Abstract
Modified atmosphere packaging (MAP) enhanced the quality and storability of Ligularia fischeri. Oxygen transmission rate (OTR) films were used as a MAP. MAP storage displayed lower fresh weight loss than perforated film. The oxygen, carbon dioxide, and ethylene concentration were properly maintained by a 10,000 cc OTR packaging film at 8 °C and 30,000 cc OTR packaging film at 24 °C. On the last day of storage, the off-odor, such as the acetaldehyde and ethanol concentration, was the lowest in the 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treatments. The 10,000 cc OTR film treatment at 8 °C and 30,000 cc OTR film treatments at 24 °C had the highest chlorophyll content, total phenolic content, leaf toughness, antioxidant activity, vitamin C, and less off-flavor. The shelf life of 10,000 cc OTR film was 13 days, at 8 °C storage temperature. At 24 °C storage temperature, the shelf life of 30,000 cc OTR film was 4 days. The MAP storage of the Ligularia treated with 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C had the highest essential oil content. These results suggest that the best MAP film for cold-chain distribution was the 10,000 cc OTR film, and the 30,000 cc OTR film was a more suitable MAP film for local distribution without the cold-chain system of L. fischeri.
Keywords: Antioxidant, Chlorophyll, Visual quality, Vitamin C
Introduction
Ligularia fischeri (Ledeb.) Turcz is a perennial plant that is distributed mainly in China, Korea, Japan, and the far-east area of Russia (Xie et al. 2010). L. fischeri, also called Gom-chi in Korean, that is a plant found usually in damp shady regions which has long been cultivated, and in Korea the leaves are consumed as a fresh vegetable (An et al. 2014).
Fruits and vegetables are highly perishable products that start metabolic processes from harvest time (Fonseca et al. 2000). Modified atmosphere packaging (MAP) provides a wide range of useful benefits for whole, prepared fruit and vegetables, such as delayed softening, reduced chlorophyll degradation, reduced enzymatic browning, and alleviation of physiological disorders (Selman 1993). The proper MAP condition increases storability, maintains visual quality and soluble solids, and retains firmness (Islam et al. 2014).
In recent years, herbal extracts and essential oils have attracted plenty of scientific interest because of their potential as sources of biologically active compounds and natural antioxidants (Bozin et al. 2006). The medicinal and aromatic properties are associated with the presence of an essential oil (Nurzynska-Wierdak et al. 2013). The fatty alcohol content depends on harvest time and cultivar (Giuffre 2014). Fresh leaves volatile aroma is one of the main factors in the taste of all edible green plants, and the volatile aroma in almost all edible green leaves is suggested to be essential oil compounds (Han et al. 2010). In Korea, the aroma components are important determinants of leafy vegetables’ market value and consumer preference. We conducted this experiment because there were not enough studies on OTR film to extend the storability and essential oil content of L. fischeri. This study was conducted to assess the quality, storability, and essential oil content of L. fischeri during MAP storage.
Materials and methods
Plant material and treatments
Plastic-house-farmer grown fresh leaves of L. fischeri were harvested in Yanggu, Gangwon-do, Korea. Leaves packaged with perforated (with 0.6 cm diameter of four hole) and non-perforated (without hole) oxygen transmission rate (OTR) film as a modified atmosphere packaging. The storage temperatures were 8 °C for perforated film; 10,000; 30,000; and 50,000 cc OTR packaging film and 24 °C for perforated film; 30,000; 50,000; 70,000 cc OTR packaging film with 90% relative humidity.
Gaseous parameters
During storage, the oxygen and carbon dioxide concentrations were analyzed by a Check Mate 9900 gas analyzer (PBI-Dansensor, Ringsted, Denmark). The ethylene, ethanol, and acetaldehyde were standardized by a Shimadzu GC-2010 gas chromatograph (Shimadzu Corporation, Tokyo, Japan) furnished by a 30 m × 0.25 mm × 0.25 µm BP20 wax column (SGE Analytical Science, Australia) and a flame ionization detector. The detector and injector were operated at 127 °C, the ovens were operated at 50 °C, and 0.67 was the carrier gas (N2) flow rate mL/s (Park et al. 2000).
The off-flavor was rated on the basis of 1–5 (1 = excellent, 2 = good, 3 = moderate, marketable, 4 = bad, and 5 = worst), and final storage and trained five panel members were employed to assess the off-flavor.
Leaf quality parameters
During storage, the L. fischeri fresh weight loss was measured by subtracting the sample weights from their previously recorded weights, and the result was presented as a percent of weight loss compared to the initial weight.
