Abstract
This study was aimed at monitoring changes in the quality of strawberry purée preserved by high pressure processing (HPP) and thermal pasteurization (TP) during cold storage (6 °C) and determining its optimal storage period. The storage period of strawberry purée treated at 500 MPa, 50 °C, 15 min based on microbiological changes was 12 weeks. During this period, purée lost 32% of polyphenols, 73% of anthocyanins and entire vitamin C. Color changes described as dE increased up to 5.05 whereas the overall sensory quality decreased by 3 points on a 9-point scale. Under similar storage conditions, TP-preserved purée lost only 28% of polyphenols and 54% of anthocyanins, and entire vitamin C. Color changes were more visible (dE = 7.21) compared to the HPP sample whereas the overall sensory quality decreased only by 2 points. Recommended cold shelf-life for the HPP product was estimated at 6 weeks, during which period HPP-preserved purée had higher content of polyphenols and colour parameters compared to TP purée.
Keywords: High pressure processing, Thermal processing, Strawberries, Anthocyanins, Polyphenols, Vitamin C, Storage period
Introduction
Traditional heat pasteurization or sterilization of fruit and vegetable products allow to produce stable and safe preserves, however, their sensory quality (color, taste, aroma, consistency) and chemical composition are subjected to significant changes (Odriozola-Serrano et al. 2008; Tiwari et al. 2009; Verbeyst et al. 2010, 2013). One of the non-thermal methods of food preservation is high pressure processing (HPP). HPP-treated food is usually characterized by a higher nutritive value and greater preservation of valuable bioactive compounds, such as polyphenols, anthocyanins, vitamins and sensory values of the raw material compared to foods preserved by thermal methods (Marszałek et al. 2015; Terefe et al. 2013a, b).
Inactivation of vegetative microflora of fruits and vegetables by HPP does not generally pose major problems and can be achieved even under relatively mild pressure and temperature conditions. Typically, significant inactivation of vegetative bacteria, yeasts and molds can be observed within minutes at room temperature and a pressure higher than 300 MPa. However, increasing the pressure to 700 MPa or higher significantly accelerates most of the inactivation reactions (Hainz and Buckow 2009). The main problem connected with the use of HPP to preserve fruit and vegetable products is incomplete inactivation of native enzymes, which can cause changes during storage.
The major enzymes that are believed to be involved in the degradation of polyphenols in horticultural products and subsequent browning discoloration are polyphenol oxidases (PPO), peroxidases (POD) and β-glucosidase (BGL). These enzymes are responsible for the negative changes of the major sensorial attributes, such as color and flavor of fruit and vegetable products. In addition to phenolic compounds, POD also act on other hydrogen donors, e.g., aromatic amines, ascorbic acid and indoles. Hydrogen peroxide, which acts as a substrate for POD, is produced in degradation reactions of PPO-catalyzed phenolics and BGL-catalyzed anthocyanins. The oxidative enzymes are the most pressure-resistant enzymes, withstanding even up to 600-800 MPa. Thus, inactivation of PPO and POD is one of the major concerns in the fruit and vegetable processing industry (Chacraborty et al. 2014).
Previous studies (Marszałek et al. 2011, 2015) had shown that important factors affecting the quality of strawberry purée subjected to HPP was the process temperature and the pressure. The degree of inactivation of PPO and POD also depended on the exposure time of the pressure applied, but none of the process parameters allowed for the complete inactivation of enzymes. Hence, taking into account kinetics of enzymatic reactions (i.e. browning), equally important or even more relevant, than the investigation of the direct impact of the HPP process on products’ quality, is the analysis of changes occurring during storage.
Strawberries are well known for their health-promoting properties, because they contain many important readily degraded antioxidants, e.g., anthocyanins, phenolic acids, flavonols, and vitamin C. The preventive role of polyphenols has already been proved against, e.g., cancer, cardiovascular diseases, arthritis or type 2 diabetes (Kaur and Kapoor 2001; Marszałek et al. 2015). Therefore, maintaining the possible highest level of this compound is desirable during storage.
HPP has been applied to preserved strawberries, strawberry purée and juice but most of this studies do not show microbial quality and physicochemical changes during long time storage (Patras et al. 2009; Cao et al. 2011, 2012; Marszałek et al. 2015; Gao et al. 2016).
This study was aimed at determining the shelf-life of strawberry purée preserved with HPP, based on the analyses of microbiological quality (yeast, molds, total bacterial count), the rate of degradation of bio-active compounds (total polyphenols, anthocyanin pigments, phenolic acids, flavonols, vitamin C) and color parameters during cold storage at 6 °C. According to authors best knowledge there is no publications on the determination of microbial and physicochemical (including main phenolic acids and flavonols) changes of strawberry purée treated by HPP enhanced with mild heating.
Materials and methods
Production of strawberry purée
Strawberries, cv. ‘Senga Sengana’ cultivar, were pre-treated (washing, destalking, freezing) at “Ulmer” Sp.j. company (Stare Zadybie, Poland). After freezing in a fluidization tunnel (UniDex, Poland), the strawberries were sorted (Niagara Sortex, Bühler, Switzerland) according to colour and size (45–55 mm) and stored at −24 °C. After defrosting (at 4 °C), they were mashed up in a food processor (CL-30, Robot Coupe, France). The resultant strawberry pulp was homogenized on colloid mill (Mz-50, Fryma, Switzerland, Ø ≤ 0.5 mm, 10,000 × RPM) at atmospheric pressure and deaerated at 0.06 MPa (LVE, Fryma, Switzerland). The product temperature did not exceed 0 °C throughout the whole processing procedure. This strawberry purée was used as a untreated sample.
Preservation of strawberry purée
Technological procedures of strawberry purée (pH 3.3) being the material for this study as well as HPP and TP processes have been described in previous paper (Marszałek et al. 2015). Samples of purée were preserved with HPP at 300 and 500 MPa for 1, 5 and 15 min at 0 and 50 °C (omitting 500 MPa, 5 min, 50 °C) and using thermal pasteurization (TP) at 90 °C for 15 min.
Data regarding the time and temperature were recorded inside the jars in the coldest spot, using a monitoring–measuring device (9004, Ellab, Denmark) designed for measuring the temperature inside a package and the temperature of the heating medium during the course of the process.
The experiment were done in duplicate. All samples were cold-stored at 6 °C, protected from light for up to 12 weeks and subjected to testing at 2-week intervals.
