iron, calcium and potassium (Tonini and Kaminski 2009). According to Xu et al. (2011), litchi is chemically characterized by the presence of phenolic compounds such as anthocyanins and flavonoids, tannins, steroids and sesquiterpenes. Phenolic substances can eliminate free radicals and act as antioxidants or other agent mechanisms, which contribute to their anti-cancer activity, anti-inflammatory, cardio protective effect and prevention of degenerative diseases (Liu et al. 2009).
Therapeutic properties of the litchi has been widely studied in the extracts obtained by conventional extraction methods, such as antioxidant activity of aqueous extract of seeds (Qi et al. 2015), anti-inflammatory (Yang et al. 2014), anticancer (Zhao et al. 2007), thrombotic and cardiovascular diseases (Sung et al. 2012), both with ethanolic extract, and antioxidant activity of the extract obtained with solvent acetone (Yang et al. 2012), and data obtained from extraction methods with supercritical or pressurized fluids are still scarce.
Oats are an annual grass belonging to the Poaceae family, being the main species grown: white (Avena sativa L.), yellow (Avena byzantina C. Koch) and black oats (Avena strigosa Schreb) (Pomeranz 1982). Food intake is strongly recommended because multiple clinical studies on the effects of oats in assisting the prevention of diseases of the digestive system, the reduction of serum cholesterol and the consequent risks reduction of coronary heart disease are reported. In addition to effects on cholesterol, oat consumption can reduce the absorption of glucose, which is beneficial for diabetics and may stimulate immune function, both in vitro and in vivo (Daou and Zhang 2012). Such factors characterize the oats and their products as functional foods. Because it is a common food in human diet, unlike the litchi, oats does not have many studies evaluating its extracts, and are even more scarce reports of various forms of extraction, such as with pressurized fluids.
Pressurized fluid extraction is a good, green, alternative for the extraction of natural products from plants. Pressurized solvents are of particular interest since its physicochemical properties (density, diffusivity, viscosity, and dielectric constant) can be adjusted by changing operating pressure and temperature, which allows control of the solvating power and selectivity of the solvent for the extraction process. The conditions used for obtaining natural extracts can significantly reduce the problems related to the thermal degradation of the compounds. It also facilitates the recovery of the solvent due to the volatility of the fluid and allows reaching good yields of clean extracts associated with a short extraction time (Mesomo et al. 2012).
Based on traditional extraction techniques, there is a great interest in the use of pressurized n-butane or highly volatile nonpolar solvents as an alternative to conventional solvents for removal of organic bioactive compounds from various vegetable matrices. In this sense, this work aims to obtaining extracts from litchi and oat seeds through pressurized n-butane extraction and evaluate the extraction parameters as well as the composition and antioxidant activity of the extracts obtained.
Experimental
Materials
Litchi seeds were purchased from Lichia.com Food Company (Taubaté, São Paulo, Brazil) and a farmer (Mato Castelhano, RS, Brazil) kindly provided the oat seeds. Seeds were dried at 40 °C for 24 h and then grinded and classified into the Tyler standard sieves with size ranging from 9 to 42 mesh. The comminuted particles were placed into glass flasks under nitrogen atmosphere with external aluminum coating to protect from light prior to extraction. For the extraction experiments, n-butane was the solvent investigated (White Martins, 99.5% of purity).
Pressurized fluid extraction
The experimental unit consisted basically of a solvent reservoir, two thermostatic baths, a syringe pump (ISCO 500D), a 130 cm3 jacketed extraction vessel, an absolute pressure transducer (Smar, LD301) equipped with a portable programmer (Smar, HT 201) with a precision of 0.12 bar, a collector vessel with a glass tube, and a cold trap (Jacques et al. 2007; Novello et al. 2015).
Amounts of around 35 g of dry finely comminuted seeds were charged into the extraction vessel. The solvent was pumped at a constant flow rate of 3 mL/min into the bed, which was supported by two 300-mesh wire disks at both ends. The solvent was kept in contact with the herbaceous matrix for at least 30 min to allow system stabilization. Then, the extract was collected by opening the micrometer valve, and the solvent mass flux was accounted for by the pump recordings. Thereafter, the mass of the extracted extract was weighed, and the glass tube was reconnected to the equipment. This procedure was performed until no significant mass was extracted, as measured on a precision scale balance (Shimadzu, Model AY220 with 0.0001 g accuracy).
