Abstract
Interleukin-6 (IL-6) is a pleiotropic cytokine with both pro- and anti-inflammatory properties which acts directly on cancer cells to promote their survival and proliferation. Elevated serum IL-6 levels negatively correlate with survival of cancer patients, which is generally attributed to the direct effects of IL-6 on cancer cells. How IL-6 modulates the host immune response in cancer patients is unclear. Here we show the IL-6 signaling response in peripheral blood T cells is impaired in breast cancer (BC) patients and is associated with blunted Th17 differentiation. The mechanism identified involved downregulation of gp130 and IL-6Rα in BC patients and was independent of plasma IL-6 levels. Importantly, defective IL-6 signaling in peripheral blood T cells at diagnosis correlated with worse relapse-free survival (RFS). These results indicate that intact IL-6 signaling in T cells is important for controlling cancer progression. Furthermore, they highlight a potential for IL-6 signaling response in peripheral blood T cells at diagnosis as a predictive biomarker for clinical outcome of BC patients.
Keywords: IL-6, STAT, gp130, prognostic, breast cancer
Introduction
Interleukin-6 (IL-6) is a pleiotropic cytokine that plays various roles on modulating the activities of tumor and immune cells (1). IL-6 signals through the common gp130 receptor and the specific IL-6Rα co-receptor to activate the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway (2). Phosphorylated STATs dimerize and translocate into the nucleus to initiate transcription of IL-6 responsive genes (3).
Within the tumor microenvironment, not only macrophages, myeloid-derived suppressor cells (MDSC) and fibroblasts but also cancer cells produce IL-6 (4). IL-6 promotes survival and proliferation of cancer cells, drives chronic inflammation that supports tumor growth, and suppresses anti-tumor T cell activity (4-8). On T cells, IL-6 functions to prevent apoptosis (9,10) and skews naïve CD4+ T cells away from becoming regulatory T (Treg) cells and towards becoming pro-inflammatory Th17 cells (11). IL-6 also regulates chemokine receptors expression to influence tissue recruitment of T cells (12). Higher frequencies of Treg and exclusion of cytotoxic T cells from the tumor are both associated with poorer outcomes in cancer patients (13,14). Thus, alterations in the response of T cells to IL-6 may contribute to deficient anti-tumor responses. However, the mechanisms connecting IL-6 associated inflammation to dysfunctional anti-tumor immune responses have yet to be fully elucidated. Alterations in cytokine levels and the ability of immune cells to appropriately respond to cytokines are likely to contribute to immunologic abnormalities in cancer patients. The prominent association of IL-6 with both inflammation and cancer argues that this pleiotropic immunomodulatory cytokine might serve as a link between cancer-associated inflammation and immune dysfunction
In this regard, we investigated the functionality of IL-6 signaling responses in peripheral blood T cells of breast cancer (BC) patients. By using phophoflow cytometry, we found that IL-6 induced phosphorylation of STAT1 and STAT3 were significantly lower in peripheral blood CD4+ naïve T cells from BC patients at diagnosis. To explore the mechanisms underlying defective responses of patient T cells to IL-6, expression levels of key components of the IL-6 signaling pathway were evaluated. BC patients had substantially decreased levels of the IL-6 co-receptors, gp130 and IL-6Rα, which further correlated with decreased responsiveness to IL-6. Interestingly, IL-6 plasma levels were not elevated in BC patients at diagnosis and IL-6 signaling responses were independent of the IL-6 plasma levels. We also found that defective IL-6 responses were associated with blunted Th17 differentiation from CD4+ naïve T cells. More importantly, defective IL-6 signaling response significantly correlated with worse relapse-free survival (RFS), indicating the potential of IL-6 signaling response in peripheral blood T cells at diagnosis as a prognostic biomarker for BC patients.
Materials and Methods
Patients
All patient blood samples were collected prior to surgery or administration of any therapy. Age-matched healthy control peripheral blood samples were obtained from the Stanford Blood Center and City of Hope Blood Donor Center. All the blood from patients and healthy donors was drawn directly into heparin-coated vacutainer tubes (BD Biosciences, San Jose, CA, USA). This study was approved by the Institutional Review Board of Stanford Medical Center and City of Hope Comprehensive Cancer Center.
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were collected by density gradient centrifugation using Ficoll-Paque and cryopreserved in 10% DMSO FBS. Cryopreserved PBMCs were thawed and rested overnight in RPMI-10% heat-inactivated FBS-1× Penicillin-Streptomycin-Glutamine at 37°C, 7.5% CO2 prior to performing assays.
IL-6 stimulation of PBMCs for phosphoflow cytometry
Briefly, 0.5 × 106 PBMCs were aliquoted into individual wells of deep-well 96-well plates. Media or IL-6 (R&D Systems, Minneapolis, MN, USA) was added to each well to obtain a final concentration of 100ng/ml for IL-6. Cells were then incubated at 37°C for 15 minutes followed by fixation with 1.5% paraformaldehyde (PFA) for 10 minutes at room temperature. Cells were washed with PBS to remove PFA, and permeabilized by the addition of 100% methanol. PBMCs were stored at -80°C until antibody staining for flow cytometry analysis.
