Abstract
When a raw sweet potato root is analysed, only sucrose, glucose and fructose are present but during cooking, starch is hydrolysed into maltose giving the sweet flavour to cooked roots. This study aimed at developing an HPTLC protocol for the rapid quantitative determination of maltose and total sugars in four commercial varieties and to compare them to 243 hybrids grouped by flesh colour (white, orange, purple). In commercial varieties, mean maltose content varied from 10.26 to 15.60% and total sugars from 17.83 to 27.77% on fresh weight basis. Hybrids showed significant variation in maltose content within each group, with means ranging from 7.65% for white-fleshed, to 8.53% in orange- and 11.98% in purple-fleshed. Total mean sugars content was 20.24, 22.11 and 26.84% respectively for white, orange and purple flesh hybrids. No significant correlations were detected between individual sugars but maltose and total sugars content were highly correlated. Compared to the best commercial variety (Baby), 25 hybrids (10.3%) presented a higher maltose content and 40 (16.5%) showed a higher total sugars content. HPTLC was observed as an attractive, cost efficient, high-throughput technique for quantitating maltose and total sugars in sweet potatoes. Perspectives for improving sweet potato quality for consumers’ requirements are also discussed.
Keywords: Clonal generation, Early screening, Hybrids, Phenotypic recurrent selection, Sweet potato
Introduction
Sweet potato (Ipomoea batatas (L.) Lam.) is the seventh most important crop in the world with 110 million tons of fresh storage roots produced annually (FAOSTAT 2015). Its roots are rich in carbohydrates and are consumed baked or boiled, or processed into animal feed, starch, flour, noodles, natural colorants, candy, and alcohol (Woolfe 1992). Breeding programmes aim at combining the chemical compositions corresponding to these different products (high or low dry matter, starch, sugars, proteins, anthocyanins and carotenes) with agronomic objectives (yield, early maturity and resistance to pests and diseases).
The sweet potato is a self-incompatible outcrossing hexaploid with 90 chromosomes. Selected parents planted in isolated crossing blocks are open-pollinated (OP) by bees and other naturally occurring insects. Phenotypic recurrent selection is commonly used as it allows minor and recessive genes to be expressed and selected with a progressive increase in a population. This approach results in the rapid capture of additive effects and the accumulation of suitable genes (Grüneberg et al. 2015). In practice, hundreds of OP hybrids are screened annually for the desirable agronomic traits and the best are planted in a new polycross block for the following cycle of open pollination.
Seedlings of OP hybrids are clonally propagated and multiplied through successive clonal generations to allow accurate phenotyping. Except for very few deleterious traits rapidly detected in the seminal generation (F1), it is necessary to assess the agronomic performance in the first clonal generation (three plants per genotype), second clonal generation (six plants) and so on. This is a fairly time consuming process because only the distal cutting can be taken from each of the plant stems. Heavy selection pressure is applied early for resistance to diseases, such as scab (Elsinoe batatas), and it takes four or five clonal generations before the quality of the roots can be properly assessed (Wilson et al. 1989). Breeders are therefore looking for efficient tools for reliable high-throughput chemotyping of progenies (Kays and Wang 2002).
Breeding for improved quality of sweet potato roots is complex as texture, mouth feel, and taste are directly related to the dry matter (DM), starch and sugar contents of the cooked roots (Champagne et al. 2009; Laurie et al. 2013). When a raw sweet potato root is analysed, only sucrose, glucose and fructose are present. During the cooking process, starch is hydrolysed into maltose by enzymes (α-amylase and β-amylase) and there are significant differences between varieties for the level of starch conversion into maltose (Walter et al. 1975; Picha 1985). Some varieties which are not sweet when raw do not stay non-sweet after cooking due to maltose. Other varieties are not sweeter after cooking because of their very low β-amylase, a trait controlled by one recessive allele (Kumagai et al. 1990). The sweet flavour of baked roots is known to depend mainly to its maltose content (Truong et al. 1986; Sun et al. 1994; Laurie et al. 2013). When screening progenies, a proper analytical technique is therefore needed to quantitate maltose and total sugars contents in cooked roots.
Microwaving sweet potato roots is a suitable cooking process for the fast evaluation of numerous progenies (Grüneberg et al. 2015) and no significant differences between baked and microwaved roots have been detected (Picha 1985; Truong et al. 1986; Lewthwaite et al. 1997). High Performance Liquid Chromatography (HPLC) is the most commonly used technique to quantitate maltose, sucrose, glucose and fructose in cooked sweet potatoes (Waramboi et al. 2011). Unfortunately, HPLC is fairly time consuming as samples have to be injected one after the other through the column and the elution time is at least 10 min per sample (Picha 1985). Gas chromatography (GC) has also been used to analyse sugars in cooked samples (Horvat et al. 1991; Kays et al. 2005) with similar technical constraints.