The visible quality was subjectively evaluated based on determinants such as freshness, mold growth, decay, shriveling, smoothness, shininess, and homogeneity. The visual quality was rated on a scale of 1–5 (1 = waste, 2 = bad, 3 = good, marketable, 4 = very good, and 5 = excellent) at storage period (Kang and Kim 2007). Trained five panel members were assessed the visual quality (Islam et al. 2014) of the L. fischeri.
The leaf color value of L. fischeri was investigated using a CR 400 chroma meter (Konica Minolta Sensing, Inc., Osaka, Japan), and a* measured the degree of redness (+a*) and greenness (−a*), while b* measured the degree of yellowness (+b*) and blueness (−b*).
The chlorophyll value was measured by a Plus chlorophyll meter of SPAD-502 model (Konica Minolta Sensing, Inc., Osaka, Japan; Martinez et al. 2015).
The leaf toughness (hardness or firmness) was analyzed using a rheometer (Sun Scientific Co. Ltd., Setagaya-ku, Japan) with the greatest force of 10 kg and a 6 mm diameter stainless-steel probe with a flat end (Islam et al. 2012). During measurement, the vegetables were placed on a plastic disk to keep them stable. The penetrating force (N) of the vegetable flesh skin and the deformation (mm) values during penetration were recorded.
The total phenolic content was analyzed according to Chandler and Dodds (1983) with slight modifications. The total phenolic content was tested by a UV spectrophotometer (Shimadzu Corporation, Tokyo, Japan) at an absorbance of 725 nm. 2 g leaf sample added in 2.5 mL of 94% ethanol, and was kept in the refrigerator at 0 °C for 48 h. The leaf extracts were homogenized by a T25 Ultra-Turrax disperser (IKA Korea Ltd., Seoul, Korea), and centrifuged for 20 min at 35,280g and at 4 °C by a Mega 17R centrifuge (Hanil Science Industrial Co., Ltd, Korea). Next, 1 mL of supernatant was moved to a test tube and 1 mL of 94% ethanol, 5 mL of distilled water, and 0.5 mL of 50% Folin–Ciocalteu reagent were added. 1 mL of 5% Na2CO3 was mixed with the samples after 5 min and kept in the dark for 60 min. Afterwards; absorbance was taken at 725 nm with 94% ethanol.
The free-radical scavenging activity was analyzed by 2-2 diphenyl picryhydrazyl (DPPH). The assay to determine the antioxidant activity was fixed on the method of Blois (1958) and Jain and Agrawal (2008) with slight modification. 200 µL of test solution with 50 µL of DPPH (0.659 mm) solution which were incubated at 25 °C for 30 min, and following which, the absorbance was read at 518 nm was measured by UV spectrophotometer. A control reaction was worked out without the test sample. The percent inhibition was calculated on the basis of the following equation: %Inhibition = (A0 − At)/A0 × 100, where A0 absorbance denotes the control (blank, without extract) and At absorbance denotes the presence of the extract.
For the vitamin C analysis, a 2 g frozen L. fischeri sample was put upon a 50 mL centrifuge tube with 20 mL 5% metaphosphoric acid. The samples were homogenized by a T25 Ultra-Turrax homogenizer (IKA Korea, Ltd., Korea) and centrifuged for 20 min at 35,280g and at 4 °C by a Mega 17R centrifuge (Hanil Science Industrial Co., Ltd, Korea). The 20 μL supernatant sample was added into the vial through a 0.20 µL regenerated cellulose (RC) membrane filter for vitamin C analysis. The vitamin C assay was operated using a Waters HPLC system (Waters Associates, Milford, MA, USA) of 717plus autosampler, a 4.6 cm × 250 mm × 5 μm ZORBAX Eclipse XDB-C18 analytical columns (Agilent, USA), a Waters 600 controller pump, and with a Waters 486 tunable of 265 nm absorbance detector. 100% MeOH: 0.1 M KH2PO4 and 1:9 was the mobile phase and 1.0 mL/min was the flow rate (Li and Chen 2001).