Analyses
Microbiological analyses
Yeasts and moulds were analyzed according to the ISO 21527–1:2008 standard. Purée samples were diluted using sterile normal saline (0.85% sodium chloride), spread plated on sterile nutrient agar with dichloran rose bengal chlortetracycline (DRBC) and incubated at 25 °C for 5–7 days.
The total microbial count (TMC) was determined according to the EN ISO 4833: 2003 standard. Samples diluted with sterile normal saline were plated count agar (PCA); plates were incubated at 30 °C for 72 h. All the experiments were conducted in duplicates and mean values of Log 10 CFU/g sample have been reported. The samples were considered microbiologically stable until the count of any of the analyzed groups of microorganisms increased by more than one logarithmic cycle, as compared to the previous sampling point (carried out in 2-week intervals), or visible symptoms of deterioration were observed.
Total content of polyphenols (TCP)
Strawberry purée (10 g) was extracted for 5 min in an ultrasound bath (40 kHz, 100 W, 25 °C, ITR, Poland) using 30 g of an extraction mixture: methanol/water/hydrochloric acid (80:19.9:0.1; v/v/v) in centrifuge flasks. Afterwards, the sample was centrifuged in a laboratory centrifuge (MPW-350R, MPW Med. Instruments, Poland) at 3.670×g and 4 °C for 5 min. Extraction was repeated 5 times, supernatants were combined and the methanol was evaporated under vacuum (B-481, Büchi, Switzerland). The residue was transferred quantitatively to a volumetric flask and filled up to 50 mL with 0.1% (v/v) o-phosphoric acid.
The total content of polyphenols was determined spectrophotometrically using the Folin–Ciocalteu method modified by Gao et al. (2000), and was expressed in milligrams of gallic acid equivalent per 100 g of the purée’s fresh weight (mg GAE/100 g FW).
HPLC analysis of phenolic acids and flavonols
Phenolic compounds were determined according to a modified method of Odriozola-Serrano et al. (2008). To determine the contents of p-hydroxybenzoic acid (p-HBA), ellagic acid (EA), quercetin (Q) and kaempferol (K), acidic hydrolysis was conducted with the method used by Da Silva Pinto et al. (2008) with slight modifications described by Marszałek et al. (2015). Forty milliliters of a methanol/6 M hydrochloric acid/water mixture (56:25:19; v/v/v) was added to 1 mL of the extract (described in TCP section). The sample was heated at 120 °C for 90 min, then cooled and neutralized with 6 M NaCl to pH ca. 3.5.
The analysis of phenolic acids and flavonols in the HPLC system equipped with a DAD SPD-10Avp detector, thermostat CTD-10AsVp and DEGASEX™ DG-4400 degasser (Shimadzu, Japan) was carried out on a reversed–phase Luna C18 column (250 × 4.6 mm, 5 µm, Phenomenex, USA) at a temperature of 30 °C, flow rate of 1 mL/min and detection at 260 nm (phenolic acids) and 360 nm (flavonols). The water/formic acid mixture (99:1, v/v) and acetonitrile were used as eluents (eluent A and B, respectively) in the following gradient program: 10% B (10 min), from 10 to 45% B (15 min), from 45 to 70% B (5 min), from 70 to 10% B (3 min), and 10% B (4 min). Selected phenolic compounds were quantified using p-HBA, EA, Q and K calibration curves. The contents of all compounds were expressed in mg/100 g FW. Peak identities were as described by Odriozola–Serrano et al. (2008).
HPLC analysis of anthocyanins
The HPLC determination of the anthocyanin content was carried out using the method described by Goiffon, et al. (1999), with modifications described by Marszałek et al. (2015). Ten milliliters of the extract (described in TCP section) were absorbed and purified on Sep–Pak C18 minicolumns (Waters, USA). Anthocyanins were eluted with 5 mL of a methanol/water/hydrochloric acid mixture (75:24.9:0.1; v/v/v), and then filtered on PTFE filters with a pore size of 0.45 µm (Waters, USA). Analyses were carried out in a gradient system using the equipment described in previous section at a temperature of 25 °C, flow rate of 1 mL/min and detection at 520 nm. A water/formic acid mixture (89:11, v/v) and acetonitrile were used as eluents (A and B, respectively) in the following program: 9% B (15 min), from 9 to 20% B (1 min), from 20 to 30% B (1 min), from 30 to 9% B (2 min), and 9% B (4 min). Monomers of the anthocyanins were identified by comparing their retention times with those of the standards and with data from the literature. Pelargonidin-3-O-gucoside (Pg-3-Glc), followed by cyanidin-3-O-glucoside (Cy-3-Glc) contents were calculated based on their standard calibration curves. Pelargonidin-3-O-rutonoside (Pg-3-Rut) was expressed as Pg-3-Glc. Total content of anthocyanins (TCA) was calculated as the sum of Pg-3-Glc, Cy-3-Glc and Pg-3-Rut. All results were expressed in mg/100 g FW.
HPLC analysis of AA and DHAA
The total vitamin C content, expressed as L-ascorbic acid (AA) and L-dehydroascorbic acid (DHAA), was determined as described by Odriozola–Serrano et al. (2007). Approximately 1 g of purée was transferred to a volumetric flask and filled to 10 mL with 0.01% of m-phosphoric acid. The extract was then filtered through PTFE 0.45 µm filters. In order to assay the DHAA, it was reduced to AA with 0.1% DL-dithiothreitol (DTT) using 1 mL of the following mixture: extract/DTT (50:50; v/v) and leaving it in the dark for 1 h. The DHAA content was calculated using the formula: DHAA = (AA + DHAA) − AA.
The HPLC analysis (2695, Waters, USA) was carried out using a reversed-phase SunFire C18 column (250 × 4.6 mm, 5 μm, Waters, USA) and a photodiode detector (DAD) (2996, Waters, USA). The analysis was carried out using the isocratic method using 0.01% m-phosphoric acid at a flow rate of 1 mL/min and column temperature of 25 °C. AA was quantified using external calibration curves. The contents of AA and DHAA were expressed in mg AA/100 g FW. Peak identities were as described by Odriozola–Serrano et al. (2007).
Determination of PPO and POD activities
The activity of selected tissue enzymes was determined as described by Terefe et al. (2010). The extraction mixture comprised 0.2 M phosphate buffer (pH = 6.5) containing 4% (w/v) polyvinylpyrrolidone (PVPP), 1% (v/v) Triton X-100 and 1 M NaCl. The strawberry purée and the mixture (4.5: 4.5 g, w/w) were treated with ultrasound (40 kHz, 100 W, 25 °C, ITR, Poland) for 3 min and centrifuged (MPW-350R, MPW Med. Instruments, Poland) at 17,700×g for 30 min at 4 °C. The supernatant, after filtration through blotting filter paper, was used to determine PPO and POD activity.