Such experimental conditions were selected after performing a full 22 factorial experimental design, considering as the main extraction variables presented in Table 1. Solvent density was estimated using the HBT (P–V–T) correlation for compressed liquids (Poling et al. 2001) or taken from experimental literature values (Sage et al. 1942), making possible to estimate the mass of solvent charged into the extraction vessel.
Table 1.
Coded and actual variable values of the 22 factorial design for oat and litchi seeds extractions
| Oat seeds | |||
| Variables | +1 | 0 | −1 |
| Pressure (bar) | 13 | 10 | 7 |
| Temperature (°C) | 45 | 35 | 25 |
| Litchi seeds | |||
| Variables | +1 | 0 | −1 |
| Pressure (bar) | 100 | 75 | 50 |
| Temperature (°C) | 70 | 60 | 50 |
Experiments were accomplished isothermally at constant pressure. A completely experimental run lasted in general 6 h, including all steps involved: sample weighing, temperature stabilization (baths, extractor), extraction, and depressurization. Based on triplicate experiments carried out for all the experimental conditions, the overall average standard deviation of the yields was determined to be about 0.2%.
The output of compressed fluids was computed based on the volume decay of the syringe pump reservoir, with a resulting accuracy of ±0.01 g in pressurized solvents delivery. With known values of pressure and temperature in the syringe pump reservoir, solvent densities were calculated and accordingly the mass of solvents charged into the extraction vessel.
Chromatographic analysis
The chemical composition of oat and litchi extracts was determined using a GC–MS (Gas Chromatograph QP5050A Shimadzu) equipped with a capillary column (DB-5 (30 m × 0.25 mm × 0.25 µm film, J&W Scientific, USA), with nonpolar characteristic. The carrier gas was helium (Praxair Industrial Gases, 99.9% purity) with a constant flow rate of 1.1 mL/min. The column was heated to 40 °C for 3 min, heated to 200 C at 4 C/min, programmed at 5 °C/min up to 220 °C and maintained at 220 °C for 5 min. The extract was diluted in n-hexane and injected at 250 °C. The identification of major compounds was based on mass spectrum of the substance compared with the database of the GC–MS system (Standard Reference Data Series of the National Institute of Standards and Technology—98 NIST MS library) and the relative amounts of individual components were calculated based on the GC peak area.
Determination of total phenolic compounds
The total phenolic content in the extracts was determined by the Folin–Ciocaulteu method based on the method described by Shahidi and Naczk (1995). Triplicate extract solutions protected from light were prepared at concentration of 0.5 mg/mL by adding water and then the Folin–Ciocalteu reagent after 3 min, together with 0.2 mL of a saturated solution of sodium carbonate. After 1 h, absorbance at 765 nm was read in a spectrophotometer. Quantification was performed based on a gallic acid standard curve being the total phenolic compounds expressed as mg gallic acid equivalent (GAE) per 100 g of dry extract.
Determination of total antioxidant activity by DPPH free radical scavenging
The DPPH (2.2-diphenyl-1-picryl hydrazyl) assays were carried out according to the method of Brand-Williams et al. (1995). The DPPH solution was prepared by dissolving 24 mg DPPH in 100 mL chloroform and diluting it until the absorbance reached the value of 1.1 ± 0.02 units at 517 nm. The DPPH solution (1.9 mL) was mixed with 50 mL of chloroform and 50 μL of Litchi chinensis Sonn. or Avena sativa L. seeds extract, and incubated for 24 h at 25 °C. A control was prepared by adding 1.9 mL of DPPH solution and 100 mL of chloroform. The absorbance was measured at 517 nm against a blank (pure chloroform) in a spectrophotometer (Biochrom Libra S22). All results were expressed as an inhibition percentage, which was calculated as a DPPH radical scavenging. The concentration of antioxidant that causes a 50% decrease in the radical absorbance (IC50) was estimated for the DPPH radical assay by non-linear regression analysis, using GraphPad Prism version 4.0 (La Jolla CA, USA). All analyses were performed in triplicate.
