Abstract
Cadherin-based adherens junctions are conserved structures that mediate epithelial cell–cell adhesion in invertebrates and vertebrates. Despite their pivotal function in epithelial integrity, adherens junctions show a remarkable plasticity that is a prerequisite for tissue architecture and morphogenesis. Epithelial cadherin (E-cadherin) is continuously turned over and undergoes cycles of endocytosis, sorting and recycling back to the plasma membrane. Mammalian cell culture and genetically tractable model systems such as Drosophila have revealed conserved, but also distinct, mechanisms in the regulation of E-cadherin membrane trafficking. Here, we discuss our current knowledge about molecules and mechanisms controlling endocytosis, sorting and recycling of E-cadherin during junctional remodeling.
E-cadherins help mediate cell–cell adhesion in epithelial cells. Despite their critical role in maintaining tissue integrity, they undergo cycles of endocytosis, sorting, and recycling during tissue remodeling and homeostasis.
The ability of epithelial cells to organize into monolayered sheets is a prerequisite for multicellularity, thereby providing tissue integrity, barrier function, and tissue polarity in metazoan organisms. Adherens junctions (AJs) are conserved key structures that mediate cell–cell adhesion in invertebrates and vertebrates. In many polarized epithelial sheets, AJs form a continuous adhesive belt at the apical–lateral interfaces of cell–cell contacts, the zonula adherens. The structural and functional core components of epithelial AJs are clusters of dimeric E-cadherin, a calcium-dependent, homophilic cell–cell adhesion receptor (Fig. 1). High-resolution microscopy analyses, however, recently revealed that E-cadherin clusters also accumulate throughout the lateral junctions below the zonula adherens (Wu et al. 2014; Yap et al. 2015).
Figure 1.
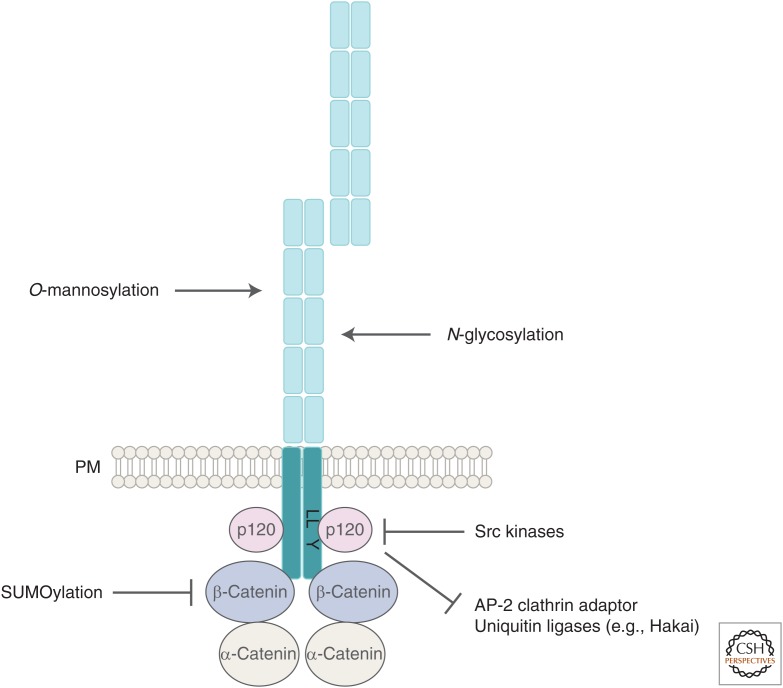
The core E-cadherin/catenin complex at adherens junctions (AJs). The stability and turnover of the core E-cadherin/catenin complex is regulated by different molecules and posttranslational modifications, for further details see main text.
Classical cadherins such as E-cadherin are single-pass membrane proteins with characteristic extracellular cadherin (EC) repeat domains that mediate trans-homophilic interactions between neighboring cells. While the numbers of ECs vary between different species, their intracellular domains are highly conserved from flies to humans and form a complex with catenins that link AJs to the actin cytoskeleton. The juxtamembrane domain of the cadherin intracellular tail interacts with p120 catenin, whereas the carboxy-terminal part directly binds β-catenin, which, in turn, binds α-catenin mediating a dynamic linkage to the actin cytoskeleton (Fig. 1) (Drees et al. 2005; Gates and Peifer 2005; Yamada et al. 2005). This dynamic interaction between AJs and the actin cytoskeleton is tightly linked to junctional maintenance, dynamics, and plasticity in developing and differentiated tissues (Michael and Yap 2013).
The dual properties of stability and plasticity of AJs were first observed in calcium chelation experiments (Kartenbeck et al. 1982). Depletion of extracellular calcium results in a rapid disruption of cell–cell adhesion in cultured epithelial monolayers because of the endocytic internalization of cadherins from AJs (Kartenbeck et al. 1982, 1991). The significance of cadherin internalization under physiological conditions is now well established. Over the last three decades, numerous studies showed that junctional proteins such as E-cadherin are dynamically turned over at the cell surface and this is fundamental for tissue remodeling during morphogenesis and tissue homeostasis (Kowalczyk and Nanes 2012; Takeichi 2014). Mammalian cell culture studies combined with in vivo models like Drosophila have led to the identification of conserved molecules and the underlying regulatory mechanisms driving cadherin trafficking in a large variety of morphogenetic and developmental processes. Here, we review our current knowledge about the proteins and the mechanisms controlling endocytosis, sorting and recycling of E-cadherin.
E-CADHERIN IS CONSTANTLY INTERNALIZED FROM THE CELL SURFACE
Dynamic changes in cell shape within tissues require a constant remodeling of cell junctions. Initial metabolic labeling experiments in cultured Madin–Darby canine kidney (MDCK) epithelial cells showed a half-life of endogenous E-cadherin at the cell surface of ∼5–10 h (McCrea and Gumbiner 1991; Troxell et al. 1999). Recent fluorescence recovery after photobleaching (FRAP) and photoconversion experiments in living epithelial layers of the Drosophila embryo confirmed a relatively slow biosynthetic turnover of E-cadherin clusters of about 1 h in vivo (Cavey et al. 2008). Thus, the comparatively slow transcriptional regulation of E-cadherin cannot account for all rapid changes in cell adhesion strength during fast cellular movements and tissue remodeling. Instead, cadherins are constantly removed from the plasma membrane through endocytosis and recycled back by exocytosis. Depending on the cellular context, E-cadherin can be internalized through different endocytic pathways. Most studies analyzed clathrin-mediated endocytosis of E-cadherin (Le et al. 1999; Palacios et al. 2002; Paterson et al. 2003). However, growth-factor-induced non-clathrin-mediated pathways of E-cadherin, including Rac1-dependent macropinocytosis, have been reported (Braga et al. 1997, 1999; Akhtar and Hotchin 2001; Lu et al. 2003; Bryant et al. 2007).
LOCAL REMOVAL OF E-CADHERIN FROM THE PLASMA MEMBRANE BY CLATHRIN-MEDIATED ENDOCYTOSIS
Unlike macropinocytosis, clathrin-mediated endocytosis allows a spatially controlled internalization. Since clathrin does not bind directly to cargo receptors, selection of cargo relies on adaptor proteins that recognize internalization motifs within the cytoplasmic region of transmembrane receptors (Kelly and Owen 2011). E-cadherin associates with several endocytic adaptors including AP-2, Dab-2, and Numb (Ling et al. 2007; Miyashita and Ozawa 2007b; Yang et al. 2007; Sato et al. 2011). A central adaptor in clathrin-mediated endocytosis is AP-2, which forms a tetrameric complex that directly binds clathrin and recruits several classes of receptors bearing an acidic dileucine internalization signal in their cytoplasmic tail (Fig. 2) (Traub 2003, 2009; Kelly and Owen 2011). Vertebrate E-cadherin contains an AP-2 binding motif and mutations in this dileucine motif affect the localization of E-cadherin by preventing its clathrin-mediated endocytosis (Miranda et al. 2001; Miyashita and Ozawa 2007a,b).
Figure 2.
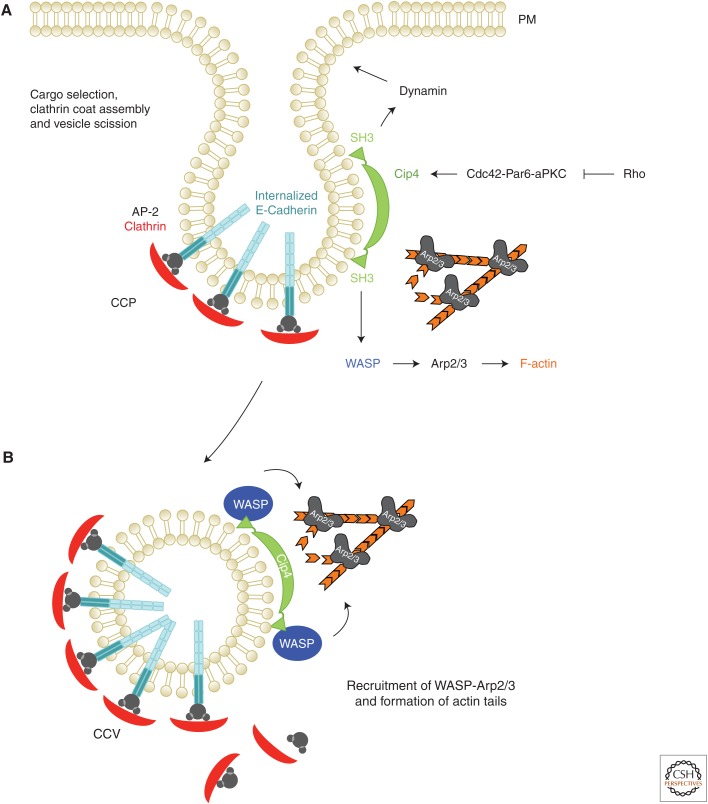
The Cdc42-Par6-aPKC polarity complex promotes E-cadherin endocytosis by recruiting the Cip4-WASP-Arp2/3 actin machinery. (A) E-cadherin is actively internalized by clathrin-mediated endocytosis. Vertebrate E-cadherin contains an AP-2 binding motif. Once E-cadherin is selected and bound by AP-2 or other cargo-adapter proteins (e.g., Numb, see main text), the clathrin coat is assembled. Cdc42–Par6–aPKC recruits Cip4 to the site of E-cadherin endocytosis. F-BAR proteins such as Cip4 are thought to facilitate the scission by recruiting Dynamin to the neck of a nascent vesicle. This recruitment requires the SH3 domain that binds to Proline-rich motifs of Dynamin but also the Arp2/3 activator Wiskott–Aldrich syndrome protein (WASP). (B) WASP-Arp2/3-mediated actin polymerization has a supportive function in vesicle budding and promotes actin-comet-tail-based movement of newly formed clathrin-coated vesicles. The GTPase Rho seems to suppress cadherin endocytosis by antagonizing Cdc42–Par6–aPKC functions (Warner and Longmore 2009).
