Abstract
Effects of enzymatic hydrolysis on the physicochemical and functional properties of egg yolk were investigated in this study. Alcalase, neutrase and flavourzyme were used to hydrolyze egg yolk. Solubility, foaming properties, emulsifying and microstructure properties of egg yolk were determined after enzymatic hydrolysis. Results showed that alcalase had better efficiency of hydrolysis than neutrase and flavourzyme. Enzymatic hydrolysis caused a marked changes in protein solubility, surface hydrophobicity, molecular weight distributions, microstructure and other functional properties. It was observed that egg yolk and its hydrolysates exhibited a relatively smooth curve over the entire pH range; egg yolk hydrolysates with high DH had higher solubility than those having lower DH. Foam capacity and stability generally increased with increasing DH although foam stability showed a decrease at 15% DH. Hydrolysates of egg yolk showed scattered and fewer aggregated particles. This study demonstrated that egg yolk hydrolysates could be an excellent emulsifying agent for food and other applications.
Keywords: Egg yolk, Enzyme, Hydrolysate, Functional properties
Introduction
Egg yolk with high nutrients and good functional properties has been widely used in the food industry, such as salad dressing and pastry food. In in vitro tests, digestibility of egg yolk protein (EYP) was better than that of milk casein with the treatment by pepsin and trypsin. In the test of protein efficiency ratio, the criteria to determine the nutritional value of proteins, EYP showed a significantly higher value than that of milk casein (Sakanaka et al. 2000). Proteins are macromolecules that play an important role in the functionality of food and pharmaceutical products. Under various processing conditions, solubility is considered as a critical functional property applied to protein ingredients in beverages, liquid foods and emulsions (Kinsella 1982). Solubility can influence other functional properties of protein, such as emulsification, foaming, as well as to customize functional proteins to meet specific needs (Strixner et al. 2013).
Protein is usually modified by enzymatic treatments to change its structure and accordingly its physicochemical and functional characteristics. Furthermore, limited enzymatic hydrolysis is an effective method to improve functionalities of food proteins without impacting its nutritive value (Kristinsson and Rasco 2000). Strixner et al. (2013) reported that solubility and surface activity of egg yolk granules was significantly improved by modification with phospholipase A2 after spray drying compared to non-modified particles. Jin et al. (2013) pointed that phospholipase A1 modification has a markedly impact on properties of egg yolk and its fractions with regard to protein solubility, emulsifying properties, and foaming properties. However, there is little information on the effect of controlled enzymatic hydrolysis of the whole egg yolk on their functional properties and surface hydrophobicity.
Previous studies reported that functional properties of the hydrolysates could be influenced by the extent of proteolytic degradation of food proteins. Limited enzymatic hydrolysis can improve the functional properties of proteins by altering the molecular size, structure, and interaction force between protein and protein interaction force of intermolecular and intramolecular (Ryan 1977). Sara and Jorge (2002) studied the influence of the degree of hydrolysis on the functional properties of the soy protein isolates and showed that the enzymatic hydrolysis of isolates increased their protein solubility and foam capacity, although it reduced the foam stability. Horiuchi and Fukushima (1978) indicated pepsin hydrolyzed egg white protein leads large concentration of protein molecules at the interface so as to form more stable foam, However, extensive hydrolysis can have negative effects on the functional properties, when degrees of hydrolysis over 12%, foam capability, stability and emulsification all decreased more than 25% than control. Thus, the proteolysis reaction must be carefully monitored and controlled for the purpose of manufacturing products with desired functionality.
However, little information is available on the effects of degree of hydrolysis (DH) on the functional properties of egg yolk hydrolysate. The objective of the present investigation was to evaluate the functional properties and surface hydrophobicity of egg yolk as influenced by the extent of enzymatic hydrolysis with three food grade proteases. Changes in microstructure of egg yolk with enzymatic treatment at different DHs were also studied using electron microscopy.
Materials and methods
Materials
Egg were obtained from a local market (Harbin, China) and stored at 4 °C in the laboratory before experimental work. All enzymes used in this study bought from YuanYe Bio-Technology Co., LTD, China. These enzymes were chosen because of their availability and their apparent suitability for industrial use. All other chemicals and reagents used were of analytical grade.
Preparation of substrates
Egg yolks were manually separated from the albumen and placed on absorbent paper to remove adhering albumen and chalazas. The vitellin membrane was punctured and the yolk was immediately collected. Then, egg yolk was defatted according to the method of Palacios and Wang (2005). In brief, fresh egg yolk protein (EYP) 500 g was mixed with 500 ml of 100% ethanol for dehydration and polar lipid extraction. The mixture was centrifuged for 15 min at 4,900g and 25 °C by using a centrifuge (Avanti JXN-26Beckman Coulter, Inc. USA). Liquid phase was removed and this process was repeated three times. The solid fraction was not-lipid EYP. Both egg yolk and egg yolk-protein were filtered and dried under reduced pressure using freeze dryer (EYELA FDU-2110, Rikakikai co. Ltd, Tokyo).