High performance thin layer chromatography (HPTLC) has been used to measure starch content in wheat flour samples hydrolysed with α-amylase. The method was shown to be a precise, selective, and sensitive for measuring glucose (Aranda et al. 2005). HPTLC has also been used to analyse different carbohydrates mixtures and for quantitative determination of sugars in microbial cultures (Bernardi et al. 2015). When HPTLC performance was compared with HPLC for the analysis of seven sugars (but not maltose) in various food samples, the two techniques were orthogonal to each other with regard to separation and detection. However, their comparison demonstrated HPTLC as better than HPLC for quantitation of sugars with regard to capability of detection, accuracy, and efficiency. Considering the reduced analysis costs and time and the less critical sample preparation, HPTLC is an attractive alternative for quantitation of sugars (Morlock and Gulnar 2012). If a reliable technique for the fast quantitation of maltose in sweet potato clones could be developed, it could serve as a tool for early selection in breeding programmes.
The objectives of the present study were: (1) to develop and test an HPTLC protocol for the fast quantitation of maltose and total sugars in cooked sweet potato roots, (2) to determine the quantitative variation between commercial roots samples of four well appreciated varieties, and (3) to compare 243 OP hybrids in their fifth clonal generation to commercial varieties for their maltose and total sugars contents. Potential applications and perspectives for sweet potato breeding programmes are discussed.
Materials and methods
Plant materials
Sweet potato OP hybrids were evaluated by the Vanuatu sweet potato breeding programme located on Efate Island, central Vanuatu (17′44°S, 168′20°E). More than two thousands OP hybrids were selected through six consecutive generations (the seminal, F1 and five clonal ones: C1 to C5). In F1, the seedlings were mostly selected for vigour and roots yield and for resistance to scab, the major pathogen. In the first clonal generation (C1), hybrids were selected again for the same traits but yield was measured on three individual plants weight (three hills trial, 1 × 1 m, two cuttings per plant). Low yielding hybrids (<one kilo per plant) were discarded. In second to fifth generations, 6–12 plants (1 × 1 m, two cuttings per plant) were evaluated for the same previous traits with an additional care to quality characteristics. Hybrids with attractive appearance of their roots shape with smooth skin were selected. In C4 and C5 (16 plants per genotype), hybrids were evaluated for all of the previous traits plus for tasting quality through consumption preference by local staff. These assessments were mainly qualitative and sensorial. Roots hardness (ease of hand-cutting the central section) was also evaluated because it is related to DM content. Watery sweet potato roots were rejected. About 90% of the hybrids were eliminated during these five successive selection steps. Quantitative evaluation is still in progress to reduce hybrids number while other populations resulting from successive polycrosses are also being screened.
In the present study, all hybrids were studied in their fifth clonal generation. Accessions were planted at the same time and harvested at optimum maturity, when plants were 4 months old, to avoid ontogenic variation. Each OP hybrid was evaluated in small plots of 12 clones (12 m2: 1 × 1 m between plants). For each hybrid, the twelve plants in the plot (3 × 4) were harvested and their roots were bulked together. One root representative of the twelve clones was selected for its good appearance, with no visible insect damage. Hybrids were grouped according to their flesh colour: white (104 hybrids), yellow and/or orange (66 hybrids) and a combination of any colour with purple (73 hybrids). In addition, four commercial varieties (Baby, Salili, Bankis and Téouma) from Port-Vila market, well appreciated to consumers in Vanuatu, were also studied to determine a benchmark for hybrids comparison. For each commercial variety, twenty roots were purchased and analysed to assess intra-variety variation.
Sample preparation
Overall, 323 different samples were prepared and analysed. After harvest, the fresh roots were hand-washed under cool running water, peeled, and a slice of 20 g was cut from the central part of the root. This slice was wrapped in a full-sized paper towel and damped with cold water. The excess water was squeezed out and the 20 g portion was put into a 100 mL beaker. Eight samples at a time were placed in a circle on a glass turntable and cooked for 4 min at 80% heat in a microwave oven (Sharp model R-42 B ST). Microwaved samples were then unwrapped and 20 mL of methanol was poured into the beaker on the cooked sweet potato. The samples were then gently smashed with a fork for approximately 10 min, until transformed into a fine and homogenous purée. The whole purée was then transferred into a 50 mL polypropylene centrifuge tube (CellStar Tubes, Greiner Bio-One GmBH, Frickenhausen, Germany) and the eight samples were centrifuged together at 4500 rpm for 10 min in a Universal 32 centrifuge (Hettich Zentrifugen, Germany). Finally, the supernatant was transferred to 9 mm wide opening screw thread vial of 2 mL in amber glass (Chromacol™, Thermo Fisher™, US) and stored in a fridge at 4 °C in the dark until analysis. The whole sample preparation process was standardised for taking a constant time of less than 1 h, for eight samples, from root cutting to extract placed into the fridge.
Standards and reagents
Maltose, sucrose, glucose and fructose HPLC grade standards were purchased from Sigma-Aldrich (St Quentin, France). Standard stock solutions were prepared by dissolving the appropriate amount of each compound in distilled water (1.0 mg/mL). Stock solutions were stored at 4 °C in the dark and were stable for several months. All solvents were of analytical grade. Acetone, acetonitrile, methanol, phosphoric acid and diphenylamine were also purchased from Sigma-Aldrich (St Quentin, France). Aniline (reagent grade ACS) was purchased from Scharlau (Scharlab S.L., 08181 Sentmemat, Spain). For derivatization after development, the aniline diphenylamine o-phosphoric acid reagent was prepared (4 mL aniline solution + 4 g diphenylamine dissolved in 60 mL acetone and 30 mL o-phosphoric acid). The aniline reagent was stable for months when stored refrigerated at 4 °C.
