Abstract
Acute lymphoblastic leukemia (ALL) is an aggressive neoplasm of B- or T-lymphoid progenitors and is the commonest childhood tumor. ALL comprises multiple subtypes characterized by distinct genetic alterations, with stereotyped patterns of aneuploidy present in many cases. Although alterations of TP53 are common in many tumors, they are infrequent in ALL, with the exception of two ALL subtypes associated with poor outcome: relapsed disease and ALL with hypodiploidy. TP53 alterations are present in almost all cases of ALL with low hypodiploidy and are associated with alterations of the lymphoid transcription factor IKZF2 and the tumor-suppressor gene loci CDKN2A and CDKN2B. Remarkably, more than half of TP53 mutations in low-hypodiploid ALL in children are present in nontumor cells, indicating that low-hypodiploid ALL is a manifestation of Li–Fraumeni syndrome. These findings have profound implications for our understanding of the genetic pathogenesis of hypodiploid ALL, suggesting that alteration of TP53 function may promote the distinctive aneuploidy characteristic of hypodiploid ALL. Moreover, the identification of hypodiploidy mandates offering testing for TP53 mutational status to patients and their relatives, with appropriate counseling and disease surveillance.
Mutations in TP53 are relatively infrequent in acute lymphoblastic leukemia (ALL), but there are exceptions. In one subtype, low-hypodiploid ALL, TP53 mutations are common, but their role in the disease remains unclear.
ACUTE LYMPHOBLASTIC LEUKEMIA
Acute lymphoblastic leukemia (ALL) is a neoplasm of B- or T-lineage lymphoid progenitors (Hunger and Mullighan 2015). Proliferation of leukemia cells, or blasts, results in bone marrow failure and death caused by anemia, hemorrhage, and/or infection, and invasion of extramedullary sites, including the central nervous system (CNS). Contemporary ALL therapy involves administration of multiple cytotoxic chemotherapeutic agents in several phases over several years, which is curative in >90% of children. However, relapse of ALL occurs in up to 20% of children and in much greater frequency in adults and is often refractory to further chemotherapy. Consequently, relapsed ALL is a leading cause of childhood cancer death. Recent years have witnessed intensive effort to define the genetic basis of leukemogenesis and treatment failure in ALL.
ALL comprises multiple distinct subtypes characterized by constellations of genetic alterations, including aneuploidy (gains or losses of whole chromosomes), chromosomal rearrangements, gains and losses of DNA, and sequence mutations (Mullighan 2013). Although ALL genomes harbor, on average, a low number of mutations, multiple cellular pathways are mutated in the majority of ALL cases, including the transcriptional regulation of lymphoid development, cell-cycle regulation, tumor suppression, cytokine receptor and Ras signaling, and epigenetic regulation. These lesions are considered to accumulate during leukemogenesis, in which a lymphoid progenitor acquires a founding lesion, commonly a chromosomal rearrangement that deregulates an oncogene (often a lymphoid transcription factor), or results in formation of a chimeric fusion gene with altered function of the partner genes. These rearrangements commonly perturb lymphoid maturation, resulting in developmental arrest, activate proliferation, and/or result in epigenetic deregulation. The nature of specific genes mutated varies significantly among different subtypes and has been reviewed in several overviews of the genetics of ALL.
These founding genetic alterations are usually insufficient to establish the leukemic clone, and additional genetic alterations are acquired—commonly focal DNA deletions and sequence mutations that further perturb these pathways. At the time a diagnosis of ALL is made, patients typically harbor multiple genetically distinct subclones that share one or more lesions, including the initiating chromosomal translocation, and can show marked diversity in the number and range of genetic lesions among subclones. The majority of children treated with contemporary therapy, which includes multiple cytotoxic chemotherapeutic agents given in rotating combinations over at least 2 years coupled with prophylaxis of CNS recurrence using intrathecal chemotherapy and/or CNS irradiation, are cured. However, up to 20% of children and a higher proportion of adults with ALL experience relapse that is commonly incurable with chemotherapy. Sequential genomic profiling studies of samples obtained at diagnosis, remission, and relapse have shown collapse of the multiclonal diversity at diagnosis and convergence to a single clone typically present at low frequency at diagnosis, which harbors genetic alterations that promote resistance to therapy. Additional genetic alterations are acquired, selected for, and predominate in the clone that emerges as dominant at relapse. Commonly mutated pathways enriched at relapse include tumor suppression (TP53), lymphoid development (IKZF1), glucocorticoid metabolism (CREBBP, NR3C1), and thiopurine metabolism (NT5C2, PRPS1) (Mullighan et al. 2008b, 2011; Ma et al. 2015). Many of these insights were only made in the last few years with the advent of genomic platforms to identify inherited and somatic genetic alterations throughout the genome. Collectively, hundreds of cases of childhood ALL have been subjected to whole-genome, exome, and/or transcriptome sequencing. These approaches have enabled a revised molecular taxonomy of acute ALL, by identifying new subtypes of ALL in cases that previously lacked recurring chromosomal alterations identifiable on karyotypic analysis. These include cases with rearrangements of the cytokine receptor gene CRLF2 (cytokine receptor-like factor 2) (Russell et al. 2009), focal alterations dysregulating expression of the ETS family transcription factor gene ERG (Mullighan et al. 2007b), and a subtype of high-risk leukemia with a diverse range of genetic alterations that activate cytokine receptor and kinase signaling known as Ph-like ALL (Roberts et al. 2014).
TP53 ALTERATIONS IN ALL
Although TP53 mutations are one of the most common somatic alterations in cancer, and are also observed in acute myeloid leukemia, they are relatively uncommon in ALL (Imamura et al. 1994; Gump et al. 2001; Hof et al. 2011; Zhang et al. 2011; Chiaretti et al. 2013). Genetic alterations disrupting tumor-suppressor genes and genes regulating the cell cycle are common in both B- and T-lineage ALL, but involve other genes, such as CDKN2A/CDKN2B (encoding the INK4/ARF family of tumor-suppressor genes), RB1, and PTEN, particularly in T-ALL (Mullighan et al. 2007a; Gutierrez et al. 2009). The reasons underlying these differences in patterns of alterations in tumor suppressors among tumor subtypes with relative sparing of TP53 in ALL are unknown.
TP53 is frequently mutated in two contexts in ALL: relapsed and low-hypodiploid ALL. TP53 mutations were first identified in relapse in T-ALL in 1994 (Hsiao et al. 1994) and were found to be absent at diagnosis but present in 28% of cases at relapse. These findings have been confirmed by multiple studies with deletions, mutations, and/or copy-neutral loss of heterozygosity (LOH) of chromosome 17p (leading to duplication of mutated TP53) present in up to one-third of relapsed ALL cases, which are also associated with a poor prognosis (Diccianni et al. 1994; Blau et al. 1997; Gump et al. 2001; Hof et al. 2011; Ma et al. 2015). The mechanistic basis for treatment resistance associated with TP53 mutations in relapsed ALL is unknown but does not appear to be related to genomic instability, as this is not evident in the majority of ALL genomes with TP53 alterations.