The essential oil content was extracted by steam distillation using a Nickerson and Likens apparatus, slightly modified per Charles and Simon’s (1990) method. For the extraction, the apparatus was joined for steam distillation by a 1,000 mL round flask with the 100 g sample. Another small 100 mL flask was set up with solvent diethyl ether (Daejung Chemical and Metals Co. Ltd., Daejung, Korea) above a water bath (C-WBE, Chang Shin Science Co., Seoul, Korea). To separate the oil, round flux as well with sample was placed on the heating mantle (220 V) which was kept in 100 °C by controlling the heating mantle temperature for 150 min. The evaporating compounds (steam-distilled compound) were collected with diethyl ether by a solvent extraction technique, and magnesium sulfate anhydrous (Junsei Chemical Co. Ltd., Tokyo, Japan) was added to the diethyle ether solution to remove hydrates and was refrigerated for 12 h at −4 °C. After removing the hydrates, the diethyle ether solution was filtered, and the steam-distilled compound was concentrated by a rotary evaporator (Eyela N-1000 rotary evaporator, Eyela digital water bath, Eyela A-3S aspirator, made in Japan). Subsequently, the essential oil was found on the inner surface of the distilled flask (Kang et al. 2003).
Statistical analysis
Statistical analyses were performed by SPSS V.16 (SPSS Inc., Chicago, USA) for the off-flavor, fresh weight loss, visual quality, color value, leaf toughness, antioxidant activity, and oxygen, carbon dioxide, ethylene, acetaldehyde, ethanol, chlorophyll, phenol, vitamin C, and essential oil content. Significant differences between the mean values were commenced using Duncan’s multiple range test in one-way ANOVA.
Results and discussion
Gaseous parameters
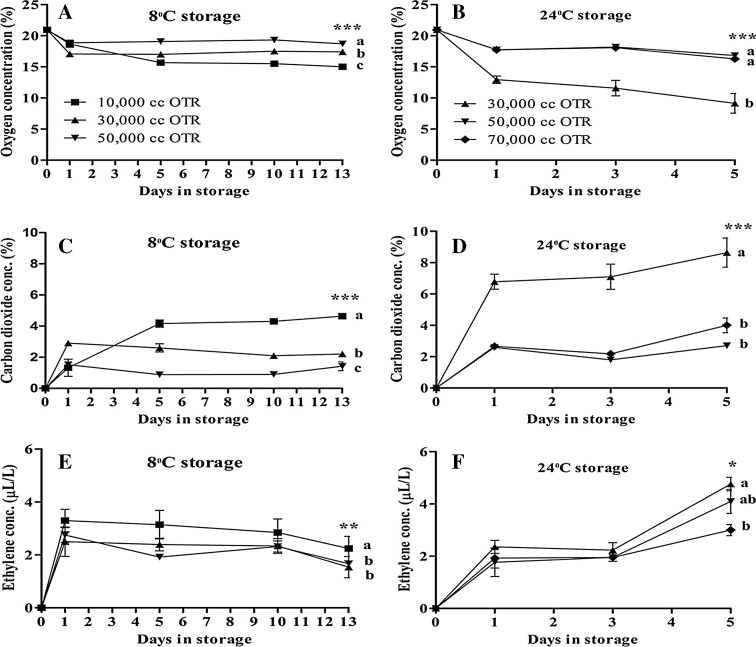
After harvesting, vegetables start producing gasses that damage their quality and storability. The optimum gaseous condition may help to increase the product’s shelf life. The oxygen concentration was the lowest in 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treatments due to less moisture loss. The needed temperature, oxygen and carbon dioxide levels differ with vegetable origin, type, variety, and growing season (Ballantyne et al. 1988). An optimum carbon dioxide and ethylene concentration was also demonstrated by the 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treatments. Ethylene is a naturally produced plant hormone that helps to plant growth and development, and storage life of many vegetables, fruits, and ornamental crops, and action of ethylene involved complicated metabolic processes of plants (Saltveit 1999). The 10,000 cc OTR film treatment that retained the highest visual quality maintained ranges of oxygen was 15.00–18.66%, carbon dioxide was 1.32–4.64%, and ethylene concentration was 1.88–3.30 µL/L at 8 °C storage period. The 30,000 cc OTR film treatment that had the highest visual quality maintained ranges of oxygen was 9.14–12.94%, carbon dioxide was 6.78–8.64%, and ethylene concentration was 2.23–4.60 µL/L at 24 °C storage period (Fig. 1).
Fig. 1.
Changes of oxygen concentration (a, b), carbon dioxide concentration (c, d), and ethylene concentration (e, f) of Ligularia fischeri at 8 °C and at 24 °C. Vertical bars represent ±SE of means (n = 5). *, **, *** Significant at p ≤ 0.05, 0.01 and 0.001, respectively of Duncan’s multiple range tests (DMRT)
Increased acetaldehyde and ethanol is not desirable for storing Ligularia because they stimulate the off-flavor. The lowest acetaldehyde and ethanol was produced by the 10,000 cc OTR film treatment at 8 °C and the 30,000 cc OTR film treatment at 24 °C due to lower respiration and ethylene production rates. Increased ethanol and acetaldehyde production is associated with respiration (Kim et al. 2005). The 50,000 cc OTR film at 8 °C and 70,000 cc OTR film at 24 °C treatments had the highest acetaldehyde and ethanol than the other treatments. The 10,000 cc OTR film at 8 °C and the 30,000 cc OTR film at 24 °C treatments had the longest shelf life by suppressing the acetaldehyde and ethanol that is needed for the greatest seller, buyer, and consumer satisfaction (Table 1).
