Abstract
Purpose
Nasogastric feeding tube is routinely positioned in intensive care units. The complications of misplacement are rare but very dangerous for the patients. The aim of this study is to estimate the diagnostic accuracy of this new technique, 4-point ultrasonography to confirm nasogastric tube placement in intensive care.
Methods
One hundred fourteen critical ill patients monitored in ICU were included. The intensivist provided in real time to perform the exam in four steps: sonography from either the right or left side of the patient’s neck to visualize the esophagus, sonography of epigastrium to confirm the passage through the esophagogastric junction and the positioning in antrum, sonography of the fundus. Finally, gastric placement of the nasogastric feeding tube was confirmed with thorax radiograph.
Results
One hundred fourteen of the gastric tubes were visualized by sonography in the digestive tract and all were confirmed by radiography (sensitivity 100%). The entire sonographic procedure, including the longitudinal and transversal scan of the esophagus, the esophagogastric junction, the antrum and the fundus, took 10 min.
Conclusions
Our pilot study demonstrated that not weighted-tip gastric tube routinely used in Intensive Care is visible with the sonography. The pilot study confirmed the high sensitivity of the sonography in the verify correct positioning of gastric tube in the adult ICU patients. The ultrasound examination seems to be easy and rapid even when performed by a intensivist whit a sonographic training of only 40 h. The sonographic exam at the bedside was performed in a shorter time than the acquisition and reporting of the X-ray.
Keywords: Enteral nutrition, Nasogastric tube, Sonography
Sommario
Obiettivo
Il sondino nasogastrico viene posizionato routinariamente in Terapia Intensiva. Le complicanze del malposizionamento sono rare ma estremamente pericolose per il paziente. Lo scopo del nostro studio è stimare l’accuratezza diagnostica di una nuova tecnica ecografica a 4 punti per confermare il posizionamento del sondino nasogastrico in Terapia Intensiva.
Metodi
Nello studio sono stati inclusi 114 pazienti ricoverati in Terapia Intensiva. L’intensivista ha provveduto in tempo reale all’esecuzione dell’esame ecografico in 4 punti: ecografia della parte destra e sinistra del collo del paziente per visualizzare l’esofago, ecografia dell’epigastrio per confermare il passaggio attraverso la giunzione esofagogastrica ed il posizionamento nell’antro, infine ecografia del fondo gastrico. Il posizionamento veniva confermato attraverso l’esecuzione della lastra del torace.
Risultati
L’ecografia ha visualizzato 114 sondini nasogastrici nel tratto digestivo e tutti sono stati confermati dalla lastra del torace (sensitività del 100%). L’intera procedura ecografica, inclusa la scansione longitudinale e trasversale dell’esofago, della giunzione esofagogastrica, dell’antro e del fondo ha necessitato di 10 minuti.
Conclusioni
Il nostro studio pilota ha dimostrato che il sondino nasogastrico non pesato in punta utilizzato routinariamente in Terapia Intensiva è visibile con l’ecografia. Lo studio pilota ha confermato l’alta sensitività dell’ecografia nella verifica del corretto posizionamento del sondino nei pazienti adulti di Terapia Intensiva. L’esame ecografico si è dimostrato facile e rapido anche se eseguito da un intensivista con un training ecografico di sole 40 ore. L’intero esame ecografico al letto del paziente è stato eseguito in un tempo minore rispetto all’acquisizione e alla refertazione della lastra del torace.
Introduction
Enteral nutrition is a fundamental part of the care of the critically ill patient. The use of the gastric tube has become routine for several reasons, not only for the administration of enteral nutrition and medications, but also for gastric decompression [1]. Gastric tube placement is a routine maneuver in the clinical practice of each Intensive Care Unit, usually performed by the nursing staff. The nursing staff provides the placement and confirmation of the correct position through the instillation of air or water. The misplacement rate appears low (between 0.5 and 1.5%), but it is difficult to estimate the exact frequency of this event [2].