The budding of clathrin-coated vesicles requires the core endocytic machinery including the GTPases Dynamin and Rab5. Reduction of these core components results in an increase of E-cadherin at the plasma membrane. Dynamin is a large, multidomain GTPase that assembles into helical structures along invaginating membranes and drives the scission of endocytic vesicles through a cycle of oligomerization and GTP hydrolysis (Fig. 2) (Schmid and Frolov 2011; Kirchhausen et al. 2014). Dynamin-mediated endocytosis is required for E-cadherin redistribution at mature AJs of MDCK and MCF7 cells, but also plays an important role in AJ turnover during Drosophila epithelial morphogenesis (Classen et al. 2005; Georgiou et al. 2008; Leibfried et al. 2008; de Beco et al. 2009; Levayer et al. 2011). A key observation of the Drosophila in vivo studies is that E-cadherin endocytosis is locally enhanced along the planar axis or along the apico-basal axis of epithelial cells and that this local E-cadherin turnover has an instructive role in tissue morphogenesis. For example, polarized endocytosis of E-cadherin is crucial for cell intercalations in the elongating Drosophila embryo whereby epithelial cells change neighbors through the shrinkage of planar polarized junctions along the dorsoventral axis. Blocking of clathrin-mediated endocytosis causes the loss of E-cadherin planar polarization and a block of cell intercalations (Levayer et al. 2011). Similar observations were made in Drosophila pupal epithelia (Classen et al. 2005; Georgiou et al. 2008; Leibfried et al. 2008; de Beco et al. 2009). Wing epithelial cells become hexagonally packed through the shrinkage of individual AJ by polarized E-cadherin turnover (Classen et al. 2005; Warrington et al. 2013). Consistently, loss of E-cadherin or dynamin function disrupts the planar polarized organization of the wing epithelium (Classen et al. 2005; Fricke et al. 2009). Polarized endocytosis is also necessary for local E-cadherin removal during embryonic wound repair (Hunter et al. 2015). Blocking of endocytosis on wounding disrupts not only AJ remodeling but also prevents the assembly of contractile actomyosin cables at the wound margin that dramatically slows down wound closure (Hunter et al. 2015). Thus, E-cadherin endocytosis appears to be a necessary step for the cytoskeletal rearrangements driving wound repair. An important requirement in coupling endocytosis-driven junctional remodeling and actomyosin contractility has also been observed in Drosophila dorsal closure and in zebrafish epiboly cell movements (Mateus et al. 2011; Song et al. 2013).
p120 CATENIN, AN INHIBITOR OF VERTEBRATE E-CADHERIN ENDOCYTOSIS
Interestingly, the acidic dileucine motif in the juxtamembrane domain of vertebrate E-cadherin overlaps with the binding site for p120 catenin, a well-known inhibitor of cadherin endocytosis (Davis et al. 2003). In the absence of p120 catenin, cadherins are rapidly internalized and degraded in the lysosome (Miyashita and Ozawa 2007b; Xiao et al. 2007). Recent data strongly suggests that p120 catenin binding masks the endocytic signal and may thereby compete for AP-2 binding to cadherins in mammals (Nanes et al. 2012; Perez-Moreno and Fuchs 2012). Supporting this notion, the binding affinities of p120 catenin and AP-2 for the acidic dileucine motif are similar (Fig. 1) (Nanes et al. 2012). However, a physiological role of AP-2 function in the initiation of clathrin-mediated endocytosis of E-cadherin has so far only been shown in Drosophila germ-band extension, a major morphogenetic movement during fly gastrulation (Levayer et al. 2011). Remarkably, Drosophila E-cadherin lacks the endocytic motif and p120 catenin function is not required for internalization of E-cadherin for degradation (Myster et al. 2003; Bulgakova and Brown 2016). Different from mice, worms and flies lacking p120 catenin are viable and fertile and display no striking defects in the structure or function of adherens junctions (Myster et al. 2003; Pettitt et al. 2003; Davis and Reynolds 2006; Perez-Moreno et al. 2006). However, loss of Drosophila p120 catenin function strongly enhances phenotypic traits in E-cadherin and β-catenin mutants, suggesting an important supportive role of p120 catenin in junctional cell adhesion (Myster et al. 2003). Surprisingly, Bulgakova and Brown (2016) recently found that Drosophila p120 catenin rather promotes clathrin-mediated endocytosis of E-cadherin and propose that the inhibitory function of p120 catenin has been newly acquired in vertebrates during evolution. Thus, the key role of p120 catenin as a master regulator of cadherin stability may represent a vertebrate-specific adaptation in response to an increased complexity in tissue morphogenesis and architecture during evolution (Carnahan et al. 2010). It also implies the existence of additional ancient key principles in regulating the stability of AJs present in invertebrate and vertebrates.
POSTTRANSLATIONAL MODIFICATIONS REGULATING E-CADHERIN TURNOVER
Phosphorylation might represent such an ancient regulatory mechanism controlling cadherin internalization. Early cell culture studies have already revealed that cell–cell junctions are prominent targets of tyrosine phosphorylation (Maher et al. 1985). Stimulation of epithelial cultures by growth factors (e.g., epithelial growth factor [EGF] or human growth factor [HGF]) promotes tyrosine phosphorylation of several junctional proteins followed by a rapid internalization of junctional complexes. Within the past decades, numerous studies in cultured cells have unraveled a series of tyrosine, but also serine phosphorylation events and identified diverse kinases and phosphatases acting on E-cadherin/catenin complex integrity (reviewed in Daniel and Reynolds 1997; Roura et al. 1999; Lilien and Balsamo 2005; Bertocchi et al. 2012; Coopman and Djiane 2016). The picture emerging from these studies of how AJ functions and phosphorylation interplay is still very incomplete, especially because many of these phosphorylation events strongly depend on the cell type and the stimulus such as growth factor treatment (reviewed in Bertocchi et al. 2012; Coopman and Djiane 2016).
Members of the Src protein family have emerged as critical cytoplasmic tyrosine kinases that affect E-cadherin dynamics and E-cadherin/catenin complex integrity in vertebrates and invertebrates. The activation of Src kinases and subsequent phosphorylation of AJ components in cultured epithelial cells generally results in a disruption of cell–cell adhesions and increased invasiveness (Behrens et al. 1993; Boyer et al. 1997; Calautti et al. 1998; Owens et al. 2000). However, depending on the signal strength, Src can also act positively on E-cadherin-mediated cell adhesion (McLachlan et al. 2007). On Src activation E-cadherin is phosphorylated at two specific tyrosine residues in the juxtamembrane domain, thereby creating a binding surface for the c-Cbl-like ubiquitin ligase Hakai (Fig. 1) (Fujita et al. 2002). Since the binding sites for Hakai and p120 catenin are closely apposed, it has been suggested that p120 catenin binding might also compete with Src or Hakai and hence might inhibit ubiquitination and degradation. Consistently, ubiquitination of the juxtamembrane domain prevents p120 catenin binding to E-cadherin and results in proteasomal degradation of E-cadherin (Hartsock and Nelson 2012). However, tyrosine phosphorylation-defective mutants of E-cadherin can still be internalized and Hakai-mediated ubiquitination might rather serve as a sorting signal for its trafficking to lysosomes (Palacios et al. 2005). Thus, ubiquitination does not seem to directly regulate the internalization of E-cadherin. Hakai is highly conserved in metazoans, and flies lacking Hakai function also show severe defects in epithelial integrity, as well as defects in cell specification and cell migration (Kaido et al. 2009). A functional interaction between Hakai and Src kinases has never been observed in Drosophila. Src42A, one of the two Src kinases in Drosophila, forms a ternary complex with E-cadherin and β-catenin (Armadillo [Takahashi et al. 2005]). Src42A activation has a dual effect on E-cadherin-based AJs. On the one hand Src42A increases E-cadherin turnover by phosphorylation of β-catenin and thereby, destabilizing AJ complex integrity during tracheal epithelial morphogenesis in Drosophila (Shindo et al. 2008). On the other hand it also results in a transcriptional activation of E-cadherin by released β-catenin that acts as a well-known transactivator of the Tcf/Lef family of transcription factors (Shindo et al. 2008; Langton et al. 2009). Recent FRAP experiments of green fluorescent protein (GFP)-marked E-cadherin at Drosophila AJs further revealed that Src42A promotes the recycling of E-cadherin and is required for polarized cell-shape changes during epithelial tube elongation (Forster and Luschnig 2012). Thus, Src-mediated tyrosine phosphorylation has an important conserved role in E-cadherin turnover. However, the functional consequences and the underlying regulatory mechanism of tyrosine phosphorylation seem to differ remarkably between different cell types and species.