Production of enzymatic hydrolysates
Egg yolk or defatted egg yolk, was dissolved in distilled water, respectively, at a concentration of 7 g protein/100 ml. Protein contents were determined using the Kjeldahl procedure. The dispersions were continuously stirred at 60 °C for 30 min before enzymatic hydrolysis with alcalase (200 U/mg), neutrase (200 U/mg) and flavourzyme (200 U/mg), respectively. The respective optimal best hydrolysis conditions were described as follows, alcalase, pH 8.5, 55 °C, neutrase, pH 7.0, 45 °C, flavourzyme, pH 7.0, 50 °C. The reaction was initiated by the addition of the enzyme to obtain a final enzyme-to-substrate ratio of 1.2% (w/w). The pH-stat technique was used, and the pH of the mixture was maintained during the reaction continued for 4 h by adding 1 M NaOH. At certain DH, the samples were cooled, adjusted to pH 7.0 with 1 M HCl or 1 M NaOH, and heated at 90 °C for 10 min with occasional agitation to inactivate the enzymes. The hydrolysates were then filtered and spray-dried. The powder was stored at −20 °C for further use. In addition, egg yolk and defatted yolk without the addition of enzyme were prepared as controls.
Determination of the DH
The degree of hydrolysis (DH), defined as the percent ratio of the number of peptide bonds broken (h) to the total numbers of bonds per unit weight (htot), was determined using the pH-stat method (Adler-Nissen 1986) and calculated by the following equation:
where B and Nb refer to the amount of NaOH during the proteolysis of the substrate and its normality, respectively; α represents the average degree of dissociation of the a-NH2 groups in the protein substrate; Mp is the mass (g) of the protein (N × 6.25); and htot is the total number of peptide bonds in the protein substrate (8.0 meq/g egg yolk protein).
Determination of functional properties
Solubility
The solubility of protein hydrolysate was measured as described by Morr et al. (1985) with slight modification. Samples (100 mg) were dissolved in 20 ml deionized water and the pH of the mixture was adjusted to 2–10 with 1 M HCl and 1 M NaOH. The suspensions were stirred for 30 min at 22 °C and then centrifuged at 4,000g for 30 min. Protein contents were determined using the Kjeldahl procedure. Solubility was expressed as the percentage nitrogen content of supernatant divided by the overall nitrogen content in the starting solution at each pH value.
Foaming properties
Foaming properties were determined by the method of Shahidi et al. (1995) with a modified procedure. 200 ml sample solution with 0.5% protein concentration of varying pH (2, 4, 6, 8, and 10) were homogenized for 2 min using shear homogenizer (16,000 r/min; IKA T18 basic; IKA-Werke GmbH & Co., Staufen, Germany) to incorporate the air at room temperature. The mixture was poured into a 500-ml graduated cylinder then foam volume was measured at 0 and 20 min after whipping.
where A is the volume before whipping (ml); B is the foam volume at 0 min after whipping (ml); C is the foam volume at 20 min after whipping (ml).
Emulsifying properties
Preparation of emulsion
The protein solutions (10 mg/ml) were prepared in sodium phosphate buffer (10 mM, pH 7.0). Oil-in-water emulsions containing 20% (v/v) soybean oil and 80% (v/v) protein dispersion were prepared at ambient temperature using an APV-1000 homogenizer (APV Gaulin, Abvertslund, Denmark) operating at 30 MPa.
Determination of droplet size distribution and mean droplet size
Particle size of emulsions was determined as described by Luo et al. (2010). Droplet size distributions of emulsion samples were determined using a Malvern Mastersizer MS2000 (Malvern Instruments Ltd., Worcestershire, UK). The optical parameters used were: refractive indices of 1.46 and 1.33 for sample and dispersant (water), respectively, and particle absorbance of 0.001. The mean particle size was characterized in terms of the surface area mean diameter d32 and volume mean diameter d43. The d32 value was used to estimate the specific surface area of freshly made emulsion, and the d43 value was used to monitor changes in droplet size. Measurements were made on three replicates.
Emulsion stability measurements
Emulsion stability was determined according to the method of Chen et al. (2011). Emulsion stability was evaluated by determining the particle size d43 values and creaming index of emulsions during the quiescent storage time of 21 days at ambient temperature. Emulsion samples were poured into 10 ml glass tubes. Subsequently, the tubes were sealed to prevent evaporation. During storage, the extent of creaming was characterized by creaming index (CI, %): , where HS is the height of serum layer, and HE is the total height of the emulsion.
Water absorption capacity (WAC)
The WAC of the sample was measured using a modified method of Lopez et al. (1991). Sample (0.5 g) was vigorously mixed with 20 ml of distilled water with a vortex mixer for 5 min (Scientific Industries, Inc. New York, USA). The slurry was then centrifuged at 2,000g for 30 min. The volume of decanted supernatant fluid was measure and WAC was expressed as ml of water absorbed per gram of sample.
Oil absorption capacity (OAC)
The OAC of sample was tested by the method of Sathe and Salunkhe (1981) with some modifications. Sample (0.5 g) and 5 ml soybean oil were added to centrifuge tubes, and mixed for 5 min with a vortex mixer. The slurry was then centrifuged at 2,000g for 30 min and the volume of oil separated from the samples was measured.
where v represents volume of retained oil; m is the weight of sample.