High-performance thin layer chromatography
Analyses were performed on Merck (Darmstardt, Germany) silica gel 60 F254 plates (glass plates 20 × 10 cm, reference 1.05642.0001), using a Camag (Muttenz, Switzerland) HPTLC system equipped with an automatic TLC sampler (ATS4), an automatic developing chamber (ADC2), a visualiser and a TLC scanner 4. All machines were controlled on-line with winCATS™ software (Camag, Switzerland). Standards and sample solutions were applied band wise (band length of 8 mm, 250 nL/s delivery speed, track distance 8.0 mm and distance from the edge of 15 mm) at a concentration rate of 1 µL per band. Twenty sweet potato extracts were applied on a single silica gel plate. After 30 s of pre-drying, plates were developed at room temperature with a mobile phase of acetonitrile: water (8.5:1.5, v/v) (10 mL) for a maximum migration distance of 85 mm. The glass tank interior was not vapour-saturated with a developing solvent prior to migration. For post-chromatographic derivatization, the plate was automatically immersed into the derivatization reagent with the TLC Immersion Device III (Camag) using a vertical speed of 3 cm/s and 1 s immersion time. Then, the plate was heated on the TLC Plate Heater III (Camag) at 110 °C for 10 min. Visual inspection and documentation of the plates were carried out in white light. Digital images of the plates were documented by the TLC Visualizer documentation system equipped with a high-resolution 12 bit CCD digital camera (Camag). For all plates, images were captured with an exposure time of 35 ms. Plates were scanned in absorbance mode at 520 nm with D2 and W lamp slit dimension 8.00 mm × 0.20 mm, scanning speed 20 mm/s, data resolution 100 µm/step.
Method validation
Peak purity tests were done by comparing UV spectra of the four individual sugars (maltose, sucrose, glucose, fructose) in standard and sample tracks. For determination of the linearity curve, different amounts of stock solutions (0.1, 0.2, 0.3, 0.4, 0.5 µL) of the four standard sugars were applied on HPTLC plates that were developed with the same mobile phase as described above and scanned at 520 nm. The calibration plots of peak area versus concentration were linear for all standards (R 2 > 0.99, P = 0.01). Linear ranges were computed using the least squares method. Repeatability was confirmed by applying five repetitions of each standard at five different concentration levels (0.1, 0.2, 0.3, 0.4, 0.5 µL). The variance among the five repetitions was expressed as the repeatability standard deviation, %RSD (AOAC 2002). Peak areas measurements (in area units, AU) were compared to individual sugars standards and corresponding values were quantitated in %FW. Equivalents for % dry weight (DW) were estimated based on a mean DM of 30.94% measured in a previous study in 240 accessions (Lebot et al. 2011). Sucrose equivalents (SE) were estimated based on the equation: SE = (1.2 × fructose) + (1 × sucrose) + (0.64 × glucose) + (0.43 × maltose) (Shallenberger 1993).
Statistical analysis
For each sample analysed (243 open pollinated hybrids roots plus 80 roots of four commercial varieties), data resulting from scanning of peak areas at 520 nm (absorbance), were entered into Excel™ spread sheet format. Statistical analyses (Spearman coefficients of correlation, mean standard deviation, and ANOVA Fisher’s test of Least Significant Difference (LSD at P < 0.05) were performed using XLStat™ software (Microsoft Corporation).
Results
Method validation
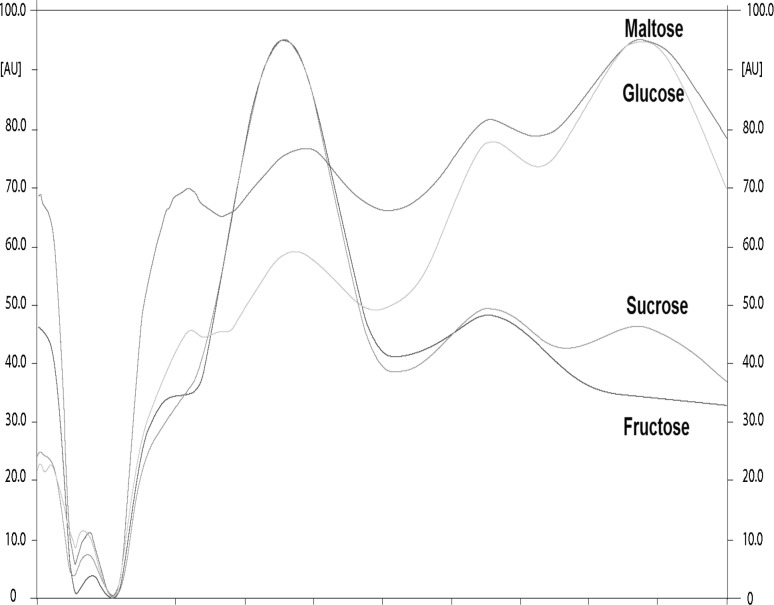
The accuracy, linearity and repeatability tests results of the HPTLC protocol are presented in Table 1. For the four standards, the R 2 coefficients were above 0.99 (P = 0.01). The precision of repetitions (%RSD), estimated by comparing the peaks areas to the concentrations applied, were lower than 3.5% for the four sugars indicating that the method was accurate (AOAC, 2002) for measuring individual sugars in sweet potato. UV spectra of the four pure standards (maltose, sucrose, glucose and fructose) are presented in Fig. 1. Maltose and glucose showed λmax at 640 nm while sucrose and fructose had λmax at 370 nm. However, different scans were conducted with different wavelengths to select the most interesting one and it was observed that by scanning at 520 nm, maltose and sucrose peaks were well separated. As, maltose and sucrose were the most important sugars in all analysed samples, it was decided to keep only one wavelength for the four sugars. Subsequently, all the studied plates were scanned at 520 nm for peaks quantification (in AU). Examples of chromatograms for the four pure standards and for six different sweet potato hybrids are presented in Fig. 2. When the extracts were applied at 0.1 µL, the bands for each sugar were well defined. In all samples, maltose and glucose were the most important sugars and sucrose and fructose were in much lower quantities. In a few hybrids (for example in lane no. 4, Fig. 2) these two sugars were very difficult to detect visually and presented very small peaks and concentrations after scanning.