The posttranslational addition of a small ubiquitin-related modifier (SUMO) protein to E-cadherin seems to be essential for E-cadherin recruitment to AJs and for the maintenance of its interaction with the actin cytoskeleton in Caenorhabditis elegans (Fig. 1) (Tsur et al. 2015). SUMO modification is a reversible process analogous to ubiquitylation: Sumoylation is mediated by the concerted actions of E1, E2, and E3 enzymes, whereas desumoylation is promoted by SUMO specific proteases (Flotho and Melchior 2013). A key finding is that sumoylation on a conserved lysine residue of the E-cadherin cytoplasmic tail reduces its interaction with β-catenin (Tsur et al. 2015). Both the loss and overexpression of SUMO proteases resulted in similar defects in AJ assembly, suggesting that balanced sumoylation-desumoylation events are important to sustain AJ plasticity. Sumoylation is essential for most organisms and mammals express three SUMO precursor proteins, whereas C. elegans, Drosophila, and yeast only express a single SUMO protein (Flotho and Melchior 2013). Thus, it will be important to determine whether transient sumoylation acts as a conserved molecular switch of AJ dynamics in other species.
LOCAL E-CADHERIN ENDOCYTOSIS DEPENDS ON THE Cdc42-Par6-aPKC POLARITY COMPLEX
The spatially controlled endocytosis of E-cadherin depends on evolutionarily conserved polarity proteins including the PDZ domain protein Par6, the atypical protein kinase C (aPKC) and the Rho-GTPase Cdc42, which is also a well-known regulator of the actin cytoskeleton. Par6 and aPKC form, together with the scaffold protein Par3 (Bazooka), the Par complex, a universal module defining apico-basal cell polarity in metazoans (Tepass 2012). Cells of the pupal thorax epithelium of Drosophila lacking either the Par6-aPKC or Cdc42 function show a defective internalization of E-cadherin characterized by malformed endocytic membrane tubules, reminiscent of vesicle scission defects in dynamin (shibire) mutant cells (Georgiou et al. 2008; Leibfried et al. 2008). The Par complex seems to act as an effector of Cdc42 in controlling endocytosis of E-cadherin, because constitutively active aPKC can partially suppress the AJ disruption of cdc42 loss-of-function (Fig. 2). An important requirement of Cdc42 and the Par6-aPKC module in the endocytic turnover of E-cadherin has also been found in mammalian cell culture and in C. elegans (Balklava et al. 2007). Recent studies in mammals as well as in flies further suggest that other polarity proteins, including Lethal giant larvae (LgL), Scribble (Scrib), and Crumbs, may also play an important role in the maintenance of AJs (Goldstein and Macara 2007; Nance and Zallen 2011). However, these effects could also be indirect because these proteins are also crucial for the maintenance of apico-basal polarity and therefore tissue integrity (Tepass and Knust 1990, 1993).
How is the Cdc42-Par6-aPKC polarity complex linked to the endocytic machinery promoting E-cadherin internalization? Cdc42 is also a well-known activator of the Wiskott-Aldrich syndrome protein family (WASP), which, in turn, promotes actin nucleation through the Arp2/3 complex, a major actin nucleator in eukaryotic cells (Fig. 2) (Symons et al. 1996; Rohatgi et al. 2000; Pollard and Beltzner 2002). Cdc42 directly binds WASP and relieves an autoinhibitory contact between the GTPase-binding domain and the carboxy-terminal Arp2/3-activating VCA module (Kim et al. 2000). On activation of WASP by Cdc42 branched actin filaments are formed at the rim of endocytic pits that are thought to facilitate the invagination and scission of the underlying membrane (Fig. 2) (Merrifield and Kaksonen 2014; Kaksonen et al. 2006). WASP-Cdc42-induced actin polymerization at endocytic sites further depends on the membrane-curvature promoted by F-BAR proteins such as Cip4 (Cdc42-interacting protein 4) (Ho et al. 2004; Tsujita et al. 2006; Takano et al. 2008). The F-BAR domain dimer of Cip4 forms a crescent-shaped surface that binds and deforms the membrane, whereas the carboxy-terminal Src homology 3 (SH3) domain binds Dynamin and WASP (Fig. 2) (Heath and Insall 2008; Aspenstrom 2009; Chen et al. 2013). Thus, F-BAR proteins such as Cip4 are prime candidates to couple WASP-Cdc42-mediated actin polymerization to Dynamin-mediated vesicle scission in endocytosis and vesicle movement (Fig. 2) (Fricke et al. 2010). In Drosophila, thorax epithelia lacking either WASP or Cip4 function display AJ breaks and the formation of long tubular endocytic structures that are very reminiscent of dynamin mutant cells in which vesicle scission is blocked (Georgiou et al. 2008; Leibfried et al. 2008). A dynamin-related phenotype has been observed on overexpression in the fly wing epithelium of a dominant-negative Cip4 protein lacking the Dynamin-interacting SH3 domain (Fricke et al. 2009). Here, Dynamin function is required for junctional remodeling during epithelial repacking (Classen et al. 2005). Thus, a model has been proposed in which activated Cdc42 acts through the Par complex and recruits Cip4-WASP to promote both Dynamin-mediated vesicle scission and branched actin nucleation at endocytic vesicles (Leibfried et al. 2008; Fricke et al. 2009). Despite the conserved function of Cip4 in regulating early steps in E-cadherin endocytosis, flies, worms, and mice lacking Cip4 function are viable and develop largely normally, suggesting additional compensatory or redundant functions that are likely fulfilled by other members of the F-BAR protein family (Fricke et al. 2009; Giuliani et al. 2009; Feng et al. 2010; Rolland et al. 2014). Supporting this notion, double mutants lacking Cip4 and the Cip4-like protein Nostrin show reduced viability and fertility (Zobel et al. 2015). Double mutant flies show defects in wing polarization defects and egg chamber encapsulation caused by an impaired turnover of E-cadherin (Zobel et al. 2015).
ENDOSOMAL SORTING OF E-CADHERIN
Once internalized by clathrin-dependent or -independent endocytosis, E-cadherin enters a Rab5 positive compartment, which is central for sorting of most cell surface transmembrane proteins (Fig. 3) (Pfeffer 2013; Wandinger-Ness and Zerial 2014). Within this early-endosomal (EE) compartment E-cadherin is either sorted back to the plasma membrane through recycling endosomes (RE) or it is sequestered into intraluminal vesicles of multivesicular endosomes (MVE) that finally fuse with lysosomes for degradation. How cadherins are selected from sorting endosomes for trafficking to recycling endosomes is not yet understood. Endosomal sorting of E-cadherin may involve the segregation of tubular endosomal subdomains that recruit diverse microtubule motors driving endosomal membrane scission and trafficking (Hunt et al. 2013; van Weering and Cullen 2014). This function has been proposed for Nostrin (Zobel et al. 2015). Nostrin localizes at distinct subdomains of Rab5/Rab11 endosomal intermediates. A cooperative recruitment model has been proposed, in which Cip4 first promotes membrane invagination and early actin-based endosomal motility, whereas Nostrin is linked to microtubules through the minus end-directed kinesin motor Khc-73 for trafficking of recycling endosomes (Zobel et al. 2015). An important role of the mammalian orthologs Kif13A and KifC3 either in the formation of recycling endosomal tubules along microtubules or in the microtubule-dependent transport to AJs has recently been identified (Meng et al. 2008; Delevoye et al. 2014).
Figure 3.
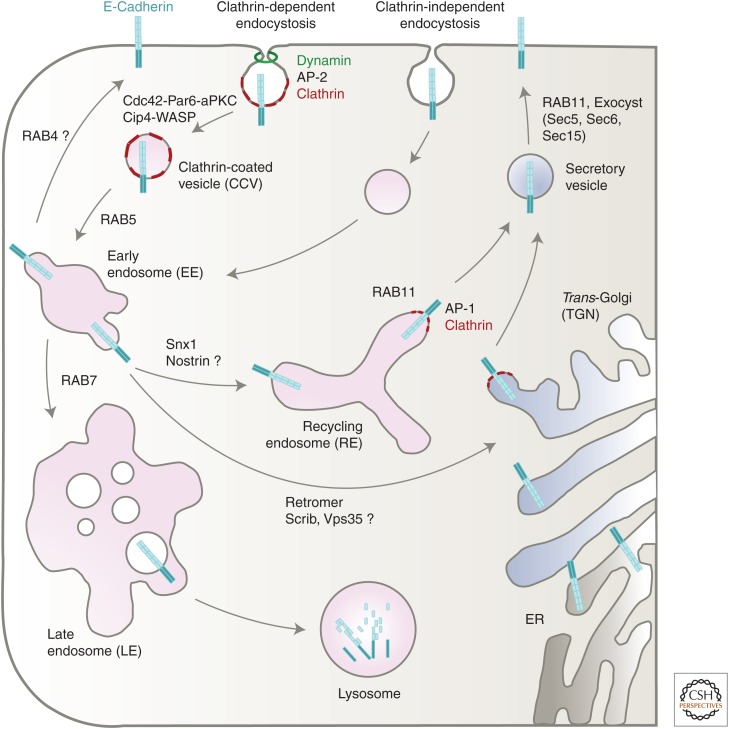
Trafficking pathways of E-cadherin. E-cadherin can undergo either clathrin-dependent or independent endocytosis. Internalized E-cadherin traffic through different endosomal compartments. Numerous molecules and endocytic machineries determine the fate of E-cadherin either to endosomal recycling back to the plasma membrane or to lysosomal degradation. Polarized sorting in both the biosynthetic pathway from the trans-Golgi network and recycling endosomes requires vesicle carriers such as AP-1 as discussed in the main text.