Flow Cytometry
Permeabilized cells were thawed and washed three times with staining buffer (PBS/2%FBS/0.5%BSA) to remove methanol. Cells were resuspended in the same volume of staining buffer and staining antibodies were added. For assessment of pSTAT1 and pSTAT3, the following staining panel was utilized: CD3-V450 (UCHT1), CD4-PerCP-Cy5.5 (SK.3), CD45RA-PE-Cy7 (L48), CD8-V500 (RPA-T8), CD16-PE (3G8), CD20-PerCP-Cy5.5 (H1), CD33-PE-Cy7 (P67.6), pSTAT1 (pY701)-AF647 (4a), and pSTAT3 (pY705)-AF488 (4/P-STAT3) antibodies (BD Biosciences, San Jose, CA, USA). Naïve CD4+ T cell population was determined by the following markers: CD3+CD4+CD45RA+. The magnitude of each individual's pSTAT1 and pSTAT3 response to IL-6 was expressed as the IL-6 induced median fluorescence intensity (MFI) minus the unstimulated MFI for pSTAT1 and pSTAT3.
For assessment of intracellular total STAT1 and STAT3 levels, LIVE/DEAD Fixable Blue Dead Cell Stain (Life Technologies, Carlsbad, CA, USA) was added to unstimulated cells for 30 minutes and washed away prior to PFA fixation and permeabilization. After washing the cells to remove methanol, the following staining panel was utilized: CD3-V500 (UCHT1), CD4-PerCP-Cy5.5 (SK.3), CD8-PacBlue (RPA-T8), CD45RA-PE-Cy7 (L48), CD62L-FITC (DREG-56), STAT1-AF647 (1/Stat1), and STAT3-PE (M59-50) antibodies (BD Biosciences). For assessment of gp130 and IL-6Rα expression levels on live PBMCs, the following staining panel was utilized: CD3-V500 (UCHT1), CD4-PerCP-Cy5.5 (SK.3), CD8-PacBlue (RPA-T8), CD45RA-PE-Cy7 (L48), CD62L-e605NC (DREG-56), gp130-PE (AM64) (BD Biosciences), and IL-6Rα-AF647 (BL-126) (Biolegend, San Diego, CA, USA) antibodies, and LIVE/DEAD Fixable Blue Dead Cell Stain (Life Technologies). Expression of total STAT1, STAT3, gp130 and IL-6Rα was expressed by subtracting the MFI of isotype stains.
Flow cytometry was performed using FACS Canto, LSR II, or Fortessa Flow Cytometers (BD Biosciences). Flow cytometry data was analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA). t tests were used to determine the statistical significance of BC patient with healthy donors (Graphpad Prism, GraphPad Software, LaJolla, CA, USA).
Plasma IL-6 ELISA
All patient plasma samples were collected prior to surgery or administration of any therapy. Plasma samples were kept frozen at -80°C then thawed shortly before determination of IL-6 level. IL-6 levels were determined by high sensitivity ELISA (eBioscience, San Diego, CA, USA) according to manufacturer's protocol.
RNA isolation and Quantitative Real Time PCR
Naïve CD4+ T cells were isolated from PBMCs with an enrichment kit (eBioscience). Total RNA was isolated from naïve CD4+ T cells using RNAzol® RT reagent (Molecular Research Center, Cincinnati, OH) according to the manufacture's instruction. cDNA was synthesized using RT2 First Strand Kit (Qiagen, Valencia, CA). For Quantitative Real Time PCR (Q-PCR), RT2 SYBR Green Master Mix (Qiagen) was used and Q-PCR was performed and analyzed using the CFX96 Real-Time PCR System (Bio-Rad, Hercules, CA). Gene expressions were normalized to GAPDH as an internal control and results are represented as fold change using the ΔΔCt method. The following primer sequences were used in the reaction: Il6r (F: TTGTTTGTGAGTGGGGTCCT; R: TGGGACTCCTGGGAATACTG), Il6st (F: AGGACCAAAGATGCCTCAAC; R: GAATGAAGATCGGGTGGATG), Adam17 (F: ACTCTGAGGACAGTTAACCAAACC; R: AGTAAAAGGAGCCAATACCACAAG).
Th17 differentiation assay
Naïve CD4+ T cells were isolated from PBMCs with an enrichment kit (eBioscience). Cells were cultured in serum-free medium with anti-CD3/CD28 beads (Life Technologies), IL-6 (30ng/ml), IL-1β (20ng/ml) (eBioscience), IL-23 (30ng/ml), TGF-β (2.25ng/ml) (Peprotech, Rocky Hill, NJ, USA), anti-IFN-γ antibody (1μg/ml) and anti-IL-4 antibody (2.5μg/ml) (Biolegend) for 7 days. Supernatants were collected after 7 days and IL-17 levels were determined by ELISA (Biolegend). Cells were stimulated with ionomycin (1μg/ml) (Life Technologies), Phorbol 12-myristate 13-acetate (PMA) (50ng/ml) (Sigma-Aldrich, St Louis, MO, USA) and Brefeldin A (BFA) (5μg/ml) (Biolegend) for 5 hrs and were analyzed by flow cytometry with anti-RORγt-AF647 (Q21-559) (BD Bioscience) and anti-IL-17A-FITC (BL168) (Biolegend) antibodies.