The serious complications correlated with the maneuver derive from the possibility of misplacement in the tracheobronchial tree, pneumothorax, pneumomediastinum, subcutaneous emphysema, pneumonia, pulmonary hemorrhage, empyema, hemothorax, bronchopleural fistula, perforation of the esophagus, or even death [2–6]. In clinical practice the difficulty appears nonspecific to patients with spontaneous breathing—they show the clinical signs of misplacement (violent cough, desaturation),—but to intubated patients and into patients with neurological impairment in whom impaired gag reflex makes the interpretation of correct placement difficult. Moreover, repeated blind-movement attempts in the hypopharynx expose the patient to risk of mucosal liberation and development of mediastinitis, a very severe complication.
It is not surprising that cases of misplacement of the gastric tube into the brain [7, 8] were also described. For all these reasons, another safe, rapid, and feasible method is necessary to document the correct placement of the tube. Sonography is increasingly used by ICU physicians to position the central venous catheter, to assess hemodynamic status, and to diagnose pneumothorax, pleural effusion or cardiac tamponade [9–11]. Although sonography has been used for nasogastric tube verification in pediatrics and neonatology [12, 13], its use in Intensive Care Unit patients has been reported only in few cases. The aim of this study is to estimate the diagnostic accuracy of this new technique, 4-point ultrasonography, to confirm nasogastric tube placement in Intensive Care.
Patients and methods
We studied all patients admitted to our Intensive Care in an 8-month period. They received a feeding tube upon admission or during their stay. Gastric tubes were routinely positioned in all the patients in whom an incapacity for spontaneous enteral feeding was expected for at least 48 h. The study considered 114 gastric tubes in 114 patients (80 men, 34 women, age range of 14–89, average age 52). 12 patients breathed spontaneously, 100 were mechanically intubated, and two were tracheotomized. All the gastric tubes were positioned by the nursing staff by measuring the distance from the tip of the patient’s nose to the earlobe and from the earlobe to the xiphoid process (NEX). The intensivist performed the sonography in real time. The intensivist involved had sonographic experience in the positioning of catheters and in the visualization of the trachea during percutaneous dilational tracheostomy, in addition to a specific 40-h training course with a radiologist. A real-time portable ultrasound unit (Philips Sparq) equipped with a convex probe with low frequency (2–5 MHz) and a linear probe with high frequency (5–7.5 MHz) was used for this study.
The intensivist performed the exam in real time using four steps: sonography from either the right or left side of the patient’s neck to visualize the esophagus, sonography of the epigastrium to confirm passage through the esophagogastric junction, the positioning in the antrum, and the sonography of the fundus. Ultrasound examinations included a transversal scan of the neck, prior to tube insertion to verify that esophagus was located behind the respiratory tract. The proximal esophagus is visible in the neck through the trachea and the left lobe of the thyroid. The cervical esophagus appears as an oval structure on transverse scans and as a long tubular structure on longitudinal scans.
If attenuated ultrasound waves in the far field and the posterior wall of the esophagus were not observed after tube insertion, the gastric tube was considered to be positioned within the cervical esophagus (Fig. 1). In the esophagogastric junction, the gastric tube was directly visualized with longitudinal and angled scans of the epigastrium (Fig. 2). Visualization of the gastric tube in separate scans of the fundus and the antrum of the stomach was attempted. If visualization of the antrum was not possible, 50 cc of normal saline were injected through the gastric tube, and if ultrasonography showed dynamic fogging in the stomach, gastric placement of the tube was verified (Fig. 3). Visualization of the gastric tube in the neck, but not in the esophagogastric junction, in the antrum or in the fundus, was not sufficient to confirm the positioning of the tube because the tube could also be coiled within the cervical esophagus and not proceed distally.
Fig. 1.
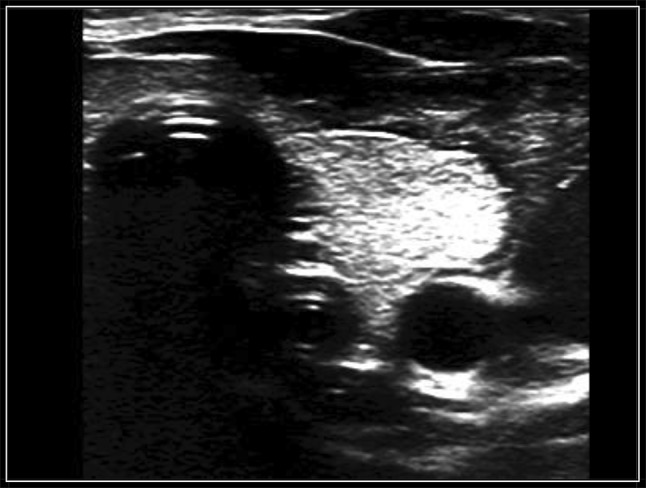
“Double trachea” image in transversal scan
Fig. 2.