Table 1.
Linearity of the HPTLC measurements
| Compound: | Pure standards at 520 nm | ||
|---|---|---|---|
| Linear equation | R 2 | RSD % | |
| Maltose | y = 1378.8x + 1995.4 | 0.9957 | 1.81 |
| Sucrose | y = 2477.1x + 4298.2 | 0.9972 | 1.97 |
| Glucose | y = 1792x + 6026.3 | 0.9924 | 2.22 |
| Fructose | y = 2607.3x + 16935 | 0.9945 | 3.16 |
Accuracy and precision of repetitions (peaks areas vs concentrations applied)
R 2, P < 0.01; RSD repeatability standard deviation (n = 5)
Fig. 1.
UV spectra of the four analytical standards. Maltose and glucose present a λmax at 640 nm. Sucrose and fructose present a λmax at 370 nm. Single scanning at 520 nm gives well separated peaks for quantitation of the four sugars (the x-axis units are in nm and the y-axis are in AU, area units)
Fig. 2.

a Peaks absorption after scanning at 520 nm. b HPTLC chromatograms of six hybrids (in lanes 1–6) and four individual pure standards (in lanes 7–10)
Commercial varieties
For each of the four commercial varieties, twenty roots were analysed to assess the extent of variation. The results are presented in Table 2. For the four varieties, maltose and sucrose are much more important than glucose and fructose, both representing together less than 2 %FW. The mean maltose content varies from 10.26 %FW for Salili to 15.60 %FW for Baby. The ratio, allowing the comparison between maltose content vs other sugars (M/(S + G + F)), is significantly higher in Salili with, however, a lower maltose content. The total sugars content varies from 17.83 %FW for Salili to 27.77 %FW for Baby but the difference between Baby and Bankis (25.59 %FW) is not significant (at P < 0.05). These results are consistent with local consumers’ knowledge reporting that Baby is sweeter and more appreciated than Salili and Téouma. Coefficients of variation (cv%) for total sugars means corresponding to twenty commercial roots analysed, ranged from 8% for Bankis to 17% for Baby. This comparatively higher cv% for Baby might be due to longer or more heterogeneous storage conditions before market sale. The total sugars values (estimated in %DW) are comparable with those obtained in previous studies which indicated that good ‘table’ varieties should not have total sugars above 10 %DW (Lebot et al. 2011). The sugars composition of Baby, a well appreciated commercial variety, can be used as a benchmark for evaluating new hybrids. In order to be able to compare our results with other studies, sucrose equivalents (SE) were computed and are presented in Table 2 (SE %FW and %DW). It appears that the four commercial varieties can be considered as “non sweet” as their SE is less than 12 %DW (Kays et al. 2005; Laurie et al. 2013).
Table 2.