Statistical Analysis
Relapse-free survival (RFS) was defined as the time from the date of diagnosis of BC to the date of cancer recurrence. Kaplan-Meier method with log-rank test was used to determine IL-6 signaling responsiveness as prognostic factors for RFS of BC patients. Multivariate Cox regression model analysis was performed to determine independence of prognostic factor. The correlation between IL-6 signaling response and clinicopathologic characteristics were evaluated with Pearson's correlation coefficient presented with r and p value. All tests with p value<0.05 were considered statistically significant.
Results
Defective IL-6 signaling responses in peripheral CD4+ T cells from BC patients
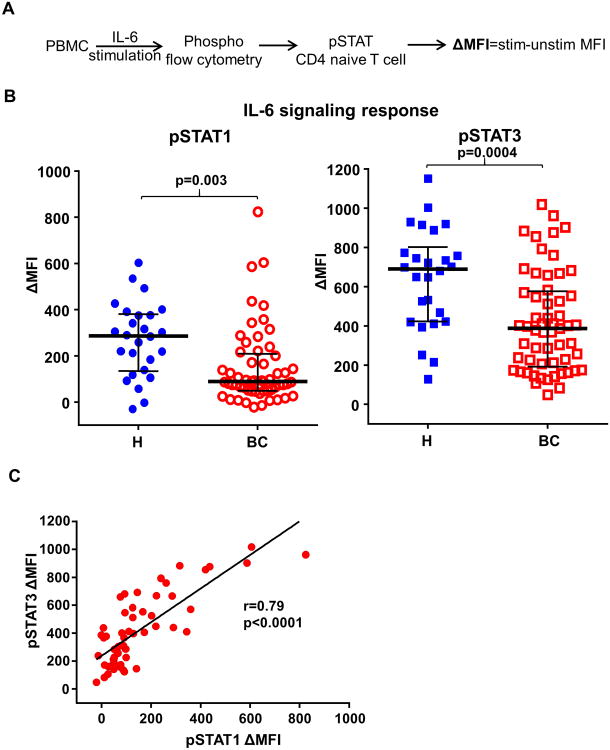
To investigate IL-6 immune biology in BC patients, we analyzed the responsiveness of peripheral blood immune cells to IL-6 in BC patients and age-matched healthy donors. Clinical and pathological characteristics of the BC patients are summarized in Table 1. Peripheral blood mononuclear cells (PBMCs) from BC patients and healthy donors were stimulated with IL-6 and phosphorylation of STAT1 and STAT3 (pSTATs) were determined by phosphoflow cytometry (15). IL-6 signaling response (ΔMFI) was represented by IL-6 stimulated minus unstimulated pSTATs median fluorescence intensity (MFI) (Fig. 1A). We examined IL-6 signaling response in T cells (CD3+), B cells (CD20+), NK cells (CD16+) and myeloid cells (CD33+) and found that IL-6 induced phosphorylation of STAT1 (p=0.003) and STAT3 (p=0.0004) in naïve CD4+ T cells from BC patients (n=57) was significantly lower than that in healthy donors (n=26) (Fig. 1B). To determine whether the observed lower IL-6 signaling response was due to reduced total available STATs, we compared the levels of total STAT1 and STAT3 in naïve CD4+ T cells by flow cytometry and found similar levels of total STAT1 and STAT3 between BC patients and healthy donors (Fig. S1A). In addition, we found similar levels of basal pSTAT1 and pSTAT3 in naïve CD4+ T cells between BC patients and healthy donors (Fig. S1B). In cancer cells, STAT1 and STAT3 are considered to play opposing roles in tumorigenesis where STAT3 is tumor-promoting and STAT1 is tumor-inhibiting (16). In contrast, we found that IL-6 induced phosphorylation of STAT1 and STAT3 are highly correlated in T cells (Fig. 1C), indicating that the IL-6-STAT pathway is coordinately dysfunctional in BC patients.
Table 1. Patient characteristics.
| Characteristics | Patients (N=57) |
|---|---|
| Age—yr | |
|
| |
| Median | 51 |
| Range | 27-85 |
|
| |
| Tumor stage— no.(%) | |
|
| |
| DCIS | 7 (123) |
| T1 | 23 (40.4) |
| T2 | 15 (26.3) |
| T3 | 8 (14.0) |
| Unknown | 4 (7.0) |
|
| |
| Grade— no.(%) | |
|
| |
| G1 | 7 (12.3) |
| G2 | 22 (33.6) |
| G3 | 28 (49.1) |
|
| |
| Nodal status—no.(%) | |
|
| |
| N0 | 29 (50.9) |
| N1-3 | 24 (42.1) |
| Unknown | 4 (7.0) |
|
| |
| Subtype— no.(%) | |
|
| |
| Luminal | 45 (79.0) |
| HER2 | 6 (10.5) |
| Triple negative | 6 (10.5) |
Fig. 1. IL-6 signaling responses are impaired in peripheral naïve CD4+ T cells from BC patients.