For the PPO activity assay, 100 µL of the supernatant was introduced into 3 mL of 0.05 M phosphate buffer (pH 6.5) containing 0.07 M catechol, and the absorbance was measured spectrophotometrically (UV-1650PC, Shimadzu, Japan) at λ = 420 nm and 25 °C for 10 min. A blank sample was prepared in the same way, by substituting the supernatant with a phosphate buffer. The PPO activity was expressed as a change in the absorbance/min/g of FW of the analyzed sample.
For the POD activity assay, 1.5 mL of 0.05 M phosphate buffer (pH = 6.5) was added to the mixture containing 200 µL of the supernatant, 200 µL of 0.05 M phosphate buffer containing 1% p-phenylenediamine (w/v) and 200 µL of 1.5% (v/v) hydrogen peroxide. Mixture absorbance was measured at λ = 485 nm, 25 °C for 10 min. The POD activity was expressed as a change in the absorbance/min/g of FW of the analyzed sample.
Changes in colour parameters
The colour of the strawberry purée was determined using a CM-3600d colorimeter (Konica Minolta, Japan), in glass cuvettes with an optical path of 10 mm. The measurement was made on the CIEL*a*b* system, using illuminant D65. The values of the absolute colour difference of the samples after preservation were calculated following equation: ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2 where L* (lightness/darkness), a* (red/green), and b* (yellow/blue).
Sensory analysis
The sensory analysis of strawberry products was conducted as described in ISO 4121:1998. An overall quality assessment was conducted using a 9-point hedonic scale; assessments were made by a trained sensory panel of nine persons (age: 28–60 years). All samples were evaluated independently in a test room complying with ISO 8589:2007 requirements.
Kinetic data analysis
The kinetics of anthocyanin degradation is usually well described as a first-order reaction (Hernández-Herrero and Frutos 2011; Patras et al. 2010; West and Mauer, 2013). The kinetic equation of this reaction is:
where C is concentration, t is time and k is the rate of reaction. Integration of this equation leads to:
Experimental data should be represented by a straight line in ln(C) versus t coordinate system. Slope of the line, determined by using linear regression, is a rate of degradation reaction. The half-lives were calculated using the relationship:
The same approach has proved to be appropriate to describe the total polyphenol content changes.
Statistical analysis
All analyses were conducted using Statistica 10 StatSoft® software. The significance of differences was determined based on the analysis of variance with Tukey’s test (p value ≤0.05). HPP and TP experiment were done in duplicate, the analysis for each experiment were done also in duplicate.
Results and discussion
Microbiological stability
Microbiological quality of preserved purée samples, the main indicator of their shelf-life, was determined based on the count of yeast and molds and the total count of microorganisms—TCM. The initial microbial load noted for untreated sample was 4.86, 4.60, and 3.82 log cfu/g, respectively for TMC, yeasts and moulds. As described in previous study (Marszałek et al. 2015), reduction in the count of yeast, and molds below 10 cfu/g was achieved after TP, and HPP at 500 MPa and 50 °C, whereas decrease of TMC was observed only for TP samples. The lack of sterility in the samples preserved with high pressure of 300 MPa at both temperatures resulted in the development of microorganisms during storage over 7.0 log cfu/g and shortened the shelf life below 4 weeks. The main cause of spoilage of the purée samples preserved with HPP at 300 MPa was ethanol fermentation induced by yeasts and in the samples preserved at 500 MPa mould growth was observed. The growth of both yeasts and molds was significantly affected by the HPP treatment time (Marszałek et al. 2015).
The count of mold and TMC not exceeded 20 and 140 cfu/g up to 12 weeks of cold storage samples preserved by HPP at 500 MPa and 50 °C for 15 min. During this time there were no yeasts detected. These samples were found to be the most microbiologically stable during the entire period of storage. These samples also showed the highest degree of PPO and POD inactivation (Marszałek et al. 2015), and thus purée preserved only under these conditions was subjected to further in-depth physicochemical analyses.
The influence of HPP on the microbiological quality of strawberry products pressurized at 220–600 MPa at room temperature and processing time from 4 to over a dozen minutes was also evaluated by other authors (Cao et al. 2012; Gao et al. 2016; Hartmann et al. 2010; Segovia-Bravo et al. 2012). Gao et al. (2016) described their strawberry products as stable after pressurization at 400 MPa for 5 min up to 45 days, whereas Cao et al. (2012) at 600 MPa for 4 min up to 6 months under refrigerated storage. This results proved that the microbial stability depended on the level of pressure as well as on the process time and temperature.
Phenolics content
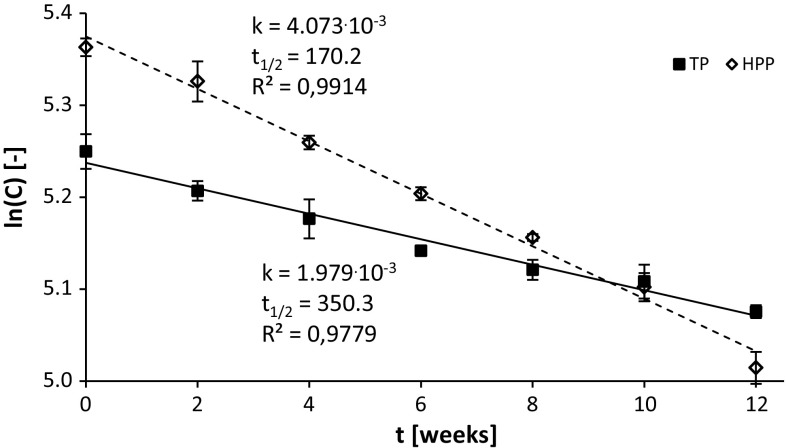
A comparison of the total content of polyphenols (TCP) in strawberry purée preserved by TP and HPP (Table 1) during storage showed that in the case of TP, the loss of these compounds after 12-week storage was 16%, whereas for HPP a 29% decrease was observed. Considering the loss of polyphenols due to the processing (14% in TP and 3% in HPP), it appears that the total losses of these compounds at the end of the storage period were similar in both pasteurized and HPP-treated purée, and amounted to 28 and 32%, respectively. In order to maintain a higher TCP content of the HPP-treated product compared to the pasteurized, the maximum period of the sample storage should not exceed 8 weeks. The half-lives of TCP were ca. 350 and 170 days for thermal pasteurization and the HPP method, respectively (Fig. 1). A Lower loss of approximately 12% of phenolics, in the pressure-preserved strawberry mousse (treated at 500 MPa for 10 min at 50 °C), stored for about 10 weeks was observed by Ferrari et al. (2011). Gao et al. (2016) reported ca. 25% loss of polyphenols in the HPP-treated (400 MPa, 5 min) strawberries stored at 4 °C for 45 days, whereas Hartmann et al. (2010) reported as much as 36% loss of this compounds in strawberry purée stored for 11 weeks at 8 °C. On the other hands Cao et al. (2012) reported only 16% loss of polyphenols HPP-treated (600 MPa, 4 min) strawberry juice stored at 4 °C for 6 month. This phenomenon could be explained by the different initial concentration of polyphenols in purées and juices.