Known integral components of the tubule-based endosomal sorting mechanism are sorting nexins (Snx). Previous work has shown that the two Snx-Bar proteins Snx4 and Snx1 are involved in E-cadherin sorting in epithelial cell culture (Bryant et al. 2007; Solis et al. 2013). Depletion of Snx1 resulted in increased intracellular accumulation and turnover of E-cadherin internalized from the cell surface of MCF-7 cells (Bryant et al. 2007). Snx1 is a conserved component of the retromer complex, an endosomal protein sorting machinery regulating the recycling of endocytosed proteins from endosomes to the trans-Golgi network (TGN) or to the plasma membrane (Fig. 3) (Cullen 2008; Wang and Bellen 2015). Whether endocytosed E-cadherin can be recycled back to the TGN via the retromer is not known. Interestingly, Drosophila Vps35 (CG5625), which is part of the cargo-selective trimer of the retromer, was recently found in a genome-wide RNAi screen for genes required for E-cadherin-dependent cell–cell adhesion (Toret et al. 2014).
E-CADHERIN IS MAINLY RECYCLED THROUGH Rab11-POSITIVE ENDOSOMES
Recycling of internalized membrane receptors can either be facilitated by a rapid Rab4-mediated route or transit through a slow Rab11-positive recycling pathway back to the plasma membrane (Fig. 3) (Stenmark 2009; Scott et al. 2014). Several studies in epithelial cell culture and in diverse Drosophila epithelial tissues showed that E-cadherin is principally transported to a Rab11-positive recycling endosomal compartment (Fig. 3) (Classen et al. 2005; Lock and Stow 2005; Bogard et al. 2007; Desclozeaux et al. 2008; Roeth et al. 2009; Pirraglia et al. 2010; Hunter et al. 2015; Le Droguen et al. 2015; Loyer et al. 2015), whereas there is only little evidence showing that E-cadherin can also pass a Rab4-dependent rapid recycling route as recently found during Drosophila leg development (de Madrid et al. 2015). Consistently, disruption of Rab11-mediated recycling results in an abnormal intracellular accumulation of E-cadherin and junctional integrity is severely impaired.
POLARIZED EXOCYTOSIS OF E-CADHERIN BY A Rab11-EXOCYST COMPARTMENT
A striking intracellular accumulation of E-cadherin in enlarged Rab11-positive vesicles was also observed in mutant cells lacking single components of the exocyst complex such as Sec5, Sec6, and Sec15, indicating a defect in the targeted delivery of E-cadherin from the basolateral domain to the apicobasal AJs (Langevin et al. 2005). Further evidence suggest that the exocyst can regulate E-cadherin recycling by acting as a direct molecular link between the Rab11-recycling endosomes and the E-cadherin/catenin complex in tissue remodeling and collective cell migration (Fig. 3) (Classen et al. 2005; Langevin et al. 2005; Blankenship et al. 2007; Wan et al. 2013).
The exocyst is an evolutionarily conserved multi-subunit protein complex that is crucial to tether secretory vesicles derived from the trans-Golgi network (TGN) or from recycling endosomes to the plasma membrane for exocytosis (Wu and Guo 2015). In yeast, the exocyst is localized to defined regions of the plasma membrane where it mediates the polarized delivery of proteins and lipids required for polarized membrane growth. This model has been adapted to epithelial cells, where targeted recycling of internalized junctional proteins to the apical membrane provides a mechanism controlling AJ remodeling during cell polarization (Grindstaff et al. 1998; Mostov et al. 2003). Consistently, Rab11 and β-catenin interact with the exocyst components Sec15, Sec5, and Sec10, respectively, and disruption of these interactions results in an intracellular accumulation of E-cadherin in recycling endosomes (Fig. 3) (Beronja et al. 2005; Langevin et al. 2005). Recycling endosomes can also function as an intermediate compartment for newly synthesized E-cadherin that is not directly transported from the TGN to the plasma membrane by the exocyst in cultured epithelial cells (Yeaman et al. 2004; Lock and Stow 2005). Remarkably, in polarized MDCK cells newly synthesized E-cadherin is transported as a complex with β-catenin and the formation of the E-cadherin/β-catenin complex is already important for efficient exit from the endoplasmic reticulum (Chen et al. 1999) Uncoupling the binding of β-catenin from E-cadherin by introducing corresponding mutations in the E-cadherin cytoplasmic tail results in an accumulation of the proteins in intracellular compartments and subsequent degradation in lysosomes (Miyashita and Ozawa 2007a). A recent in vivo study in the Drosophila follicular epithelium further supports a model in which the Rab11-exocyst interaction regulates targeting of vesicles with endocytosed as well as newly synthesized E-cadherin (Woichansky et al. 2016). The investigators further propose the existence of two exocytosis pathways for de novo synthesized E-cadherin, first a Rab11-independent pathway for exocytosis to the basal-lateral region, and second a Rab11-dependent pathway that targets exocytosis to apico-lateral AJs (Woichansky et al. 2016). In the same study a so far uncharacterized GTPase, RabX1, has been identified as a new critical component regulating E-cadherin recycling. In rabX1 mutant epithelia endocytosed E-cadherin is not properly recycled, but rather accumulates together with Rab5 and Rab11 in a large compartment (Woichansky et al. 2016).
SORTING OF NEWLY SYNTHESIZED E-CADHERIN FROM THE TRANS-GOLGI NETWORK AND Rab11 RECYCLING ENDOSOMES
In a number of ways sorting at the TGN resembles the initial steps in endocytosis. Post-Golgi vesicles are coated by clathrin and polarized sorting requires clathrin adaptors of the family of heterotetrameric AP complexes such as AP-1 (Fig. 3) (Bonifacino 2014). Different from endocytic AP-2, AP-1 complexes localize at the TGN and REs and control either the biosynthetic sorting at the TGN (AP-1A) or the RE sorting (AP-1B) to the basolateral surface (Folsch et al. 2003; Gravotta et al. 2012; Folsch 2015). In MDCK cells, double knockdown of AP-1A and AP-1B results in missorting of many basolateral proteins including E-cadherin, and causes a dramatic loss of cell polarity (Gravotta et al. 2012). Interestingly, similar to AP-2, AP-1 complexes recognize as a basolateral sorting signal the same dileucine-based motif in the cytoplasmic tail of E-cadherin (Lock and Stow 2005; Ling et al. 2007; Mattera et al. 2011). Thus, AP-1 and AP-2 recognize almost identical sets of dileucine motif-containing membrane cargo proteins, but function at different intracellular sites (Fig. 3). Knockout studies in mammals, zebrafish, Drosophila, and C. elegans further confirmed an important conserved role of AP-1 in E-cadherin trafficking and loss of AP-1 function results in strong reduction of E-cadherin-mediated cell adhesion and epithelial disorganization (Shim et al. 2000; Zizioli et al. 2010; Burgess et al. 2011; Takahashi et al. 2011; Shafaq-Zadah et al. 2012; Zhang et al. 2012; Hase et al. 2013; Gariano et al. 2014; Gillard et al. 2015; Loyer et al. 2015).
Unlike vertebrates, invertebrates only contain a single AP-1 complex and Drosophila and C. elegans E-cadherin lack the dileucine-based AP-1-sorting signal in their cytoplasmic tails (Boehm and Bonifacino 2001). Thus, in invertebrates, interactions between E-cadherin and AP-1 complexes might not be direct, but may instead be mediated by adaptors such as phosphatidylinositol phosphate 4 kinases (PI4K) (Ling et al. 2007). Mammalian PIPKIγ directly binds both E-cadherin and the μ subunit of AP-1, thus it might act as a signaling scaffold that links AP-1 complexes to E-cadherin. Depletion of PIPKIγ or disruption of PIPKIγ binding to either E-cadherin or AP complexes results in defective E-cadherin transport and blocks AJ assembly (Ling et al. 2007). Phosphatidylinositol-4-phosphate (PI4P) is a critical lipid involved in the progressive assembly of AP-1-specific clathrin coats at the TGN (Wang et al. 2003; Santiago-Tirado and Bretscher 2011).
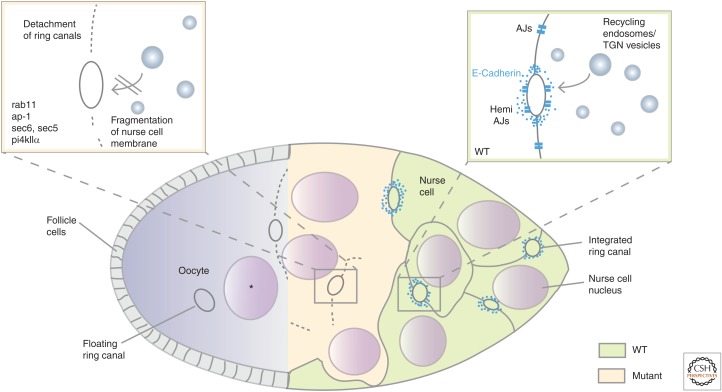
In the Drosophila germline, loss of AP-1, Rab11, PI4KIIα, or of exocyst components disrupts nurse cell plasma membrane integrity and causes a striking multinucleation phenotype (Fig. 4) (Murthy and Schwarz 2004; Murthy et al. 2005; Bogard et al. 2007; Tan et al. 2014; Loyer et al. 2015). It has been suggested that this common phenotype is caused by a defective intracellular trafficking of membrane components, but the exact underlying cellular defect is not known thus far. A detailed phenotypic analysis of AP-1 mutant germline, however, recently revealed that AP-1 regulates the trafficking of E-cadherin to the ring canals (Fig. 4). These stable intercellular bridges between nurse cells and the oocyte are composed of noncontractile actin bundles, which are anchored to the plasma membrane by adhesive E-cadherin clusters or semicircular adherens junctions (Fig. 4) (Loyer et al. 2015). Thus, the loss of nurse cell membranes in all these mutants is likely caused by the detachment of the plasma membrane from ring canals followed by fragmentation of the membrane (Fig. 4) (Loyer et al. 2015).