(A) Schematic representation of the experimental overview. Peripheral blood mononuclear cells (PBMCs) obtained from BC patients (BC) and from healthy donors (H) were stimulated with IL-6 at 100 ng/ml for 15mins. IL-6 induced phosphorylation of STAT1 and STAT3 (pSTATs) in naïve CD4+ T cells (CD3+CD4+CD45RA+) were determined by phosphoflow cytometry with anti-pSTAT1 (pY701) and anti-pSTAT3 (pY705) antibodies. IL-6 signaling responses are represented by ΔMFI (medium fluorescence intensity) which is the IL-6 stimulated MFI minus unstimulated MFI of pSTAT1 or pSTAT3. (B) IL-6 induced phosphorylation of STAT1 (p=0.003) and STAT3 (p=0.0004) in peripheral naïve CD4+ T cells were compared between BC patients (n=57, median age 51, range 27-85) and age-matched healthy donors (n=26, median age 53, range 30-72). Unpaired t test. (C) The association between IL-6 induced pSTAT1 and pSTAT3 in naïve CD4+ T cells from BC patients were determined by Pearson's correlation coefficient test (r=0.79, p<0.0001).
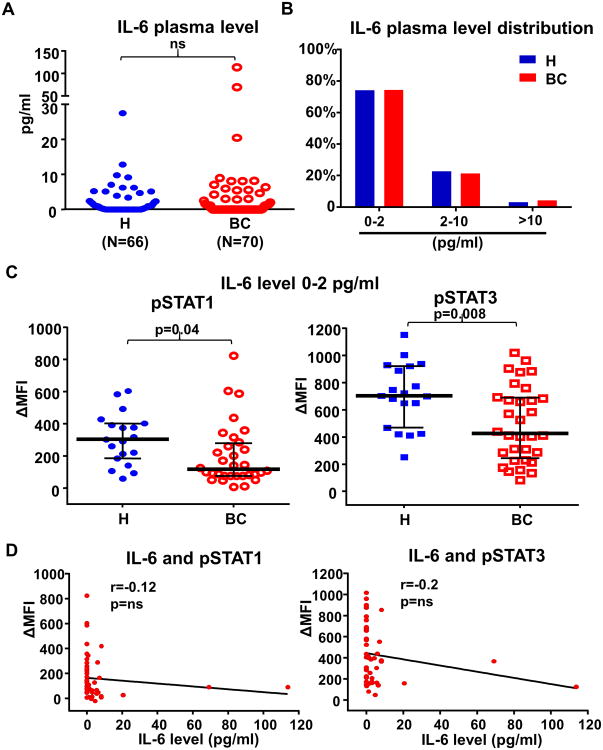
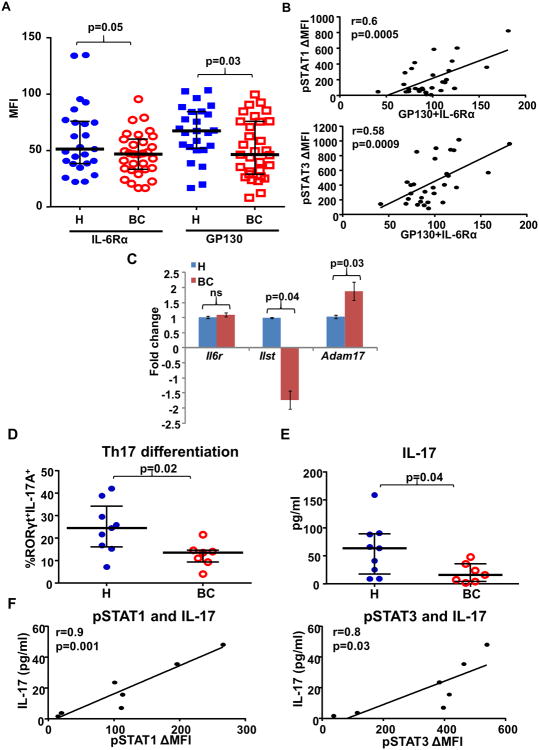
Elevated circulating IL-6 levels have been observed in advanced metastatic BC patients which negatively correlate with patient outcome (17,18). To investigate whether impaired IL-6 signaling responses observed in BC naïve CD4+ T cells were related to soluble IL-6 levels, we compared plasma IL-6 levels between BC patients (n=70) and age-matched healthy donors (n=66) by ELISA. All plasma samples from BC patients were collected at diagnosis prior to surgery or any therapy. Interestingly, we found that plasma IL-6 levels were not significantly elevated in this cohort of BC patients (mean 4.2pg/ml, median 0pg/ml) as compared to healthy donors (mean 2.0pg/ml, median 0.25pg/ml) (Fig. 2A). As normal plasma IL-6 levels are generally in the range of 0-2 pg/ml (19), we further categorized patients' plasma IL-6 levels into three ranges (0-2 pg/ml, 2-10 pg/ml, >10pg/ml) and found similar distributions between BC patients and healthy donors (Fig. 2B). Importantly, we compared the IL-6 signaling responses in T cells between healthy donors and BC patients who had normal IL-6 plasma levels (0-2pg/ml) at diagnosis. IL-6 induced phosphorylation of STAT1 (p=0.04) and STAT3 (p=0.008) in naïve CD4+ T cells from BC patients was still significantly lower than that in healthy donors even though they all had plasma IL-6 levels in the normal range (Fig. 2C). Moreover, we found no significant correlation between plasma IL-6 levels and IL-6 induced pSTATs in T cells (Fig. 2D). To investigate whether the impaired IL-6 signaling response was caused by reduced levels of the IL-6 receptor complex, we compared the cell surface levels of IL-6Rα and gp130 in naïve CD4+ T cells between BC patients and healthy donors by flow cytometry. Indeed, we found that IL-6Rα (p=0.05) and gp130 (p=0.03) levels were both lower in BC patients than in healthy donors (Fig. 3A). In addition, IL-6 induced pSTATs significantly correlate with the level of IL-6Rα plus gp130 (pSTAT1: p=0.0005; pSTAT3: p=0.0009) (Fig. 3B). To address whether these changes were regulated at the transcriptional level, we measured the mRNA levels of IL-6Rα and gp130 in CD4+ naïve T cells by qPCR. Indeed, mRNA levels of gp130 (Il6st) (p=0.04) were significantly lower