Comparison of maltose and total sugars contents in four commercial varieties (n = 20 roots each)
| Variety | Flesh colour | Maltose | Sucrose | Glucose + fructose | Ratio M/(S + G + F) | Total sugars %FW | Total sugars %DW* | SE %FW | SE %DW* | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baby | Orange | Mean | 15.60a | 11.40a | 0.77b | 1.28a | 27.77a | 8.63a | 19.53a | 6.06a |
| S.D. | 3.5 | 1.26 | 0.45 | 0.23 | 4.65 | 1.44 | 3.01 | 0.94 | ||
| cv % | 22.44 | 11.04 | 58.07 | 18.35 | 16.73 | 16.73 | 15.44 | 15.44 | ||
| Bankis | Yellow | Mean | 13.95a | 9.89a | 1.74a | 1.21a | 25.59a | 7.95a | 19.10a | 5.93a |
| S.D. | 1.49 | 0.86 | 0.64 | 0.16 | 1.93 | 0.6 | 1.58 | 0.49 | ||
| cv % | 10.69 | 8.65 | 36.48 | 13.27 | 7.54 | 7.54 | 8.28 | 8.28 | ||
| Téouma | Yellow | Mean | 11.37ab | 9.90a | 1.53a | 1.03b | 22.79a | 7.08a | 17.59b | 5.46b |
| S.D. | 1.88 | 1.71 | 0.57 | 0.28 | 2.24 | 0.69 | 2.08 | 0.65 | ||
| cv % | 16.51 | 17.24 | 37.35 | 51.67 | 9.81 | 9.81 | 9.84 | 9.84 | ||
| Salili | White | Mean | 10.26b | 6.78b | 0.79b | 1.39a | 17.83b | 5.54b | 11.91b | 3.70b |
| S.D. | 1.71 | 1.58 | 0.94 | 0.30 | 2.28 | 0.71 | 1.56 | 0.48 | ||
| cv % | 16.67 | 23.28 | 119.47 | 21.38 | 12.79 | 12.79 | 13.06 | 13.06 |
Means with different letters within each column are significantly different at P < 0.05
* Estimated based on a mean DM of 30.94% FW for 240 breeding clones (Lebot et al. 2011)
Open pollinated hybrids
The results obtained for 243 OP hybrids in their fifth generation are presented in Table 3. Great variation for maltose content was observed within each group (cv% values), with mean values ranging from 7.65% for white-fleshed to 8.23% in orange-fleshed and 11.98% in purple-fleshed hybrids with a significant difference (at P < 0.05). The variation in total sugars content (cv%) was lower within each group, with mean values of 20.24 %FW, 22.11 and 26.84 %FW in white-, orange- and purple-fleshed hybrids, respectively. Compared to the benchmark (Baby), most hybrids presented lower maltose and total sugars contents. For each of the 243 hybrids, maltose and total sugars contents are presented in Fig. 3. If benchmarks of 15.6% for maltose and of 27.7% for total sugars (values for Baby) are used, it appears that only 25 hybrids (10.3%) present higher maltose and 40 (16.5%) higher total sugars content (Fig. 3). The most preferred commercial variety (Baby) is an orange-fleshed variety but the analysis of 66 orange-fleshed hybrids revealed tremendous variation for maltose (1.36–15.95 %FW) and total sugars (10.31–34.34 %FW). On average, purple-fleshed hybrids presented a significantly higher mean maltose and total sugars contents (11.98 and 26.84 %FW) but the range of variation within this group is also very important with cv% of 41.45 for maltose and 21.11 for total sugars (Table 3). Correlation coefficients between individual sugars were computed but no significant correlations were observed. Maltose and total sugars content are highly correlated (0.799, P = 0.01) (Fig. 3).
Table 3.
Comparison of maltose and total sugars content in 243 hybrids in their fifth clonal generation grouped according to their root flesh colours
| Flesh colour: | Maltose | Sucrose | Glucose + fructose | Ratio M/(S + G + F) | Total S %FW | Total S %DW* | SE %FW | SE %DW* | |
|---|---|---|---|---|---|---|---|---|---|
| White | Min | 0.66 | 0.89 | 0.60 | 0.04 | 2.31 | 0.72 | 2.31 | 0.72 |
| n = 104 | Max | 21.50 | 11.53 | 13.81 | 2.14 | 36.14 | 11.22 | 34.53 | 10.73 |
| Mean | 7.65b | 7.80a | 4.79b | 0.67b | 20.24b | 6.29b | 18.95b | 5.88b | |
| S.D. | 4.19 | 1.77 | 2.84 | 0.43 | 4.88 | 1.51 | 4.91 | 1.53 | |
| cv % | 54.73 | 22.65 | 59.18 | 63.96 | 24.10 | 24.10 | 25.92 | 25.92 | |
| Orange | Min | 1.36 | 4.26 | 0.84 | 0.08 | 10.31 | 3.20 | 10.21 | 3.17 |
| n = 66 | Max | 15.95 | 13.56 | 15.38 | 1.45 | 34.34 | 10.66 | 35.30 | 10.96 |
| Mean | 8.23b | 8.70a | 5.17b | 0.63b | 22.11b | 6.87b | 20.73b | 6.44b | |
| S.D. | 3.79 | 2.06 | 3.19 | 0.34 | 4.69 | 1.45 | 5.10 | 1.58 | |
| cv % | 46.03 | 23.68 | 61.66 | 53.19 | 21.19 | 21.19 | 24.62 | 24.62 | |
| Purple | Min | 2.16 | 3.84 | 1.41 | 0.14 | 15.79 | 4.90 | 13.08 | 4.06 |
| n = 73 | Max | 24.39 | 14.18 | 16.99 | 2.04 | 39.85 | 12.38 | 39.46 | 12.25 |
| Mean | 11.98a | 8.33a | 6.52a | 0.86a | 26.84a | 8.33a | 24.18a | 7.51b | |
| S.D. | 4.96 | 2.01 | 3.30 | 0.43 | 5.67 | 1.76 | 5.58 | 1.73 | |
| cv % | 41.45 | 24.13 | 50.62 | 49.56 | 21.11 | 21.11 | 23.07 | 23.07 |
Means with different letters within each column are significantly different at P < 0.05
* Estimated based on a mean DM of 30.94% FW for 240 breeding clones (Lebot et al. 2011)
Fig. 3.
