Abstract
Satureja avromanica is an indigenous plant which is frequently used as a spice in Avraman-Kurdistan region of Iran. The present study aimed to investigate the chemical composition, antimicrobial and antioxidant properties of the S. avromanica. In addition, rosmarinic acid and total phenolic content of S. avromanica was assessed by spectrophotometric method and HPTLC. The essential oil and methanolic extract were isolated by hydrodistillation and maceration methods, respectively. A total of 32 compounds representing 98.6% of the essential oil were identified by GC–MS and GC–FID. The main constituents were n-pentacosane (23.8%), spathulenol (11.5%), β-bourbonen (11.3%) and n-docosane (11.0%). The antibacterial activity of samples were carried out by disc diffusion method and evaluate the minimal inhibitory concentration (MIC) essential oil and methanolic extract were found to be effective against Staphylococcus aureus, Bacillus cereus and Bacillus pumilus. The highest scavenging activity was found for methanolic extract of S. avromanica (21.58 µg/mL) and the total phenolics of methanolic extract of S. avromanica was 95.3 mg GAE/g. The rosmarinic acid content of S. avromanica methanolic extract was 0.83 mg/g plant. Antioxidant activity and rosmarininc acid content of S. avromanica suggests that the essential oil and methanolic extract of S. avromanica has great potential for application as a natural antimicrobial and antioxidant agent to preserve food.
Keywords: Satureja avromanica, Spice, Antimicrobial, Antioxidant, Essential oil, Rosmarinic acid
Introduction
Recently, there is a growing interest in natural substances exhibiting antioxidant and antimicrobial properties, especially those of plant origin, that are supplied to human and animal organisms as food components or as specific pharmaceutics (Dastan et al. 2014; Ghahremani-majd et al. 2012; Ozkan et al. 2007; Mohammadi et al. 2012).
Synthetic antimicrobial chemicals are sometimes associated with adverse effects including allergic reaction, hypersensitivity and immunity suppression. Therefore, there has been a growing interest in research concerning alternative natural antimicrobial, antifungal and antioxidant agents, including natural products from medicinal plants.
The essential oils and extracts from various species of plants are relatively less damaging to human health. As well as many applications including raw and processed food preservation, medicine and pharmaceuticals were reported for medicinal plants (Cakir et al. 2005; Gao et al. 2011).
The role of active oxygen species and free radicals is becoming increasingly recognized in the pathogenesis of many human diseases, including aging, cancer and atherosclerosis (Özer et al. 2007; Naseri et al. 2013).
The genus Satureja L., (Lamiaceae) contains about 200 species of herbs, shrubs and mainly aromatic plants with wide distribution in the Mediterranean area, Asia and boreal America (Senatore et al. 1998). From ages past, Satureja species have been used as culinary herbs, spices and flavorings and in Iranian folk medicine the aerial parts of several species of this genus are used to treat various diseases such as urinary tract infections, upper respiratory tract infections, gastroenteritis, wounds and diarrhea (Behravan et al. 2004). Satureja species has been extensively investigated as a source of natural products with potential antimicrobial, antioxidant, analgesic, antiseptic, antiviral, antiproliferative, antiprotozoal, antidiarrheal, anti-inflammatory, antinociceptive and vasodilatory activities (Azaz et al. 2005; Eftekhar et al. 2009; Gohari et al. 2005). S. avromanica, one of the species in the Satureja genus, is frequently used as a spice in Avraman-Kurdistan region (West of Iran).
The chemical components of the essential oil and extract of plants allowed their use in traditional medicine and as a food preservative. Various pharmaceutical and biological activities like, antibacterial, antifungal, antioxidant, antiinflammatory, antiviral, anticancer, antimutagenic, antidiabetic and antiprotozoal properties are assigned to them (Raut and Karuppayil 2014; Shan et al. 2007; Singh et al. 2006; Mumivand et al. 2010).
Rosmarinic acid, an economically important metabolite is a biologically active phenolic acid from Satureja species (Lamiaceae). This compound showed various biological properties including antioxidative, anti-inflammatory, antibacterial, antimutagen, and antiviral activities (Tepe and Sokmen 2007).
To our knowledge, the phytochemical study and biological activities of the S. avromanica have not been investigated. Therefore, the present study aimed to investigate the chemical composition, antimicrobial and antioxidant properties of the S. avromanica. In addition, rosmarinic acid and total phenolic content of methanolic extract from S. avromanica was assessed that has not been reported to date.