Table 1.
Changes of acetaldehyde concentration, ethanol concentration, and off-flavor of Ligularia fischeri on the 13th storage day at 8 °C and 5th storage day at 24 °C temperature
| Storage tempt. | Treatments | Acetaldehyde conc. (μL/L) | Ethanol conc.(μL/L) | Off-flavor |
|---|---|---|---|---|
| 8 °C | 10,000 cc OTR | 4.17b1 | 51.06b | 2.70b |
| 30,000 cc OTR | 5.66b | 86.85ab | 3.20a | |
| 50,000 cc OTR | 11.75a | 141.86a | 3.40a | |
| p value | ** | *** | ** | |
| 24 °C | 30,000 cc OTR | 11.52b | 74.21b | 3.30b |
| 50,000 cc OTR | 14.15ab | 104.75ab | 3.60ab | |
| 70,000 cc OTR | 17.06a | 117.78a | 3.80a | |
| p value OTR | * | * | * |
*, **, *** Significant at p ≤ 0.05, 0.01 and 0.001, respectively
1Mean separation within columns by Duncan’s multiple range tests (DMRT)
The 10,000 cc OTR film at 8 °C and the 30,000 cc OTR film at 24 °C treatments had suppressed acetaldehyde and ethanol, which resulted in the best visual quality for market value and maintained the lowest off-flavor. Consumers desire vegetables with low or no off-flavor (Table 1). A high off-flavor can damage the cell tissue and shorten the shelf life of Ligularia.
Leaf quality parameters
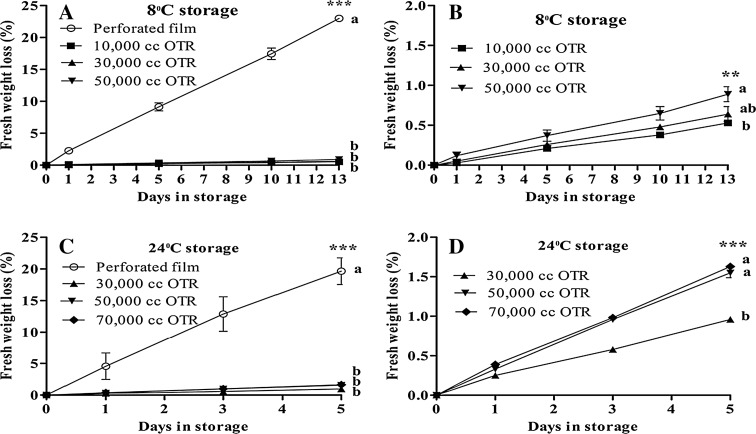
Moisture keeps vegetables fresh for the consumer. The highest fresh weight loss resulted from the perforated film reaching 22.98% at 8 °C and 19.66% at 24 °C storage temperature on the last storage day. The MAP storage resulted in the lowest fresh weight loss (0.53–0.89% at 8 °C and 0.96–1.63% at 24 °C) compared with the perforated film (Fig. 2). There was a significant difference with the treatments compared with the perforated film. The MAP storage had the lowest fresh weight loss due to less transpiration or moisture loss from the vegetables. The weight loss adversely affects the appearance, texture, and flavor, all factors that determine the quality of fruits and vegetables (Manolopoulou and Varzakas 2013). Higher fresh weight loss from Ligularia decreases the quality and market value. A weight loss greater than 5% would decrease the fruits and vegetables retail value (Ohta et al. 2002). MAP storage maintains the quality by reducing fresh weight loss. The 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treatments had the lowest fresh weight loss due to less transpiration or moisture loss.
Fig. 2.