Surface hydrophobicity
Surface hydrophobicity of the protein hydrolysates was determined according to the method of Paraman et al. (2008), using 1-anilino-8-naphthalenesulphonate (ANS) as the hydrophobic fluorescence probes. In brief, 8 mM solution of ANS and protein solution (4 ml) with various concentrations from 1 to 5 mg/ml were prepared in 10 mM phosphate buffer (pH 7.0).Then 20 ml of freshly prepared ANS was added to 4 ml of samples. The mixtures were shaken with a vortex mixer for 5 s and stored for 10 min in the dark. Fluorescence intensity (FI) was measured at 390 nm (excitation) and 470 nm (emission) at room temperature, with a constant excitation and emission slit of 5 nm. The final FI value at each protein concentration was obtained by subtracting the FI of the blank from the FI of each sample in butter. The initial slope of the curve of the fluorescence intensity versus the protein concentration plot was calculated by linear regression analysis and used as the index of protein hydrophobicity. All the determinations were conducted in triplicate.
Scanning electron microscopy (SEM)
The surface and bulk structures of the hydrolysates were observed using a scanning electron microscope (SEM; Qumnta 200, FEI, Hong Kong). The powder samples were sprinkled on aluminum stubs using double-sided adhesive tape. Excess particles were removed by directing a jet of dry air at the surface of the stub. The samples were then coated with gold in a sputter coater to produce the conductive surface.
Statistical analysis
All the experiments were performed in triplicate and statistical analysis was carried out using SPSS (Version 17.0). An analysis of variance (ANOVA) was performed on the data, and the significance of differences between means was determined by the least significant difference test procedure at P < 0.05.
Results and discussion
Hydrolysis of egg yolk
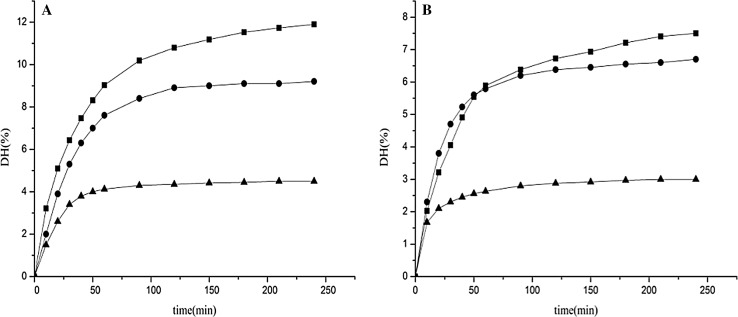
Hydrolysis of egg yolk and defatted yolk was carried out by the pH–stat method (Fig. 1). Rapid hydrolysis was noted during the initial 60 min and then the speed of the hydrolysis was slower until the end of the reaction and prolonging the reaction time did not produce any significant improvement in the DH after 150 min. That suggested that the maximum peptide cleavage during hydrolysis occurred within 30 min. The results were in agreement with that of fish protein hydrolysates (Sathivel et al. 2003) and wheat gluten (Kong et al. 2007).
Fig. 1.
Degree of hydrolysis (DH) of egg yolk (a) and defatted yolk (b) hydrolyzed with alcalase, neutrase or flavourzyme (alcalase (field square), neutrase (field circle), flavourzyme (field triangle))
In the hydrolysis experiments, the criterion for selection was the ability of the enzyme to obtain the desired degree of hydrolysis at a low concentration at the same time. Enzymes were used under the optimal conditions. The decrease of the hydrolysis’ rate for the progressing of the hydrolysis might be due to the decrease in available specific peptide bonds for hydrolysis. And during the hydrolysis, the completion between the peptides and the native protein was formed constantly with the same amount of substrate and enzyme, a higher value of DH with alcalase was observed than neutrase or flavourzyme, during the whole period of hydrolysis, which implied that Alkaline proteases indicated higher activities than neutral proteases, and demonstrate that the effectiveness of enzymatic hydrolysis was related to the type of enzyme. Thus, alcalase was chosen to produce yolk hydrolysates for the functional properties analysis.
All the enzymatic hydrolysates of egg yolk and yolk defatted were compared, suggesting that DHs of egg yolk were higher than those from defatted yolk. These results were similar to Klompong et al. (2007) who showed that hydrolysates produced from defatted mince had a lower DH than those from the original mince. The reaction of enzyme and insoluble protein particles was carried out rapidly, and then polypeptide chains bound to the surface were hydrolyzed. The denser core protein were cleaved more slowly (Benjakul and Morrissey 1997). Defatted yolk was denatured by the alcohol treatment. Polar solvent may make conformation of protein structure change irreversibly, resulting in aggregation and protein precipitation (Wang and Wang 2009). Therefore, the effect of the enzyme on the defatted protein substrates was not obvious. Due to the weak hydrolysis of defatted yolk, the original egg yolk without fat extracted was utilized in further studies.
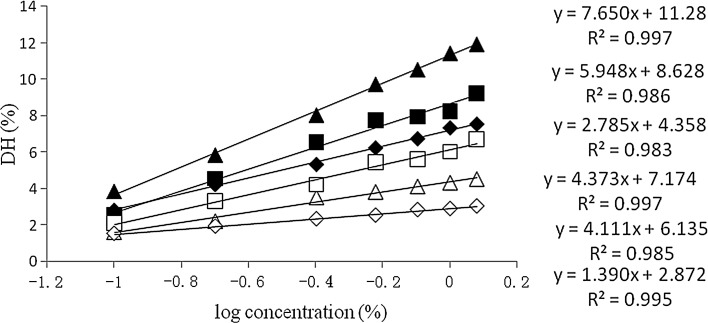
The linear relationship of log10 (enzyme concentration) and DH was presented in Fig. 2. This figure shows the effects of enzyme species, enzyme concentration, defatted on the protein hydrolysis degree. Similar results were found previously by Benjakul and Morrissey (1997) for Pacific whiting solid wastes. In order to achieve ideal DH, the accurate concentration of enzyme could be calculated basing on the regression.