Table 1.
Changes in total content of phenolic acids, flavonols and total polyphenols in pasteurized (90 °C, 15 min) strawberry purée and purée preserved with HPP (500 MPa, 15 min, 50 °C) during storage (mg/100 g FW)
| TP | HPP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-HBA | EA | Q | K | TCP | p-HBA | EA | Q | K | TCP | |
| Untreated | 1.19 ± 0.02de | 33.54 ± 3.65d | 6.23 ± 0.68b | 2.75 ± 0.16a | 221.0 ± 3.3a | 1.19 ± 0.02b | 33.54 ± 3.65d | 6.23 ± 0.68d | 2.75 ± 0.16a | 221.0 ± 3.33a |
| Weeks of storage | ||||||||||
| 0 | 1.85 ± 0.08ab | 43.77 ± 2.51c | 9.61 ± 0.46a | 3.04 ± 0.25a | 190.6 ± 3.6b | 1.82 ± 0.11a | 39.43 ± 2.94cd | 9.25 ± 0.28c | 2.44 ± 0.34a | 213.4 ± 2.0b |
| 2 | 1.72 ± 0.58abc | 42.71 ± 2.93c | 9.41 ± 1.91a | 2.87 ± 0.17a | 182.5 ± 1.9c | 1.83 ± 0.66a | 48.03 ± 3.73b | 12.90 ± 1.27a | 2.37 ± 0.43a | 205.7 ± 4.5c |
| 4 | 2.02 ± 0.12a | 47.91 ± 2.25c | 9.26 ± 0.42a | 3.12 ± 0.49a | 177.1 ± 3.8d | 1.76 ± 0.39a | 43.87 ± 5.05bc | 10.66 ± 1.09bc | 2.47 ± 0.17a | 192.4 ± 1.4d |
| 6 | 1.56 ± 0.18bcd | 59.53 ± 2.79b | 9.11 ± 0.56a | 2.62 ± 0.27ab | 171.0 ± 0.6e | 1.36 ± 0.21a | 46.47 ± 3.53b | 11.07 ± 0.42b | 1.78 ± 0.41b | 182.0 ± 1.3e |
| 8 | 1.34 ± 0.09cde | 61.06 ± 6.26b | 9.69 ± 0.65a | 2.83 ± 0.44a | 167.5 ± 1.8ef | 1.18 ± 0.08a | 50.27 ± 1.94ab | 10.42 ± 1.04bc | 1.50 ± 0.10bc | 173.5 ± 2.4f |
| 10 | 1.07 ± 0.29e | 68.28 ± 2.08a | 10.41 ± 1.16a | 1.96 ± 0.81b | 165.4 ± 3.0f | 1.49 ± 0.63a | 56.49 ± 5.33a | 9.67 ± 0.98bc | 1.17 ± 0.07c | 164.4 ± 1.6g |
| 12 | 1.12 ± 0.14e | 61.99 ± 2.49b | 10.57 ± 1.86a | 3.07 ± 0.12a | 160.1 ± 1.1g | 1.37 ± 0.37a | 56.40 ± 2.59a | 9.99 ± 1.40bc | 1.20 ± 0.11c | 150.6 ± 1.4h |
Mean values denoted with the same letter do not differ significantly during storage at p value ≤0.05
Data mean ± SD (n = 4), FW fresh weight, p-HBA p-hydroxybenzoic acid, EA ellagic acid, Q quercetin, K kaempferol, TCP total content of polyphenols
Fig. 1.
Kinetics of changes of the total content of polyphenols (TCP) during storage of puree preserved with TP and HPP (500 MPa, 15 min, 50 °C). Slopes of the lines are equal to reaction rates. Data mean and standard deviations (n = 4); TP thermal pasteurization (dotted line); HPP high pressure processing (solid line); k kinetic rate constant [days−1]; t1/2: half-life time [days]
Analysis of the HPP-treated samples of strawberry purée showed that the free p-hydroxybenzoic acid was not present, while the levels of free ellagic acid and free flavonols were very low and ranged from 1.6 to 3.7% of TCP, depending on the sample (Marszałek et al. 2015). Hence, when discussing changes in the content of phenolic compounds and flavonols during storage, attention should be paid to the total content of these compounds, determined after acidic hydrolysis. Treating the samples with either method increased the total content of p-HBA from 1.2 to ca. 1.8 mg/100 g FW. No significant changes were recorded in the p-HBA content during storage of the HPP-treated samples. The content of p-HBA in the stored TP-treated purée remained unchanged until week 4, followed by a successive decline by 25%, until week 12. Odriozola-Serrano et al. (2008) reported a similar trend during the 8-week cold storage of strawberry juice preserved by the continuous-flow pasteurization (90 °C, 60 s). These authors found an 8% increase in the p-HBA content after processing, and ca. 45% loss of this compound at the end of the storage period. The content of ellagic acid increased until the 10th week of storage, regardless of the preservation technique, and then stabilized in the HPP-treated samples, while slightly declined in TP samples. The increasing content of phenolic acids during storage could have been associated with the active enzymatic system in the pressurized purée, and to heat treatment in the case of pasteurization, which could lead to the release of these compounds in hydrolysis reactions. Ellagic acid may be also released from high molecular ellagitannins. Acidic environment is another factor facilitating the release of phenolic acids (purée pH was 3.3), which enhance the hydrolysis. Odriozola-Serrano et al. (2008) also reported an increase in the EA content till the 4th week of storage, and then a slow gradual decrease, in the pasteurized strawberry juice (90 °C, 60 s).