Materials and methods
Plant material
The aerial parts of S. avromanica were collected in September 2014 from Avraman Mountains (Kurdistan Province, Iran) and dried in shadow. Voucher specimens have been deposited at the Herbarium of the Research Institute of Forests and Rangelands Researches by Hossein Maroufi, Sanadaj, Iran, under voucher no. (8504) (Maroofi 2010) (Fig. 1).
Fig. 1.

Satureja avromanica Maroofi
Preparation of the methanolic extracts
The air dried plant (100 g) was extracted successively with 500 mL of methanol by using a Soxhlet extractor (ISOPAD, Heidelberg, Germany) for 24 h in a hot water bath at a temperature not exceeding the boiling point of the solvent (Ahmadi et al. 2010). The resulting extract was filtered using Whatman filter paper (no. 1) and then concentrated in vacuo at 40 °C using a rotary evaporator (Heidolph, Laborota 4000, Schwabach, Germany). The residues obtained were stored at 4 °C until tested and analyzed.
Extraction of the essential oil
The essential oil of air dried plant (100 g) was isolated by hydrodistillation for 3 h, using a Clevenger-type apparatus as previously reported (Pezhmanmehr et al. 2010). The obtained essential oil was dried over anhydrous sodium sulfate and, after filtration, stored at 4 °C until tested and analyzed.
Analysis of the essential oil
GC–FID and GC–MS analysis
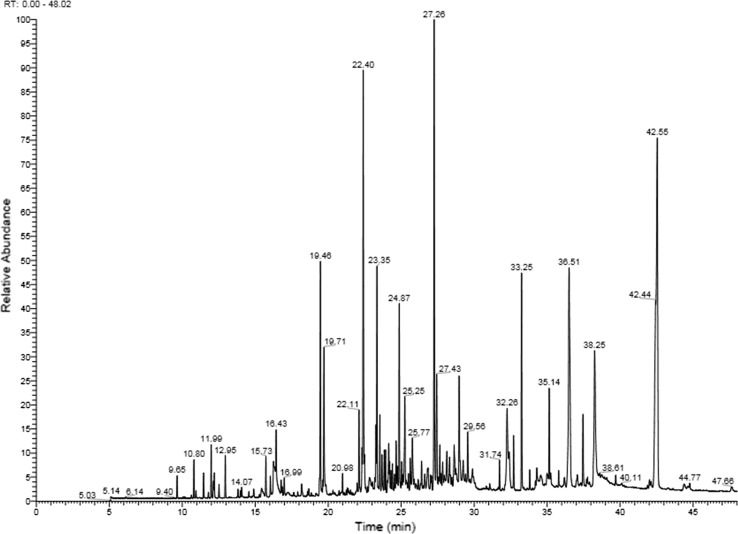
GC analysis was carried out on a Thermoquest–Finnigan Trace gas chromatograph with a flame ionization detector (FID). The analysis was performed by using fused silica capillary DB-5 column (60 m × 0.25 mm; film thickness 0.25 μm). The oven temperature was programed from 60 to 250 °C at the rate of 5 °C/min, and finally held isothermally for 10 min. Nitrogen was used as carrier gas at a flow rate of 1.1 mL/min. The injector and detector temperatures were kept at 250 and 300 °C, respectively. GC–MS analysis was carried out on a Thermoquest–Finnigan gas chromatograph equipped with above mentioned column, used under the same conditions coupled to a TRACE mass spectrometer. Helium was used as the carrier gas at a flow rate of 1.1 mL/min with a split ratio of 1:50. The quadrupole mass spectrometer was scanned over 45–465 amu (atomic mass unit) with an ionizing voltage of 70 eV. Ion source and interface temperatures were kept 200 and 250 °C, respectively (Fig. 2).
Fig. 2.
GC–MS spectrum of S. avromanica essential oil
Identification of compounds
The constituents of the essential oil were identified by calculation of their retention indices under temperature programmed conditions for homologous series of n-alkanes (C6–C24) and the essential oil on a DB-5 column under the same chromatographic conditions. For identification of individual compounds, their mass spectra was compared by internal reference mass spectra library or with authentic compounds and confirmed by comparison of their retention indices with authentic compounds or with those of reported in the literature (Adams 2001b; Shibamoto 1987).