The second context in which TP53 mutations are common is low-hypodiploid ALL (Holmfeldt et al. 2013). Hypodiploid ALL comprises up to 5% of childhood ALL cases and is further stratified according to the severity of aneuploidy, with several stereotyped patterns of chromosomal loss identified: near haploidy (24–31 chromosomes), low hypodiploidy (32–39 chromosomes), and high hypodiploidy (40–44 chromosomes). As described below, each of these is characterized by distinct genetic mutations uncommon in other forms of ALL, one of the most notable being TP53 mutations in low-hypodiploid ALL.
HYPODIPLOID ALL
Previous cytogenetic analyses of hypodiploid ALL cases subdivided tumors based on chromosome number and found natural groupings of 24–30 and 32–41 chromosomes based on the patterns of aneuploidy (Pui et al. 1990; Heerema et al. 1999; Harrison et al. 2004; Nachman et al. 2007). These two subtypes of hypodiploid ALL are associated with very poor outcome in the majority of studies. Less common are hypodiploid cases with a higher modal chromosome number, either high-hyperdiploid cases with 42–44 chromosomes or near-diploid cases, which are frequently associated with the formation of dicentric chromosomes.
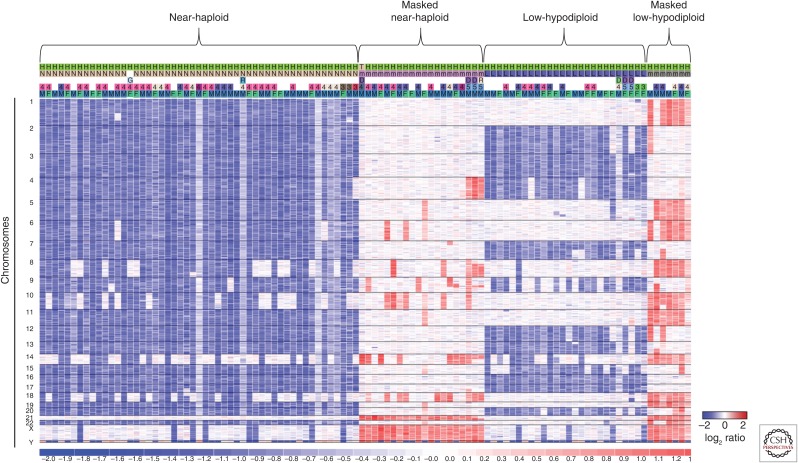
The pattern of aneuploidy within low-hypodiploid ALL cases is not random, showing consistency in the chromosomes that are commonly lost and those that are rarely or never lost. The chromosomes most frequently lost in low-hypodiploid cases are 2, 4, 7, 9, 15, and 20 with 3, 12, 13, 16, and 17 being universally lost (Fig. 1). Chromosomes 5, 8, 14, 18, 19, 22, X, and Y are rarely lost, whereas chromosome 21 is universally retained. The lack of aneuploidy of chromosome 21 is further notable for not being present in any form of acute leukemia, with gain commonly observed in both ALL with high hyperdiploidy, as well as hypodiploidy.
Figure 1.
Hypodiploid ALL tumors show a nonrandom pattern of aneuploidy. Data is shown as a log2 ratio DNA copy-number heatmap of single-nucleotide polymorphism (SNP) 6.0 microarray data from hypodiploid ALL primary tumor samples. This analysis was performed with a total of 50 near-haploid tumors, 18 of which had duplicated their genome (referred to as masked), and 26 low-hypodiploid tumors, eight of which were masked. The pattern of aneuploidy is remarkably consistent among tumor subtypes, showing nonrandom loss of specific chromosomes. The masked cases show two sets of the same chromosomes that typically remain diploid in nonmasked cases. (Data from Holmfeldt et al. 2013.)
Identification of hypodiploid ALL can be challenging as these tumors have a tendency to duplicate their aneuploid genome, referred to as masking (Harrison et al. 2004; Carroll et al. 2009). Masking has been observed in both low-hypodiploid and near-haploid cells, causing them to present a hyperdiploid karyotype, which requires careful cytogenetic and structural analysis to identify as masked hypodiploid. The patterns of chromosomal gain may suggest masking as typical high-hyperdiploid ALL has trisomy of chromosomes, including 4, 10, 14, 17, and 21, whereas masking typically results in tetrasomy of chromosomes that were diploid in the originating nonhypodiploid clone. Masking may result in most cells in a patient having a hyperdiploid karyotype with no evidence of the nonmasked hypodiploid clone or, more commonly, the presence of both masked and nonmasked clones. This phenomenon may be suggested by analysis of the DNA index, which may show peaks representing both masked and nonmasked clones, as well as analysis of genome-wide single-nucleotide polymorphism (SNP) data on microarray or genome sequencing analysis, in which widespread LOH is observed in diploid chromosomes, indicating loss and subsequent reduplication. One of the few immortal hypodiploid cell lines, MHH-CALL-2, shows this phenomenon (Tomeczkowski et al. 1995). This cell line was characterized as hyperdiploid with a karyotype of 52 chromosomes. SNP array analysis using the Illumina platform revealed that the disomic chromosomes showed LOH (Aburawi et al. 2011). This contrasts with the much lower frequency of copy-neutral chromosomal LOH observed in high-hyperdiploid ALL (Paulsson et al. 2010). The distinction of masked hypodiploid and hyperdiploid ALL is of great clinical significance as hyperdiploid ALL has a favorable prognosis, whereas hypodiploid ALL has a substantially inferior outcome and is classified as a high-risk entity in most risk classification algorithms (Pui et al. 1987, 1990; Raimondi et al. 2003; Moorman et al. 2010).
THE GENETIC BASIS OF HYPODIPLOID ALL
Although hypodiploidy has been recognized for many years, the nature of any associated genomic alterations, the factors driving the generation of aneuploidy, and the biologic basis of poor outcome have been poorly understood.
To address these questions, a collaborative study initiated by St. Jude Children’s Research Hospital and the Children’s Oncology Group performed detailed genomic analysis of hypodiploid ALL. One hundred and twenty-six tumors were studied by microarray profiling of gene expression and DNA copy-number alterations, candidate gene sequencing, and whole-genome sequencing (WGS), including exome and/or WGS of a subset of the tumors as part of the St. Jude Children’s Research Hospital–Washington University Pediatric Cancer Genome project. These analyses provided the first detailed understanding of the genomic landscape of this disease (Holmfeldt et al. 2013).
This study confirmed that the previous cytogenetic subclassification of hypodiploid ALL was appropriate in that low-hypodiploid (32–39 chromosomes) and near-haploid (24–31 chromosomes) cases have distinct transcriptomic profiles and patterns of genetic alteration, and should be considered distinct diseases (Table 1). Specifically, near-haploid tumors have a high frequency of alterations activating Ras signaling, including NRAS- or KRAS-activating sequence mutations in 18% of cases or deletions or mutations leading to loss of NF1 function (44%). The NF1 mutations were notable for their high frequency and the identification of a recurrent deletion of exons 15–35 involving the Ras-GAP domain required for negative regulation of Ras activity. Moreover, because of aneuploidy involving chromosome 17, which harbors NF1, the genetic alterations result in biallelic inactivation of NF1. These Ras pathway alterations were mutually exclusive, suggesting each is sufficient to promote constitutive Ras signaling, cell growth, and proliferation.