The level of quercetin increased significantly after preservation using both HPP and TP, followed by a successive increase in the HPP-treated purée until the 2nd week of storage. These results indicate that the bound quercetin is released from high-molecular compounds, and then, as a less stable monomer, is subjected to degradation, especially in the presence of active tissue enzymes. The content of this compound in the TP samples remained unchanged during storage. The content of kaempferol did not increase as a result of preservation, and only a minor loss was recorded after 4 weeks of storage in HPP- and TP-treated samples. During the 8 week storage of the flow-pasteurized strawberry juice (90 °C, 60 s), Odriozola-Serrano et al. (2008) reported 45 and 20% loss of quercetin and kaempferol, respectively, which could also result from incomplete inactivation of enzymes responsible for the degradation of these compounds. The study of Terefe et al. (2010) demonstrated that the PPO present in the strawberry purée exhibited a high heat stability and might retain as much as 72% of the activity even after heating at 100 °C for 30 min. The HPP-treated strawberry purée, showed the activity of PPO and POD at the level of 28 and 50%, respectively, compared to the non-preserved sample (Marszałek et al. 2015).
Anthocyanins content
The results presented in Table 2 showed that, although HPP itself did not lead to anthocyanin degradation, these labile compounds were degraded during the long-term storage. Loss of anthocyanins in the purée preserved with HPP upon storage was 69% after 12 weeks, while in the pasteurized samples only a 19% decrease was observed during this period. Taking into account significant differences resulting from the preservation process, the total losses of these compounds after the 12-week storage reached 73% in the HPP-treated purée and 54% in the pasteurized purée compared to the raw material. Gao et al. (2016) reported similar losses of anthocyanins in the HPP-treated (400 MPa, 5 min) strawberries stored at 4 °C for 45 days. Significantly lower losses when compared to the aforementioned values were reported by Ferrari et al. (2011), who recorded only 35% loss of anthocyanin pigments after ca. 10 weeks of storage at 4 °C of the HPP-treated strawberry mousse (500 MPa, 10 min, 50 °C). Cao et al. (2012) noted ca. 30% loss of anthocyanins in HPP treated strawberry juice (600 MPa, 4 min) stored at 4 °C for 6 months. On the other hand, Hartmann et al. (2010) reported as much as 54% loss of anthocyanins in the pasteurized purée after 11 weeks of storage at 8 °C.
Table 2.
Changes in contents of anthocyanins in pasteurized purée (90 °C, 15 min) and purée preserved with HPP (500 MPa, 15 min, 50 °C) during storage (mg/100 g FW)
| TP | HPP | |||||||
|---|---|---|---|---|---|---|---|---|
| Cy-3-Glc | Pg-3-Glc | Pg-3-Rut | Total | Cy-3-Glc | Pg-3-Glc | Pg-3-Rut | Total | |
| Untreated | 7.81 ± 0.01a | 80.79 ± 4.81a | 4.03 ± 0.71a | 92.60 ± 5.49a | 7.81 ± 0.01a | 80.79 ± 4.81a | 4.03 ± 0.71a | 92.60 ± 5.49a |
| Weeks of storage | ||||||||
| 0 | 4.36 ± 0.11b | 45.97 ± 1.68b | 2.04 ± 0.08b | 52.36 ± 1.86b | 6.86 ± 0.08b | 70.13 ± 1.52b | 2.80 ± 0.07b | 79.79 ± 1.61b |
| 2 | 4.01 ± 0.06c | 42.67 ± 1.23bc | 1.97 ± 0.07b | 48.66 ± 1.33bc | 6.82 ± 0.31b | 65.66 ± 1.41c | 2.36 ± 0.13b | 74.83 ± 1.71c |
| 4 | 3.84 ± 0.04cd | 40.75 ± 0.69cd | 1.85 ± 0.03b | 46.45 ± 0.69cd | 5.09 ± 0.12c | 52.53 ± 0.76d | 1.83 ± 0.11c | 59.45 ± 0.98d |
| 6 | 4.00 ± 0.14c | 41.92 ± 1.15c | 1.90 ± 0.05b | 47.82 ± 1.32c | 4.52 ± 0.12d | 46.75 ± 1.17e | 1.67 ± 0.09cd | 52.94 ± 1.36e |
| 8 | 3.76 ± 0.13cd | 40.05 ± 1.13cd | 1.73 ± 0.05b | 45.54 ± 1.29cd | 3.37 ± 0.18e | 36.76 ± 1.37f | 1.30 ± 0.02de | 41.43 ± 1.43f |
| 10 | 3.96 ± 0.48c | 39.29 ± 1.29cd | 1.72 ± 0.01b | 44.97 ± 1.53cd | 2.92 ± 0.17f | 31.06 ± 0.98g | 1.10 ± 0.01ef | 35.08 ± 1.15g |
| 12 | 3.62 ± 0.08d | 37.37 ± 1.78d | 1.66 ± 0.05b | 42.65 ± 1.91d | 1.95 ± 0.18g | 22.24 ± 1.17h | 0.78 ± 0.04f | 24.96 ± 1.37h |
Mean values denoted with the same letter do not differ significantly at p value ≤0.05
Data mean ± SD (n = 4), FW fresh weight, TP thermal pasteurization, HPP high pressure processing, Cy-3-Glc cyanidin-3-glucoside, Pg-3-Glc pelargonidin-3-glucoside, Pg-3-Rut pelargonidin-3-rutinoside, total total content of anthocyanins
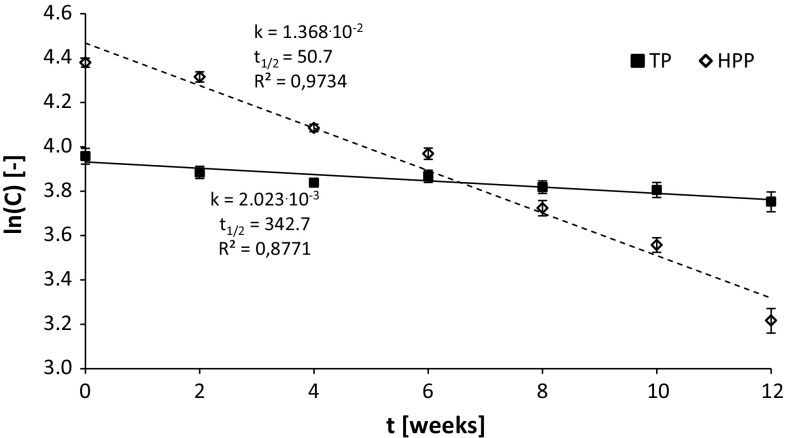
Among the monomers investigated in our study, the most unstable during storage was Pg-3-Rut, the content of which decreased to 19 and 41% of initial content after 12 weeks in the purée after HPP and TP, respectively. The average content of other monomers analyzed reached 26 and 46% of the initial value for HPP- and TP-treated purées, respectively. Changes in the content of anthocyanin pigments during storage indicated that the rate of degradation of these compounds in the pasteurized purée was significantly slower than in the HPP-treated purée (Table 2). These differences were probably mainly due to the activity of tissue enzymes in the pressurized purée. After the 8-week storage of the flow-pasteurized strawberry juice (90 °C, 60 s), Odriozola-Serrano et al. (2008) reported 66, 68 and 53% loss of Cy-3-Glc, Pg-3-Glc and Pg-3-Rut, respectively. Faster degradation of anthocyanins in the analyzed purée may result from a higher content of L-ascorbic acid, which was reported to facilitate the degradation of these pigments (Veridiana et al. 2007). Hydrogen peroxide is formed during the oxidation of vitamin C, the presence of which is necessary for enzymatic reaction of POD. The higher activity of POD can also be caused by the synergistic effect of the PPO reaction products as substrates for POD reactions (Terefe et al. 2013a, b). Strawberry juice obtained using a more complex and invasive technological process had probably a lower content of vitamin C compared to purée. The assessment of purée quality based on the half-life of anthocyanin pigments demonstrated a clear superiority of the pasteurized purée compared to the high-pressure-treated purée in terms of anthocyanin stability. For the HPP-preserved purée, the half-life of total anthocyanins calculated using a lineal regression reached ca. 50 days, whereas the half-life of this compounds in the pasteurized purée was about 340 days (Fig. 2). Shorter half-life of approximately 30 days was reported by Marszałek et al. 2011 on strawberry juices and nectars preserved by HPP.