Figure 4.
Polarized trafficking of Drosophila E-cadherin is required for the maintenance of ring canal anchoring to plasma membrane. Drosophila eggs arise from individual units called egg chambers consisting of two cell types, the germline cyst and the surrounding somatic monolayered follicle epithelium. The 16-cell germline cyst consists of one oocyte (blue) and 15 nurse cells (green and orange) that provide nutrients for oocyte maturation. Cytoplasmic bridges between nurse cells and the oocyte support the growth of the oocyte. These bridges are formed by F-actin-rich ring canals that allow the directed microtubule-mediated transport of all macromolecules and organelles from the nurse cells into the oocyte. E-cadherin clusters form circular ‘‘hemi-adherens junctions’’ around the ring canals mediating the anchoring of ring canals into the nurse cell membrane. During oocyte maturation, the volume of the oocyte increases by four orders of magnitude resulting in a dramatic increase of plasma membrane tension. A continuous secretory transport of E-cadherin is required to maintain ring canal anchoring to the plasma membrane in wild type (WT). In nurse cells mutant for Rab11, AP-1, Sec5, Sec6 or PI4KIIα (orange) the polarized trafficking of E-cadherin to ring canals is disrupted. Due to the increasing membrane tension ring canals detach from the membrane resulting in an immediate fragmentation of the membrane. As a consequence, floating ring canals and nurse cell nuclei in the oocyte (*) can be frequently observed in mutant egg chambers defective for E-cadherin trafficking.
Ring canals are also formed at E-cadherin-positive boundaries in mammalian germ cell cysts, but their functions are not well understood (Pepling et al. 1999; Mork et al. 2012). Unlike in flies, where these intercellular bridges allow the transport of nutrients to the oocyte, in mammals the ring canals are thought to play a role in the synchronization of mitotic divisions and the entry into meiosis (Haglund et al. 2011).
A number of additional players have also been identified that control E-cadherin trafficking and cell adhesion, such as Scribble (Scrib), a conserved polarity protein and tumor suppressor that defines the basolateral domain in epithelial cells (Navarro et al. 2005; Qin et al. 2005; Dow et al. 2007). A more recent study suggests that Scrib may stabilize E-cadherin/p120 catenin binding and blocks retrieval of E-cadherin to the Golgi (Lohia et al. 2012). An additional function of Scrib in blocking retromer-mediated diversion of E-cadherin to the Golgi has been discussed (Lohia et al. 2012). A substantial body of evidence also indicates an important regulatory role of glycosyltransferases involved in the remodeling of N-glycans on E-cadherin, a process that dramatically affects E-cadherin stability and localization (Liwosz et al. 2006; Zhao et al. 2008; Zhou et al. 2008; Pinho et al. 2011). N-glycosylation of E-cadherin has also been shown to be an essential posttranslational modification in the lateral epidermis during Drosophila germband extension (Zhang et al. 2014). Mutations in the xiantuan gene (xit) encoding a conserved ER glycosyl-transferase affect glycosylation and the intracellular distribution of E-cadherin, but not the total amount of E-cadherin protein. The phenotypic analysis of xit mutants further suggests that N-glycosylation is important for the distribution and clustering of E-cadherin within the plasma membrane. A similar role for O-mannosylation in E-cadherin distribution was recently described in mouse embryos (Lommel et al. 2013; Vester-Christensen et al. 2013).
CONCLUSIONS AND PERSPECTIVES
Cell biological approaches in cultured epithelial cells together with in vivo studies in genetically tractable model organisms such as Drosophila and C. elegans have greatly advanced our understanding of the molecular regulation of AJ dynamics and homeostasis. Here, we highlighted conserved players and mechanisms that regulate E-cadherin endocytosis, sorting, exocytosis, and recycling. Recent quantitative proteomic approaches using the proximity biotinylation technique further suggest a remarkable molecular complexity and identified additional conserved regulatory hubs controlling E-cadherin-mediated cell adhesion (Guo et al. 2014; Van Itallie et al. 2014a,b). These approaches expand the inventory of the E-cadherin interaction network of hundreds of uncharacterized proteins at the AJs. Numerous cytoskeletal proteins were found, in addition to many trafficking, signaling proteins, metabolic enzymes, and transcription factors. The candidates identified are unlikely to be unique to AJs and future studies need to address their functional and physiological relevance in E-cadherin-mediated cell adhesion. Subcellular localization data might be the first step to exclude potential false-positives of these initial screens. However, many of the most abundant candidates in the E-cadherin interactome, such as Filamin-A, Scrib, Discs large (Dlg), and Annexins, are multifunctional and/or are active in multiple subcellular compartments. A recent genome-wide RNAi screen for genes required for calcium- and cadherin-dependent cell–cell adhesion is a new step forward to identify conserved protein hubs that functionally complement the proximity biotinylation approaches mentioned above (Toret et al. 2014). In this study, the Vale and Nelson groups identified about 400 proteins that regulate the core E-cadherin/catenin adhesion complex using nonmotile and non-extracelluar matrix (ECM)-adherent Drosophila S2 cells in which all components required for integrin dependent-cell adhesion, cell spreading, and cell migration were eliminated. Selected candidates were further validated for defects in cell–cell adhesion in mammalian MDCK epithelial cells and in Drosophila for defects in E-cadherin-dependent oocyte positioning on germline-targeted RNAi. As might be expected, the largest group of proteins found in the E-cadherin interactome as well as in the RNAi screen are actin regulators including subunits of the WAVE regulatory complex (WRC), a major activator of the Arp2/3 complex that drives cell movements in most eukaryotes (Pollitt and Insall 2009). AJs are known to be tightly coupled to the actin cytoskeleton and this functional interplay is crucial for the junctional integrity, but also provides the mechanical forces coordinating individual cell-shape changes during tissue rearrangements in morphogenesis. However, the exact function of the Arp2/3 complex that is recruited to E-cadherin junctional complexes (Kovacs et al. 2002), in controlling E-cadherin dynamics in morphogenetic movements and epithelial remodeling, is still not well understood. Addressing how actin polymerization exactly acts on AJ integrity, internalization or intracellular trafficking of E-cadherin will be important for understanding tissue morphogenesis. Combining cell culture approaches with in vivo animal models has been applied very successfully. Advances in high-resolution light microscopy and in three-dimensional (3D) electron tomography will help us to better analyze the functional consequences of E-cadherin trafficking within a cell and within an animal. This will be not only important for a better understanding of E-cadherin biology, but also tissue remodeling in development and disease.
ACKNOWLEDGMENTS
We thank Meike Bechtold, Veit Riechman, and Stefan Luschnig for helpful discussions and critical reading of the manuscript. This work was supported by a grant to S.B. from the cluster of excellence “Cells in Motion” (CIM), and by grants to S.B. from the Heisenberg Program of the Deutsche Forschungsgemeinschaft (DFG) and the SPP1464 priority program of the DFG.