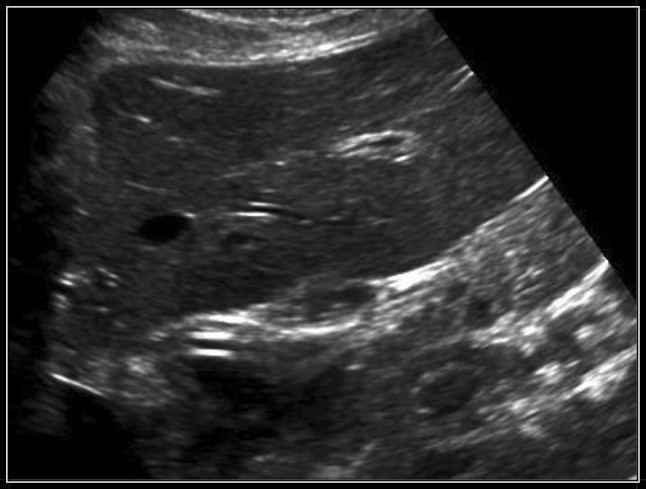
Passage of the tube through the esophagogastric junction
Fig. 3.
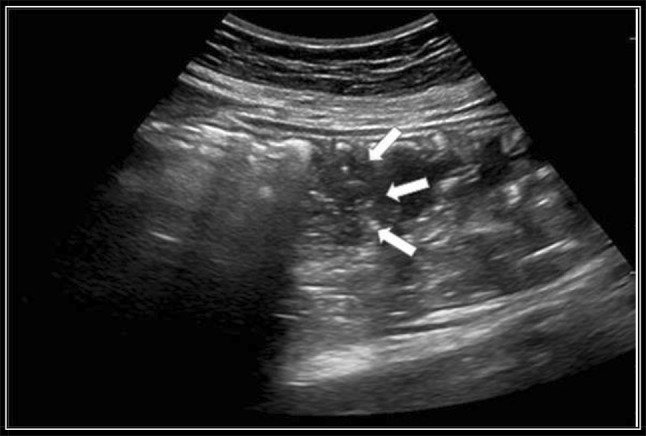
“Fogging” effect (arrows) after the injection of 50 mL of normal saline
The antrum is a landmark located posterior to the left hepatic lobe and anterior to the pancreas (the antrum is delimited anterior to the pancreas in a transversal epigastric section). In a fasting patient the antrum appears like a finger glove in a transversal section, with hypoechogenic walls and homogeneous (air–liquid) content. In a medium-sagittal epigastric section, the antrum has an ovoid aspect, often described as a “bulls-eye pattern”. When the volume increases (gastric secretions, water), the antrum appears round with thin walls. It is evident that it’s more difficult to visualize the antrum with sonography without previous preparation. The gastric fundus is located in the left superior abdominal quadrant, under the diaphragm, anterior to the left kidney and posterior to the spleen. The fundus can be difficult to visualize because of its deep position and the acoustic windows of the ribs. We facilitated the visualization of the fundus through two approaches: a left lateral intercostal trans-splenic section or a longitudinal section over the medium-axillary line (Fig. 4).
Fig. 4.