Results and discussion
Extraction yield
Results of the experimental design for extraction of oat seeds demonstrated that increasing temperature and pressure caused no significant effect on the extraction yield shown in Table 2. Temperatures from 25 to 70 °C can be used, which makes the extraction technique with n-butane most attractive since it reduces the risk of loss of thermo sensitive compounds; which may occur in other extraction techniques such as, e.g., n-hexane by soxhlet solvent extraction, which requires higher temperatures (usually above 60 °C). The oat mass extract obtained with n-butane pressure varied between 1.16 and 1.41 g, affording a yield of 2.90–3.53 wt%.
Table 2.
Matrix 22 of the experimental design for litchi extraction after 90 min of extraction and oat extraction after 120 min of extraction using pressurized n-butane as solvent
| Oat extract | Litchi extract | ||||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Pressure (bar) | Extract mass (g) | Yield (wt%) | Temperature (°C) | Pressure (bar) | Extract mass (g) | Yield (wt%) |
| 25 (−1) | 7 (−1) | 1.33 | 3.32 | 50 (−1) | 50 (−1) | 0.08 | 0.27 |
| 45 (+1) | 7 (−1) | 1.16 | 2.90 | 70 (+1) | 50 (−1) | 0.09 | 0.29 |
| 25 (−1) | 13 (+1) | 1.22 | 3.05 | 50 (−1) | 100 (+1) | 0.07 | 0.24 |
| 45 (+1) | 13 (+1) | 1.21 | 3.03 | 70 (+1) | 100 (+1) | 0.09 | 0.29 |
| 35 (0) | 10 (0) | 1.27 | 3.17 | 60 (0) | 75 (0) | 0.10 | 0.35 |
| 35 (0) | 10 (0) | 1.41 | 3.53 | 60 (0) | 75 (0) | 0.09 | 0.29 |
| 35 (0) | 10 (0) | 1.21 | 3.02 | 60 (0) | 75 (0) | 0.10 | 0.35 |
For the extraction of the litchi seeds it was observed that an increase in pressure and temperature does not improve the extraction yield. The maximum amount of extract was 0.10 g with a yield of 0.35 wt% under the conditions 60 °C and 75 bar. In other extraction conditions, the yields obtained were less than 0.10 wt% (Table 2).
Chemical composition of litchi and oat extracts
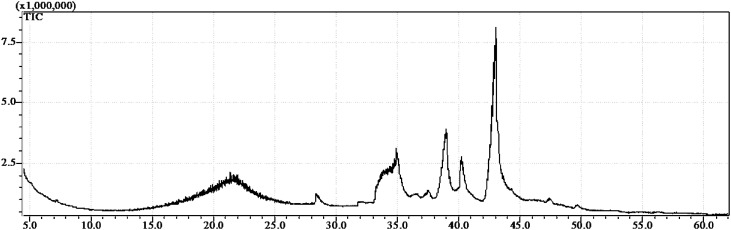
Through the GC–MS analysis it was identified three major compounds, with match probability >95%, in the litchi seed extract obtained with pressurized n-butane (Fig. 1): benzene-1,2-dicarboxylic acid (Rt = 40.5 min) a lesser amount (3.0%), followed by palmitic acid (Rt = 39.0 min) (8.2%) and at the highest concentration, oleic acid (Rt = 43.0 min) (37.9%).
Fig. 1.
Chromatogram of litchi extract using pressurized n-butane at 50 °C and 50 bar
The peak in approximately 34 min, also observed in the oat extract, was identified as diisobutylphthalate and most probably is due to solvent (n-hexane) contamination used in samples dilution. In fact chromatographic analyses of the solvent (blank test) demonstrated the presence of such contaminant. Its characteristic shape, with wide base not compatible to its intensity (height) suggests overlap of compounds, whose resolution and characterization is negatively affected by the presence of diisobutylphthalate as contaminant.