Comparison of maltose (blue bars) and total sugars (red bars) contents for 243 hybrids (white = 104, orange = 66, purple = 73). The benchmarks corresponding to the maltose and total sugars values for the commercial variety Baby are indicated (black horizontal lines). 25 hybrids (10.3%) present higher maltose content 40 hybrids (16.5%) present higher total sugars content (the x-axis represents the different accessions analysed and the y-axis represents the sugars contents in %) (color figure online)
Discussion
Staple-type varieties
There is a variation in maltose content between the twenty roots of the same commercial variety within reasonable range (Table 2). It has already been observed that there is a variation in the level of sweetness within an individual variety because of production and postharvest factors (Adu-Kwarteng et al. 2014). It is still unclear, however, if this variation is due to lower levels of sugars in the raw root or to reduced starch hydrolysis depending on storage conditions. The four commercial varieties used for benchmarking in this study can be considered as “non sweet”. For comparison, the total sugars concentration of cultivar Jewel, widespread in the US, has been found to be 39 %FW after baking (Koehler and Kays 1991). Total sugars content measured on seven Taiwanese cultivars (Lai et al. 2013) varied from 15.1 to 19.1 %DW and are higher than those found for the four commercial varieties analysed here (ranging from 5.54 to 8.63 %DW (Table 2). Our values are, however, comparable to those presented by four staple-type varieties in Ghana (ranging from 7.36 to 10.34 %DW) (Adu-Kwarteng et al. 2014).
Sweet potato varieties can be classified into four classes based on their sugar concentration and changes during cooking: (1) low sugars/low starch (−/−), (2) low sugars/high starch (−/+), (3) high sugars/low starch (+/−) and (4) high sugars/high starch (+/+) (Morrison et al. 1993). In Vanuatu and in the Pacific, where sweet potato consumption per capita and per year is very high compared to other countries in the World (Bourke 2009; FAOSTAT 2015), the focus is on class 2 (−/+). Kays et al. (2005) analysed 272 clones originating from 34 different countries and compared the relative sweetness of their baked roots as sucrose equivalents (SE). The majority (93%) of the sweet potato varieties tested were classified as moderate and only 4.4% as very high. Maltose accounted for 42% of the total SE and the results presented here give comparable values for maltose analysed with HPTLC. However, our SE %DW values are on average quite low (<12%) and most hybrids can be considered as “non sweet”. Our results are, however, consistent with a previous survey conducted on 164 uncooked varieties originating from five different South Pacific countries which reported low mean value for total sugars (2.38 %FW) (Bradbury and Holloway 1988).
Comparison with other analytical techniques
On average, the chemical composition of cooked sweet potato roots presents higher levels of maltose, lower levels of sucrose and very low (and equivalent) levels of glucose and fructose (Tables 2, 3). This has been reported in previous studies using HPLC (Picha 1985; Lewthwaite et al. 1997; Lai et al. 2013) and the results obtained in the present study using HPTLC confirm this decreasing order of proportions between the four sugars. In New Zealand for example, the sugars content of ten different varieties measured by HPLC and expressed as %FW, varied for maltose from 2.4 to 6.2%, for sucrose from 1.1 to 4.3, for glucose from 0.3 to 1.7 and for fructose from 0.2 to 1.4 (Lewthwaite et al. 1997). These figures are slightly lower than our values for individual and total sugars expressed as %FW (Tables 2, 3). Our higher standard deviations of the means are most likely due to the greater number of hybrids analysed in our study. A limited number of varieties were analysed by HPLC, ranging from six varieties (Picha 1985), four (Truong et al. 1986), ten (Lewthwaite et al. 1997), 25 (Waramboi et al. 2011), and seven varieties (Lai et al. 2013).
Depending on genotypes, it has been shown that starch is highly and positively correlated with DM content of the raw root (0.497 at P = 0.01). The total sugars content, which is the most important factor determining quality and consumers’ acceptance, is negatively correlated with %DM and starch (−0.325, −0.812, respectively) (Lebot et al. 2011). In a breeding programme, a severe early selection focus on high %DM and starch conducted throughout the first clonal generations of the evaluation process would most likely reduce the total sugars content of the selected clones. The results presented in Table 3 confirm that most hybrids have low total sugars values. Even if there is still, remarkable variation within each group, the maximum values remain rather low with 36.14 %FW for white-fleshed hybrids and 34.34 and 39.85 %FW for orange- and purple-fleshed hybrids, respectively. When converted into SE values, these hybrids can be considered as “non sweet” (Kays et al. 2005). Maltose has been shown to be positively and significantly related with %DM of the raw root (Lewthwaite et al. 1997). Other studies have shown that varieties with high DM content have high sucrose and low glucose and fructose levels (Zhang et al. 2002; Mcharo and LaBonte 2007). In the present study, no significant correlations were detected between individual sugars.
It appeared, difficult to compare different studies results. For example, the US cultivar Beauregard analysed with HPLC in New Zealand presented 2.44 %FW maltose, 1.31% sucrose, 1.72% glucose and 1.22% fructose, giving a total sugars content of 7.45 %FW which can be converted to SE of 26.58 %DW (Lewthwaite et al. 1997). The same cultivar presented SE of 43.5 %DW when analysed in the US by GC (Kays et al. 2005). Different growing conditions, post-harvest time and storage, analytical protocols for extraction and for chromatographic analysis may have led to different results for the same clone.