Fig. 2.
Relationship between DH and log enzyme concentration of alcalase in egg yolk (field triangle) and defatted yolk (field diamond) and of neutrase in egg yolk (field square) and defatted yolk (open square) and of flavourzyme in egg yolk (open triangle) and defatted yolk (open diamond). Different amounts of enzyme were added to the homogenate of egg yolk or defatted yolk. The reaction was run for 240 min under respective optimum hydrolysis conditions
Functional properties
Solubility
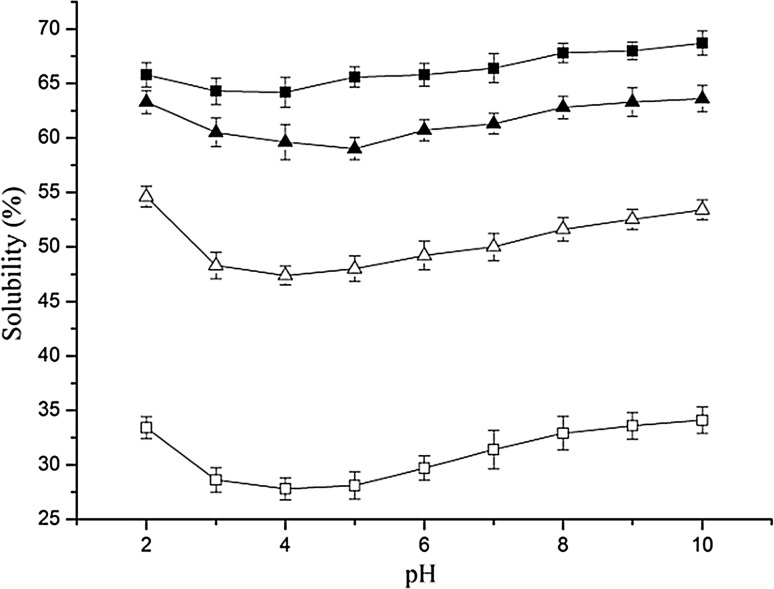
Protein solubility enhancement is key in improving the appearance and taste of the final product. The solubility curves of egg yolk and hydrolysates with different DHs in the pH range of 2–10 are shown in Fig. 3. Distinctly, the egg yolk and its hydrolysates exhibited a relatively flat curve over the entire pH range, and no apparent isoelectric point was observed. This is in agreement with Kong et al. (2007) who reported similar pH-independent solubility curves for wheat gluten hydrolysates, although the non-hydrolyzed wheat gluten was pH-dependent. The solubility of all the hydrolysates with different DHs was more than 50% over a wide pH range and was remarkably higher than the untreated one (p < 0.05). Egg yolk hydrolysates with high DH had higher solubility than those possessing lower DH, which is consistent with earlier report (Zhao et al. 2011). Molecular size hydrophobicity, polar and ionizable groups were altered by enzymatic hydrolysis (Mutilangi et al. 1996). Smaller peptides from hydrolysate with high DH were considered to have more polar residues which could enhance the quantity of hydrogen bonds with water resulting in increase of protein solutions (Liu et al. 2010), compared to low hydrolyzed. Enzymatic hydrolysis could reduce molecular weight and form more hydrophilic groups as well as more solvated polypeptide units, which were contributed to the high solubility of egg yolk hydrolysates (González-Tello et al. 1994).
Fig. 3.
Solubility of egg yolk hydrolysates prepared using Alcalase with different DHs as influenced by pH: Control (open square), DH 5% (open triangle), 10% (field triangle) and 15% (field square). Bars represent standard deviations from triplicate determinations
Foaming properties
Foaming capacity (FC) and foaming stability (FS) results of all hydrolysates at pH 2, 4, 6, 8, 10 are presented in Table 1. FC of enzymatically hydrolysed egg yolk showed a large increase, compared to the control by exposing more hydrophobic residues and increasing capacity to reduced surface tension. Proteins in dispersions generate low surface tension at the water–air interface, hence creating foaming capacity (Surowka and Fik 1992). Enzymatic hydrolysis reduced molecular weight, enhanced the solubility and hydrophobicity, increased the flexibility of protein, which facilitated protein molecules disperse more rapidly to air–water interface to encapsulate air particles to enhance interfacial membrane and foaming (Chau and Cheung 1998). Foam stability increased by the increase in DH, then a decrease was found with DH 15%. The reduced molecular by hydrolysis will make amphiphilic peptides more flexible, which in turn increase viscosity of the aqueous phase, protein concentration and film thickness (Phillips et al. 1994). Klompong et al. (2007) indicated more microscopic peptides could not form a stable foam system, and this may have caused lower foam stability of hydrolysates at 15% DH in this study. The foaming properties of all hydrolysates were affected by pH. FC and FS decreasing was observed at pH 2–4, followed by a marked increase as the pH increased until a maximum value was obtained at pH 8. Subsequently, a slight decrease was observed at pH 10. Net charge affected the surface adsorption of the proteins in the air–water interface. In highly acidic and alkaline regions, with the net charge increased, foaming property of hydrolysates was improved (Sorgentini and Wagner 2002). Moreover, increase in protein solubility away from isoelectric pH could be conducive to higher foaming property (Aluko and Yada 1995).