Table 1.
Frequency of genetic lesions in hypodiploid ALL
| Gene | Near-haploid | Low-hypodiploid | Near-diploid |
|---|---|---|---|
| CDKN2A/B | 22.1 | 23.5 | 77.3 |
| PAX5 | 5.9 | 5.9 | 59.1 |
| FLT3 | 8.8 | 0 | 0 |
| NF1 | 44.1 | 5.9 | 4.5 |
| KRAS | 2.9 | 0 | 9.1 |
| NRAS | 14.7 | 0 | 18.2 |
| PAG1 | 10.3 | 2.9 | 0 |
| PTPN11 | 1.5 | 0 | 9.1 |
| Total Ras pathway | 82.3 | 8.8 | 31.8 |
| IKZF1 | 4.4 | 2.9 | 9.1 |
| IKZF2 | 1.5 | 52.9 | 0 |
| IKZF3 | 13.2 | 2.9 | 0 |
| RB1 | 8.8 | 41.2 | 0 |
| TP53 | 2.9 | 91.2 | 4.5 |
| Histone cluster, 6p22 | 19.1 | 2.9 | 9.1 |
Numbers represent the percentage of cases with copy-number alterations or sequence mutations within the specified gene. Deletion of the CDKN2A/B locus is commonly observed in numerous B-ALL subtypes. The Ras pathway-activating mutations in near-haploid tumors are typically found in other ALL subtypes at relapse—NRAS, in particular. IKZF1 deletion, although recurrent in many subtypes of B-ALL, is rare in hypodiploid ALL. The TP53 alterations present in virtually all low-hypodiploid cases are rarely seen in other ALL subtypes.
Alterations of the Ikaros family of transcription factors was also a hallmark of hypodiploid ALL. This family comprises multiple genes, including IKZF1 (Ikaros), IKZF2 (Helios), and IKZF3 (Aiolos), which encode zinc-finger-containing, DNA-binding transcription factors temporally regulated during hematopoietic and lymphoid development (Cortes et al. 1999). The most extensively studied is the founding member, IKZF1 (Ikaros), which is required for development of all lymphoid lineages (Georgopoulos et al. 1994) and is commonly mutated in other high-risk subtypes of ALL, particularly BCR-ABL1 (Philadelphia chromosome, or Ph-positive) and Ph-like ALL (Mullighan et al. 2008a, 2009; Roberts et al. 2012, 2014). In contrast, IKZF1 alterations were uncommon in hypodiploid ALL, but near-haploid tumors showed recurrent homozygous loss of IKZF3 (Aiolos) in 13% of cases compared with 3% of low-hypodiploid cases.
The mutational profile of low-hypodiploid ALL was characterized by a relatively low frequency of Ras pathway alterations (9% of cases), IKZF2 (Helios) alterations in 65% of cases, and a high frequency of TP53 alterations, as discussed below. Alterations in the TP53 pathway were uncommon in near-haploid ALL (11%). Gene expression profiling by unsupervised hierarchical clustering and principal component analysis revealed discrete clustering of subtypes that were independent of the pattern of aneuploidy, further illustrating the molecular difference between near-haploid and low-hypodiploid ALL. The role and cooperativity between the alterations in Ras signaling, Ikaros-family alterations, and TP53 alterations in leukemogenesis and their potential relationship to the highly stereotyped, severe aneuploidy characteristic of this disease remain poorly understood but are the subject of investigation. In particular, although there is now strong evidence supporting a role of IKZF1 alterations in treatment resistance in B-ALL (Schjerven et al. 2013; Schwickert et al. 2014; Churchman et al. 2015), the role of IKZF2 and IKZF3 alterations in leukemogenesis has not been formally explored.
TP53 ALTERATIONS IN HYPODIPLOID ALL
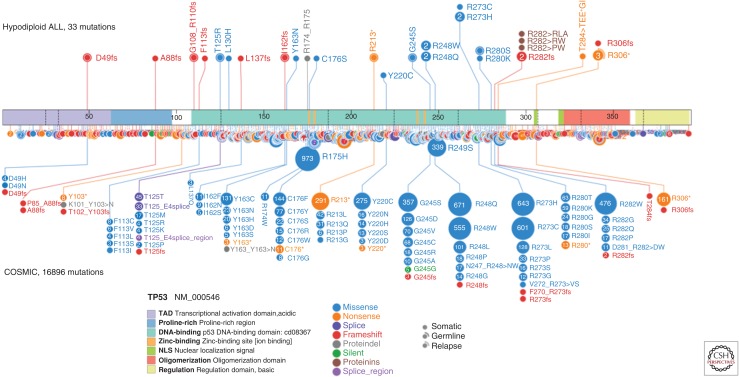
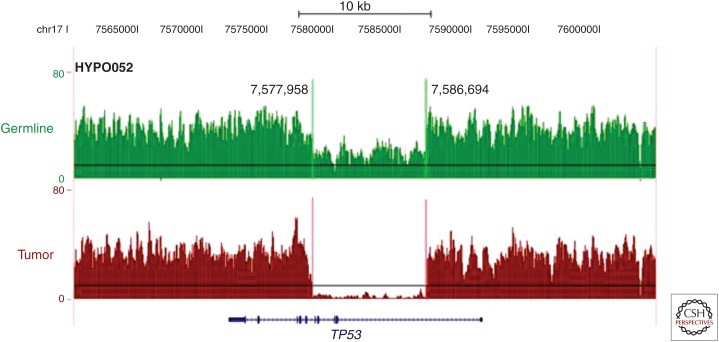
A striking finding from this study was alteration of TP53 in 91% of childhood low-hypodiploid cases, which was present in only 8% (8/106) of non-low-hypodiploid cases. Of the cases with TP53 alteration, 97% were in the form of sequence mutations that resulted in insertion-deletion or single-nucleotide variations with clustering at the proximal and distal regions of the DNA-binding domain, as well as in the nuclear localization sequence (NLS) (Fig. 2). As in the case of the NF1 and Ikaros family alterations, aneuploidy and loss of the nonmutated chromosome resulted in biallelic alteration of TP53. A single case was found on reverse transcription and polymerase chain reaction (PCR) of tumor RNA to express no wild-type TP53, but it did express an aberrantly spliced isoform lacking exons 2–6. This case was not subjected to WGS at the time of the original study (Holmfeldt et al. 2013), but subsequent sequencing identified a focal deletion of TP53 resulting in this aberrant splicing that was not evident on microarray-based DNA copy-number alteration analysis because of relatively poor coverage of the TP53 locus (Fig. 3) (Zhang et al. 2015). Thus, the previously high frequency of TP53 alteration in low-hypodiploid ALL is an underestimate, and most or all low-hypodiploid ALL cases likely have biallelic alterations of TP53.
Figure 2.
Schematic of TP53 mutation in hypodiploid ALL and the Catalog of Somatic Mutations in Cancer (COSMIC) database. Hypodiploid mutations are shown on the top of the figure and all TP53 mutations from the COSMIC database are shown on the bottom, with mutations of the same residues selected as those mutated in hypodiploid ALL. The majority of mutations are missense mutations and they are located within the DNA-binding domain. The nonsense R306* mutation occurs in the bipartite nuclear localization sequence (NLS) domain.