Antimicrobial activity
The essential oil and methanolic extract from S. avromanica were tested individually against a range of 11 microorganisms, including Bacillus subtilis (ATCC 465), Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29737), Escherichia coli (ATCC 25922), Staphylococcus epidermidis (ATCC 12228), Klebsiella pneumoniae (ATCC 10031), Pseudomonas aeruginosa (ATCC 85327), Bacillus cereus (PTCC 1015), Bacillus pumilus (PTCC 1274), Saccharomyces cerevisiae (ATCC 9763) and Candida albicans (ATCC 10231).
Disc diffusion assay
The antibacterial activity of samples were carried out by disc diffusion method (Eftekhar et al. 2009). Briefly, 0.1 mL of a suspension of the test microorganism (108 cells mL−1) was spread on Mueller–Hinton Agar plates and Sabouraud Dextrose Agar for bacteria and fungi, respectively. About 10 μL of samples was injected to the sterile disc papers (Whatman No. 1, diameter 6 mm). Then the prepared discs were placed on the culture medium. Then the inoculated plates were incubated at 37 °C for 24 h for the bacteria and at 30 °C for 48 h for the fungi. The diameter of the clear zone around the disc was measured and reported in millimeters as its antimicrobial activity. Triplicate tests were performed in all experiments.
Determination of minimum inhibitory concentration (MIC)
The minimal inhibitory concentration (MIC) values were also studied for the essential oil and methanolic extract of plant (Eftekhar et al. 2009). Serial twofold dilutions of the samples were made in Sabouraud Dextrose Broth with 0.5% Tween 80 for the fungi and Mueller–Hinton Broth containing 0.5% Tween 80 for the bacteria in 96-well micro titer plates. Fresh microbial suspensions prepared from overnight grown cultures in the same media were added to give a final concentration of 5 × 105 organisms mL−1. A positive control (containing inoculums but no essential oil or methanolic extracts) and negative control (containing essential oil and extracts but no inoculums) were included on each microplate. The contents of the wells were mixed and the microplates were incubated at 37 °C for 24 h for the bacteria and 30 °C for 48 h for the fungi. The MIC was defined as the lowest concentration of the compounds to inhibit the growth of microorganisms.
Antioxidant properties
The capacity of S. avromanica essential oil and methanolic extract to scavenge DPPH were determined according to the technique reported by Esmaeili et al. (2010). The 1.5 mL of various concentrations of essential oil and methanolic extract in ethanol was added to 1.5 mL of a 0.25 mM ethanol solution of DPPH. The mixture was shaken vigorously in the darkness and allowed to reach a steady state at room temperature for 30 min. Then, decolorization of DPPH was determined by measuring the absorbance at 517 nm against a blank with a Varian spectrophotometer (Cary-100 Bio Varian, Melbourne, Australia). The DPPH radicals scavenging activity was calculated using the following equation: Scavenging activity (%) = [(A0 − A1/A0) × 100], where A0 is the absorbance of the control (blank, without essential oil and extract) and A1 is the absorbance in the presence of the essential oil and extract or standard sample. Tests were carried out in triplicate and butylated hydroxytoluene (BHT) was used as positive control.
Rosmarinic acid measurement
Rosmarinic acid content of S. avromanica methanolic extract was determined using a high Performance thin-layer Chromatography (HPTLC) method (Ebrahimi et al. 2008). Briefly Separation was performed on 20 cm × 10 cm silica gel TLC Aluminum plates with fluorescence indicator F254 (Merck, Darmstadt, Germany) with toluene–ethyl acetate–formic acid (5:4:1) as mobile phase. The determination was carried out by using the densitometry absorbance mode at 329 nm using a CAMAG TLC Scanner 3. The amount of rosmarinic acid in S. avromanica methanolic extract was determined according to a calibration curve (Y = 94.35X × 1284.2) with 0.9901 as correlation coefficient for. 1–150 µg/mL of rosmarinic acid from Sigma-Aldrich Company used for achieve calibration curve. Tests were carried out in triplicate.
Determination of total phenolics
The total phenolics content (TPC) of S. avromanica methanolic extract was determined according to the Folin–Ciocalteu procedure (Ghahremani-majd et al. 2012). Total phenols content was expressed as mg gallic acid equivalents per g plant extract (mg (GAE)/g sample).