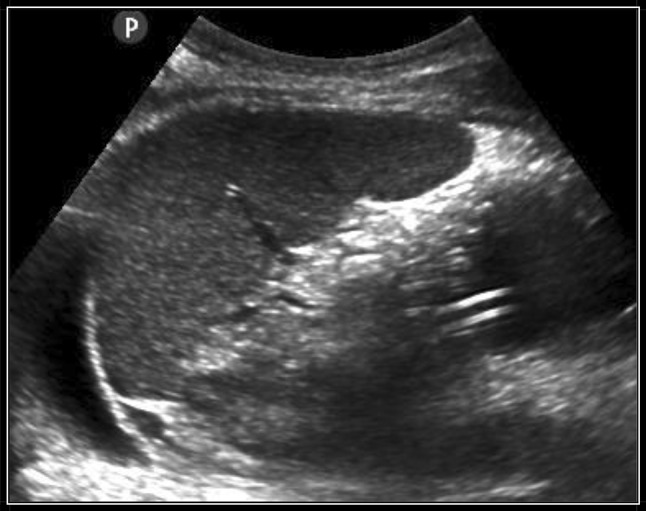
Image of the tube in the gastric fundus in sagittal scan
Results
The tip of the gastric tube was never positioned in the respiratory tract. 114 of the gastric tubes were visualized in the digestive tract using sonography and all were confirmed by radiography (sensitivity 100%). It was necessary to inject 50 cc of normal saline for a better visualization of the stomach in the longitudinal scans of six patients. The clinical and demographic characteristics of the patients are described in Table 1. The entire sonographic procedure, including the longitudinal and transversal scan of the esophagus, the esophagogastric junction, the antrum and the fundus, took 10 min. The entire radiography, from the request for the X-ray to the radiologic referral, took about 60 min.
Table 1.
Demographic and clinic characteristics of 114 patients
| Characteristics | n = 114 |
|---|---|
| Age (years) | |
| Range of age | 14–89 |
| Average | 52 |
| Gender (M/F) | 80/34 |
| Diagnosis | |
| NCH | 42 |
| Sepsis | 20 |
| Heart | 24 |
| Other | 28 |
| Intubated/tracheostomy/ | 100/2 |
| Not intubated | 12 |
Discussion
Surprisingly, the positioning of the gastric tube, which is often used in clinical practice, has not yet been standardized. In the USA, the placement of the gastric tube is regularly controlled by checking X-ray images [14, 15]. In many countries in Europe, as well as in many institutions in Italy, correct placement is verified using air insufflation [16]. Several methods are suggested to verify the placement of gastric tube, such as the auscultation of bubbling sounds, the sampling of pH aspirates from the feeding tube, a chest X-ray and colorimetric capnography [17]. Auscultation with a stethoscope confirms gurgling sounds in the epigastrium when air or water is injected after gastric tube insertion. The uncertainty of this method was documented by American group Metheny in 1990 following a study [18].
In 2011, the National Patient Safety Agency (NPSA) issued an alert about the misplacement of gastric tubes [19]. The alert confirmed that nutrition through a misplaced gastric tube must be a “never event.” The alert recommended testing with pH indicator paper as the first line check. It advised checking X-ray images as the second line test, when the result is unclear or there is no opportunity to obtain a sample of aspirate. The determination of pH value, considering a value lower than 5 as the cut-off for the correct placement, is not indicated in patients who take some medications, such as H2 blockers, and doesn’t distinguish between intestinal and tracheal placement [20]. In the case of correct positioning, the pH measurement was between 0 and 6.0 in 85% of patients, whereas in the intestinal fluids the pH was greater than 6.0. In this study of 605 patients, four cases of incorrect positioning in the respiratory tract were described (2 in the tracheobronchial tree, 2 in the pleural space); in all four aspirates the pH value was greater than 6.5 [13].
In another study that considered the connection between pH and bilirubin, it was possible to distinguish between tracheobronchial aspirate and gastric and intestinal fluid [15] using a pH value greater than 5.0 and a bilirubin value smaller than 5 mg%.
Radiography remains the gold standard in the confirmation of tube and line position, drawing attention to the necessity of prompt examination and standard interpretation [19, 21, 22]. Radiographic exposure must be adequate to allow the tube to be visible to the bottom of the film, which should be centered lower than normal to show the abdomen as far as possible below the diaphragm [23, 24]. A case report published in 2006 describes a case of “nutrothorax”, the serious complication of blind nasogastric feeding tube insertion in a 65-year-old female patient [25]. However, interpretation of the X-ray can be difficult for the radiologist in some situations. Radiography also exposes the patient to ionizing radiation and, in any case, the nasogastric tube could be dislocated from the first position or clogged during the recovery, making it necessary to repeat the radiographs with a greater radiologic risk for the patient and an increase in costs [26].