Figure 3.
Cryptic germline TP53 alterations in hypodiploid ALL. Germline DNA is shown in green and tumor DNA is shown in brown as a wiggle plot of whole-genome sequencing (WGS) read depth spanning the TP53 gene shown on the University of California at Santa Cruz (UCSC) genome browser. One low-hypodiploid tumor (HYPO052) that was originally reported as an aberrantly spliced isoform was later found to contain an 8.7-kb focal deletion of exon 2–exon 5 on deeper sequencing analysis (Holmfeldt et al. 2013). The original estimate of ∼90% of low-hypodiploid cases possessing TP53 alteration is likely an underestimation, with a higher number of cases having germline mutations (data from Zhang et al. 2015).
Importantly, 43% of pediatric low-hypodiploid ALL cases were found to have TP53 mutations in matched nontumor cells, suggesting that these mutations may be inherited. Moreover, many of the mutations identified in nontumor cells had previously been identified in Li–Fraumeni syndrome (LFS), such as TP53 p.Arg248Trp. For most cases, blood or bone marrow obtained at remission was used as the source of nontumor DNA; thus, the mutations may represent mutations that are either inherited or acquired de novo in the germline or hematopoietic lineage. However, several kindreds have been reported in which familial TP53 mutations are associated with hypodiploid ALL and other malignancies. In this study, one childhood case of low-hypodiploid ALL harbored a p.Gly302fs mutation that was homozygous in tumor samples and heterozygous on skin biopsy. This patient had a family history of cancer, including glioblastoma multiforme diagnosed in the father at age 31 years. Sequencing of the father’s DNA revealed the identical TP53 frameshift mutation. A second kindred has been reported with five individuals with ALL across three generations, several of which were low hypodiploid and each of which harbored a germline TP53 p.Arg306X mutation (Powell et al. 2013). Thus, low-hypodiploid ALL in children should be considered a manifestation of LFS, and clinical testing and genetic counseling should be offered to patients and their relatives following a diagnosis of low-hypodiploid ALL.
The near-universal alteration of TP53 in low-hypodiploid ALL was further confirmed in 10 of 11 (91%) adult cases that harbored mutation of TP53, all of which were located in the DNA-binding domain. Whereas half of these mutations had previously been associated with LFS, the TP53 mutations were somatic in all adult hypodiploid ALL cases. The high frequency of TP53 mutations in low-hypodiploid ALL has been confirmed in an independent study, in which 27 of 29 cases harbored a TP53 sequence mutation (Muhlbacher et al. 2014). The majority of patients in this cohort were adult, and germline mutational status was not reported.
The sequence mutations observed in both childhood and adult hypodiploid ALL showed clustering within the DNA-binding domain (Fig. 2). Three cases harbored frameshift mutations upstream of the DNA-binding domain (Asp49fs, Ala88fs, and Gly108_Arg110fs), which are likely to eliminate protein expression. Truncating mutations at p.Arg306 were observed in four cases. Although germline and somatic mutations at this residue have been described previously (Petitjean et al. 2007), mutations at this site are disproportionately more frequent in hypodiploid ALL. While truncating mutations of TP53 have been shown to result in loss-of-function, p.Arg306 is located distal to the DNA-binding domain in a basic p.Lys305-Arg306 domain that forms part of the bipartite NLS required for nuclear localization of TP53 (Liang and Clarke 1999, 2001). Analysis of a low-hypodiploid xenograft containing the p.Arg306* mutation identified elevated TP53 expression (Holmfeldt et al. 2013). This mutation was also identified in a case of endometrioid adenocarcinoma, in which the mutation was present only within the serous component of a mixed epithelial carcinoma, and was accompanied by elevated TP53 expression in serous, but not endometrioid cells in the tumor (Sholl et al. 2012). Moreover, the p.Gly302fs mutation identified in the kindred described in this study was also accompanied by elevated TP53 expression in the brain tumor sample. There is currently little direct knowledge regarding the functional consequence of these truncating mutations at or near the NLS on TP53 intracellular localization, cell-cycle arrest, and apoptosis in response to DNA damage. However, the observation that missense TP53 mutations are associated with elevated TP53 protein expression in hypodiploid leukemic cells, and the dearth of TP53 deletions without concomitant sequence mutation in this disease, strongly suggests a gain or change in TP53 function for many of the mutations. The high frequency of TP53 alterations in low-hypodiploid ALL indicates that children with hypodiploid ALL and their families should be offered genetic counseling and TP53 mutational testing. This also offers the opportunity for clinical surveillance of children within families of known TP53 mutation carriers for early detection of low-hypodiploid ALL (Villani et al. 2011; McBride et al. 2014).
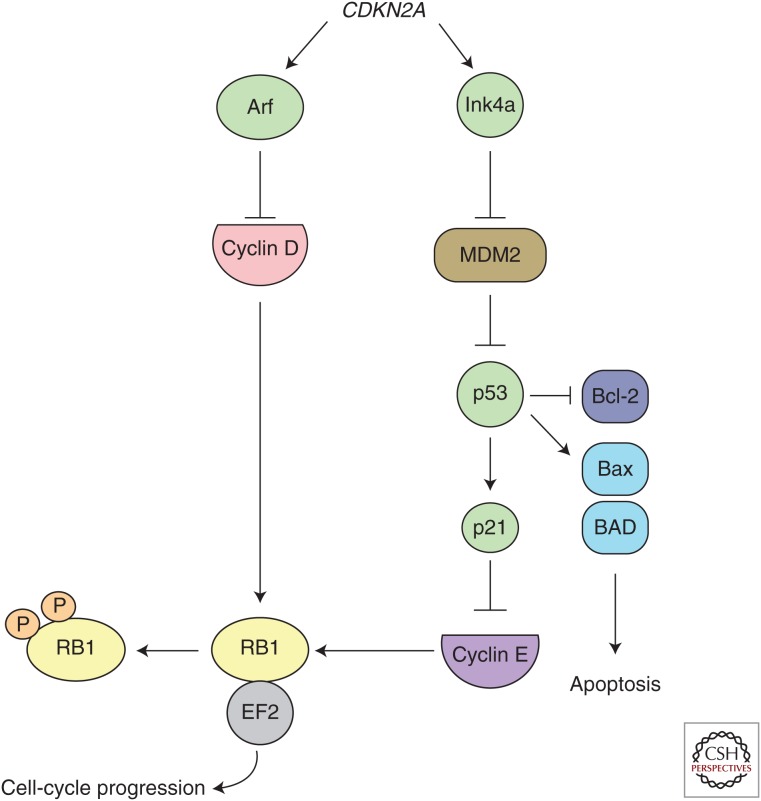
Additional recurring genetic alterations involving cell-cycle regulatory genes and tumor suppressors were also common in hypodiploid ALL. Loss of RB1 was present in 41% of cases (14/34) compared with 9% (6/68) of near-haploid cases (Holmfeldt et al. 2013). Most cases with RB1 alterations had concomitant TP53 mutations, suggesting distinct and cooperative effects in leukemogenesis. Alterations of CDKN2A/CDKN2B, encoding the INK4/ARF family of tumor suppressors (Sherr 2001), were present in 22.1% and 23.5% of near-haploid and low-hypodiploid cases, respectively. Despite 62% of low-hypodiploid ALL cases containing either RB1 or CDKN2A/B alterations, only one case harbored concomitant TP53, RB1, and CDKN2A/B alterations, suggesting that the products of RB1 and CDKN2A/B are epistatic within the RB1 tumor-suppressor pathway in this context (Fig. 4). Although RB1 is a well-known tumor suppressor that is often observed inactivated in many solid tumors (Lohmann 1999; Deshpande and Hinds 2006; Chen et al. 2014) and T-lineage ALL (Mullighan et al. 2007a), it is otherwise uncommonly mutated in B-ALL (Zhang et al. 2011; Schwab et al. 2013).