Footnotes
Editors: Carien M. Niessen and Alpha S. Yap
Additional Perspectives on Cell–Cell Junctions available at www.cshperspectives.org
REFERENCES
- Akhtar N, Hotchin NA. 2001. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell 12: 847–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenstrom P. 2009. Roles of F-BAR/PCH proteins in the regulation of membrane dynamics and actin reorganization. Int Rev Cell Mol Biol 272: 1–31. [DOI] [PubMed] [Google Scholar]
- Balklava Z, Pant S, Fares H, Grant BD. 2007. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol 9: 1066–1073. [DOI] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. 1993. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 120: 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U. 2005. Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J Cell Biol 169: 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocchi C, Vaman Rao M, Zaidel-Bar R. 2012. Regulation of adherens junction dynamics by phosphorylation switches. J Signal Transduct 2012: 125295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JT, Fuller MT, Zallen JA. 2007. The Drosophila homolog of the Exo84 exocyst subunit promotes apical epithelial identity. J Cell Sci 120: 3099–3110. [DOI] [PubMed] [Google Scholar]
- Boehm M, Bonifacino JS. 2001. Adaptins: The final recount. Mol Biol Cell 12: 2907–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogard N, Lan L, Xu J, Cohen RS. 2007. Rab11 maintains connections between germline stem cells and niche cells in the Drosophila ovary. Development 134: 3413–3418. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS. 2014. Adaptor proteins involved in polarized sorting. J Cell Biol 204: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer B, Roche S, Denoyelle M, Thiery JP. 1997. Src and Ras are involved in separate pathways in epithelial cell scattering. EMBO J 16: 5904–5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. 1997. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J Cell Biol 137: 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Del Maschio A, Machesky L, Dejana E. 1999. Regulation of cadherin function by Rho and Rac: Modulation by junction maturation and cellular context. Mol Biol Cell 10: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Kerr MC, Hammond LA, Joseph SR, Mostov KE, Teasdale RD, Stow JL. 2007. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J Cell Sci 120: 1818–1828. [DOI] [PubMed] [Google Scholar]
- Bulgakova NA, Brown NH. 2016. Drosophila p120–catenin is crucial for endocytosis of the dynamic E-cadherin-Bazooka complex. J Cell Sci 129: 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess J, Jauregui M, Tan J, Rollins J, Lallet S, Leventis PA, Boulianne GL, Chang HC, Le Borgne R, Kramer H, et al. 2011. AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila. Mol Biol Cell 22: 2094–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti E, Cabodi S, Stein PL, Hatzfeld M, Kedersha N, Paolo Dotto G. 1998. Tyrosine phosphorylation and src family kinases control keratinocyte cell–cell adhesion. J Cell Biol 141: 1449–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan RH, Rokas A, Gaucher EA, Reynolds AB. 2010. The molecular evolution of the p120–catenin subfamily and its functional associations. PLoS ONE 5: e15747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M, Rauzi M, Lenne PF, Lecuit T. 2008. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453: 751–756. [DOI] [PubMed] [Google Scholar]
- Chen YT, Stewart DB, Nelson WJ. 1999. Coupling assembly of the E-cadherin/β-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J Cell Biol 144: 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Aardema J, Corey SJ. 2013. Biochemical and functional significance of F-BAR domain proteins interaction with WASP/N-WASP. Semin Cell Dev Biol 24: 280–286. [DOI] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S. 2005. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell 9: 805–817. [DOI] [PubMed] [Google Scholar]
- Coopman P, Djiane A. 2016. Adherens Junction and E-Cadherin complex regulation by epithelial polarity. Cell Mol Life Sci 73: 3535–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ. 2008. Endosomal sorting and signalling: An emerging role for sorting nexins. Nat Rev Mol Cell Biol 9: 574–582. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. 1997. Tyrosine phosphorylation and cadherin/catenin function. BioEssays 19: 883–891. [DOI] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. 2003. A core function for p120–catenin in cadherin turnover. J Cell Biol 163: 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Reynolds AB. 2006. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120–catenin in the mouse salivary gland. Dev Cell 10: 21–31. [DOI] [PubMed] [Google Scholar]
- de Beco S, Gueudry C, Amblard F, Coscoy S. 2009. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc Natl Acad Sci 106: 7010–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Madrid BH, Greenberg L, Hatini V. 2015. RhoGAP68F controls transport of adhesion proteins in Rab4 endosomes to modulate epithelial morphogenesis of Drosophila leg discs. Dev Biol 399: 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye C, Miserey-Lenkei S, Montagnac G, Gilles-Marsens F, Paul-Gilloteaux P, Giordano F, Waharte F, Marks MS, Goud B, Raposo G. 2014. Recycling endosome tubule morphogenesis from sorting endosomes requires the kinesin motor KIF13A. Cell Rep 6: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, Le HT, Stow JL. 2008. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am J Physiol Cell Physiol 295: C545–C556. [DOI] [PubMed] [Google Scholar]
- Dow LE, Kauffman JS, Caddy J, Zarbalis K, Peterson AS, Jane SM, Russell SM, Humbert PO. 2007. The tumour-suppressor scribble dictates cell polarity during directed epithelial migration: Regulation of Rho GTPase recruitment to the leading edge. Oncogene 26: 5692–5692. [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. 2005. α-catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell 123: 903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Hartig SM, Bechill JE, Blanchard EG, Caudell E, Corey SJ. 2010. The Cdc42-interacting protein-4 (CIP4) gene knock-out mouse reveals delayed and decreased endocytosis. J Biol Chem 285: 4348–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotho A, Melchior F. 2013. Sumoylation: A regulatory protein modification in health and disease. Annu Rev Biochem 82: 357–385. [DOI] [PubMed] [Google Scholar]
- Folsch H. 2015. Role of the epithelial cell-specific clathrin adaptor complex AP-1B in cell polarity. Cell Logist 5: e1074331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H, Pypaert M, Maday S, Pelletier L, Mellman I. 2003. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol 163: 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster D, Luschnig S. 2012. Src42A-dependent polarized cell shape changes mediate epithelial tube elongation in Drosophila. Nat Cell Biol 14: 526–534. [DOI] [PubMed] [Google Scholar]
- Fricke R, Gohl C, Dharmalingam E, Grevelhorster A, Zahedi B, Harden N, Kessels M, Qualmann B, Bogdan S. 2009. Drosophila Cip4/Toca-1 integrates membrane trafficking and actin dynamics through WASP and SCAR/WAVE. Curr Biol 19: 1429–1437. [DOI] [PubMed] [Google Scholar]
- Fricke R, Gohl C, Bogdan S. 2010. The F-BAR protein family Actin’ on the membrane. Commun Integr Biol 3: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. 2002. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 4: 222–231. [DOI] [PubMed] [Google Scholar]
- Gariano G, Guarienti M, Bresciani R, Borsani G, Carola G, Monti E, Giuliani R, Rezzani R, Bonomini F, Preti A, et al. 2014. Analysis of three µ1-AP1 subunits during zebrafish development. Dev Dyn 243: 299–314. [DOI] [PubMed] [Google Scholar]
- Gates J, Peifer M. 2005. Can 1000 reviews be wrong? Actin, α-Catenin, and adherens junctions. Cell 123: 769–772. [DOI] [PubMed] [Google Scholar]
- Georgiou M, Marinari E, Burden J, Baum B. 2008. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol 18: 1631–1638. [DOI] [PubMed] [Google Scholar]
- Gillard G, Shafaq-Zadah M, Nicolle O, Damaj R, Pecreaux J, Michaux G. 2015. Control of E-cadherin apical localisation and morphogenesis by a SOAP-1/AP-1/clathrin pathway in C. elegans epidermal cells. Development 142: 1684–1694. [DOI] [PubMed] [Google Scholar]
- Giuliani C, Troglio F, Bai Z, Patel FB, Zucconi A, Malabarba MG, Disanza A, Stradal TB, Cassata G, Confalonieri S, et al. 2009. Requirements for F-BAR proteins TOCA-1 and TOCA-2 in actin dynamics and membrane trafficking during Caenorhabditis elegans oocyte growth and embryonic epidermal morphogenesis. PLoS Genet 5: e1000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. 2007. The PAR proteins: Fundamental players in animal cell polarization. Dev Cell 13: 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta D, Carvajal-Gonzalez JM, Mattera R, Deborde S, Banfelder JR, Bonifacino JS, Rodriguez-Boulan E. 2012. The clathrin adaptor AP-1A mediates basolateral polarity. Dev Cell 22: 811–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. 1998. Sec6/8 complex is recruited to cell–cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93: 731–740. [DOI] [PubMed] [Google Scholar]
- Guo Z, Neilson LJ, Zhong H, Murray PS, Zanivan S, Zaidel-Bar R. 2014. The F-BAR protein family Actin’ on the membrane. Commun Integr Biol 3: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund K, Nezis IP, Stenmark H. 2011. Structure and functions of stable intercellular bridges formed by incomplete cytokinesis during development. Commun Integr Biol 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ. 2012. Competitive regulation of E-cadherin juxtamembrane domain degradation by p120–catenin binding and Hakai-mediated ubiquitination. PLoS ONE 7: e37476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase K, Nakatsu F, Ohmae M, Sugihara K, Shioda N, Takahashi D, Obata Y, Furusawa Y, Fujimura Y, Yamashita T, et al. 2013. AP-1B-mediated protein sorting regulates polarity and proliferation of intestinal epithelial cells in mice. Gastroenterology 145: 625–635. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Insall RH. 2008. F-BAR domains: Multifunctional regulators of membrane curvature. J Cell Sci 121: 1951–1954. [DOI] [PubMed] [Google Scholar]
- Ho HY, Rohatgi R, Lebensohn AM, Le M, Li J, Gygi SP, Kirschner MW. 2004. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell 118: 203–216. [DOI] [PubMed] [Google Scholar]