Table 1.
Effect of pH on foaming properties, Water/oil absorption capacity and surface hydrophobicity of egg yolk hydrolysates with different degree hydrolysis
| Samples | pH | FC (%) | FS (%) | WAC (ml/g) | OAC (ml/g) | H 0 |
|---|---|---|---|---|---|---|
| Control | 2.0 4.0 6.0 8.0 10.0 |
29.94 ± 0.07d
17.03 ± 0.04e 55.77 ± 0.03c 86.30 ± 0.02a 64.20 ± 0.02b |
16.2 ± 0.04d
9.29 ± 0.03e 24.34 ± 0.03b 35.10 ± 0.04a 22.5 ± 0.02c |
3.88 ± 0.03d | 3.42 ± 0.02d | 788.00 ± 1.63d |
| DH5% | 2.0 4.0 6.0 8.0 10.0 |
36.27 ± 0.02d
22.27 ± 0.03e 60.44 ± 0.04c 92.58 ± 0.05a 69.55 ± 0.02b |
20.32 ± 0.02d
13.52 ± 0.03e 26.61 ± 0.04b 40.86 ± 0.05a 24.35 ± 0.04c |
4.35 ± 0.01c | 4.53 ± 0.02c | 1238.33 ± 3.30c |
| DH10% | 2.0 4.0 6.0 8.0 10.0 |
51.06 ± 0.03d
35.07 ± 0.02e 76.52 ± 0.04c 107.16 ± 0.03a 86.29 ± 0.01b |
25.20 ± 0.03d
21.07 ± 0.03e 30.21 ± 0.02c 44.52 ± 0.02a 32.02 ± 0.06c |
4.57 ± 0.02b | 4.90 ± 0.01a | 1358.33 ± 2.88a |
| DH15% | 2.0 4.0 6.0 8.0 10.0 |
59.30 ± 0.03d
44.50 ± 0.02e 82.41 ± 0.03c 112.01 ± 0.07a 95.31 ± 0.04b |
21.61 ± 0.03d
19.29 ± 0.03e 27.71 ± 0.04c 39.59 ± 0.05a 30.26 ± 0.05b |
4.85 ± 0.03a | 4.72 ± 0.01b | 1254.33 ± 3.09b |
All data are expressed by mean values of triplicates ± standard deviation
Emulsifying capability and emulsion stability
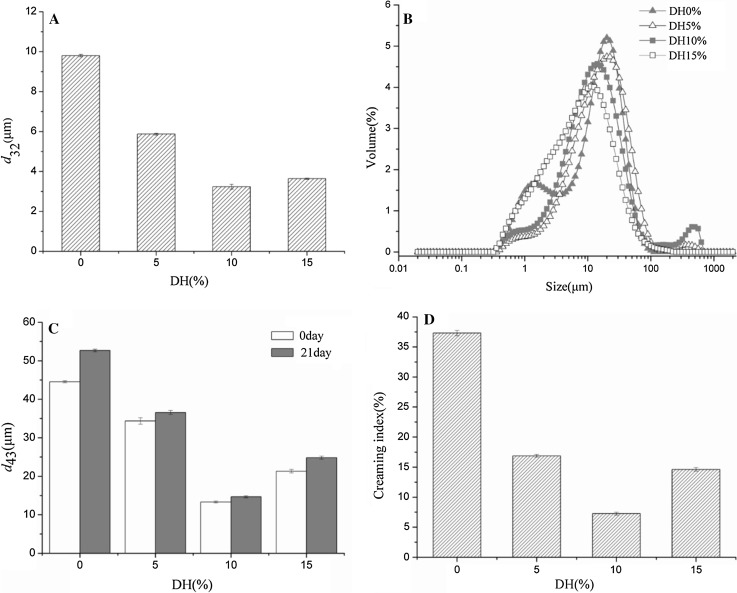
Various d32 values of hydrolysates in response to different DHs ranging from 5 to 15 were presented in Fig. 4a. After enzymatic treatment, all hydrolysates had lower d32 compared with original egg yolk. However, with the increase of DH value, d32 of all hydrolysates decreased initially, and then increased at high DH values. Hydrolyzed egg yolk exhibited a limited improvement with minimum d32 value of 3.24 μm. Lower d32 values signify higher emulsifying activity (Chen et al. 2011). These results suggest that enzymatic hydrolysis with alcalase gave much better effect on improving the emulsifying capability of egg yolk. The solubility and hydrophobicity of protein were improved by hydrolysis, which may cause the enhancement of emulsifying activity. Besides, solubility was an important prerequisite for film formation, which inhibits coalescence of the oil droplet.
Fig. 4.