Importance of maltose for hybrid selection
Dry matter, starch and sugars contents do not exclusively control sweet potato taste and flavour. The evaluation of new varieties must be conducted for taste by panel of expert consumers (Laurie et al. 2013) and it was known that different sugars differ in consumers’ perceived sweetness. Once selected, the best varieties are compared for their agronomic performances in multi environment trials that also include taste rating of the cooked roots evaluated by farmers and extension personnel for tastiness (i.e. flavour, sweetness, dry texture) (Laurie et al. 2013). Fructose, sucrose and glucose are sweeter than maltose by factors 5×, 3× and 2×, respectively (Biester et al. 1925; Shallenberger 1993) but maltose is the most important sugar contributing to cooked sweet potato taste (Lewthwaite et al. 1997; Laurie et al. 2013). When individual sugars are added to a purée produced from sweet potato baked root, panellists consistently rank the sugars in the same order of preference: 1-maltose, 2-sucrose and 3-fructose (Koehler and Kays 1991). If different varieties are assessed for sensory characteristics and consumer acceptability, significant correlations of maltose content with starch and DM contents are observed. Maltose content has a positive correlation with sweet flavour and sweet potato-like flavour (Laurie et al. 2013). Maltose is also the primary precursor of the numerous volatile aromatic compounds produced by cooking (Sun et al. 1994).
Conclusion
No significant correlations between root flesh colour, maltose and total sugars contents were observed however significant variation existed within each group of genotypes. Maltose was a major component of quality and new tool, such as the HPTLC protocol described here, was needed for rapid quantitative analysis of maltose in sweet potato breeding clones. Pure analytical standard for maltose can be applied on each silica gel plate of 20 tracks allowing accurate quantitation of 19 hybrids per plate in less than 1 h with only 10 mL of solvents. HPTLC is, therefore, an eco-friendly and cost efficient analytical technique for high-throughput analysis of maltose and total sugars in early clonal generations of sweet potato breeding programmes.
Acknowledgements
This study would not have been possible without the financial support of the Agence Nationale pour la Recherche (ANR, France, through the Project No. ANR-10-STRA-007) and the technical assistance of the Department of Agriculture and Rural Development (DARD, Vanuatu). Special thanks are due to Charles Rogers for germplasm field maintenance and to Juanita Stevens for laboratory assistance in preparing the samples and extracts.
Compliance with ethical standards
Conflict of interest
The author declares that there are no potential conflicts of interest.
References
- Adu-Kwarteng E, Sakyi-Dawson EO, Ayernor GS, Truong VD, Shih FF, Daigle K. Variability of sugars in staple-type sweet potato (Ipomoea batatas) cultivars: the effects of harvest time and storage. Int J Food Prop. 2014;17:410–420. doi: 10.1080/10942912.2011.642439. [DOI] [Google Scholar]
- AOAC (2002) Guidelines for single laboratory validation of chemicals methods for dietary supplements and botanicals. http://www.aoac.org/aoac_prod_imis/AOAC.../SLV_Guidelines_Dietary_Supplements.pdf. Accessed Mar 2015
- Aranda MB, Vega MH, Villages RF. Routine method for quantification of starch by planar chromatography (HPTLC) J Planar Chrom. 2005;16:285–289. doi: 10.1556/JPC.18.2005.4.6. [DOI] [Google Scholar]
- Bernardi T, Pedrini P, Marchetti MG, Tamburini E. Separation and quantitative determination of carbohydrates in microbial submerged cultures using different planar chromatography techniques (HPTLC, AMD, OPLC) J Anal Bioanal Tech. 2015;6:247. [Google Scholar]
- Biester A, Wood MW, Wahlin CS. Carbohydrate studies 1. The relative sweetness of pure sugars. Am J Physiol. 1925;73:387–396. [Google Scholar]
- Bourke M. Sweetpotato in Oceania, Chap 22. In: Loebenstein G, Thottappilly G, editors. The sweetpotato. Berlin: Springer; 2009. pp. 489–502. [Google Scholar]
- Bradbury JH, Holloway WD (1988) Chemistry of tropical root crops: significance for nutrition and agriculture in the Pacific. ACIAR Monograph No 6
- Champagne A, Legendre L, Lebot V. Chemotype profiling to guide breeders and explore traditional selection of root crops in Vanuatu, South Pacific. J Agric Food Chem. 2009;57:10363–10370. doi: 10.1021/jf903321e. [DOI] [PubMed] [Google Scholar]
- FAOSTAT (2015) Database food and agriculture organization of the United Nations. http://www.fao.org/3/a-i4691e.pdf. Accessed Apr 2016