in T cells from BC patients (n=4) than in healthy donors (n=4), but not IL-6Rα (Il6r) (Fig. 3C). IL-6Rα on the cell surface is known to be subjected to proteolytic cleavage by a metallopeptidase ADAM 17 (20). Intriguingly, we found that mRNA levels of ADAM17 were significantly higher (p=0.03) in T cells from BC patients than healthy donors (Fig. 3C). These data indicate that impaired IL-6 signaling responses in T cells from BC patients are caused by reductions in both chains of the IL-6 receptor complex via two distinct mechanisms: gp130 via reduced transcription, and IL-6Rα via enhanced cleavage by ADAM17.
Fig. 2. Impaired IL-6 signaling responses in naïve CD4+ T cells are not correlated with IL-6 plasma levels.
(A) IL-6 plasma levels in healthy donors (mean 2.0 pg/ml, median 0.25 pg/ml) and BC patients (mean 4.2 pg/ml, median 0 pg/ml) were determined by ELISA. Age-matched healthy donors (n=66, median age 58, range 18-72) were compared to BC patients (n=70, median age 50, range 27-85). All BC patient plasma was collected at diagnosis prior to surgery or any therapy. (B) IL-6 plasma level distributions (subdivided into 0-2pg/ml, 2-10pg/ml and >10pg/ml) in the healthy donors and BC patients. (C) Among the healthy donors and BC patients with normal IL-6 plasma levels (0-2 pg/ml), IL-6 induced phosphorylation of STAT1 (p=0.04) and STAT3 (p=0.008) in peripheral naïve CD4+ T cells were compared. (D) The relationship between IL-6 plasma levels and IL-6 induced pSTAT1 and pSTAT3 in naïve CD4+ T cells from the BC patients was examined by Pearson's correlation coefficient test. ns=not significant.
Fig. 3. Impaired IL-6 signaling responses in naïve CD4+ T cells are associated with lower IL-6 receptor levels and defective Th17 differentiation.
(A) Surface expression levels of IL-6Rα (p=0.05) and gp130 (p=0.03) on naïve CD4+ T cells from healthy donors (n=25) and BC patients (n=31) were determined by flow cytometry with anti-IL-6Rα and anti-gp130 antibodies. (B) The associations between IL-6 induced pSTATs and the expression levels of gp130 plus IL-6Rα were determined by Pearson's correlation coefficient test (pSTAT1: r=0.6, p=0.0005; pSTAT3: r=0.58, p=0.0009). (C) Total RNA was extracted from isolated CD4+ naïve T cells and analyzed for the relative fold change by Q-PCR. mRNA levels of IL-6Rα (Il6r) (p=ns), gp130 (Il6st) (p=0.04) and ADAM17 (Adam17) (p=0.03) were compared between healthy donors (n=4) and BC patients (n=4). (D) Naïve CD4+ T cells were isolated from fresh PBMCs and were cultured in Th17 differentiation medium for 7 days. RORγt+ IL-17A+ cells identified Th17 cells by flow cytometry. The percentages of differentiated Th17 cells were compared between BC patients (n=7) and age-matched healthy donors (n=8). (p=0.02). (E) Supernatants were collected after 7 days of Th17 differentiation and the levels of IL-17 were determined by ELISA (pg/ml/1×106 cells). The levels of IL-17 were compared between BC patients (n=7) and age-matched healthy donors (n=9). (p=0.04). (F) Among the BC patients (n=7), the associations between IL-6 induced pSTATs and level of IL-17 were determined by Pearson's correlation coefficient test. (pSTAT1: r=0.9, p=0.001; pSTAT3: r=0.8, p=0.03). All the blood from BC patients were collected at diagnosis prior to surgery or any therapy.