Changes of fresh weight loss (a–d) of Ligularia fischeri at 8 °C and at 24 °C. Vertical bars represent ±SE of means (n = 5). As OTR films graphs line are not clearly visible with combined perforated film (a, c) so b, d produce separately only for OTR films. **, *** Significant at p ≤ 0.01 and 0.001, respectively of Duncan’s multiple range tests (DMRT)
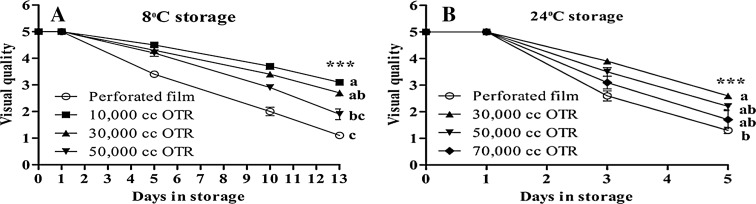
Consumer choice mainly depends on visual quality. Good visual quality vegetables can get the best market value. During the storage period, the 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C attained the highest visual quality. MAP storage retained the visual quality and improved the shelf life of Ligularia by reducing respiration and ethylene production. The lowest visual quality resulted from the perforated film during the storage period at both storage temperatures. We agree with Islam et al. (2014) that the visual quality and shelf life of perforated film is worse than MAP because of the moisture loss. At 8 °C storage temperature, the shelf life of perforated, 10,000 cc OTR film, 30,000 cc OTR film, and 50,000 cc OTR film was almost 6, 13, 12, and 9 days, accordingly. The shelf life of perforated film, 30,000 cc OTR film, 50,000 cc OTR film, and 70,000 cc OTR film was around 2, 4, 3, and 3 days, respectively at 24 °C storage temperature (Fig. 3).
Fig. 3.
Changes of visual quality (a, b) of Ligularia fischeri at 8 °C and at 24 °C.Visual quality scores were 5 excellent, 4 very good, 3 good, marketable, 2 bad, 1 waste. Vertical bars represent ±SE of means (n = 5). *** Significant at p ≤ 0.001 of Duncan’s multiple range tests (DMRT)
Appearance is the most important attribute selected by the consumer and it plays a major part in deciding which food products to purchase (Jacxsens et al. 2002). The 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treatments had the highest visual quality because the packaging film maintained vegetable freshness.
Color is another important factor when consumers choose vegetables. Vegetables that are greener are more desirable to consumers and have greater market value. On the last storage day, the L* and b* values decreased in all treatments compared with the initial values. The MAP and 8 °C storage resulted in more greenness than the 24 °C storage. The 10,000 cc OTR film at 8 °C and 30,000 cc OTR film treatment at 24 °C had the most greenness compared to other films. The 10,000 cc OTR film and 30,000 cc OTR film treatments that had longer shelf life maintained higher chlorophyll content and lower L* values than the initial values (Table 2). The 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treatments suppressed the most leaf yellowing by reducing respiration and ethylene. Leaves yellowing responsible due to chlorophyll degradation that is the most obvious visible symptom of leafy vegetable senescence (Buchanan-Wollaston 1997). As leaf yellowing depends on chlorophyll degradation, the 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treatments showed the highest chlorophyll content. We agree with Watada (1986) that green color loss is initiated or accelerated when the plant tissue is exposed to ethylene.
Table 2.
Changes of color L*, a*, b* and chlorophyll of Ligularia fischeri at initial day, on the 13th storage day at 8 °C and 5th storage day at 24 °C temperature
| Storage tempt. | Treatments | Color | Chlorophyll (SPAD) | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| 8 °C | Initial | 31.20 ± 0.47 | −9.25 ± 0.21 | 21.36 ± 0.07 | 39.92 ± 0.79 |
| Perforated | 37.46a1 | −8.72a | 10.60a | 32.71b | |
| 10,000 cc OTR | 36.96a | −8.35a | 8.99b | 37.21a | |
| 30,000 cc OTR | 37.35a | −8.32a | 8.25b | 37.04a | |
| 50,000 cc OTR | 37.28a | −8.50a | 8.10b | 34.38ab | |
| p value | NS | NS | * | ** | |
| 24 °C | Perforated | 36.92a | −10.20a | 11.35a | 30.36b |
| 30,000 cc OTR | 33.74b | −9.85a | 9.14ab | 36.74a | |
| 50,000 cc OTR | 33.97b | −9.89a | 8.44b | 36.52a | |
| 70,000 cc OTR | 36.69a | −10.53a | 11.17a | 29.72b | |
| p value | ** | NS | * | *** | |
NS not significant
*, **, *** Significant at p ≤ 0.05, 0.01 and 0.001, respectively
1Mean separation within columns by Duncan’s multiple range tests (DMRT). Initial value indicated mean ± SE (n = 5)