In a case report published by Swartzlander and coll [21]. in 2012, which was about a pregnant woman (aged 37) with emesis gravidarum, in need of nutritional support and undergoing repeated attempts to place a feeding tube, sonography played a fundamental role in avoiding the continuous exposure of the fetus to ionizing radiation. Sonography is increasingly used by ICU physicians to position a central venous catheter, to assess hemodynamic status and to diagnose pneumothorax, pleural effusion or cardiac tamponade [9–11]. In the Hernandez-Socorro and coll. study in 1996, sonography was used to confirm the weighted tip of a naso-enteric tube feeding during the passing of pylorus [27].
In another pilot study from Lock and coll. in 2003, following 55 patients recovering in medical intensive care, the placement of feeding tubes was verified with insufflation of 50 mL of air. In 43 of 60 feeding tubes (72%), correct placement was verified with sonography [28]. In the prospective study of Vigneau and colleagues in 2005, the use of sonography was considered in adult patients for the verification of weighted-tip nasogastric tube, in comparison with radiography [29]. The study included 35 patients in the ICU and demonstrated that sonography was faster than conventional radiography and had a high sensitivity to the position of the feeding tube in 97% of cases. The weighted-tip nasogastric tubes have a metal tip that facilities placement and recognition, though the metal in sonography appears hyperechogenic with a posterior cone. Vigneau and coll. suggested that the findings needed to be confirmed in a larger ICU population and also in surgical or trauma patients, in whom ultrasonic access to gastric tubes is more difficult. They noted that only weighted-tip feeding tubes are visible with sonography. In an Intensive Care setting, however, no weighted-tip feeding tubes are used, and tubes can also be visualized with sonography in a simple and rapid matter.
In a recent study, Gok and coll. were able to obtain the “real time” imaging of the passage of the nasogastric tube through the esophagus in a high percentage of patients, using ultrasonography and a metal guide [30]. In a prospective multicenter study from two French cities, conducted by Chenatia and coll. [31] and involving 130 patients who were intubated and mechanically ventilated in a prehospital setting, sonography seemed to be a valid and feasible method to confirm the correct positioning of the gastric tubes with a sensitivity of 98.3% and a specificity of 100%. The limitation of this prospective study, in addition to the small number of patients, was that almost all the patients studied in the prehospital setting were not fasting, making gastric content sonographically detectable and thus facilitating the visualization of the tip of the gastric tube. The study of Kim and colleagues, which compared the efficacy of the auscultation method with the pH measure of gastric content and sonography in non-intubated patients with neurological impairments, lighted the weaknesses of the sonographic exam [32]. In this prospective study, the examination was performed with the patients in a supine position. In cases of obese or excessively mobile patients, it was difficult to posteriorly observe the gastric tube in the cervical region. In some patients, the vision of the gastric tube with the transabdominal scansion was also difficult because of the large volume of gas in the gastrointestinal tract. Obviously controlling the positioning of the gastric tube with sonography has the advantage of reducing complications, sparing time for other procedures and decreasing radiation exposure. In some cases, however, the sonographic exam is difficult due to the features and the conditions of the patients. For this reason we need facilitating maneuvers to obtain and interpret the images.
Our pilot study demonstrated that gastric tubes without weighted tips, routinely used in Intensive Care, are visible with sonography, in contrast to the results of Vigneau’s study [29]. Moreover, we used a gastric tube without a metal guide, and the ultrasonographic images were clearly visible. The pilot study confirmed the high sensitivity of sonography in the verification of the correct positioning of the gastric tube in the adult ICU patients. The ultrasound examination seemed to be easy and fast even when performed by an intensivist with a sonographic training of only 40 h. The sonographic exam at the bedside was performed in a shorter time than the acquisition and reporting of the X-ray. The main difficulties were found in the visualization of the esophagogastric junction and the antrum in the transabdominal longitudinal scan in obese patients because of the interposition of gas. The position in the left lateral decubitus, if possible, and the injection of 50 cc of normal saline, facilitated the ultrasound exam. In contrast, in this category of patients, ultrasound exams of the neck and visualizations of the cervical esophagus in axial and longitudinal scans seem to be particularly advantageous and easy, turning the patient’s head to the contra lateral side.