Figure 4.
Parallel p53 and RB1 tumor-suppressor pathways. The Arf and Ink4a proteins encoded by the CDKN2A gene regulate p53 and RB1 function, respectively. Ink4A inhibits cyclin D phosphorylation of RB1 to prevent cell-cycle progression. Arf blocks MDM2-mediated inhibition of p53, leading to apoptosis through BH3-only proteins.
Loss of TP53 in tumors is widely thought to induce genomic instability (Hanel and Moll 2012). Mutation of TP53 has also been associated with aneuploidy and catastrophic DNA rearrangements known as chromothripsis, as was the situation in a recent analysis of medulloblastoma cases (Thompson and Compton 2010; Stephens et al. 2011; Rausch et al. 2012). However, several observations suggest that TP53 alterations in hypodiploid ALL do not result in genome instability. First, low-hypodiploid ALL is not as severely aneuploid as near-haploid ALL, in which TP53 is infrequently mutated, albeit with only one copy per cell. This suggests that mechanisms other than inactivation of TP53 may be more responsible for the aneuploidy observed in hypodiploid ALL. Second, evidence of chromothripsis is lacking in low-hypodiploid ALL genome sequences. Their genomes do not contain clustered breakpoints or concentrated structural rearrangements, which are considered a hallmark of chromosome instability (Jones and Jallepalli 2012). Overall, the genomes of low-hypodiploid ALL are relatively stable, showing a consistent pattern of aneuploidy that is conserved at relapse. Xenografts of low-hypodiploid ALL maintain this pattern of aneuploidy and mutation even after successive passages (Holmfeldt and Mullighan 2015).
MODELING OF HYPODIPLOID ALL
Currently, there are no preclinical models of hypodiploid ALL. The hypodiploid ALL literature consists largely of clinical case reports, patient sample analysis, retrospective analysis of clinical outcome, and in vitro study of the two hypodiploid cell lines NALM-16 and MHH-CALL-2, both of which are of the near-haploid subtype (Pui et al. 1990; Ma et al. 1998; Heerema et al. 1999; Ramos et al. 2000; Das et al. 2003; Raimondi et al. 2003; Harrison et al. 2004; Morrissette et al. 2006; Nachman et al. 2007; Aburawi et al. 2011; Safavi et al. 2013; Muhlbacher et al. 2014; Mehta et al. 2015). The consensus of these studies is that hypodiploid ALL is associated with poor outcome. One exception is a recent report from the St. Jude Total Therapy XV study incorporating minimal residual disease (MRD) monitoring and risk directed therapy, in which undetectable levels of leukemic cells in the bone marrow (MRD) early in therapy was associated with a favorable outcome (Mullighan et al. 2015). The lack of hypodiploid ALL preclinical models has greatly limited our ability to assess the efficacy of new therapeutic agents and characterize the molecular phenotype of both near-haploid and low-hypodiploid subtypes.
The description of the genomic landscape of 126 hypodiploid ALL patients has provided insights into the genetic basis of this disease that may be exploited to improve outcome with targeted therapy. However, the diversity of genomic alterations, many of which have not been studied in lymphoid leukemogenesis, aneuploidy, and lack of a known driving oncogene, result in challenges for the generation of faithful models and testing of preclinical therapeutic approaches. Moreover, the distinct genomic lesions observed in each subtype mandate the generation of distinct engineered and patient-derived xenograft (PDX) mouse models.
PDX models may be the most clinically relevant for the testing of new therapeutics. One of the advantages of PDX models is the maintenance of the characteristic aneuploidy of the tumor, which is exceptionally challenging to recapitulate in genetically engineered mouse models (GEMMs). Use of PDX models for preclinical testing of therapeutic agents should incorporate tumors representing the diversity of genetic lesions found in hypodiploid patients as much as possible to both correlate the drug mechanism with the tumor genomic profile and define the extent of activity of the compound within each subtype. This requires detailed characterization of the phenotype, genomic landscape, and signaling pathway activation of each xenograft. The observation that both near-haploid and low-hypodiploid xenografts show constitutive Ras activation and ex vivo sensitivity to PI3K inhibitors suggests both subtypes may be dependent on Ras-mediated growth mechanisms (Holmfeldt et al. 2013). Constitutive Ras activation was expected for near-haploid tumors containing Ras-activating mutations; however, it was surprising for low-hypodiploid tumors lacking such alterations. Targeted inhibition of PI3K/MAPK signaling may, therefore, be a promising treatment to inhibit the oncogenic effect of Ras. This dual blockade approach is being investigated in several cancer types, including colorectal, melanoma, ovarian, and pancreatic tumors (Britten 2013).
GEMMs offer opportunities to study the interaction of specific genetic lesions in the development of B-ALL through analysis of discrete complements of lesions within mice of identical genetic backgrounds. This will be of great interest to compare the effects of alterations of the different Ikaros family members on lymphoid maturation, tumor development, and chromatin remodeling but will likely require directed deletion in the B-lymphoid lineage in view of the propensity of mice to develop T-lymphoid neoplasms on perturbation of Ikzf1 (Winandy et al. 1995). The immunophenotype of hypodiploid ALL typically observed is that of a pre-B cell that lacks expression of a mature B-cell receptor (Pui et al. 1990). It is likely that induction of B-ALL will depend on the stage of maturation at which Ikzf2/3 alterations, and other lesions, are acquired, as suggested by immunophenotypic and antigen receptor recombination data indicative of different maturational states of near-haploid and low-hypodiploid ALL. Specifically, low-hypodiploid tumors expressed significantly less CD19 compared with near-haploid and near-diploid tumors, as well as much less frequent antigen receptor rearrangements (Holmfeldt et al. 2013). Gene set enrichment analysis revealed a pro-B-cell expression profile for low-hypodiploid cells that was not seen in near-haploid cells, suggesting a less mature lymphoid progenitor cell of origin for low-hypodiploid compared with near-haploid cells. Hypodiploid ALL therefore, represents a biologically relevant model to study how loss of Ikzf2/3 affects B-cell development and promotes persistent growth of immature B cells.