Since IL-6 is critical for Th17 differentiation (11), we examined whether dysfunctional IL-6 signaling responses in naïve T cells from BC patients was associated with impaired Th17 differentiation. Naïve CD4+ T cells were isolated from fresh PBMCs and cultured in Th17 differentiation medium for 7 days. BC patient samples (n=7) exhibited fewer differentiated Th17 cells (RORγt+IL-17A+) (p=0.02) (Fig. 3D) with lower IL-17 secretion levels (p=0.04) (Fig. 3E) than age-matched healthy donors (n=9). Among the BC patients, IL-6 induced pSTATs significantly correlated with levels of IL-17 production (pSTAT1: p=0.001; pSTAT3: p=0.03) (Fig. 3F).
IL-6 signaling responses in peripheral CD4+ T cells as prognostic marker
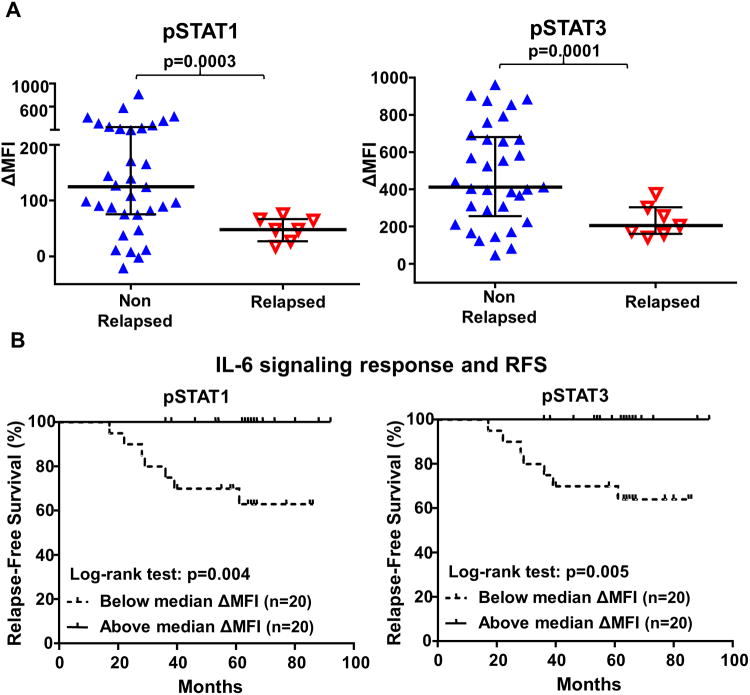
To evaluate the clinical significance of IL-6 signaling responsiveness, we compared the IL-6 induced pSTATs responses in peripheral naïveCD4+ T cells between relapsed and non-relapsed BC patients. Only patients with blood collected at diagnosis prior to surgery or any therapy who had been clinically followed for at least 36 months were selected for this analysis. The median follow-up time of BC patients (n=40) was 63 months (range 17-92 months). We found that IL-6 induced phosphorylation of STAT1 (p=0.0003) and STAT3 (p=0.0001) in peripheral blood naïve T cells at diagnosis were significantly lower in patients who went on to relapse than those who remained disease-free (Fig. 4A). Kaplan-Meier survival analysis was performed to determine the relationship between IL-6 signaling responses and relapse-free survival (RFS). To divide BC patients (n=40) into two populations in an unbiased way, median ΔMFI of IL-6 induced pSTAT1 or pSTAT3 was used as the cut-off. BC patients with pSTAT1 (p=0.004) or pSTAT3 (p=0.005) ΔMFI below the median (n=20) had significantly worse RFS than those above the medianΔMFI (n=20) (Fig. 4B), indicating that lower IL-6 signaling responses predict worse RFS. Intriguingly, none of the patients with IL-6 signaling responses above the median experienced relapse over 100 months (Fig. 4B). To understand if the IL-6 signaling response changes over time amongst relapsed BC patients, we compared the IL-6 signaling response between patients with blood collected at diagnosis (n=7) versusat time of relapse (n=7) and found no significant difference (Fig. S2). We also examined IL-6 signaling responses in patients who achieved remission after relapse (n=5). There was a trend towards higher IL-6 induced pSTAT1 (p=0.1) and pSTAT3 (p=0.2) responses in some relapsed patients who achieved remission (Fig. S2), indicating that impaired IL-6 signaling in T cells is a persistent defect during cancer progression but may return to normal in some relapsed patients who achieved remission. In a multivariate analysis adjusted for age, tumor stage, grade, nodal status and subtype of BC patients, IL-6 induced phosphorylation of STAT1 (p=0.001) or STAT3 (p=0.005) still retained the prognostic significance for RFS, indicating that IL-6 signaling responses could be a predictor of clinical outcome independent of these clinicopathologic characteristics (Table 2). The associations between IL-6 signaling response in T cells and clinicopathologic characteristics of BC patients was also evaluated and no significant correlations were found between IL-6 signaling responses and age, tumor stage, grade, T status or subtype (Table S1). Therefore, these findings suggest that IL-6 signaling responsiveness in peripheral naïve CD4+ T cells could potentially be developed into a prognostic blood test to predict the clinical outcome of BC patients.
Fig. 4. IL-6 signaling responses in peripheral blood CD4+ naïve T cells at diagnosis are correlated with clinical outcome.