Fig. 2.
Kinetics of changes of the total content of anthocyanins during storage of puree preserved with TP and HPP (500 MPa, 15 min, 50 °C). Slopes of the lines are equal to reaction rates. Data mean and standard deviations (n = 4); TP thermal pasteurization (dotted line); HPP high pressure processing (solid line); k kinetic rate constant [days−1]; t1/2: half-life [days]
AA and DHAA content
The loss of L-ascorbic acid in the purée samples was high during the first 2 weeks of storage (Table 3), as only 44 and 67% of AA remained in the pasteurized and HPP-treated purée, respectively. The DHAA was subjected to rapid degradation. No vitamin C was detected in the thermally-pasteurized purée after 8 weeks of storage, while complete degradation was observed after 4 weeks of storage for HPP-treated purée. Cao et al. (2012) noted ca. 40 and 50% loss of vitamin C in HPP-treated (600 MPa, 4 min) cloudy and clear strawberry juice stored at 4 °C for 6 months. The study of Hartmann et al. (2010) on the loss of vitamin C during storage of pasteurized strawberry purée demonstrated a 60% decrease of AA content after 11 weeks of storage at 8 °C, whereas Odriozola-Serrano et al. (2008) reported ca. 80% loss of AA after 8 weeks of storage of pasteurized strawberry juice. Strawberry juices are significantly poorer in phenolic compounds (including anthocyanin pigments) and may contain even only half of these compounds compared to the raw material (Oszmiański et al. 2007), which may positively affect the vitamin C stability.
Table 3.
Changes in contents of L-ascorbic acid L-dehydroascrobic acid and in ΔE color coefficient in pasteurized purée (90 °C, 15 min) and purée preserved with HPP (500 MPa, 15 min, 50 °C) during storage (mg/100 g FW)
| TP | HPP | |||||||
|---|---|---|---|---|---|---|---|---|
| AA | DHAA | AA + DHAA | ΔE | AA | DHAA | AA + DHAA | ΔE | |
| Untreated | 37.49 ± 0.77a | 15.89 ± 0.72a | 53.38 ± 0.95a | – | 37.49 ± 0.77a | 15.89 ± 0.72a | 53.38 ± 0.95a | – |
| Weeks of storage | ||||||||
| 0 | 14.93 ± 0.16b | 5.55 ± 0.39b | 20.48 ± 0.33b | 3.03 ± 0.03f | 16.28 ± 0.28b | 16.28 ± 0.28a | 37.88 ± 0.18b | 2.25 ± 0.03g |
| 2 | 6.90 ± 0.11c | 2.67 ± 0.19c | 9.57 ± 0.10c | 4.32 ± 0.04d | 10.96 ± 0.10c | 10.96 ± 0.10b | 11.56 ± 0.12c | 1.56 ± 0.04f |
| 4 | 6.52 ± 0.11d | 2.47 ± 0.03d | 8.99 ± 0.14d | 4.44 ± 0.03c | 0.78 ± 0.00d | 0.78 ± 0.00c | 1.51 ± 0.03d | 1.17 ± 0.06e |
| 6 | 2.04 ± 0.03e | 1.69 ± 0.16e | 3.74 ± 0.14e | 4.05 ± 0.05e | nd | nd | nd | 3.52 ± 0.04d |
| 8 | 0.83 ± 0.16f | nd | 0.83 ± 0.16f | 4.41 ± 0.06c | nd | nd | nd | 3.66 ± 0.05c |
| 10 | nd | nd | nd | 5.81 ± 0.05b | nd | nd | nd | 4.32 ± 0.06b |
| 12 | nd | nd | nd | 7.21 ± 0.04a | nd | nd | nd | 5.05 ± 0.03a |
Data mean ± SD (n = 4), FW fresh weight, TP thermal pasteurization, HPP high pressure processing, AA L-ascorbic acid, DHAA L-dehydroascorbic acid, nd not detected
ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2, where L* (lightness/darkness), a* (red/green), and b* (yellow/blue). Mean values denoted with the same letter do not differ significantly at p value ≤0.05
Color parameters
The greatest changes in color parameters in the TP purée occurred as a result of the preservation process (Table 3). Barba et al. (2013) demonstrated that if ΔE coefficient is higher than 1.5, changes in the sample color are noticeable by an inexperienced observer. According to this assumption, the pasteurized purée was also characterized by significant changes in color during storage. These changes were attributed to the darkening (decrease of parameter L*) and to a decrease in the intensity of red (parameter a*) and yellow color (parameter b*). The initial increase in the value of ΔE coefficient in the HPP treated sample could be due to the increased intensity of the color resulting from the extraction of anthocyanin pigments from pulp to intracellular juice under pressure. The colour changes of TP sample during storage up to 8 weeks were almost unnoticable, whereas after 12 weeks of storage, the value of ΔE for the pasteurized purée reached 7.2. Changes in the color of HPP-treated purée could not be noticeable by an inexperienced observer until even 4 weeks whereas over this period changes of colour were significant. Color changes are mainly attributable to the activity of tissue enzymes (polyphenol oxidases and peroxidases) and to Maillard non-enzymatic browning reactions induced by, e.g., increased temperature and low pH (Rodrigo et al. 2007; Gössinger et al. 2009). Products of enzymatic oxidation of phenolic compounds have been previously shown to facilitate degradation of anthocyanins, leading to the formation of numerous polymeric compounds, which may precipitate from the solution (Cao et al. 2011).