- Hunt SD, Townley AK, Danson CM, Cullen PJ, Stephens DJ. 2013. Microtubule motors mediate endosomal sorting by maintaining functional domain organization. J Cell Sci 126: 2493–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MV, Lee DM, Harris TJ, Fernandez-Gonzalez R. 2015. Polarized E-cadherin endocytosis directs actomyosin remodeling during embryonic wound repair. J Cell Biol 210: 801–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaido M, Wada H, Shindo M, Hayashi S. 2009. Essential requirement for RING finger E3 ubiquitin ligase Hakai in early embryonic development of Drosophila. Genes Cells 14: 1067–1077. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG. 2006. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 7: 404–414. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J, Schmid E, Franke WW, Geiger B. 1982. Different modes of internalization of proteins associated with adhaerens junctions and desmosomes: Experimental separation of lateral contacts induces endocytosis of desmosomal plaque material. EMBO J 1: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J, Schmelz M, Franke WW, Geiger B. 1991. Endocytosis of junctional cadherins in bovine kidney epithelial (MDBK) cells cultured in low Ca2+ ion medium. J Cell Biol 113: 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BT, Owen DJ. 2011. Endocytic sorting of transmembrane protein cargo. Curr Opin Cell Biol 23: 404–412. [DOI] [PubMed] [Google Scholar]
- Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. 2000. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 404: 151–158. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Owen D, Harrison SC. 2014. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb Perspect Biol 6: a016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. 2002. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol 12: 379–382. [DOI] [PubMed] [Google Scholar]
- Kowalczyk AP, Nanes BA. 2012. Adherens junction turnover: Regulating adhesion through cadherin endocytosis, degradation, and recycling. Subcell Biochem 60: 197–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y. 2005. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev Cell 9: 365–376. [DOI] [PubMed] [Google Scholar]
- Langton PF, Colombani J, Chan EH, Wepf A, Gstaiger M, Tapon N. 2009. The dASPP-dRASSF8 complex regulates cell–cell adhesion during Drosophila retinal morphogenesis. Curr Biol 19: 1969–1978. [DOI] [PubMed] [Google Scholar]
- Le Droguen PM, Claret S, Guichet A, Brodu V. 2015. Microtubule-dependent apical restriction of recycling endosomes sustains adherens junctions during morphogenesis of the Drosophila tracheal system. Development 142: 363–374. [DOI] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. 1999. Recycling of E-cadherin: A potential mechanism for regulating cadherin dynamics. J Cell Biol 146: 219–232. [PMC free article] [PubMed] [Google Scholar]
- Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. 2008. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol 18: 1639–1648. [DOI] [PubMed] [Google Scholar]
- Levayer R, Pelissier-Monier A, Lecuit T. 2011. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat Cell Biol 13: 529–540. [DOI] [PubMed] [Google Scholar]
- Lilien J, Balsamo J. 2005. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of β-catenin. Curr Opin Cell Biol 17: 459–465. [DOI] [PubMed] [Google Scholar]
- Ling K, Bairstow SF, Carbonara C, Turbin DA, Huntsman DG, Anderson RA. 2007. Type I γ phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with µ1B adaptin. J Cell Biol 176: 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liwosz A, Lei TL, Kukuruzinska MA. 2006. N-glycosylation affects the molecular organization and stability of E-cadherin junctions. J Biol Chem 281: 23138–23149. [DOI] [PubMed] [Google Scholar]
- Lock JG, Stow JL. 2005. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell 16: 1744–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohia M, Qin Y, Macara IG. 2012. The Scribble polarity protein stabilizes E-cadherin/p120-catenin binding and blocks retrieval of E-cadherin to the Golgi. PLoS ONE 7: e51130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommel M, Winterhalter PR, Willer T, Dahlhoff M, Schneider MR, Bartels MF, Renner-Muller I, Ruppert T, Wolf E, Strahl S. 2013. Protein O-mannosylation is crucial for E-cadherin-mediated cell adhesion. Proc Natl Acad Sci 110: 21024–21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyer N, Kolotuev I, Pinot M, Le Borgne R. 2015. Drosophila E-cadherin is required for the maintenance of ring canals anchoring to mechanically withstand tissue growth. Proc Natl Acad Sci 112: 12717–12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Ghosh S, Wang Z, Hunter T. 2003. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell 4: 499–515. [DOI] [PubMed] [Google Scholar]
- Maher PA, Pasquale EB, Wang JYJ, Singer SJ. 1985. Phosphotyrosine-containing proteins are concentrated in focal adhesions and intercellular-junctions in normal-cells. Proc Natl Acad Sci 82: 6576–6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateus AM, Gorfinkiel N, Schamberg S, Martinez Arias A. 2011. Endocytic and recycling endosomes modulate cell shape changes and tissue behaviour during morphogenesis in Drosophila. PLoS ONE 6: e18729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R, Boehm M, Chaudhuri R, Prabhu Y, Bonifacino JS. 2011. Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J Biol Chem 286: 2022–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea PD, Gumbiner BM. 1991. Purification of a 92-kDa cytoplasmic protein tightly associated with the cell–cell adhesion molecule E-cadherin (uvomorulin). Characterization and extractability of the protein complex from the cell cytostructure. J Biol Chem 266: 4514–4520. [PubMed] [Google Scholar]
- McLachlan RW, Kraemer A, Helwani FM, Kovacs EM, Yap AS. 2007. E-cadherin adhesion activates c-Src signaling at cell–cell contacts. Mol Biol Cell 18: 3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Mushika Y, Ichii T, Takeichi M. 2008. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell–cell contacts. Cell 135: 948–959. [DOI] [PubMed] [Google Scholar]
- Merrifield CJ, Kaksonen M. 2014. Endocytic accessory factors and regulation of clathrin-mediated endocytosis. Cold Spring Harb Perspect Biol 6: a016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael M, Yap AS. 2013. The regulation and functional impact of actin assembly at cadherin cell–cell adhesions. Semin Cell Dev Biol 24: 298–307. [DOI] [PubMed] [Google Scholar]
- Miranda KC, Khromykh T, Christy P, Le TL, Gottardi CJ, Yap AS, Stow JL, Teasdale RD. 2001. A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem 276: 22565–22572. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Ozawa M. 2007a. A dileucine motif in its cytoplasmic domain directs β-catenin-uncoupled E-cadherin to the lysosome. J Cell Sci 120: 4395–4406. [DOI] [PubMed] [Google Scholar]
- Miyashita Y, Ozawa M. 2007b. Increased internalization of p120–uncoupled E-cadherin and a requirement for a dileucine motif in the cytoplasmic domain for endocytosis of the protein. J Biol Chem 282: 11540–11548. [DOI] [PubMed] [Google Scholar]
- Mork L, Tang H, Batchvarov I, Capel B. 2012. Mouse germ cell clusters form by aggregation as well as clonal divisions. Mech Develop 128: 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K, Su T, ter Beest M. 2003. Polarized epithelial membrane traffic: Conservation and plasticity. Nat Cell Biol 5: 287–293. [DOI] [PubMed] [Google Scholar]
- Murthy M, Schwarz TL. 2004. The exocyst component Sec5 is required for membrane traffic and polarity in the Drosophila ovary. Development 131: 377–388. [DOI] [PubMed] [Google Scholar]
- Murthy M, Ranjan R, Denef N, Higashi ME, Schupbach T, Schwarz TL. 2005. Sec6 mutations and the Drosophila exocyst complex. J Cell Sci 118: 1139–1150. [DOI] [PubMed] [Google Scholar]
- Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M. 2003. Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J Cell Biol 160: 433–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J, Zallen JA. 2011. Elaborating polarity: PAR proteins and the cytoskeleton. Development 138: 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA, Kowalczyk AP. 2012. p120–catenin binding masks an endocytic signal conserved in classical cadherins. J Cell Biol 199: 365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le Bivic A, et al. 2005. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene 24: 4330–4339. [DOI] [PubMed] [Google Scholar]
- Owens DW, McLean GW, Wyke AW, Paraskeva C, Parkinson EK, Frame MC, Brunton VG. 2000. The catalytic activity of the Src family kinases is required to disrupt cadherin-dependent cell–cell contacts. Mol Biol Cell 11: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios F, Schweitzer JK, Boshans RL, D’Souza-Schorey C. 2002. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol 4: 929–936. [DOI] [PubMed] [Google Scholar]
- Palacios F, Tushir JS, Fujita Y, D’Souza-Schorey C. 2005. Lysosomal targeting of E-cadherin: A unique mechanism for the down-regulation of cell–cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol 25: 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS. 2003. Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: The initial fate of unbound E-cadherin. J Biol Chem 278: 21050–21057. [DOI] [PubMed] [Google Scholar]
- Pepling ME, de Cuevas M, Spradling AC. 1999. Germline cysts: A conserved phase of germ cell development? Trends Cell Biol 9: 257–262. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. 2012. Guilt by association: What p120–catenin has to hide. J Cell Biol 199: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. 2006. p120–catenin mediates inflammatory responses in the skin. Cell 124: 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J. 2003. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol 162: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. 2013. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol 25: 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho SS, Seruca R, Gartner F, Yamaguchi Y, Gu J, Taniguchi N, Reis CA. 2011. Modulation of E-cadherin function and dysfunction by N-glycosylation. Cell Mol Life Sci 68: 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirraglia C, Walters J, Myat MM. 2010. Pak1 control of E-cadherin endocytosis regulates salivary gland lumen size and shape. Development 137: 4177–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Beltzner CC. 2002. Structure and function of the Arp2/3 complex. Curr Opin Struct Biol 12: 768–774. [DOI] [PubMed] [Google Scholar]