Results and discussion
Chemical compositions of the essential oil
Essential oil compositions of the S. avromanica have not been reported earlier. Hydrodistillation of dried aerial parts of S. avromanica had a light yellow color essential oil in 0.8% yield (v/w%) relative to dry weight of plant. Thirty-two compounds representing 98.6% of the essential oil were identified. The compounds were identified by GC–MS and quantified by GC–FID. A list of identified compounds is presented in Table 1. The compounds are listed according to their elution from the DB-5 column.
Table 1.
Essential oil compositions of S. avromanica
| No. | Compound | RI (lit)a | RI (Cal)b | Percentage (% ± SDc) |
|---|---|---|---|---|
| 1 | p-Cymene | 1025 | 1020 | 0.8 ± 0.0 |
| 2 | γ-Terpinene | 1060 | 1054 | 0.6 ± 0.0 |
| 3 | Linaloole | 1097 | 1095 | trd |
| 4 | Camphore | 1146 | 1145 | tr |
| 5 | iso-Menthone | 1163 | 1158 | 0.5 ± 0.0 |
| 6 | neo-Menthol | 1166 | 1161 | 2.7 ± 0.2 |
| 7 | Terpinene-4-ol | 1177 | 1174 | 3.4 ± 0.2 |
| 8 | Geraniol | 1253 | 1250 | tr |
| 9 | Carvenone | 1258 | 1253 | tr |
| 10 | Bornyl acetate | 1289 | 1287 | 5.4 ± 0.4 |
| 11 | Thymol | 1290 | 1289 | tr |
| 12 | Carvacrol | 1299 | 1297 | tr |
| 13 | dihydroedulan | 1305 | 1312 | 3.6 ± 0.3 |
| 14 | α-Copaene | 1377 | 1374 | 1.4 ± 0.1 |
| 15 | β-Cubebene | 1388 | 1387 | 2.1 ± 0.2 |
| 16 | β-Bourbonen | 1388 | 1389 | 11.3 ± 0.8 |
| 17 | Vanillin | 1394 | 1393 | tr |
| 16 | trans-Caryophyllene | 1419 | 1417 | 4.1 ± 0.3 |
| 18 | β-gurjunene | 1434 | 1432 | 1.2 ± 0.1 |
| 19 | Aromadendrene | 1441 | 1439 | 0.6 ± 0.0 |
| 20 | γ-Muurolene | 1480 | 1477 | 0.7 ± 0.0 |
| 21 | γ-Himachalene | 1483 | 1481 | tr |
| 22 | Germacrene D | 1485 | 1484 | 3.6 ± 0.2 |
| 23 | Bicyclogermacrene | 1500 | 1500 | 3.2 ± 0.3 |
| 24 | Cubebol | 1515 | 1514 | tr |
| 25 | δ-Cadinene | 1523 | 1522 | 1.1 ± 0.0 |
| 26 | Spathulenol | 1578 | 1577 | 11.5 ± 0.6 |
| 27 | Globulol | 1585 | 1590 | 2.9 ± 0.0 |
| 28 | Virdiflorol | 1593 | 1592 | 0.6 ± 0.0 |
| 29 | β-Eudesmol | 1651 | 1649 | 2.5 ± 0.1 |
| 30 | Valerianol | 1658 | 1653 | tr |
| 31 | n-Docosane | 2200 | 2205 | 11.0 ± 0.5 |
| 32 | n-Pentacosane | 2500 | 2504 | 23.8 ± 1.1 |
| Monoterpene hydrocarbons | 1.4 | |||
| Oxygenated monoterpenes | 15.6 | |||
| Sesquiterpene hydrocarbons | 29.3 | |||
| Oxygenated Sesquiterpene | 17.5 | |||
| n-Alkan | 34.8 | |||
| Total identified | 98.6 |
The major compounds were n-pentacosane (23.8%), spathulenol (11.5%), β-bourbonen (11.3%), n-docosane (11.0%), bornyl acetate (5.4%) and trans-caryophyllene (4.1%). The predominant essential oil were n-alkan with 34.8% followed by 29.3% sesquiterpene hydrocarbons. Inspection of the literature revealed that thymol and carvacrol are the main components in the essential oil of Satureja species but according to Table 1, the amount of thymol and carvacrol in S. avromaica essential oil were in trace (Farsam et al. 2004; Gohari et al. 2006; Hadian et al. 2010; Novak et al. 2006; Oke et al. 2009; Sefidkon and Jamzad 2004). The essential oil composition of S. avromanica was qualitatively more similar to S. isophylla so that the amount of thymol and carvacrol in S. isophylla were 0.0 and 1.0%, respectively (Sefidkon and Jamzad 2006). These results can be used for further study on the taxonomy of Satureja genus.