Sensory quality
Sensorial panel noted no significant changes of the quality of strawberry purée after pressurization, whereas thermally treated samples noted significant lower scores. During storage, sensorial quality expressed on hedonic scale deteriorated significantly in the HPP samples up to 6 weeks, whereas in the pasteurized purée, no changes were observed throughout the storage period with the same storage period (Table 4). Despite these differences, the color of purée after HPP were better evaluated by sensory panel. In terms of taste, HPP samples were rated better than TP samples directly after the processing and for the first 2 weeks of storage, but later the changes were insignificant. The aroma of pasteurized purée was better evaluated from 4th to 12th week of storage, compared to the HPP-treated purée. The sensory panel noted that consistency of pasteurized purée remained unchanged over the entire storage period, whereas that of the HPP-treated purée was rapidly deteriorating. Initially, thick purée became watery after 4 weeks of storage. These rapid changes in consistency were probably caused by the activity of tissue enzymes, in particular pectinolytic enzymes. The overall sensory assessment of the products showed that purée preserved with HPP was superior to the pasteurized product only until ca. the 4th week of storage. Afterwards, enzymatic changes contributed to a significant deterioration of the sensory quality of the HPP-treated purée.
Table 4.
Changes in sensory quality in pasteurized purée (90 °C, 15 min) and purée preserved with HPP (500 MPa, 15 min, 50 °C) during storage (overall quality assessment in a 9-point hedonic scale)
| TP | HPP | |
|---|---|---|
| Untreated | 9.0 ± 0.0a | 9.0 ± 0.0a |
| Weeks of storage | ||
| 0 | 7.8 ± 0.4b | 8.5 ± 0.4ab |
| 2 | 7.8 ± 0.4b | 8.3 ± 0.4bc |
| 4 | 7.8 ± 0.4b | 7.6 ± 0.4cd |
| 6 | 7.8 ± 0.4b | 7.3 ± 0.4d |
| 8 | 7.8 ± 0.4b | 6.4 ± 0.4e |
| 10 | 7.0 ± 0.0c | 6.3 ± 0.4e |
| 12 | 7.0 ± 0.0c | 6.0 ± 0.0e |
Data mean ± SD (n = 9), FW fresh weight, TP thermal pasteurization, HPP high pressure processing
Mean values denoted with the same letter do not differ significantly at p value ≤0.05
Conclusion
This study was aimed at determining the optimal shelf-life of strawberry purée preserved with TP and HPP. HPP was an effective method for extending the shelf-life of strawberry purée, and enabled to obtain product of higher quality compared to TP. The period of microbiological shelf-life during cold storage (6 °C) of purée preserved at 500 MPa for 15 min at 50 °C was 12 weeks. However, undesirable quality changes were observed at that time, probably related to the insufficient inactivation of strawberry tissue enzymes. For this reason, recommended shelf-life of HPP-preserved strawberry purée should not exceed 6 weeks. During this period, the polyphenols and anthocyanins level detected in HPP preserved purée was significantly higher than that of the thermally-preserved purée. These results indicate that the HPP method is noteworthy especially for premium quality products of a relatively short term of storage.
Acknowledgements
The Project was financed from funds of National Research Center (Grant No. NN 312 252540), and co-financed from European Union funds under the European Social Fund (Contract No. 78/ES/ZS-II/W-2151.1/11). Authors would like to thank Prof. Monika Fonberg-Broczek (UNIPRESS, Poland) for the possibility of using a pressure cabinet.
References
- Barba FJ, Esteve MJ, Frigola A. Physicochemical and nutritional characteristics of blueberry juice after high pressure processing. Food Res Intern. 2013;50:545–549. doi: 10.1016/j.foodres.2011.02.038. [DOI] [Google Scholar]
- Cao X, Zhang Y, Zhang F, Wang Y, Yi J, Liao X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J Sci Food Agric. 2011;91:877–885. doi: 10.1002/jsfa.4260. [DOI] [PubMed] [Google Scholar]
- Cao X, Bi X, Huang W, Wu J, Hu X, Liao X. Changes of quality of high hydrostatic pressure processed cloudy and clear strawberry juices during storage. Innov Food Sci Emerg Technol. 2012;16:181–190. doi: 10.1016/j.ifset.2012.05.008. [DOI] [Google Scholar]
- Chacraborty S, Kaushik N, Rao PS, Mishra HN. High-pressure inactivation of enzymes: a review on its recent applications on fruit purees and juices. Compr Rev Food Sci F. 2014;13:578–596. doi: 10.1111/1541-4337.12071. [DOI] [PubMed] [Google Scholar]
- Da Silva Pinto M, Lajolo FM, Genovese MI (2008) Bioactive compounds and quantification of total ellagic acid in strawberries (Fragaria × ananassa Duch). Food Chem 107(4):1629–1635. doi:10.1016/j.foodchem.2007.10.038
- Ferrari G, Maresca P, Ciccarone R. The effects of high hydrostatic pressure on the polyphenols and anthocyanins in red fruit products. Proc Food Sci. 2011;1:847–853. doi: 10.1016/j.profoo.2011.09.128. [DOI] [Google Scholar]
- Gao X, Ohlander M, Jeppson N, Bjork L, Trajkovski V. Changes in antioxidant effect and their relationship to phytonutrients in fruit of sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem. 2000;48:1845–1890. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- Gao G, Pengyan R, Cao X, Yan B, Liao X, Sun Z, Wang Y. Comparing quality changes of cupped strawberry treated by high hydrostatic pressure and thermal processing during storage. Food Bioprod Process. 2016;100:221–229. doi: 10.1016/j.fbp.2016.06.017. [DOI] [Google Scholar]