Leaf toughness, which helps increase shelf life, is an important aspect of leafy vegetables. The 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treatments maintained the highest leaf toughness, total phenolic content, antioxidant activity, and vitamin C. Leaf toughness, antioxidants, and vitamin C decreased, and the total phenolic content increased in all treatments of both storage temperatures during the storage period compared to the initial day. However, the leaf toughness, antioxidant activity, and vitamin C were higher in the MAP storage at both storage temperatures than in the perforated film. MAP storage influenced the firmness because the treatments might help the metal binding stabilize the cell-wall structure by the pectin network. The increased toughness is an important factor in degrading the quality of green asparagus spears and, consequently, decreases their market value (Chiu and Sung 2013). The 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treatments had the highest phenol content among the treatments because of lower respiration. The 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treatments had the highest antioxidant and vitamin C content. Antioxidants neutralize chemically active metabolic products, such as free radicals, inducing serious cellular damage (Ercisli et al. 2012). The 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treated Ligularia will have the best shelf life. The L. fischeri showed that strong antioxidant activity may be due to antioxidant vitamins, phenolic compounds, or a combination thereof (Choi et al. 2007). Moreover, phenolic compounds act as primary antioxidants that produce a sour or astringent taste (Ercisli et al. 2008), and its variation depends on storage temperatures. At 8 °C, the Ligularia had higher phenolic compounds compared with those stored at 24 °C. This relates to the work of Karaman et al. (2014) who determined that high temperatures might cause degradation of phenolic compounds. Vitamin C is highly affected by storage temperatures. Moreover, the vitamin C content varies due to environmental conditions, cultivar, and ripeness stage (Baltacioglu and Artik 2013). Consumers take into consideration the aroma components when choosing ready-to-eat vegetables. Essential oil is a fragrant, light yellow liquid. The highest essential oil content increases the product fragrance, quality, and shelf life. We agree with Gharibzahedi et al. (2014) that the oil stability can be indicated to high antioxidant activity due to the phenol content in oil. The 10,000 cc OTR film at 8 °C and 30,000 cc OTR film at 24 °C treatments retained more essential oil compared to the other treatments (Table 3). The greatest essential oil yield in L. fischeri was 0.12% in 10,000 cc OTR film at 8 °C and 0.15% in 30,000 cc OTR film at 24 °C. The essential oil can reduce the antifungal activity and increase shelf life. The physicochemical characteristic of the oil change (Karaman et al. 2014) due to oxidative rancidity in the storage period (Akhtar et al. 1985) can be maintained by OTR packaging film.
Table 3.
Changes of leaf toughness, total phenolic content, antioxidant activity (% scavenging activity) and vitamin C of Ligularia fischeri at initial day, on the 13th storage day at 8 °C and 5th storage day at 24 °C temperature
| Storage tempt. | Treatments | Leaf toughness (N) | Total phenolic (GAEmg/100gFW) | Antioxidant activity (% SA) | Vitamin C (mg/100FW) | Essential oils content (% of fresh weight) |
|---|---|---|---|---|---|---|
| 8 °C | Initial | 2.38 ± 0.14 | 151.05 ± 10.90 | 58.82 ± 4.04 | 2.04 ± 0.17 | 0.19 ± 0.01 |
| Perforated | 1.38b1 | 327.1b | 40.14b | 0.86b | 0.09a | |
| 10,000 cc OTR | 2.23a | 443.3a | 53.85a | 1.16a | 0.12a | |
| 30,000 cc OTR | 2.19a | 379.2ab | 47.71ab | 0.97ab | 0.11a | |
| 50,000 cc OTR | 1.76ab | 358.9ab | 45.37ab | 0.91ab | 0.10a | |
| p value | *** | * | * | * | NS | |
| 24 °C | Perforated | 1.73b | 162.9b | 35.24b | 0.78b | 0.10b |
| 30,000 cc OTR | 2.16a | 211.3a | 47.59a | 1.02a | 0.15a | |
| 50,000 cc OTR | 2.03ab | 167.8ab | 45.13ab | 0.92ab | 0.13ab | |
| 70,000 cc OTR | 1.86ab | 156.8b | 40.27ab | 0.83b | 0.11ab | |
| p value | * | * | * | * | ** |
*, **, *** Significant at p ≤ 0.05, 0.01 and 0.001, respectively
1Mean separation within columns by Duncan’s multiple range tests (DMRT). Initial value indicated mean ± SE (n = 5)
Conclusion
The MAP storage of L. fischeri treated with 10,000 cc OTR packaging film at 8 °C and 30,000 cc OTR packaging film at 24 °C had the longest shelf life, and the highest chlorophyll content, leaf toughness, and total phenolic content and antioxidant activities, and lower L* value, acetaldehyde and ethanol content, and off-flavor. The MAP storage of L. fischeri treated with 10,000 cc OTR film had the most essential oil content at 8 °C. Moreover, the L. fischeri treated with 30,000 cc OTR film at 24 °C had the most essential oil content. Therefore, this is a valuable starting point in designing a commercial application of modified atmosphere packaging storage to extend the shelf life of L. fischeri and maintain postharvest quality and essential oil content.