Antimicrobial activity
It was previously reported that the essential oil and extracts of some members of the Satureja genus, including S. spicigera, S. cuneifolia, S. bachtiarica and S. hortensis had antimicrobial activity (Eftekhar et al. 2009; Falsafi et al. 2015; Oke et al. 2009; Zeidán-Chuliá et al. 2015). Antimicrobial activities of the essential oil and methanolic extract of S. avromanica were determined by the application of disc diffusion and MIC tests against six Gram-positive and three Gram-negative bacteria, as well as two fungi. As can be seen from Table 2, the Gram-positive bacteria were found to be the most sensitive strains. The results indicated that the methanolic extract had moderate to high inhibitory activity against the B. pumilus and B. cereus. Furthermore, we saw a resistance of Candida albicans and Pseudomonas aeruginosa against the essential oil and methanolic extract of plant.
Table 2.
Antibacterial activities of essential oil and methanolic extract of S. avromanica
| Strain | Essential oil | Methanolic extract | Ampicillin | ||
|---|---|---|---|---|---|
| MIC | IZ | MIC | IZ | IZ | |
| Bacillus subtilis | – | – | 15 | 12 | 14 |
| Staphylococcus aureus | 7.5a | 18b | 15 | 12 | 13 |
| Enterococcus faecalis | <15 | 9 | 15 | 11 | 11 |
| Escherichia coli | <15 | 10 | <15 | 10 | 11 |
| Staphylococcus epidermidis | – | – | 7.5 | 18 | nt |
| Klebsiella pneumoniae | <15 | 9 | – | – | nt |
| Pseudomonas aeruginosa | – | – | – | – | 10 |
| Bacillus cereus | 7.5 | 20 | 7.5 | 18 | nt |
| Bacillus pumilus | 7.5 | 18 | 7.5 | 20 | 15 |
| Saccharomyces cerevisiae | <10 | 10 | 15 | 11 | 18 |
| Candida albicans | – | – | – | – | 18 |
– Inactive, nt not tested
aMinimum inhibitory concentration values as mg mL−1
bZone of inhibition (in mm) includes diameter of the disc (6 mm)
The Gram-positive B. cereus, B. pumilus and S. aureus were found to be the most susceptible strains by essential oil. S. aureus is a food borne pathogen that causes gastroenteritis and poisoning and threat the global public health (Burt and Reinders 2003). Elimination and controlling the growth of S. aureus are important for the food production industries. Therefore, S. avromanica can be used as a natural preservative against S. aureus for the food production industries.
Antioxidant activity
Antioxidants are practical ingredients of foodstuffs and are widely used in food industry to protect them against deterioration and lose of quality. Antioxidant agents are effective following different mechanisms such as free radical scavenging, quenching, or inhibition of reaction mechanisms. In this study, the potential antioxidant activity of S. avromanica essential oil and methanolic extract were documented using DPPH radical scavenging activity.
According to the findings presented in Table 3, the highest scavenging activity was found for methanolic extract of S. avromanica (21.58 µg/mL), followed by essential oil of S. avromanica (111.34 µg/mL), in comparison to Butylated hydroxytoluene (BHT) with IC50 of 22.45 µg/mL. It is widely accepted that the antioxidant activity of a plant extract is correlated to its phenolic content (Elmastaş et al. 2006). The total phenolics of methanolic extract of S. avromanica was measured using Folin–Ciocalteu’s assay (95.3 mg GAE/g sample).
Table 3.
Rosmarinic acid content, antioxidant capacities and total phenolics content of essential oil and methanolic extract of S. avromanica
| Sample | Rosmarinic acid content (mg rosmarinic acid/g sample) | Antioxidant activity IC50 value (µg mL−1) | Total phenolics content (mg GAE/g sample) |
|---|---|---|---|
| Methanolic extract | 0.83 ± 0.07 | 21.58 ± 1.2 | 95.3 ± 2.1 |
| Essential oil | – | 111.34 ± 2.3 | – |
| Standard antioxidant (BHT)a | 22.45 ± 0.3 |
Values expressed are mean ± SD (standard deviation) of three parallel experiments
aBHT Butylated hydroxytoluene
Rosmarinic acid content
Rosmarinic acid is one of the main phenolic compounds of Satureja species, which has been chosen as a target component accounting for its recognized bioactive properties (Zelić et al. 2005). According to literature, plant phenolic compounds are the highly effective free radical scavengers and antioxidants. The phenolic compounds exhibit considerable free radical-scavenging activities, through their reactivity as electron- or hydrogen-donating agents, and metal ion chelating properties (Esmaeili et al. 2010; Rice-Evans et al. 1997).