An important challenge is the modeling of aneuploidy. There are few established models of B-progenitor ALL, the majority being driven by potent oncogenes (e.g., BCR-ABL1 and MLLrearrangement). The mechanism of induction of the stereotyped aneuploidy and sequence of acquisition relative to concomitant genetic alterations are unknown, although it is likely that aneuploidy is an initiating event (Safavi et al. 2013). The observation that many cases of low-hypodiploid ALL appear to result from inherited TP53 mutation for which cells become homozygous because of loss of one copy of chromosome 17 suggests that loss of TP53 function may be a necessary early event in the pathogenesis of this disease. For those patients without inherited TP53 mutation, especially adult cases, it is not known at what stage of B-cell development the somatic TP53 mutations are acquired that facilitate hypodiploid ALL.
The modeling of hypodiploid TP53 alterations in GEMMs should consider the diversity of TP53 alterations, including mutations within different regions of the gene that may affect protein function, stability, and/or localization, as well as loss of expression as a result of frameshift mutation or aberrant RNA splicing. It is important to distinguish between altered p53 function (in the case of point mutations) versus complete loss of p53 (in the case of deletion or proximal frameshift mutations). Nearly all cases sequenced were homozygous for the alteration of TP53 because of aneuploidy. For mouse models to accurately represent TP53 alterations observed in hypodiploid ALL, both deletion and mutation of TP53 should be considered. A high percentage of low-hypodiploid cases contained missense mutations of TP53, 79% of which occurred within the DNA-binding domain (Fig. 2). Several GEMMs currently exist that carry some of these most common LFS mutations; however, no mouse has been created with the Arg306* truncating mutation observed in four hypodiploid cases that would allow for the study of the functional consequence of carboxyl-terminus loss immediately after the DNA-binding domain.
A recent study on the homozygous deletion of TP53 in mouse pre-B cells using Mb1-Cre expression induced B-cell lymphoid tumors harboring a variety of clonal translocations that appeared to be caused by dysfunctional repair of double-strand breaks caused by RAG (recombination-activating gene) and AID (activation-induced deaminase) activity (Rowh et al. 2011). This illustrates the role TP53 plays in detecting and mediating repair of aberrant RAG (recombination-activating gene)/AID (activation-induced deaminase) activity. Igh locus translocations similar to those observed in this model have not been observed in hypodiploid ALL patients; however, the focal deletions of NF1 in some cases contained the heptamer footprint of RAG-mediated recombination. Considering that low-hypodiploid tumors consistently presented with recurrent lesions in tumor-suppressor genes in addition to TP53, such as RB1, CDKN2A/B, and IKZF2, the comodeling of other lesions with TP53 mutation may more accurately recapitulate this disease. Furthermore, it may be that hypodiploid ALL transformation initiates at an earlier stage of B-cell development than that of pre-B, and conditional induction of lesions at various stages should be investigated. Although a majority of low-hypodiploid cases (62%) are presented with alteration in either RB1 or CDKN2A/B, in addition to TP53 (Holmfeldt et al. 2013), the combination of all three of these alterations was only observed in a single patient, suggesting that RB1 and CDKN2A/B inactivation are epistatic within the Rb1 tumor-suppressor pathway and are not necessary to combine within a mouse model.
CONCLUSION
Compared with many types of cancer, however, ALL tends to have relatively few genomic alterations and is often characterized by oncogenic fusion genes (Zhang et al. 2011). Although TP53 is one of the most well-known tumor-suppressor genes, it is relatively rare to find TP53 mutations in ALL at diagnosis. It was, therefore, quite surprising to discover that low-hypodiploid ALL cases harbor near-universal TP53 alteration (Holmfeldt et al. 2013). This distinguishes low-hypodiploid ALL as a manifestation of LFS.
TP53 plays a critical role in protecting the cell from the transformative effects of DNA damage by inducing cell-cycle arrest and apoptosis. Loss of TP53 function is associated with genomic instability and the development of aneuploidy in cancer (Thompson and Compton 2010; Hanel and Moll 2012). However, there is insufficient evidence that loss of TP53 is the cause of aneuploidy in hypodiploid ALL as shown by the lack of TP53 lesions in near-haploid tumors. Furthermore, low-hypodiploid cells do not display characteristic features of genomic instability, such as chromosomal translocations mediated by aberrant RAG/AID activity, which is a hallmark of impaired double-strand break response (Holmfeldt et al. 2013). So, although the TP53 alteration appears to be an essential component of the pathogenesis of low-hypodiploid ALL, the functional role of TP53 alterations in this disease remains unclear.
Although multiple studies have reported an association among TP53 alterations with poor outcome in ALL, remarkably few data exist regarding the mechanistic basis of the role of such alterations in leukemogenesis and drug resistance (Hof et al. 2011; Chiaretti et al. 2013). The recent discovery of near-universal TP53 alteration in low-hypodiploid ALL has revealed a key role for this gene in leukemogenesis. Therefore, detailed studies of the role of loss of TP53 function will likely provide unique insights into the role of this gene in cancer development.
Footnotes
Editors: Guillermina Lozano and Arnold J. Levine
Additional Perspectives on The p53 Protein available at www.perspectivesinmedicine.org
REFERENCES
- Aburawi HE, Biloglav A, Johansson B, Paulsson K. 2011. Cytogenetic and molecular genetic characterization of the “high hyperdiploid” B-cell precursor acute lymphoblastic leukaemia cell line MHH-CALL-2 reveals a near-haploid origin. Br J Haematol 154: 275–277. [DOI] [PubMed] [Google Scholar]
- Blau O, Avigad S, Stark B, Kodman Y, Luria D, Cohen IJ, Zaizov R. 1997. Exon 5 mutations in the p53 gene in relapsed childhood acute lymphoblastic leukemia. Leuk Res 21: 721–729. [DOI] [PubMed] [Google Scholar]
- Britten CD. 2013. PI3K and MEK inhibitor combinations: Examining the evidence in selected tumor types. Cancer Chemother Pharmacol 71: 1395–1409. [DOI] [PubMed] [Google Scholar]
- Carroll AJ, Heerema NA, Gastier-Foster JM, Astbury C, Pyatt R, Reshmi SC, Borowitz MJ, Devidas M, Linda S, Loh ML, et al. 2009. Masked hypodiploidy: Hypodiploid acute lymphoblastic leukemia (ALL) in children mimicking hyperdiploid ALL: A report from the Children’s Oncology Group (COG) AALL03B1 Study. 51st ASH Annual Meeting and Exposition, Abstract 1580. New Orleans. [Google Scholar]