- Pollitt AY, Insall RH. 2009. WASP and SCAR/WAVE proteins: The drivers of actin assembly. J Cell Sci 122: 2575–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Capaldo C, Gumbiner BM, Macara IG. 2005. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol 171: 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeth JF, Sawyer JK, Wilner DA, Peifer M. 2009. Rab11 helps maintain apical crumbs and adherens junctions in the Drosophila embryonic ectoderm. PLoS ONE 4: e7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Ho HY, Kirschner MW. 2000. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol 150: 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland Y, Marighetti P, Malinverno C, Confalonieri S, Luise C, Ducano N, Palamidessi A, Bisi S, Kajiho H, Troglio F, et al. 2014. The CDC42-interacting protein 4 controls epithelial cell cohesion and tumor dissemination. Dev Cell 30: 553–568. [DOI] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. 1999. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem 274: 36734–36740. [DOI] [PubMed] [Google Scholar]
- Santiago-Tirado FH, Bretscher A. 2011. Membrane-trafficking sorting hubs: Cooperation between PI4P and small GTPases at the trans-Golgi network. Trends Cell Biol 21: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Watanabe T, Wang S, Kakeno M, Matsuzawa K, Matsui T, Yokoi K, Murase K, Sugiyama I, Ozawa M, et al. 2011. Numb controls E-cadherin endocytosis through p120 catenin with aPKC. Mol Biol Cell 22: 3103–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL, Frolov VA. 2011. Dynamin: Functional design of a membrane fission catalyst. Annu Rev Cell Dev Biol 27: 79–105. [DOI] [PubMed] [Google Scholar]
- Scott CC, Vacca F, Gruenberg J. 2014. Endosome maturation, transport and functions. Semin Cell Dev Biol 31: 2–10. [DOI] [PubMed] [Google Scholar]
- Shafaq-Zadah M, Brocard L, Solari F, Michaux G. 2012. AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development 139: 2061–2070. [DOI] [PubMed] [Google Scholar]
- Shim J, Sternberg PW, Lee JH. 2000. Distinct and redundant functions of µ1 medium chains of the AP-1 clathrin-associated protein complex in the nematode Caenorhabditis elegans. Mol Biol Cell 11: 2743–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo M, Wada H, Kaido M, Tateno M, Aigaki T, Tsuda L, Hayashi S. 2008. Dual function of Src in the maintenance of adherens junctions during tracheal epithelial morphogenesis. Development 135: 1355–1364. [DOI] [PubMed] [Google Scholar]
- Solis GP, Hulsbusch N, Radon Y, Katanaev VL, Plattner H, Stuermer CAO. 2013. Reggies/flotillins interact with Rab11a and SNX4 at the tubulovesicular recycling compartment and function in transferrin receptor and E-cadherin trafficking. Mol Biol Cell 24: 2689–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Eckerle S, Onichtchouk D, Marrs JA, Nitschke R, Driever W. 2013. Pou5f1-dependent EGF expression controls E-cadherin endocytosis, cell adhesion, and zebrafish epiboly movements. Dev Cell 24: 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. 2009. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Bio 10: 513–525. [DOI] [PubMed] [Google Scholar]
- Symons M, Derry JMJ, Karlak B, Jiang S, Lemahieu V, McCormick F, Francke U, Abo A. 1996. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell 84: 723–734. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Takahashi F, Ui-Tei K, Kojima T, Saigo K. 2005. Requirements of genetic interactions between Src42A, armadillo and shotgun, a gene encoding E-cadherin, for normal development in Drosophila. Development 132: 2547–2559. [DOI] [PubMed] [Google Scholar]
- Takahashi D, Hase K, Kimura S, Nakatsu F, Ohmae M, Mandai Y, Sato T, Date Y, Ebisawa M, Kato T, et al. 2011. The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology 141: 621–632. [DOI] [PubMed] [Google Scholar]
- Takano K, Toyooka K, Suetsugu S. 2008. EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization. EMBO J 27: 2817–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. 2014. Dynamic contacts: Rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Bio 15: 397–410. [DOI] [PubMed] [Google Scholar]
- Tan JL, Oh KR, Burgess J, Hipfner DR, Brill JA. 2014. PI4KIII α is required for cortical integrity and cell polarity during Drosophila oogenesis. J Cell Sci 127: 954–966. [DOI] [PubMed] [Google Scholar]
- Tepass U. 2012. The apical polarity protein network in Drosophila epithelial cells: Regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu Rev Cell Dev Biol 28: 655–685. [DOI] [PubMed] [Google Scholar]
- Tepass U, Knust E. 1990. Phenotypic and developmental analysis of mutations at the Crumbs-Locus, a gene required for the development of epithelia in Drosophila-Melanogaster. Roux Arch Dev Biol 199: 189–206. [DOI] [PubMed] [Google Scholar]
- Tepass U, Knust E. 1993. Crumbs and Stardust act in a genetic pathway that controls the organization of epithelia in Drosophila-Melanogaster. Dev Biol 159: 311–326. [DOI] [PubMed] [Google Scholar]
- Toret CP, D’Ambrosio MV, Vale RD, Simon MA, Nelson WJ. 2014. A genome-wide screen identifies conserved protein hubs required for cadherin-mediated cell–cell adhesion. J Cell Biol 204: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM. 2003. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol 163: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM. 2009. Tickets to ride: Selecting cargo for clathrin-regulated internalization. Nature reviews Molecular cell biology 10: 583–596. [DOI] [PubMed] [Google Scholar]
- Troxell ML, Chen YT, Cobb N, Nelson WJ, Marrs JA. 1999. Cadherin function in junctional complex rearrangement and posttranslational control of cadherin expression. Am J Physiol 276: C404–C418. [DOI] [PubMed] [Google Scholar]
- Tsujita K, Suetsugu S, Sasaki N, Furutani M, Oikawa T, Takenawa T. 2006. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol 172: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsur A, Bening Abu-Shach U, Broday L. 2015. ULP-2 SUMO protease regulates E-cadherin recruitment to adherens junctions. Dev Cell 35: 63–77. [DOI] [PubMed] [Google Scholar]
- Van Itallie C, Tietgens A, Aponte A, Fredriksson K, Fanning A, Gucek M, Anderson J. 2014a. Biotin ligase tagging identifies E-cadherin protein neighbors, including lipoma preferred partner, which modulates epithelial cell–cell and cell–substrate adhesion. FASEB J 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie CM, Tietgens AJ, Aponte A, Fredriksson K, Fanning AS, Gucek M, Anderson JM. 2014b. Biotin ligase tagging identifies proteins proximal to E-cadherin, including lipoma preferred partner, a regulator of epithelial cell–cell and cell–substrate adhesion. J Cell Sci 127: 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weering JR, Cullen PJ. 2014. Membrane-associated cargo recycling by tubule-based endosomal sorting. Semin Cell Dev Biol 31: 40–47. [DOI] [PubMed] [Google Scholar]
- Vester-Christensen MB, Halim A, Joshi HJ, Steentoft C, Bennett EP, Levery SB, Vakhrushev SY, Clausen H. 2013. Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc Natl Acad Sci 110: 21018–21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan P, Wang D, Luo J, Chu D, Wang H, Zhang L, Chen J. 2013. Guidance receptor promotes the asymmetric distribution of exocyst and recycling endosome during collective cell migration. Development 140: 4797–4806. [DOI] [PubMed] [Google Scholar]
- Wandinger-Ness A, Zerial M. 2014. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 6: a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Bellen HJ. 2015. The retromer complex in development and disease. Development 142: 2392–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. 2003. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114: 299–310. [DOI] [PubMed] [Google Scholar]
- Warner SJ, Longmore GD. 2009. Cdc42 antagonizes Rho1 activity at adherens junctions to limit epithelial cell apical tension. J Cell Biol 187: 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington SJ, Strutt H, Strutt D. 2013. The Frizzled-dependent planar polarity pathway locally promotes E-cadherin turnover via recruitment of RhoGEF2. Development 140: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woichansky I, Beretta CA, Berns N, Riechmann V. 2016. Three mechanisms control E-cadherin localization to the zonula adherens. Nat Commun 7: 10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Guo W. 2015. The Exocyst at a Glance. J Cell Sci 128: 2957–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SK, Gomez GA, Michael M, Verma S, Cox HL, Lefevre JG, Parton RG, Hamilton NA, Neufeld Z, Yap AS. 2014. Cortical F-actin stabilization generates apical-lateral patterns of junctional contractility that integrate cells into epithelia. Nat Cell Biol 16: 167–178. [DOI] [PubMed] [Google Scholar]
- Xiao K, Oas RG, Chiasson CM, Kowalczyk AP. 2007. Role of p120–catenin in cadherin trafficking. Biochim Biophys Acta 1773: 8–16. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. 2005. Deconstructing the cadherin-catenin-actin complex. Cell 123: 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DH, Cai KQ, Roland IH, Smith ER, Xu XX. 2007. Disabled-2 is an epithelial surface positioning gene. J Biol Chem 282: 13114–13122. [DOI] [PubMed] [Google Scholar]
- Yap AS, Gomez GA, Parton RG. 2015. Adherens junctions revisualized: Organizing cadherins as nanoassemblies. Dev Cell 35: 12–20. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. 2004. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J Cell Sci 117: 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kim A, Abraham N, Khan LA, Hall DH, Fleming JT, Gobel V. 2012. Clathrin and AP-1 regulate apical polarity and lumen formation during C. elegans tubulogenesis. Development 139: 2071–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kong D, Reichl L, Vogt N, Wolf F, Grosshans J. 2014. The glucosyltransferase Xiantuan of the endoplasmic reticulum specifically affects E-Cadherin expression and is required for gastrulation movements in Drosophila. Dev Biol 390: 208–220. [DOI] [PubMed] [Google Scholar]
- Zhao HB, Liang YL, Xu ZB, Wang LY, Zhou F, Li ZX, Jin JW, Yang Y, Fang ZY, Hu YL, et al. 2008. N-glycosylation affects the adhesive function of E-Cadherin through modifying the composition of adherens junctions (Ajs) in human breast carcinoma cell Line MDA-MB-435. J Cell Biochem 104: 162–175. [DOI] [PubMed] [Google Scholar]
- Zhou F, Su JM, Fu L, Yang Y, Zhang LN, Wang LY, Zhao HB, Zhang DC, Li ZX, Zha XL. 2008. Unglycosylation at Asn-633 made extracellular domain of E-cadherin folded incorrectly and arrested in endoplasmic reticulum, then sequentially degraded by ERAD. Glycoconjugate J 25: 727–740. [DOI] [PubMed] [Google Scholar]
- Zizioli D, Forlanelli E, Guarienti M, Nicoli S, Fanzani A, Bresciani R, Borsani G, Preti A, Cotelli F, Schu P. 2010. Characterization of the AP-1 µ1A and µ1B adaptins in zebrafish (Danio rerio). Dev Dyn 239: 2404–2412. [DOI] [PubMed] [Google Scholar]
- Zobel T, Brinkmann K, Koch N, Schneider K, Seemann E, Fleige A, Qualmann B, Kessels MM, Bogdan S. 2015. Cooperative functions of the two F-BAR proteins Cip4 and Nostrin in the regulation of E-cadherin in epithelial morphogenesis. J Cell Sci 128: 499–515. [DOI] [PubMed] [Google Scholar]