Therefore, it would be valuable to determine the rosmarinic acid content of S. avromanica. Rosmarinic acid content of S. avromanica methanolic extract measured using a high Performance thin-layer Chromatography (HPTLC) method. As shown in Table 3 the rosmarinic acid content of S. avromanica methanolic extract was 0.83 mg rosmarinic acid/g plant. According literature the rosmarinic acid content of Satureja species inclouding S. isophylla, S. bachtiarica, S spicigera, S. intermedia, S. edmondi, S. atropatana, S. mutica, S. macrantha and S. sahandica was (1.11, 0.75, 0.73, 0.33, 0.29, 0.27, 0.15. 0.09 and 0.03 mg rosmarinic acid/g plant, respectively). So this species has significant amount of rosmarinic acid (Ghorbanpour et al. 2016).
According to significant amount of rosmarininc acid in S. avromaica, using of S. avromaica as a spice and biological activity of rosmarininc acid, S. avromanica can be used in pharmaceutical and food industries.
Conclusion
A knowledge of wild foods and endemic plant species with their chemical compositions and biological properties could be of great value to further understand and make better use of these foods. To our knowledge, this study can be considered as the first detailed document on the phytochemical study and biological activities of the S. avromanica endemic of Iranica flora. The data of this study including antimicrobial activity, antioxidant activity and rosmarininc acid content of plant can be considered in foods and industries for application as a natural antimicrobial and antioxidant agent to preserve food and a source of rosmarinic acid. Therefore, the other properties of this plant, such as the its nutritive value and interactions with other food ingredients should be studied in the future.
References
- Adams RP. Identification of essential oils by gas chromatography quadrupole mass spectrometry. Carol Stream: Allured Publishing Corporation; 2001. [Google Scholar]
- Ahmadi F, Sadeghi S, Modarresi M, Abiri R, Mikaeli A. Chemical composition, in vitro anti-microbial, antifungal and antioxidant activities of the essential oil and methanolic extract of Hymenocrater longiflorus Benth., of Iran. Food Chem Toxicol. 2010;48:1137–1144. doi: 10.1016/j.fct.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Azaz AD, Kürkcüoglu M, Satil F, Can Baser KH, Tümen G. In vitro antimicrobial activity and chemical composition of some Satureja essential oils. Flavour Frag J. 2005;20:587–591. doi: 10.1002/ffj.1492. [DOI] [Google Scholar]
- Behravan J, Ramezani M, Kasaian J, Sabeti Z. Antimycotic activity of the essential oil of Satureja mutica Fisch and CA Mey from Iran. Flavour Frag J. 2004;19:421–423. doi: 10.1002/ffj.1328. [DOI] [Google Scholar]
- Burt SA, Reinders RD. Antibacterial activity of selected plant essential oils against Escherichia coli O157: H7. Lett Appl Microbiol. 2003;36:162–167. doi: 10.1046/j.1472-765X.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- Cakir A, Kordali S, Kilic H, Kaya E. Antifungal properties of essential oil and crude extracts of Hypericum linarioides Bosse. Biochem Syst Ecol. 2005;33:245–256. doi: 10.1016/j.bse.2004.08.006. [DOI] [Google Scholar]
- Dastan D, Salehi P, Gohari AR, Ebrahimi SN, Aliahmadi A, Hamburger M. Bioactive sesquiterpene coumarins from Ferula pseudalliacea. Planta Med. 2014;80:1118–1123. doi: 10.1055/s-0034-1382996. [DOI] [PubMed] [Google Scholar]
- Ebrahimi S, Kiyanpour V, Hadian J, Salehi P, Asghari B. Determination of rosmarinic acid content in some Iranian Satureja species by HPTLC. Planta Med. 2008;74:PC101. [Google Scholar]