The effect of hydrolysis degree on the emulsifying properties of egg yolk hydrolysate with alcalase a d32, b droplet size distributions, c d43 of hydrolysates stored at 0 and 21 days, d CI
The particle size distributions of the emulsions were shown in Fig. 4b. Better emulsifying properties were characterized with lower peak value and fewer numbers of peaks. Untreated egg yolk showed a bimodal distribution, and peak appeared in 2 and 25 μm. The particle size distributions of emulsions stabilized with hydrolysates were monomodal and no noticeable difference was observed as the DH raised from 10 to 15. This observation also suggests that hydrolysis can enhance the emulsifying properties of egg yolk.
The stability of emulsions formed by egg yolk and its hydrolysates was investigated over a storage period of 21 days by the changes in the particle size d43 value (Fig. 4c) and the creaming index (Fig. 4d). Enzymatic hydrolysis induced a significant decrease in the d43 values and the creaming index. Therefore, after enzymatic hydrolysis processing, emulsions stabilized by limited hydrolysate egg yolk were better than that stabilized by egg yolk. Higher emulsion stability were observed at DH 10% DH. Results suggest that the hydrolysates with a higher solubility and smaller molecular size might rapidly diffuse and adsorb at interface, however, the effect of small peptides on reduction of interfacial tension and stability of emulsion was not significant, since they cannot unfold and re-orient at the interface due to charge repulsions (Gbogouri et al. 2004). During storage, emulsion droplets would undergo aggregation and then the particle size increases, usually resulting in creaming. In this study, it was observed that larger droplet with lower surface charge of the emulsion stabilized by egg yolk without enzymatic treatment combined with the droplet aggregation, promoted the considerable creaming during storage. These results suggest that emulsion formed by hydrolysate with DH 10% could be a suitable emulsifier as well as provide excellent long term stability against flocculation and creaming.
Water and oil absorption capacity (WAC/OAC)
The water and oil absorption capacity of egg yolk hydrolysates were shown in Table 1. The water capacity of egg yolk increased with enzymatic hydrolysis with alcalase and was highest at 15% DH. Hydrolyzed protein was dissociated into smaller subunits with more water binding sites. Moreover, non-protein components in the samples to the surrounding water (e.g. carbohydrates, starches, etc.) increased by removal of protein. The two aspects above were both contributed to the improvement of WAC (Guan et al. 2007).
Results from OAC results showed a steady increase in OAC level as DH increased till DH reach 10% then decreased at DH 15%. Similar results were obtained by Yust et al. (2010). This may be due to that the hydrolysis of egg yolk proteins exposed non-polar side chains that bind hydrocarbon moieties of oil, and the unfolding of protein structure and exposure of more hydrophobic groups made the physical entrapment of oil, resulting in increasing oil absorption capacity. The highest oil absorption capacity was obtained with DH 10%. If the DH increases further, the oil absorption capacity decreases, which may be attributed to the major exposure of ionic groups after hydrolysis. Oil absorption capacity (OAC) represents the ability of proteins to interact with lipid materials, which are important functional properties used in the food industry (Adler-Nissen 1986).
Surface hydrophobicity
Alcalase modification greatly improved (P < 0.01) the surface hydrophobicity of egg yolk from 788 for the control to 1238, 1358, 1254 for hydrolysates with DH 5, 10, 15% (Table 1). The explanation for this phenomenon is that enzymatic hydrolysis occurred from the exterior to the interior of the protein molecules, resulting in the exposure of hydrophobic clusters in the inner part of protein (Panyam and Kilara 1996). Significant difference was observed between hydrolyzed samples (P < 0.01). However, further hydrolysis (DH 15%), on the contrary, decreased the H0. The phenomena could be explained in the hydrolysate with more small peptides, which could have fewer hydrophobic binding sites than larger peptides. This result revealed that the surface hydrophobicity of protein could be decreased by excessive enzyme hydrolysis.
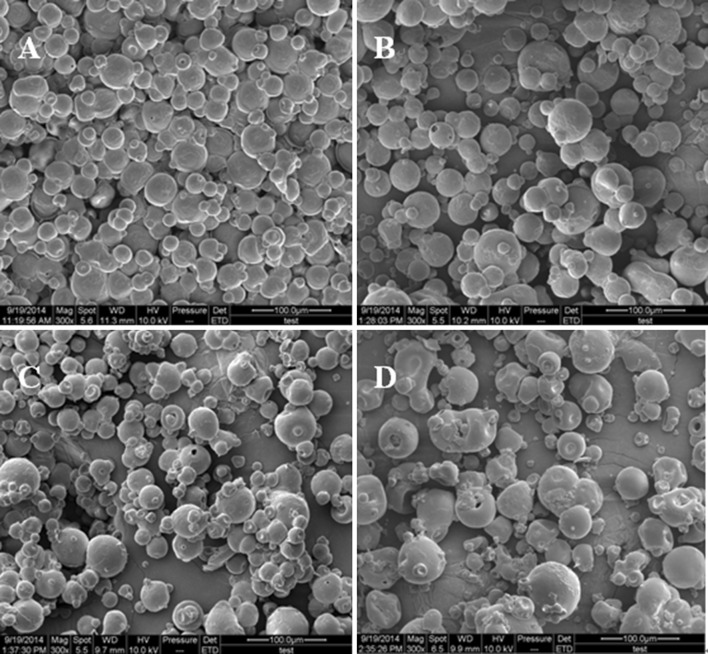
Scanning electron microscopy (SEM)
Figure 5a–d showed the SEM pictures of untreated egg yolk and hydrolysate with different DHs, respectively. The untreated sample presented spherical and relatively smooth surface and tight structure can be observed. It was observed that with the increasing DH, average size of hydrolysates was reduced. Hydrolysates particles scatter and fewer aggregations occur. When DH went up to 10 and 15%, the particle surface was rougher and shows the presence of small concave holes. These observations indicate that alcalase may effectively act on the egg yolk protein, and the way it functions to protein is likely to start from the surface and proceeds inside gradually. It is hypothesized that these changes can improve the interactions between proteins and water molecules, resulting in the higher solubility.