In the oat extract, besides diisobutylphthalate, two other major compounds were identified: palmitic acid (C16:0) was found in a lower proportion (~14%), and oleic acid (C18:1) in higher proportion (~66%).
Determination of total phenolic compounds
The content of phenolic compounds of the extracts obtained for four different assays, ranging between 126.40 and 93.32 mg GAE/100 g (Table 3). The best results were noticed for the assay at 50 °C and 50 bar with 126.4 mg GAE/100 g and for the assay of 50 °C and 100 bar to 124.96 mg GAE/100 g. Thus, the increased content of phenolic compounds found in the litchi fractions suggests that heating does not favor the release of phenolic compounds.
Table 3.
Total phenolic compounds in oat and litchi extracts
| Litchi extract | Oat extract | ||
|---|---|---|---|
| Assay | mg GAE/100 g of dry extract | Assay | mg GAE/100 g of dry extract |
| 50 °C and 50 bar | 126.40 | 25 °C and 7 bar | 50.50 |
| 50 °C and 100 bar | 124.96 | 45 °C and 7 bar | 52.70 |
| 70 °C and 100 bar | 104.48 | 45 °C and 13 bar | 53.70 |
| 60 °C and 75 bar | 93.32 | 35 °C and 10 bar | 52.60 |
| 35 °C and 10 bar | 46.90 | ||
Results obtained here by extraction with n-butane are higher than those found by Su et al. (2014), who reported 86.11 mg GAE/100 g of phenolic compounds in the extract using a solvent mixture of acetone:water (80:20, v/v), which clearly shows that pressurized n-butane can extract a proper amount of litchi seed phenolic compounds, and consequently may improve the bioactivity of the extract.
According to Naczk and Shahidi (1989), the phenolic compounds are covalently conjugated to the cell wall plant components, such as cellulose, and because of this combination, phenol compounds cannot be extracted by the so-called conventional extraction methods that commonly employ liquid organic solvents. Among the compounds already identified, we can mention the work by Prasad et al. (2009), who identified four phenolic compounds in the litchi seed, namely gallic acid, procyanidin B2, (−)-gallocatechin, (−)-epicatechin and (−)-epicatechin-3-gallate compounds.
Among the five samples of oat extracts investigated, all presented results with the contents of phenolic compounds, and values were obtained between 46.9 and 53.7 mg GAE/100 g of dry matter (Table 3). It can be observed from this table that the different extraction conditions did not cause significant changes in phenolic content, as also observed for the extraction yield.
The amount of phenolic compounds found in both extracts studied in our work is far superior to other vegetable extracts consumed directly in its raw state that have the most important nutrients (Ballus et al. 2015).
Determination of antioxidant activity of oat and litchi extracts
For the antioxidant activity of the oat extract it can be observed that the condition of 50 °C and 100 bar presented the highest antioxidant activity—78.36%, followed by the condition of 60 °C and 75 bar and 50 °C and 50 bar, with 65.28 and 63.71%, respectively (Table 4). It is well known that antioxidant activity values above 50% are considered significant and accordingly the oat extract obtained from pressurized n-butane possess high antioxidant capacity.
Table 4.
Antioxidant activity of oat and litchi extracts against the radical DPPH
| Oat | Litchi | ||
|---|---|---|---|
| Extraction assay | (wt%) | Extraction assay | (wt%) |
| 50 °C and 100 bar | 78.36 | 25 °C and 7 bar | 10.63 |
| 70 °C and 100 bar | 24.37 | 45 °C and 7 bar | 22.71 |
| 60 °C and 75 bar | 65.28 | 45 °C and 13 bar | 26.39 |
| 50 °C and 50 bar | 63.71 | 35 °C and 10 bar | 22.97 |
| 35 °C and 10 bar | 14.25 | ||
It is interesting to compare the litchi antioxidant capacity extracted with n-butane with other solvents. For instance, Queiroz et al. (2015) reported the litchi antioxidant capacity of 47.92% for aqueous extract, 27.67% for acetone extract, 39.12% for the extract with 70:30 (v/v) acetone:ethanol and 78.42% for the methanol extract. It can be stated that n-butane has better ability to extract compounds with antioxidant activity than water, ethanol and acetone, while methanol is equivalent.