(A) IL-6 induced phosphorylation of STAT1 (p=0.0003) and STAT3 (p=0.0001) in peripheral naïve CD4+ T cells were compared between the non-relapsed and relapsed BC patients, all of whom had been clinically followed for at least 36 months. All the blood from BC patients were collected at diagnosis prior to surgery or any therapy. (B) Kaplan-Meier survival analysis was performed to compare relapse-free survival (RFS) between BC patients with lower and higher IL-6 signaling response (pSTAT1 p=0.004, pSTAT3 p=0.005). The median IL-6 induced phosphorylation of STAT1 or STAT3 (ΔMFI) was used as the cut-off to divide BC patients into lower and higher IL-6 signaling response groups.
Table 2. Univariate and multivariate analysis for relapse-free survival by Cox regression.
| Variables | Univariate | Multivariate |
|---|---|---|
|
| ||
| p-value | p-value* | |
| pSTAT1 | 0.006 | 0.001 |
| pSTAT3 | 0.015 | 0.005 |
Note:
adjusting for age, tumor stage, grade, nodal status and subtype
Discussion
IL-6 is an inflammation-associated cytokine produced primarily by tumor cells, tumor stroma and tumor-associated myeloid cells (21). Despite the well documented role for the IL-6-STAT3 axis in promoting tumor growth through its direct activities on tumor cells, little is known about the role IL-6 plays in immune modulation in cancer patients. To interrogate the effects of cancer on IL-6 immune biology, STAT signaling responses to IL-6 were examined in BC patient PBMC populations. In response to IL-6, compared with healthy individuals, T cells from BC patients were found to be defective in their ability to phosphorylate both STAT1 and STAT3. We also found lower IL-6 signaling response in CD4+ naïve T cells from melanoma (Mel), gastrointestinal (GI) and lung cancer (LC) patients (Fig. S3), suggesting that dysregulated IL-6 signaling in peripheral blood T cells may be a more general phenomenon in cancer patients. Importantly, there was lower individual and combined expression of the IL-6 receptor complex components, gp130 and IL-6Rα, in T cells from BC patients compared with healthy controls. Thus, modulation of IL-6 pathway regulators, particularly the lower expression of the IL-6R complex, contributes to the loss of IL-6 responsiveness in BC patient immune cells.
The tumor microenvironment is considered to be a chronically inflamed setting. IL-6 is systemically upregulated in cancer and IL-6 levels negatively associate with the survival of patients with various cancer types (17,22-24). In healthy adults, IL-6 circulation levels over 10 pg/ml are considered abnormally elevated (21). Our findings that IL-6 signaling responses were defective in BC patients with normal IL-6 plasma level suggest that IL-6 related immune function could be dysregulated in cancer patients with normal IL-6 circulation level.
Within the tumor microenvironment, IL-6 is well-established as a pro-tumor cytokine and high expression levels of IL-6 are found within human BC tumors (25-27). Previous studies demonstrated that chronic exposure to IL-6 causes reduced levels of gp130 on T cells (28-30). It was also reported that steroid hormones were able to affect IL-6 signaling pathway (31). While our data showed that defective IL-6 signaling response was not dependent on serum IL-6 level, it is possible that trafficking through the tumor region with high local IL-6 levels may be sufficient to cause IL-6 receptor down-regulation in T cells from BC patients. Intriguingly, the two chains of the IL-6 receptor complex were reduced via two distinct mechanisms: gp130 via reduced transcription, and IL-6Rα via enhanced cleavage by ADAM17. gp130 cytokines have pleiotropic roles in immune cell functions while the effects of gp130 deficiencies in the immune compartment in cancer models have not to our knowledge been studied. Thus, downregulation of gp130 expression will likely result in loss of the pleiotropic balance of gp130 cytokine responses in immune cells, the outcome of which will also depend on the integration of responses to other differentially expressed cytokines and aberrant signaling pathways.
IL-6 functions include promoting T cell survival, mediating helper T cell differentiation decisions by promoting Th2 over Th1 induction and Th17 over Treg induction, and regulating chemokine receptor expression, thereby influencing T cell recruitment to tissues (32,33). Therefore, loss of IL-6 responses may result in dysfunctional T cell survival as well as altered helper T cell differentiation and recruitment during inflammatory conditions. In the presence of IL-6 and TGFβ and IL-1β, naïve T cells can differentiate into Th17 cells, which are characterized by expression of the master transcription factor RORγt (34). Th17 cells are found to negatively correlate with the presence of Treg cells and positively correlate with effector immune cells, including cytotoxic CD8+ T cells and NK cells (35,36). The anti-tumor role of Th17 cells is at least partially due to their capacity to recruit effector cytotoxic T cells. The findings that Th17 differentiations from CD4+ naïve T cells from BC patients were defective and correlated with IL-6 signaling responses suggest that IL-6 response in peripheral T cell may be linked with the Th17/Treg differentiation in BC patients.