- Grüneberg WJ, Ma D, Mwanga ROM, Carey EE, Huamani K, Diaz F, Eyzaguirre R, Guaf E, Jusuf M, Karuniawan AK, et al. Advances in sweetpotato breeding from 1993 to 2012. In: Low J, Nyongesa M, Quinn S, Parker M, et al., editors. Potato and sweetpotato in Africa transforming the value chains for food and nutrition security. Wallingford: CABI; 2015. pp. 1–77. [Google Scholar]
- Horvat RJ, Arrendale GG, Dull GW, Chapman JR, Kays SJ. Volatile constituents and sugars of three diverse cultivars of sweet potatoes [Ipomoea batatas (L) Lam] J Food Sci. 1991;56(3):714–715, 742. doi: 10.1111/j.1365-2621.1991.tb05363.x. [DOI] [Google Scholar]
- Kays SJ, Wang Y (2002) Sweetpotato quality: its importance, assessment and selection in breeding programs. In: Ames T (eds) Proc 1st IS on sweetpotato. Acta Hort 583, ISHS 2002, pp 187–193
- Kays SJ, Wang Y, McLaurin WJ. Chemical and geographical assessment of the sweetness of the cultivated sweetpotato clones of the world. J Am Soc Hort Sci. 2005;130(4):591–597. [Google Scholar]
- Koehler PE, Kays SJ. Sweet potato flavour: quantitative and qualitative assessment of optimum sweetness. J Food Quality. 1991;14:241–249. doi: 10.1111/j.1745-4557.1991.tb00065.x. [DOI] [Google Scholar]
- Kumagai T, Umemura Y, Baba T, Iwanaga M. The inheritance of β-amylase null in storage roots Ipomoea batatas (L) Lam. Theor Appl Genet. 1990;79:369–376. doi: 10.1007/BF01186081. [DOI] [PubMed] [Google Scholar]
- Lai YC, Huang CL, Chan CF, Lien CY, Liao WC. Studies of sugar composition and starch morphology of baked sweet potatoes (Ipomoea batatas (L) Lam) J Food Sci Technol. 2013;50(6):1193–1199. doi: 10.1007/s13197-011-0453-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie SM, Faber M, Calitz FJ, Moelich EI, Muller N, Labuschagne MT. The use of sensory attributes, sugar content, instrumental data and consumer acceptability in selection of sweet potato varieties. J Sci Food Agric. 2013;93(7):1610–1619. doi: 10.1002/jsfa.5932. [DOI] [PubMed] [Google Scholar]
- Lebot V, Ndiaye A, Malapa R. Phenotypic characterization of sweet potato [Ipomoea batatas (L) Lam] genotypes in relation to prediction of chemical quality constituents by NIRS equations. Plant Breed. 2011;130(4):457–463. doi: 10.1111/j.1439-0523.2010.01840.x. [DOI] [Google Scholar]
- Lewthwaite SL, Sutton KH, Triggs CM. Free sugar composition of sweetpotato cultivars after storage. New Zeal J Crop Hort Sci. 1997;25:33–41. doi: 10.1080/01140671.1997.9513984. [DOI] [Google Scholar]
- Mcharo M, LaBonte D (2007) Genotypic variation among sweet potato clones for beta-carotene and sugar contents. In: Proceedings of the 13th Symposium of ISTRC, Tanzania, November, pp 746–754
- Morlock GE, Gulnar S. Comparison of two orthogonal liquid chromatographic methods for quantification of sugars in food. J Liq Chromatogr Relat Technol. 2012;34(10–11):902–919. [Google Scholar]
- Morrison TA, Pressey R, Kays SJ. Changes in α- and β-amylase activities during storage of sweetpotato lines with varying starch hydrolysis potential. J Am Soc Hort Sci. 1993;118:236–242. [Google Scholar]
- Picha DH. HPLC determination of sugars in raw and baked sweet potatoes. J Food Sci. 1985;50(1189–1190):1210. [Google Scholar]
- Shallenberger RS. Taste chemistry. London: Blackie Academic; 1993. [Google Scholar]
- Sun YB, Severson RF, Kays SJ. Effects of heating temperature and microwave pretreatment on the formation of sugars and volatiles in Jewel sweet potato. J Food Qual. 1994;14:447–456. doi: 10.1111/j.1745-4557.1994.tb00165.x. [DOI] [Google Scholar]
- Truong VD, Biermann CJ, Marlett JA. Simple sugars, oligosaccharides and starch concentrations in raw and cooked sweet potato. J Agric Food Chem. 1986;34:421–425. doi: 10.1021/jf00069a010. [DOI] [Google Scholar]
- Walter WM, Jr, Purcell AE, Nelson AM. Effects of amyolytic enzymes on “moistness” and carbohydrates changes of baked sweet potato cultivars. J Food Sci. 1975;40:793–796. doi: 10.1111/j.1365-2621.1975.tb00558.x. [DOI] [Google Scholar]
- Waramboi JG, Dennien S, Gidley MJ, Sopade PA. Characterisation of sweetpotato from Papua New Guinea and Australia: physicochemical, pasting and gelatinisation properties. Food Chem. 2011;126:1759–1770. doi: 10.1016/j.foodchem.2010.12.077. [DOI] [PubMed] [Google Scholar]
- Wilson JE, Pole FS, Smith NEJM, Taufatofua P. Sweet potato breeding. Western Samoa: IRETA-USP Agro-Facts Publication; 1989. p. 39p. [Google Scholar]
- Woolfe JA. Sweet potato, an untapped food resource. Cambridge: Cambridge University Press; 1992. p. 643p. [Google Scholar]
- Zhang Z, Wheatley CC, Corke H. Biochemical changes during the storage of sweet potato roots differing in dry matter content. Postharv Biol Technol. 2002;24:317–325. doi: 10.1016/S0925-5214(01)00149-1. [DOI] [Google Scholar]