For the extract obtained from litchi seeds, antioxidant activity is considered low to moderate, with the highest percentage at 45 °C and 13 bar—26.39%. The highest antioxidant activity found for oat extract when compared to litchi extract can be related to the greater amount of phenolic compounds of the oat extract.
These results indicate that the extraction with n-butane does not favor the extraction of antioxidants, it is reported that oats are a source of many compounds exhibiting this activity. Vitamin E, phytic acid and phenolic compounds are the most abundant avenanthramides antioxidants in oats. These antioxidants are concentrated in the outer layers of the grain, and various in vitro assays have been used to evaluate the activity of oat extracts (Peterson et al. 2002).
Conclusion
In this work the extraction of litchi (Litchi chinensis Sonn.) and oat (Avena sativa L.) seeds were investigated using pressurized n-butane as solvent, resulting in extraction yields up to 3.53 wt%. GC–MS chemical analysis of litchi seed extract revealed the presence of beceno-1, 2-dicarboxylic acid o-phthalic acid, palmitic acid and oleic acid, while for oat seed extract diisobutylphthalate, palmitic acid and oleic acid were experimentally found. In terms of antioxidant activity, DPPH radical scavenging method provided inhibition percentages up to 78% for oat seed extract and 26% for litchi seed extract. n-Butane presented to be a promising, green, alternative solvent to conventional extraction methods, as very mild operating conditions and eco-friendly solvent can be used to provide good results without any solvent residues in the final product.
Acknowledgements
The authors thank CNPq, CAPES, FAPERGS, Transfertech Gestão de Inovações LTDA and URI Erechim for financial support and scholarships.
References
- Ballus CA, Quirantes-Piné R, Bakhouche A, Silva LF, Oliveira AF, Coutinho EF, Croce DM, Segura-Carretero A, Godoy HT. Profile of phenolic compounds of Brazilian virgin olive oils by rapid resolution liquid chromatography coupled to electrospray ionization time-of-flight mass spectrometry (RRLC–ESI-TOF-MS) Food Chem. 2015;170:366. doi: 10.1016/j.foodchem.2014.08.054. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Daou C, Zhang H. Oat beta-glucan: its role in health promotion and prevention of diseases. Compr Rev Food Sci Food Saf. 2012;11:355. doi: 10.1111/j.1541-4337.2012.00189.x. [DOI] [Google Scholar]
- Jacques RA, Krause LC, Freitas LS, Dariva C, Oliveira JV, Caramão EB. Influence of drying methods and agronomic variables on the chemical composition of mate tea leaves (Ilex paraguariensis A. St.-Hil) obtained from high-pressure CO2 extraction. J Agric Food Chem. 2007;55:10081. doi: 10.1021/jf071544o. [DOI] [PubMed] [Google Scholar]
- Lima RAZ, Abreu CMP, Asmar SA, Corrêa AD, Santos CD. Embalagens e recobrimento em lichias (Litchi chinensis Sonn.) armazenadas sob condições não controladas. Ciênc Agrotecnol Lavras. 2010;34:914. doi: 10.1590/S1413-70542010000400017. [DOI] [Google Scholar]
- Liu SC, Lin JT, Wang CK, Chen HY, Yang DJ. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem. 2009;114:577. doi: 10.1016/j.foodchem.2008.09.088. [DOI] [Google Scholar]
- Mesomo MC, Scheer AP, Perez E, Ndiaye PM, Corazza ML. Ginger (Zingiber officinale R.) extracts obtained using supercritical CO2 and compressed propane: kinetics and antioxidant activity evaluation. J Supercrit Fluids. 2012;71:102. doi: 10.1016/j.supflu.2012.08.001. [DOI] [Google Scholar]