- Eftekhar F, Raei F, Yousefzadi M, Ebrahimi SN, Hadian J. Antibacterial activity and essential oil composition of Satureja spicigera from Iran. Z Naturforsch C. 2009;64:20–24. doi: 10.1515/znc-2009-1-204. [DOI] [PubMed] [Google Scholar]
- Elmastaş M, Gülçin İ, Beydemir Ş, İrfan Küfrevioğlu Ö, Aboul-Enein HY. A study on the in vitro antioxidant activity of juniper (Juniperus communis L.) fruit extracts. Anal Lett. 2006;39:47–65. doi: 10.1080/00032710500423385. [DOI] [Google Scholar]
- Esmaeili MA, Sonboli A, Noushabadi MA. Antioxidant and protective properties of six Tanacetum species against hydrogen peroxide-induced oxidative stress in K562 cell line: A comparative study. Food Chem. 2010;121:148–155. doi: 10.1016/j.foodchem.2009.12.022. [DOI] [Google Scholar]
- Falsafi T, Moradi P, Mahboubi M, Rahimi E, Momtaz H, Hamedi B. Chemical composition and anti-Helicobacter pylori effect of Satureja bachtiarica Bunge essential oil. Phytomedicine. 2015;22:173–177. doi: 10.1016/j.phymed.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Farsam H, Amanlou M, Radpour M, Salehinia A, Shafiee A. Composition of the essential oils of wild and cultivated Satureja khuzistanica Jamzad from Iran. Flavour Frag J. 2004;19:308–310. doi: 10.1002/ffj.1300. [DOI] [Google Scholar]
- Gao C, Tian C, Lu Y, Xu J, Luo J, Guo X. Essential oil composition and antimicrobial activity of Sphallerocarpus gracilis seeds against selected food-related bacteria. Food Control. 2011;22:517–522. doi: 10.1016/j.foodcont.2010.09.038. [DOI] [Google Scholar]
- Ghahremani-majd H, Dashti F, Dastan D, Mumivand H, Hadian J, Esna-Ashari M. Antioxidant and antimicrobial activities of Iranian mooseer (Allium hirtifolium Boiss) populations. Hortic Environ Biotechnol. 2012;53:116–122. doi: 10.1007/s13580-012-0131-2. [DOI] [Google Scholar]
- Ghorbanpour M, Hadian J, Hatami M, Salehi-Arjomand H, Aliahmadi A. Comparison of chemical compounds and antioxidant and antibacterial properties of various Satureja species growing wild in Iran. J Med Plants. 2016;3:58–72. [Google Scholar]
- Gohari AR, Hadjiakhoondi A, Shafiee A, Ebrahimi ES, Mozaffarian V-A. Chemical composition of the essential oils of Satureja atropatana and Satureja mutica growing wild in Iran. J Essent Oil Res. 2005;17:17–18. doi: 10.1080/10412905.2005.9698817. [DOI] [Google Scholar]
- Gohari AR, Hadjiakhoondi A, Sadat-Ebrahimi E, Saeidnia S, Shafiee A. Composition of the volatile oils of Satureja spicigera C. Koch Boiss. and S. macrantha CA Mey from Iran. Flavour Frag J. 2006;21:510–512. doi: 10.1002/ffj.1613. [DOI] [Google Scholar]
- Hadian J, Ebrahimi SN, Salehi P. Variability of morphological and phytochemical characteristics among Satureja hortensis L. accessions of Iran. Ind Crop Prod. 2010;32:62–69. doi: 10.1016/j.indcrop.2010.03.006. [DOI] [Google Scholar]
- Hsu CS. Analytical advances for hydrocarbon research. Berlin: Springer Science & Business Media; 2003. [Google Scholar]
- Maroofi H. Two new plant species from Kurdistan province, West of Iran. Iran J Bot. 2010;16:76–80. [Google Scholar]
- Mohammadi M, Yousefi M, Habibi Z, Dastan D. Chemical composition and antioxidant activity of the essential oil of aerial parts of Petasites albus from Iran: a good natural source of euparin. Nat Prod Res. 2012;26:291–297. doi: 10.1080/14786410903374819. [DOI] [PubMed] [Google Scholar]
- Mumivand H, Rustaii A-R, Jahanbin K, Dastan D. Essential oil composition of Pulicaria dysenterica (L.) Bernh from Iran. J Essent Oil Bear Plants. 2010;13:717–720. doi: 10.1080/0972060X.2010.10643884. [DOI] [Google Scholar]