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (114072-03) and 2015 Research Grant from Kangwon National University (No. 520150119).
References
- Akhtar P, Asghar A, Sheikh AS. Effect of proxy radical scavengers on fluorescent light induced oxidation in some edible oils. J Pure Appl Sci. 1985;4:1–7. [Google Scholar]
- An S, Park HS, Kim GH. Evaluation of the antioxidant activity of cooked Gomchwi (Ligularia fischeri) using the myoglobin methods. Prev Nutr Food Sci. 2014;19(1):34–39. doi: 10.3746/pnf.2014.19.1.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne A, Stark R, Selman JD. Modified atmosphere packaging of shredded lettuce. Int J Food Sci Technol. 1988;23:267–274. doi: 10.1111/j.1365-2621.1988.tb00578.x. [DOI] [Google Scholar]
- Baltacioglu H, Artik N. Study of postharvest changes in the chemical composition of persimmon by HPLC. Turk J Agric For. 2013;37:568–574. doi: 10.3906/tar-1210-21. [DOI] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Bozin B, Mimica-Dukic N, Simin N, Anackov G. Characterization of the volatile composition of essential oil of some Lamiaceae species and the antimicrobial and antioxidant activities of the entire oils. J Agric Food Chem. 2006;54:1822–1828. doi: 10.1021/jf051922u. [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V. The molecular biology of leaf senescence. J Exp Bot. 1997;48:181–199. doi: 10.1093/jxb/48.2.181. [DOI] [Google Scholar]
- Chandler SF, Dodds JH. The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of Solanum laciniatum. Plant Cell Rep. 1983;2:205–208. doi: 10.1007/BF00270105. [DOI] [PubMed] [Google Scholar]
- Charles DJ, Simon JE. Comparison of extraction methods for the rapid determination of essential oil content and composition of basil. J Am Soc Hortic Sci. 1990;115(3):458–462. [Google Scholar]
- Chiu KY, Sung JM. Quality of low temperature heat-shocked green asparagus spears during short-term storage. Afr J Agric Res. 2013;8(28):3849–3856. doi: 10.5897/AJAR2012.6697. [DOI] [Google Scholar]
- Choi EM, Ding Y, Nguyen HT, Park SH, Kim YH. Antioxidant activity of Gomchi (Ligularia fischeri) leaves. Food Sci Biotechnol. 2007;16(5):710–714. [Google Scholar]
- Ercisli S, Akbulut M, Ozdemir O, Sengul M, Organ E. Phenolic and antioxidant diversity among persimmon (Diospyrus kaki L.) genotypes in Turkey. Int J Food Sci Nutr. 2008;59(6):477–482. doi: 10.1080/09637480701538262. [DOI] [PubMed] [Google Scholar]
- Ercisli S, Tosun M, Karlidag H, Dzubur A, Hadziabulic S, Aliman Y. Color and antioxidant characteristics of some fresh fig (Ficus carica L.) genotypes from northeastern Turkey. Plant Foods Hum Nutr. 2012;67:271–276. doi: 10.1007/s11130-012-0292-2. [DOI] [PubMed] [Google Scholar]
- Fonseca SC, Oliveira FAR, Lino IBM, Brecht JK, Chau KV. Modeling O2 and CO2 exchange for development of perforation mediated modified atmosphere packaging. J Food Eng. 2000;43:9–15. doi: 10.1016/S0260-8774(99)00122-3. [DOI] [Google Scholar]
- Gharibzahedi SMT, Mousavi SM, Hamedi M, Khodaiyan F. Determination and characterization of kernel biochemical composition and functional compounds of Persian walnut oil. J Food Sci Technol. 2014;51(1):34–42. doi: 10.1007/s13197-011-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffre AM. Evolution of fatty alcohols in olive oils produced in Calabria (Southern Italy) during fruit ripening. J Oleo Sci. 2014;63(5):485–496. doi: 10.5650/jos.ess13212. [DOI] [PubMed] [Google Scholar]
- Han SS, Sa JY, Lee KC. A comparison the volatile aroma compounds between Ligularia fischeri and Ligularia fischeri var. spiciformis leaves. J For Sci. 2010;26(3):209–217. [Google Scholar]
- Islam MZ, Kim YS, Kang HM. Effect of temperature on the quality and storability of cherry tomato during commercial handling condition. J Bio Environ Control. 2012;21(2):88–94. [Google Scholar]