- Goiffon JP, Mouly PP, Gaydou EM. Anthocyanic pigment determination in red fruit juices, concentrated juices and syrups using liquid chromatography. Anal Chim Acta. 1999;382:39–50. doi: 10.1016/S0003-2670(98)00756-9. [DOI] [Google Scholar]
- Gössinger M, Mortiz S, Hermes M, Wendelin S, Scherbichler H, Halbwirth H, Stich K, Berghofer E. Effects of processing parameters on colour stability of strawberry nectar from purée. J Food Eng. 2009;90:171–178. doi: 10.1016/j.jfoodeng.2008.06.018. [DOI] [Google Scholar]
- Hainz V, Buckow R. Food preservation by high pressure. J Verbrauch Lebensm. 2009;5:73–81. doi: 10.1007/s00003-009-0311-x. [DOI] [Google Scholar]
- Hartmann A, Patz C, Andlauert H, Dietrich H, Ludwig M. Change of ascorbic acid and phenolic compounds during the processing of strawberry purée and juice. Fruit Process. 2010;20(3):102–109. [Google Scholar]
- Hernández-Herrero JA, Frutos JM. Degradation kinetics of pigments, colour and stability of the antioxidant capacity in juice model systems from six anthocyanin sources. J Food Sci Technol. 2011;46:2550–2557. doi: 10.1111/j.1365-2621.2011.02780.x. [DOI] [Google Scholar]
- Kaur C, Kapoor HC. Antioxidants in fruits and vegetables. The millennium’s health. Intern J Food Sci Technol. 2001;33:703–725. doi: 10.1046/j.1365-2621.2001.00513.x. [DOI] [Google Scholar]
- Marszałek K, Mitek M, Skąpska S. Application of high hydrostatic pressure (UHP) to stabilize strawberry juices and nectars. ZYWN-Nauk Technol Jakosc. 2011;1(74):113–123. [Google Scholar]
- Marszałek K, Mitek M, Skąpska S. The effect of thermal pasteurization and high pressure processing at cold and mild temperatures on the chemical composition, microbial and enzyme activity in strawberry purée. Innov Food Sci Emerg Technol. 2015;27:48–56. doi: 10.1016/j.ifset.2014.10.009. [DOI] [Google Scholar]
- Odriozola-Serrano I, Soliva-Fortuny R, Martin-Belloso O. Phenolic acids, flavonoids, vitamin C and antioxidant capacity of strawberry juices processed by high-intensity pulsed electric fields or heat treatments. Euro Food Res Technol. 2008;228:239–248. doi: 10.1007/s00217-008-0928-5. [DOI] [Google Scholar]
- Odriozola–Serrano I, Harnandes-Jover T, Martin-Belloso O. Comparative evaluation of UV–HPLC methods and reducing agents to determine vitamin C in fruits. Food Chem. 2007;105:1151–1158. doi: 10.1016/j.foodchem.2007.02.037. [DOI] [Google Scholar]
- Oszmiański J, Wojdyło A, Matuszewski P. In polyphenols compound changes In the industrial production process of concentrated strawberry juice. ZYWN-Nauk Technol Jakosc. 2007;1(50):94–104. [Google Scholar]
- Patras A, Brunton NP, Da Pieve S, Butler F. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Innov Food Sci Emerg Technol. 2009;10(3):308–313. doi: 10.1016/j.ifset.2008.12.004. [DOI] [Google Scholar]
- Patras A, Brunton NB, O’Donnell C, Tiwari BK. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci Technol. 2010;21:3–11. doi: 10.1016/j.tifs.2009.07.004. [DOI] [Google Scholar]
- Rodrigo D, Loey A, Hendrickx M. Combined thermal and high pressure colour degradation of tomato purée and strawberry juice. J Food Eng. 2007;79:553–560. doi: 10.1016/j.jfoodeng.2006.02.015. [DOI] [Google Scholar]
- Segovia-Bravo KA, Guignon B, Bermejo-Prada A, Sanz PD, Otero L. Hyperbaric storage at room temperature for food preservation: a study in strawberry juice. Innov Food Sci Emerg Technol. 2012;15:14–22. doi: 10.1016/j.ifset.2012.02.005. [DOI] [Google Scholar]
- Terefe NS, Yang YH, Knoerzer K, Buckow R, Versteeg C. High pressure and thermal inactivation kinetics of polyphenol oxidase and peroxidase in strawberry purée. Innov Food Sci Emerg Technol. 2010;11:52–60. doi: 10.1016/j.ifset.2009.08.009. [DOI] [Google Scholar]
- Terefe NS, Buckow R, Versteeg C. Quality-related enzymes in fruit and vegetable products: effects of novel food processing technologies, part 1: high pressure technologies. Crit Rev Food Sci Nutr. 2013;54(1):24–63. doi: 10.1080/10408398.2011.566946. [DOI] [PubMed] [Google Scholar]
- Terefe NS, Kleintschek T, Gamage T, Fanning KJ, Netzel G, Versteeg C, Netzel M. Comparative effects of thermal and high pressure processing on phenolic phytochemicals in different strawberry cultivars. Innov Food Sci Emerg Technol. 2013;19:57–65. doi: 10.1016/j.ifset.2013.05.003. [DOI] [Google Scholar]
- Tiwari BK, O’Donnell CP, Cullen PJ. Effect of non-thermal processing technologies on the anthocyanin content of fruit juices. Trends Food Sci Technol. 2009;20:137–145. doi: 10.1016/j.tifs.2009.01.058. [DOI] [Google Scholar]
- Verbeyst L, Oey I, Plancken VI, Hendrickx M, Loey A. Kinetic study on the thermal and pressure degradation of anthocyanins in strawberries. Food Chem. 2010;123:269–274. doi: 10.1016/j.foodchem.2010.04.027. [DOI] [Google Scholar]
- Verbeyst L, Bogaerts R, Plancken I, Hendrickx M, Loey A. Modeling of vitamin C degradation during thermal and high-pressure treatments of red fruit. Food Bioproc Technol. 2013;6(4):1015–1023. doi: 10.1007/s11947-012-0784-y. [DOI] [Google Scholar]
- Veridiana V, Rosso D, Mercadante AZ. The high ascorbic acid content in the main cause of the low stability of anthocyanin extracts from acerola. Food Chem. 2007;103:935–943. doi: 10.1016/j.foodchem.2006.09.047. [DOI] [Google Scholar]
- West ME, Mauer LJ. Color and chemical stability of a variety of anthocyanins and ascorbic acid in solution and powder forms. J Agric Food Chem. 2013;61:4169–4179. doi: 10.1021/jf400608b. [DOI] [PubMed] [Google Scholar]