- Naseri M, Monsef-Esfehani H, Saeidnia S, Dastan D, Gohari A. Antioxidative coumarins from the roots of Ferulago subvelutina. Asian J Chem. 2013;25:1875. [Google Scholar]
- Novak J, Bahoo L, Mitteregger U, Franz C. Composition of individual essential oil glands of savory (Satureja hortensis L., Lamiaceae) from Syria. Flavour Frag J. 2006;21:731–734. doi: 10.1002/ffj.1725. [DOI] [Google Scholar]
- Oke F, Aslim B, Ozturk S, Altundag S. Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia Ten. Food Chem. 2009;112:874–879. doi: 10.1016/j.foodchem.2008.06.061. [DOI] [Google Scholar]
- Özer H, Sökmen M, Güllüce M, Adigüzel A, Sahin F, Sökmen A, Kiliç H, Baris Ö. Chemical composition and antimicrobial and antioxidant activities of the essential oil and methanol extract of Hippomarathrum microcarpum (Bieb.) from Turkey. J Agric Food Chem. 2007;55:937–942. doi: 10.1021/jf0624244. [DOI] [PubMed] [Google Scholar]
- Ozkan G, Simsek B, Kuleasan H. Antioxidant activities of Satureja cilicica essential oil in butter and in vitro. J Food Eng. 2007;79:1391–1396. doi: 10.1016/j.jfoodeng.2006.04.020. [DOI] [Google Scholar]
- Pezhmanmehr M, Dastan D, Ebrahimi SN, Hadian J. Essential oil constituents of leaves and fruits of Myrtus communis L. from Iran. J Essent Oil Bear Plants. 2010;13:123–129. doi: 10.1080/0972060X.2010.10643800. [DOI] [Google Scholar]
- Raut JS, Karuppayil SM. A status review on the medicinal properties of essential oils. Ind Crop Prod. 2014;62:250–264. doi: 10.1016/j.indcrop.2014.05.055. [DOI] [Google Scholar]
- Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- Sefidkon F, Jamzad Z. Essential oil composition of Satureja spicigera (C. Koch) Boiss. from Iran. Flavour Frag J. 2004;19:571–573. doi: 10.1002/ffj.1357. [DOI] [Google Scholar]
- Sefidkon F, Jamzad Z. Essential oil analysis of Iranian Satureja edmondi and S. isophylla. Flavour Frag J. 2006;21:230–233. doi: 10.1002/ffj.1562. [DOI] [Google Scholar]
- Senatore F, Urrunaga Soria E, Urrunaga Soria R, Della Porta G, De Feo V. Essential oils from two Peruvian Satureja species. Flavour Frag J. 1998;13:1–4. doi: 10.1002/(SICI)1099-1026(199801/02)13:1<1::AID-FFJ672>3.0.CO;2-4. [DOI] [Google Scholar]
- Shan B, Cai Y-Z, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int Food Microbiol. 2007;117:112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Shibamoto T. Retention indices in essential oil analysis in capillary gas chromatography. In: Sandra P, Bicchic A, editors. Essential oil analysis. New York: Alfred Heuthig-Verlag; 1987. pp. 259–275. [Google Scholar]
- Singh G, Marimuthu P, de Heluani CS, Catalan CA. Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components. J Agric Food Chem. 2006;54:174–181. doi: 10.1021/jf0518610. [DOI] [PubMed] [Google Scholar]
- Tepe B, Sokmen A. Production and optimisation of rosmarinic acid by Satureja hortensis L. callus cultures. Nat Prod Res. 2007;21:1133–1144. doi: 10.1080/14786410601130737. [DOI] [PubMed] [Google Scholar]
- Zeidán-Chuliá F, Keskin M, Könönen E, Uitto V-J, Söderling E, Moreira JCF, Gürsoy UK. Antibacterial and antigelatinolytic effects of Satureja hortensis L. essential oil on epithelial cells exposed to Fusobacterium nucleatum. J Med Food. 2015;18:503–506. doi: 10.1089/jmf.2013.0052. [DOI] [PubMed] [Google Scholar]
- Zelić B, Hadolin M, Bauman D, Vasić-Rački D. Recovery and purification of rosmarinic acid from rosemary using electrodialysis. Acta Chim Slov. 2005;52:126–130. [Google Scholar]