- Islam MZ, Mele MA, Lee HJ, Lee KS, Hong SM, Jeong MJ, Kim IS, Hong SK, Choi IL, Baek JP, Kang HM. Selection of non-perforated breathable film to enhance storability of cherry tomato for modified atmosphere storage at different temperatures. Prot Hortic Plant Fac. 2014;23(2):116–122. doi: 10.12791/KSBEC.2014.23.2.116. [DOI] [Google Scholar]
- Jacxsens L, Devlieghere F, Debevere J. Predictive modeling for packaging design: equilibrium modified atmosphere packages of fresh-cut vegetables subjected to a simulated distribution chain. Int J Food Microbiol. 2002;73:331–341. doi: 10.1016/S0168-1605(01)00669-9. [DOI] [PubMed] [Google Scholar]
- Jain PK, Agrawal RK. Antioxidant and free radical scavenging properties of developed mono-and poly herbal formulations. Asian J Exp Sci. 2008;22(3):213–220. [Google Scholar]
- Kang HM, Kim IS. Effect of vitamin C treatments on the storability of baby vegetables in MA storage. J Bio Environ Control. 2007;16(4):420–425. [Google Scholar]
- Kang HM, Na CW, Park KW. Effects of strength of nutrient solution on the growth and essential oil content of Marjoram (Orignum majorana) and Oregno (Origanum vulgare) J Bio Environ Control. 2003;12(4):235–239. [Google Scholar]
- Karaman S, Toker OS, Ozturk I, Yalcin H, Kayacier A, Dogan M, Sagdic O. A response surface methodology study on the effects of some phenolics and storage period length on vegetable oil quality: change in oxidation stability parameters. Turk J Agric For. 2014;38:759–772. doi: 10.3906/tar-1403-98. [DOI] [Google Scholar]
- Kim JG, Luo Y, Saftner RA, Gross KC. Delayed modified atmosphere packaging of fresh-cut Romaine lettuce: effects on quality maintenance and shelf life. J Am Soc Hortic Sci. 2005;130(1):116–123. [Google Scholar]
- Li HB, Chen F. Simultaneous determination of nine water-soluble vitamins in pharmaceutical preparations by high-performance liquid chromatography with diode array detection. J Sep Sci. 2001;24:271–274. doi: 10.1002/1615-9314(20010401)24:4<271::AID-JSSC271>3.0.CO;2-L. [DOI] [Google Scholar]
- Manolopoulou E, Varzakas TH. Effect of modified atmosphere packaging (MAP) on the quality of ‘ready-to-eat’ shredded cabbage. Int J Agric Food Res. 2013;2(3):30–43. [Google Scholar]
- Martinez F, Palencia P, Weiland CM, Alonso D, Oliveira JA. Influence of nitrification inhibitor DMPP on yield, fruit quality and SPAD values of strawberry plants. Sci Hortic. 2015;185:233–239. doi: 10.1016/j.scienta.2015.02.004. [DOI] [Google Scholar]
- Nurzynska-Wierdak R, Borowski B, Dzida K, Zawislak G, Kowalski R. Essential oil composition of sweet basil cultivars as affected by nitrogen and potassium fertilizer. Turk J Agric For. 2013;37:427–436. [Google Scholar]
- Ohta H, Shiina T, Sasaki K. Dictionary of freshness and shelf life of food. Tokyo: In Science Forum Co., Ltd.; 2002. [Google Scholar]
- Park KW, Kang HM, Kim CH. Comparison of storability on film sources and storage temperature for fresh Japanese mint in MA storage. J Bio Environ Control. 2000;9(1):40–46. [Google Scholar]
- Saltveit ME. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol Technol. 1999;15:279–292. doi: 10.1016/S0925-5214(98)00091-X. [DOI] [Google Scholar]
- Selman JD. Practical experiences of sensory investigations into the packaging of vegetables. Packag Technol Sci. 1993;6:183–193. doi: 10.1002/pts.2770060403. [DOI] [Google Scholar]
- Watada AE. Effects of ethylene on the quality of fruits and vegetables. Food Technol. 1986;40(5):82–85. [Google Scholar]
- Xie WD, Weng CW, Lia X, Row KH. Eremophila sesquiterpenoids from Ligularia fischeri. Helv Chim Acta. 2010;93:1983–1989. doi: 10.1002/hlca.201000010. [DOI] [Google Scholar]