Since BC is a heterogeneous disease with varied presentation, morphology and clinical behavior, a major challenge is the outcome prediction for early stage BC patients. Currently, the risk of BC progression is evaluated based on clinical and pathologic parameters (37) which can only be obtained after invasive biopsy or surgery and have limited predictive power. More informative prognostic tests for BC patients at diagnosis are needed. In this study, the demonstration that IL-6 signaling responses predict clinical outcome indicates that IL-6 signaling responses in peripheral T cells may have promise as noninvasive blood-based predictive biomarkers for BC patient outcomes.
Supplementary Material
Acknowledgments
We would like to thank Michele Kirschenbaum for consenting patients and acquiring samples at City of Hope. We would like to thank David Dunkley for organizing the collection of age-matched healthy blood. Research reported in this publication included work performed in the Analytical Cytometry Core supported by National Cancer Institutes of Health under award number P30CA33572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported by the Department of Defense Breast Cancer Research Program (BCRP) (U.S. Army Medical Research and Materiel Command) under W81XWH-10-1-0616 (A.K.Miyahira) and W81XWH-06-1-0417 (P.P.Lee), NIH Molecular and Cellular Immunobiology Training Grant No. 5 T32 AI07290-24 (A.K.Miyahira), and NIH R01 CA130817 (P.P.Lee).
Footnotes
No potential conflicts of interest were disclosed.
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. The Biochemical journal. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends in molecular medicine. 2008;14:109–19. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Seminars in immunology. 2014;26:38–47. doi: 10.1016/j.smim.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Seminars in immunology. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nature reviews Cancer. 2014;14:736–46. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 7.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Teague TK, Marrack P, Kappler JW, Vella AT. IL-6 rescues resting mouse T cells from apoptosis. J Immunol. 1997;158:5791–6. [PubMed] [Google Scholar]
- 10.Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–60. [PubMed] [Google Scholar]
- 11.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. European journal of immunology. 2010;40:1830–5. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 12.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–57. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 14.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 15.Critchley-Thorne RJ, Simons DL, Yan N, Miyahira AK, Dirbas FM, Johnson DL, et al. Impaired interferon signaling is a common immune defect in human cancer. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9010–5. doi: 10.1073/pnas.0901329106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avalle L, Pensa S, Regis G, Novelli F, Poli V. STAT1 and STAT3 in tumorigenesis: A matter of balance. JAKSTAT. 2012;1:65–72. doi: 10.4161/jkst.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–6. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 18.Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88:1721–6. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 20.Schumacher N, Meyer D, Mauermann A, von der Heyde J, Wolf J, Schwarz J, et al. Shedding of Endogenous Interleukin-6 Receptor (IL-6R) Is Governed by A Disintegrin and Metalloproteinase (ADAM) Proteases while a Full-length IL-6R Isoform Localizes to Circulating Microvesicles. J Biol Chem. 2015;290:26059–71. doi: 10.1074/jbc.M115.649509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heikkila K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. European journal of cancer. 2008;44:937–45. doi: 10.1016/j.ejca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 22.Hoejberg L, Bastholt L, Schmidt H. Interleukin-6 and melanoma. Melanoma research. 2012;22:327–33. doi: 10.1097/CMR.0b013e3283543d72. [DOI] [PubMed] [Google Scholar]
- 23.Knupfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients--a summary of published results. International journal of colorectal disease. 2010;25:135–40. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer treatment reviews. 2012;38:904–10. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast cancer research and treatment. 2007;102:129–35. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 26.Dethlefsen C, Hojfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast cancer research and treatment. 2013;138:657–64. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- 27.Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848–62. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang XJ, Taga T, Yoshida K, Saito M, Kishimoto T, Kikutani H. gp130, the cytokine common signal-transducer of interleukin-6 cytokine family, is downregulated in T cells in vivo by interleukin-6. Blood. 1998;91:3308–14. [PubMed] [Google Scholar]
- 29.Hidalgo E, Essex SJ, Yeo L, Curnow SJ, Filer A, Cooper MS, et al. The response of T cells to interleukin-6 is differentially regulated by the microenvironment of the rheumatoid synovial fluid and tissue. Arthritis and rheumatism. 2011;63:3284–93. doi: 10.1002/art.30570. [DOI] [PubMed] [Google Scholar]
- 30.Oberg HH, Wesch D, Grussel S, Rose-John S, Kabelitz D. Differential expression of CD126 and CD130 mediates different STAT-3 phosphorylation in CD4+CD25- and CD25high regulatory T cells. Int Immunol. 2006;18:555–63. doi: 10.1093/intimm/dxh396. [DOI] [PubMed] [Google Scholar]
- 31.Canellada A, Alvarez I, Berod L, Gentile T. Estrogen and progesterone regulate the IL-6 signal transduction pathway in antibody secreting cells. J Steroid Biochem Mol Biol. 2008;111:255–61. doi: 10.1016/j.jsbmb.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clinical immunology. 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silver JS, Hunter CA. gp130 at the nexus of inflammation, autoimmunity, and cancer. J Leukoc Biol. 2010;88:1145–56. doi: 10.1189/jlb.0410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Frontiers in immunology. 2014;5:276. doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nature reviews Immunology. 2010;10:248–56. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rakha EA. Pitfalls in outcome prediction of breast cancer. Journal of clinical pathology. 2013;66:458–64. doi: 10.1136/jclinpath-2012-201083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.