Fig. 5.
SEM of hydrolysates with different DHs. a Control, b DH5%, c DH10%, d DH15%
Conclusion
Defatted yolk is less susceptible to the proteases used, which could be due to the presence of alcohol denatured proteins during the process of delipidation. The experiments demonstrated that alcalase treatment with the DH range of 5–15% could change the microstructure of egg yolk and improve markedly surface hydrophobicity and functional properties of egg yolk, such as the solubility, foam properties, emulsifying properties, and water and oil absorption capacity. Additionally, solubility and foam properties were affected by pH. These results presented that egg yolk hydrolysates could be used in food products. Further study on the bioactivities of egg yolk hydrolysates is recommended.
Acknowledgments
This study was funded by the Modern Agro-industry Technology Research System of China (Grant No. CARS-41-K25) and National Natural Science Foundation of China (Grant No. 31470094). The authors also sincerely appreciate the invaluable inputs by the editors and reviewers which greatly improved the quality of the manuscript.
References
- Adler-Nissen J. Enzymic hydrolysis of food proteins. New York: Elsevier Applied Science Publishers; 1986. [Google Scholar]
- Aluko RE, Yada RY. Structure-function relationships of cowpea globulin isolate: influence of pH and NaCl on physicochemical and functional properties. Food Chem. 1995;53(3):259–265. doi: 10.1016/0308-8146(95)93931-G. [DOI] [Google Scholar]
- Benjakul S, Morrissey MT. Protein hydrolysates from pacific whiting solid wastes. J Agric Food Chem. 1997;45(9):3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Chau CF, Cheung PCK. Functional properties of flours prepared from three Chinese indigenous legume seeds. Food Chem. 1998;61(4):429–433. doi: 10.1016/S0308-8146(97)00091-5. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen J, Ren J, et al. Effect of ultrasound pretreatment on the enzymatic hydrolysis of soy protein isolates and on the emulsifying properties of hydrolysates. J Agric Food Chem. 2011;59:2600–2609. doi: 10.1021/jf103771x. [DOI] [PubMed] [Google Scholar]
- Gbogouri GA, Linder M, Fanni J, Parmentier M. Influence of hydrolysis degree on the functional properties of salmon byproduct hydrolysates. J Food Sci. 2004;69:615–622. doi: 10.1111/j.1365-2621.2004.tb09909.x. [DOI] [Google Scholar]
- González-Tello P, Camacho F, Jurado E, Páez MP, Guadix EM. Enzymatic hydrolysis of whey proteins. ii. Molecular-weight range. Biotechnol Bioeng. 1994;44(4):529–532. doi: 10.1002/bit.260440416. [DOI] [PubMed] [Google Scholar]
- Guan X, Yao H, Chen Z, Shan L, Zhang M. Some functional properties of oat bran protein concentrate modified by trypsin. Food Chem. 2007;101(1):163–170. doi: 10.1016/j.foodchem.2006.01.011. [DOI] [Google Scholar]
- Horiuchi T, Fukushima D. Studies on enzyme-modified proteins as foaming agents: effect of structure on foam stability. Food Chem. 1978;3:35–41. doi: 10.1016/0308-8146(78)90045-6. [DOI] [Google Scholar]
- Jin YG, Huang D, Ding T, MA MH, OH DH. Effect of phospholipase A1 on the physicochemical and functional properties of hen’s egg yolk, plasma and granules. J Food Biochem. 2013;37(1):70–79. doi: 10.1111/j.1745-4514.2011.00608.x. [DOI] [Google Scholar]
- Kinsella JE. Relationships between structure and functional properties of food proteins. Food proteins. 1982;1:51–103. [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102(4):1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Kong X, Zhou H, Qian H. Enzymatic hydrolysis of wheat gluten by proteases and properties of the resulting hydrolysates. Food Chem. 2007;102:759–763. doi: 10.1016/j.foodchem.2006.06.062. [DOI] [Google Scholar]
- Kristinsson HG, Rasco BA. Fish protein hydrolysates: production, biochemical, and functional properties. Crit Rev Food Sci Nutr. 2000;40(1):43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kong B, Xiong YL, Xia X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118(2):403–410. doi: 10.1016/j.foodchem.2009.05.013. [DOI] [Google Scholar]
- Lopez OP, Falomir CO, Olivares-Vasquez MR. Chickpea protein isolates: physicochemical, functional and nutritional characterization. J Food Sci. 1991;56:726–729. doi: 10.1111/j.1365-2621.1991.tb05367.x. [DOI] [Google Scholar]