- Chen X, Bahrami A, Pappo A, Easton J, Dalton J, Hedlund E, Ellison D, Shurtleff S, Wu G, Wei L, et al. 2014. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep 7: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaretti S, Brugnoletti F, Tavolaro S, Bonina S, Paoloni F, Marinelli M, Patten N, Bonifacio M, Kropp MG, Sica S, et al. 2013. TP53 mutations are frequent in adult acute lymphoblastic leukemia cases negative for recurrent fusion genes and correlate with poor response to induction therapy. Haematologica 98: e59–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchman ML, Low J, Qu C, Paietta EM, Kasper LH, Chang Y, Payne-Turner D, Althoff MJ, Song G, Chen SC, et al. 2015. Efficacy of retinoids in IKZF1-mutated BCR-ABL1 acute lymphoblastic leukemia. Cancer Cell 28: 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes M, Wong E, Koipally J, Georgopoulos K. 1999. Control of lymphocyte development by the Ikaros gene family. Curr Opin Immunol 11: 167–171. [DOI] [PubMed] [Google Scholar]
- Das PK, Sharma P, Koutts J, Smith A. 2003. Hypodiploidy of 37 chromosomes in an adult patient with acute lymphoblastic leukemia. Cancer Genet Cytogenet 145: 176–178. [DOI] [PubMed] [Google Scholar]
- Deshpande A, Hinds PW. 2006. The retinoblastoma protein in osteoblast differentiation and osteosarcoma. Curr Mol Med 6: 809–817. [DOI] [PubMed] [Google Scholar]
- Diccianni MB, Yu J, Hsiao M, Mukherjee S, Shao LE, Yu AL. 1994. Clinical significance of p53 mutations in relapsed T-cell acute lymphoblastic leukemia. Blood 84: 3105–3112. [PubMed] [Google Scholar]
- Georgopoulos K, Bigby M, Wang JH, Molnar A, Wu P, Winandy S, Sharpe A. 1994. The Ikaros gene is required for the development of all lymphoid lineages. Cell 79: 143–156. [DOI] [PubMed] [Google Scholar]
- Gump J, McGavran L, Wei Q, Hunger SP. 2001. Analysis of TP53 mutations in relapsed childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 23: 416–419. [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Sanda T, Grebliunaite R, Carracedo A, Salmena L, Ahn Y, Dahlberg S, Neuberg D, Moreau LA, Winter SS, et al. 2009. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood 114: 647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanel W, Moll UM. 2012. Links between mutant p53 and genomic instability. J Cell Biochem 113: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Moorman AV, Broadfield ZJ, Cheung KL, Harris RL, Reza Jalali G, Robinson HM, Barber KE, Richards SM, Mitchell CD, et al. 2004. Three distinct subgroups of hypodiploidy in acute lymphoblastic leukaemia. Br J Haematol 125: 552–559. [DOI] [PubMed] [Google Scholar]
- Heerema NA, Nachman JB, Sather HN, Sensel MG, Lee MK, Hutchinson R, Lange BJ, Steinherz PG, Bostrom B, Gaynon PS, et al. 1999. Hypodiploidy with less than 45 chromosomes confers adverse risk in childhood acute lymphoblastic leukemia: A report from the children’s cancer group. Blood 94: 4036–4045. [PubMed] [Google Scholar]
- Hof J, Krentz S, van Schewick C, Korner G, Shalapour S, Rhein P, Karawajew L, Ludwig WD, Seeger K, Henze G, et al. 2011. Mutations and deletions of the TP53 gene predict nonresponse to treatment and poor outcome in first relapse of childhood acute lymphoblastic leukemia. J Clin Oncol 29: 3185–3193. [DOI] [PubMed] [Google Scholar]
- Holmfeldt L, Mullighan CG. 2015. Generation of human acute lymphoblastic leukemia xenografts for use in oncology drug discovery. In Current protocols in pharmacology. Wiley, New York. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmfeldt L, Wei L, Diaz-Flores E, Walsh M, Zhang J, Ding L, Payne-Turner D, Churchman M, Andersson A, Chen SC, et al. 2013. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet 45: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao MH, Yu AL, Yeargin J, Ku D, Haas M. 1994. Nonhereditary p53 mutations in T-cell acute lymphoblastic leukemia are associated with the relapse phase. Blood 83: 2922–2930. [PubMed] [Google Scholar]
- Hunger SP, Mullighan CG. 2015. Acute lymphoblastic leukemia in children. N Engl J Med 373: 1541–1552. [DOI] [PubMed] [Google Scholar]
- Imamura J, Miyoshi I, Koeffler HP. 1994. p53 in hematologic malignancies. Blood 84: 2412–2421. [PubMed] [Google Scholar]
- Jones MJ, Jallepalli PV. 2012. Chromothripsis: Chromosomes in crisis. Dev Cell 23: 908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SH, Clarke MF. 1999. A bipartite nuclear localization signal is required for p53 nuclear import regulated by a carboxyl-terminal domain. J Biol Chem 274: 32699–32703. [DOI] [PubMed] [Google Scholar]
- Liang SH, Clarke MF. 2001. Regulation of p53 localization. Eur J Biochem 268: 2779–2783. [DOI] [PubMed] [Google Scholar]
- Lohmann DR. 1999. RB1 gene mutations in retinoblastoma. Hum Mutat 14: 283–288. [DOI] [PubMed] [Google Scholar]
- Ma SK, Chan GC, Wan TS, Lam CK, Ha SY, Lau YL, Chan LC. 1998. Near-haploid common acute lymphoblastic leukaemia of childhood with a second hyperdiploid line: A DNA ploidy and fluorescence in-situ hybridization study. Br J Haematol 103: 750–755. [DOI] [PubMed] [Google Scholar]
- Ma X, Edmonson M, Yergeau D, Muzny DM, Hampton OA, Rusch M, Song G, Easton J, Harvey RC, Wheeler DA, et al. 2015. Rise and fall of subclones from diagnosis to relapse in pediatric B-acute lymphoblastic leukaemia. Nat Commun 6: 6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride KA, Ballinger ML, Killick E, Kirk J, Tattersall MH, Eeles RA, Thomas DM, Mitchell G. 2014. Li–Fraumeni syndrome: Cancer risk assessment and clinical management. Nat Rev Clin Oncol 11: 260–271. [DOI] [PubMed] [Google Scholar]
- Mehta PA, Zhang MJ, Eapen M, He W, Seber A, Gibson B, Camitta BM, Kitko CL, Dvorak CC, Nemecek ER, et al. 2015. Transplantation outcomes for children with hypodiploid acute lymphoblastic leukemia. Biol Blood Marrow Transplant 21: 1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman AV, Chilton L, Wilkinson J, Ensor HM, Bown N, Proctor SJ. 2010. A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood 115: 206–214. [DOI] [PubMed] [Google Scholar]
- Morrissette JJ, Halligan GE, Punnett HH, McKenzie AS, Guerrero F, de Chadarevian JP. 2006. Down syndrome with low hypodiploidy in precursor B-cell acute lymphoblastic leukemia. Cancer Genet Cytogenet 169: 58–61. [DOI] [PubMed] [Google Scholar]
- Muhlbacher V, Zenger M, Schnittger S, Weissmann S, Kunze F, Kohlmann A, Bellos F, Kern W, Haferlach T, Haferlach C. 2014. Acute lymphoblastic leukemia with low hypodiploid/near triploid karyotype is a specific clinical entity and exhibits a very high TP53 mutation frequency of 93%. Genes Chromosomes Cancer 53: 524–536. [DOI] [PubMed] [Google Scholar]
- Mullighan CG. 2013. Genomic characterization of childhood acute lymphoblastic leukemia. Semin Hematol 50: 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, et al. 2007a. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 446: 758–764. [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Miller CB, Su X, Radtke I, Dalton J, Song G, Zhou X, Pui C-H, Shurtleff SA, Downing JR. 2007b. ERG deletions define a novel subtype of B-progenitor acute lymphoblastic leukemia. Blood 110 (Abstr 691). [Google Scholar]
- Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, Ma J, White D, Hughes TP, Le Beau MM, Pui CH, et al. 2008a. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 453: 110–114. [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA, Downing JR. 2008b. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science 322: 1377–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, Ma J, Liu W, Cheng C, Schulman BA, et al. 2009. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med 360: 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullighan CG, Zhang J, Kasper LH, Lerach S, Payne-Turner D, Phillips LA, Heatley SL, Holmfeldt L, Collins-Underwood JR, Ma J, et al. 2011. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature 471: 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullighan CG, Jeha S, Pei D, Payne-Turner D, Coustan-Smith E, Roberts KG, Waanders E, Choi JK, Ma X, Raimondi SC, et al. 2015. Outcome of children with hypodiploid ALL treated with risk-directed therapy based on MRD levels. Blood 126: 2896–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman JB, Heerema NA, Sather H, Camitta B, Forestier E, Harrison CJ, Dastugue N, Schrappe M, Pui CH, Basso G, et al. 2007. Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood 110: 1112–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson K, Forestier E, Lilljebjorn H, Heldrup J, Behrendtz M, Young BD, Johansson B. 2010. Genetic landscape of high hyperdiploid childhood acute lymphoblastic leukemia. Proc Natl Acad Sci 107: 21719–21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. 2007. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: Lessons from recent developments in the IARC TP53 database. Hum Mutat 28: 622–629. [DOI] [PubMed] [Google Scholar]
- Powell BC, Jiang L, Muzny DM, Trevino LR, Dreyer ZE, Strong LC, Wheeler DA, Gibbs RA, Plon SE. 2013. Identification of TP53 as an acute lymphocytic leukemia susceptibility gene through exome sequencing. Pediatr Blood Cancer 60: E1–E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pui CH, Williams DL, Raimondi SC, Rivera GK, Look AT, Dodge RK, George SL, Behm FG, Crist WM, Murphy SB. 1987. Hypodiploidy is associated with a poor prognosis in childhood acute lymphoblastic leukemia. Blood 70: 247–253. [PubMed] [Google Scholar]
- Pui CH, Carroll AJ, Raimondi SC, Land VJ, Crist WM, Shuster JJ, Williams DL, Pullen DJ, Borowitz MJ, Behm FG, et al. 1990. Clinical presentation, karyotypic characterization, and treatment outcome of childhood acute lymphoblastic leukemia with a near-haploid or hypodiploid less than 45 line. Blood 75: 1170–1177. [PubMed] [Google Scholar]
- Raimondi SC, Zhou Y, Mathew S, Shurtleff SA, Sandlund JT, Rivera GK, Behm FG, Pui CH. 2003. Reassessment of the prognostic significance of hypodiploidy in pediatric patients with acute lymphoblastic leukemia. Cancer 98: 2715–2722. [DOI] [PubMed] [Google Scholar]
- Ramos ML, Palacios JJ, Fournier BG, Martinez JL, Martinez-Lopez J, Conde MC, Izquierdo AM, Garcia MM, Miranda EB. 2000. Prognostic value of tumoral ploidy in a series of Spanish patients with acute lymphoblastic leukemia. Cancer Genet Cytogenet 122: 124–130. [DOI] [PubMed] [Google Scholar]
- Rausch T, Jones DT, Zapatka M, Stutz AM, Zichner T, Weischenfeldt J, Jager N, Remke M, Shih D, Northcott PA, et al. 2012. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell 148: 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KG, Morin RD, Zhang J, Hirst M, Zhao Y, Su X, Chen SC, Payne-Turner D, Churchman ML, Harvey RC, et al. 2012. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell 22: 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D, McCastlain K, Ding L, Lu C, Song G, et al. 2014. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med 371: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowh MA, DeMicco A, Horowitz JE, Yin B, Yang-Iott KS, Fusello AM, Hobeika E, Reth M, Bassing CH. 2011. Tp53 deletion in B lineage cells predisposes mice to lymphomas with oncogenic translocations. Oncogene 30: 4757–4764. [DOI] [PubMed] [Google Scholar]
- Russell LJ, Capasso M, Vater I, Akasaka T, Bernard OA, Calasanz MJ, Chandrasekaran T, Chapiro E, Gesk S, Griffiths M, et al. 2009. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood 114: 2688–2698. [DOI] [PubMed] [Google Scholar]
- Safavi S, Forestier E, Golovleva I, Barbany G, Nord KH, Moorman AV, Harrison CJ, Johansson B, Paulsson K. 2013. Loss of chromosomes is the primary event in near-haploid and low-hypodiploid acute lymphoblastic leukemia. Leukemia 27: 248–250. [DOI] [PubMed] [Google Scholar]
- Schjerven H, McLaughlin J, Arenzana TL, Frietze S, Cheng D, Wadsworth SE, Lawson GW, Bensinger SJ, Farnham PJ, Witte ON, et al. 2013. Selective regulation of lymphopoiesis and leukemogenesis by individual zinc fingers of Ikaros. Nat Immunol 14: 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab CJ, Chilton L, Morrison H, Jones L, Al-Shehhi H, Erhorn A, Russell LJ, Moorman AV, Harrison CJ. 2013. Genes commonly deleted in childhood B-cell precursor acute lymphoblastic leukemia: Association with cytogenetics and clinical features. Haematologica 98: 1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwickert TA, Tagoh H, Gultekin S, Dakic A, Axelsson E, Minnich M, Ebert A, Werner B, Roth M, Cimmino L, et al. 2014. Stage-specific control of early B cell development by the transcription factor Ikaros. Nat Immunol 15: 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. 2001. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol 2: 731–737. [DOI] [PubMed] [Google Scholar]
- Sholl AB, Aisner DL, Behbakht K, Post MD. 2012. Novel TP53 gene mutation and correlation with p53 immunohistochemistry in a mixed epithelial carcinoma of the endometrium. Gynecol Oncol Case Rep 3: 11–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, Pleasance ED, Lau KW, Beare D, Stebbings LA, et al. 2011. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SL, Compton DA. 2010. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol 188: 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomeczkowski J, Yakisan E, Wieland B, Reiter A, Welte K, Sykora KW. 1995. Absence of G-CSF receptors and absent response to G-CSF in childhood Burkitt’s lymphoma and B-ALL cells. Br J Haematol 89: 771–779. [DOI] [PubMed] [Google Scholar]
- Villani A, Tabori U, Schiffman J, Shlien A, Beyene J, Druker H, Novokmet A, Finlay J, Malkin D. 2011. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li–Fraumeni syndrome: A prospective observational study. Lancet Oncol 12: 559–567. [DOI] [PubMed] [Google Scholar]
- Winandy S, Wu P, Georgopoulos K. 1995. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell 83: 289–299. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mullighan CG, Harvey RC, Wu G, Chen X, Edmonson M, Buetow KH, Carroll WL, Chen IM, Devidas M, et al. 2011. Key pathways are frequently mutated in high-risk childhood acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood 118: 3080–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Walsh MF, Wu G, Edmonson MN, Gruber TA, Easton J, Hedges D, Ma X, Zhou X, Yergeau DA, et al. 2015. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med 373: 2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]