- Naczk M, Shahidi F. The effect of methanol-ammonia-water treatment on the content of phenolic acids of canola. Food Chem. 1989;31:159. doi: 10.1016/0308-8146(89)90026-5. [DOI] [Google Scholar]
- Novello Z, Scapinello J, Magro JD, Zin G, Di Luccio M, Tres MV, Oliveira JV. Extraction, chemical characterization and antioxidant activity of andiroba seeds oil obtained from pressurized n-butane. Ind Crops Prod. 2015;76:697. doi: 10.1016/j.indcrop.2015.07.075. [DOI] [Google Scholar]
- Peterson DM, Hahn MJ, Emmons CL. Oat avenanthramides exhibit antioxidant activities in vitro. Food Chem. 2002;79:473. doi: 10.1016/S0308-8146(02)00219-4. [DOI] [Google Scholar]
- Poling BE, Prausnitz JM, O’Connell JP. The properties of gases and liquids. 5. New York: McGraw-Hill; 2001. [Google Scholar]
- Pomeranz Y. Advances in cereal science and technology. St. Paul: Am. Assoc. Cereal Chem; 1982. [Google Scholar]
- Prasad KN, Yang B, Chen Y, Zhao M, Ashraf M, Jiang Y. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem. 2009;116:1. doi: 10.1016/j.foodchem.2009.01.079. [DOI] [Google Scholar]
- Qi S, Huang H, Huang J, Wang Q, Wei Q. Lychee (Lichti chinensis Sonn.) seed water extract as potential antioxidant and anti-obese natural additive in meat products. Food Control. 2015;50:195. doi: 10.1016/j.foodcont.2014.08.047. [DOI] [Google Scholar]
- Queiroz ER, Abreu CMP, Oliveira KS, Ramos VO, Frágas RM. Bioactive phytochemicals and antioxidant activity in fresh and dried lychee fractions. Rev Ciênc Agron. 2015;46:163. doi: 10.1590/S1806-66902015000100019. [DOI] [Google Scholar]
- Sage B, Reamer H, Olds R, Lacey W. Phase equilibria in hydrocarbon systems. Ind Eng Chem. 1942;34:1108. doi: 10.1021/ie50393a021. [DOI] [Google Scholar]
- Shahidi F, Naczk M. Food phenolics: sources, chemistry, effects, applications. Lancaster: Technomic Publishing Company, Incorporated; 1995. [Google Scholar]
- Su D, Huihui T, Zhang R, Wei Z, Deng Y, Guo J. Structural elucidation and cellular antioxidant activity evaluation of major antioxidant phenolics in lychee pulp. Food Chem. 2014;158:385. doi: 10.1016/j.foodchem.2014.02.134. [DOI] [PubMed] [Google Scholar]
- Sung YY, Yang WK, Kim HK. Antiplatelet, anticoagulant and fibrinolytic effects of Litchi chinensis Sonn. extract. Mol Med Rep. 2012;5:721. doi: 10.3892/mmr.2011.735. [DOI] [PubMed] [Google Scholar]
- Tonini H, Kaminski PE. Processo Tradicional da Extração e Usos do Óleo da Andiroba em Roraima. Boa Vista: EMBRAPA (Empresa Brasileira de Pesquisa Agropecuária); 2009. [Google Scholar]
- Xu X, Haihui Xie, Liangxiong Xu, Xiaoyi Wei. A novel cyclopropyl-containing fatty acid glucoside from the seeds of Litchi chinensis. Fitoterapia. 2011;82:485. doi: 10.1016/j.fitote.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Yang D, Chang Y, Chen C, Liu S, Hsu C, Lin J. Antioxidant effect and active components of litchi (Litchi chinensis Sonn.) flower. Food Chem Toxicol. 2012;50:3056. doi: 10.1016/j.fct.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Yang D, Chang Y, Lin H, Chen Y, Hsu S, Lin J. Inhibitory effect of litchi (Litchi chinensis Sonn.) flower on lipopolysaccharide-induced expression of proinflammatory mediators in RAW264.7 cells through NF-κB, ERK, and JAK2/STAT3 inactivation. J Agric Food Chem. 2012;62:3458. doi: 10.1021/jf5003705. [DOI] [PubMed] [Google Scholar]
- Zhao M, Yang B, Wang J, Liu Y, Yu L, Jiang Y. Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn.) pericarp. Int Immunopharmacol. 2007;7:162. doi: 10.1016/j.intimp.2006.09.003. [DOI] [PubMed] [Google Scholar]