- Luo D, Zhao Q, Zhao M, et al. Effects of limited proteolysis and high pressure homogenisation on structural and functional characteristics of glycinin. Food Chem. 2010;122(1):25–30. doi: 10.1016/j.foodchem.2010.02.011. [DOI] [Google Scholar]
- Morr V, German B, Kinsella JE, Regenstein JM, Van Buren JP, Kilara A, et al. A collaborative study to develop a standardized food protein solubility procedure. J Food Sci. 1985;50:1715–1718. doi: 10.1111/j.1365-2621.1985.tb10572.x. [DOI] [Google Scholar]
- Mutilangi WAM, Panyam D, Kilara A. Functional properties of hydrolysates from proteolysis of heat-denatured whey protein isolate. J Food Sci. 1996;61:270–274. doi: 10.1111/j.1365-2621.1996.tb14174.x. [DOI] [Google Scholar]
- Palacios LE, Wang T. Egg-yolk lipid fractionation and lecithin characterization. J Am Oil Chem Soc. 2005;82(8):571–578. doi: 10.1007/s11746-005-1111-4. [DOI] [Google Scholar]
- Panyam D, Kilara A. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci Technol. 1996;7(4):120–125. doi: 10.1016/0924-2244(96)10012-1. [DOI] [Google Scholar]
- Paraman I, Hettiarachchy NS, Schaefer C. Preparation of rice endosperm protein isolate by alkali extraction. Cereal Chem. 2008;85(1):76–81. doi: 10.1094/CCHEM-85-1-0076. [DOI] [Google Scholar]
- Phillips LG, Whitehead DM, Kinsella JE. Structure-function properties of food proteins. San Diego: Academic Press; 1994. [Google Scholar]
- Ryan DS. Determinants of the functional properties of proteins and protein derivatives in foods. In: Feeney RE, Whitaker JR, editors. Food proteins Improvement through chemical and enzymatic modification, Advances in chemistry series. Washington DC: American Chemical Society; 1977. pp. 67–91. [Google Scholar]
- Sakanaka S, Kitahata K, Mitsuya T, Gutierrez MA, Juneja LR. Protein quality determination of delipidated egg-yolk. J Food Compos Anal. 2000;13:773–781. doi: 10.1006/jfca.2000.0914. [DOI] [Google Scholar]
- Sara E, Jorge R. Hydrolysates of native and modified soy protein isolates: structural characteristics solubility and foaming properties. J Food Res Int. 2002;35:511–518. doi: 10.1016/S0963-9969(01)00149-1. [DOI] [Google Scholar]
- Sathe SK, Salunkhe DK. Functional properties of the great northern bean (Phaseolus vulgaris L.) proteins: emulsion, foaming, viscosity, and gelation properties. J Food Sci. 1981;46(1):71–81. doi: 10.1111/j.1365-2621.1981.tb14533.x. [DOI] [Google Scholar]
- Sathivel S, Bechtel PJ, Babbitt J, Smiley S, Crapo C, Reppond KD, et al. Biochemical and functional properties of Herring (Clupeaharengus) byproduct hydrolysates. J Food Sci. 2003;68:2196–2200. doi: 10.1111/j.1365-2621.2003.tb05746.x. [DOI] [Google Scholar]
- Shahidi F, Xiao-Quing H, Synowiecki J. Production and characteristics of protein hydrolysates from capelin (Mallotusvillosus) Food Chem. 1995;53:285–293. doi: 10.1016/0308-8146(95)93934-J. [DOI] [Google Scholar]
- Sorgentini DA, Wagner JR. Comparative study of foaming properties of whey and isolate soybean proteins. Food Res Int. 2002;35(8):721–729. doi: 10.1016/S0963-9969(02)00067-4. [DOI] [Google Scholar]
- Strixner T, Würth R, Kulozik U. Combined effects of enzymatic treatment and spray drying on the functional properties of egg yolk main fractions granules and plasma. Drying Technol. 2013;31(13–14):1485–1496. doi: 10.1080/07373937.2013.790411. [DOI] [Google Scholar]
- Surowka K, Fik M. Studies on the recovery of proteinaceous substances from chicken heads. I. An application of neutrase to the production of protein hydrolysate. Int J Food Sci Technol. 1992;27:9–20. doi: 10.1111/j.1365-2621.1992.tb01173.x. [DOI] [Google Scholar]
- Wang G, Wang T. Egg yolk protein modification by controlled enzymatic hydrolysis for improved functionalities. Int J Food Sci Technol. 2009;44(4):763–769. doi: 10.1111/j.1365-2621.2008.01894.x. [DOI] [Google Scholar]
- Yust MDM, Pedroche J, Millán-Linares MDC, Alcaide-Hidalgo JM, Millán F. Improvement of functional properties of chickpea proteins by hydrolysis with immobilised alcalase. Food Chem. 2010;122(4):1212–1217. doi: 10.1016/j.foodchem.2010.03.121. [DOI] [Google Scholar]
- Zhao G, Liu Y, Zhao M, Ren J, Yang B. Enzymatic hydrolysis and their effects on conformational and functional properties of peanut protein isolate. Food Chem. 2011;127(4):1438–1443. doi: 10.1016/j.foodchem.2011.01.046. [DOI] [Google Scholar]