Conclusion
In the present study, the ultrasound exam appears to be a simple and rapid method for the recognition of the correct positioning of the nasogastric tube in critically ill patients. This method is more rapid than the conventional X-ray and can be obtained after a short period of training. The facility of execution of a transversal and longitudinal scan of the neck and abdomen appears evident, and could potentially extend to all medical and nursing staff. The X-ray remains the gold standard in cases in which a clear ultrasonographic image is not obtained due to an anatomical feature of the patient, or the presence of gas.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Gubler C, Bauerfeind P, Vavricka SR, Mullhaupt B, Fried M, Wildi SM. Bedside sonographic control for positioning enteral feeding tubes: a controlled study in intensive care unit patient. Endoscopy. 2006;38(12):1256–1260. doi: 10.1055/s-2006-944888. [DOI] [PubMed] [Google Scholar]
- 2.Bankier AA, Wiesmayr MN, Henk C, et al. Radiographic detection of intrabronchial malposition of nasogastric tubes and subsequent complications in intensive care unit patients. Intensive Care Med. 1997;23:406–410. doi: 10.1007/s001340050348. [DOI] [PubMed] [Google Scholar]
- 3.Harris MR, Huseby JS. Pulmonary complications from nasoenteral feeding tube insertion in an intensive care unit: incidence and prevention. Crit Care Med. 1989;17:917–919. doi: 10.1097/00003246-198909000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Miller KS, Tomlison JR, Sahn SA. Pleuropulmonary complications of enteral tube feedings. Chest. 1985;88:230–233. doi: 10.1378/chest.88.2.230. [DOI] [PubMed] [Google Scholar]
- 5.Duthorn L, Steinberg HS, Hauser H, Neeser G, Pracki P. Accidental intravascular placement of a feeding tube. Anesthesiology. 1998;89:251–253. doi: 10.1097/00000542-199807000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Merchant FJ, Nichols RL, Bombeck CT. Unusual complication from insertion of nasogastric esophageal intubation-erosion into an aberrant right subclavian artery. J Cardiovasc Surg. 1977;18:147–150. [PubMed] [Google Scholar]
- 7.Fremstad JD, Martin SH. Lethal complication from insertion of nasogastric tube after severe basilar skull fracture. J Trauma Inj Infect Crit Care. 1978;18:820–822. doi: 10.1097/00005373-197812000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Gregory JA, Turner PT, Reynolds AF. A complication of nasogastric intubation: intracranial penetration. J Trauma Inj Infect Crit Care. 1978;18:823–824. doi: 10.1097/00005373-197812000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Chaney JC. Derdak. Minimally invasive hemodynamic monitoring for the intensivist: current and emerging technology. Crit Care Med. 2002;30:2338–2345. doi: 10.1097/00003246-200210000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein D, Meziere G, Bidermann P, Gepner A. The comet-tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med. 1999;25:383–388. doi: 10.1007/s001340050862. [DOI] [PubMed] [Google Scholar]
- 11.Timsi JF, Farkas JC, Boyer JM, Martin JB, Misset B, Renaud B, Carlet J. Central vein catheter-related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsis. Chest. 1998;114:2017–2213. doi: 10.1378/chest.114.1.207. [DOI] [PubMed] [Google Scholar]
- 12.Greenberger M, Bejar R, Asser S. Confirmation of transpyloric feeding tube placement by ultrasonography. J Pediatr. 1993;122:413–415. doi: 10.1016/S0022-3476(05)83429-8. [DOI] [PubMed] [Google Scholar]
- 13.Maruyama K, Shiojima T, Koizumi T. Sonographic detection of a malpositioned feeding tube causing esophageal perforation in a neonate. J Clin Ultrasound. 2003;31:108–110. doi: 10.1002/jcu.10133. [DOI] [PubMed] [Google Scholar]
- 14.Metheny N. Minimizing respiratory complications of nasoenteric tube feedings: state of science. Heart Lung. 1993;22:213–233. [PubMed] [Google Scholar]
- 15.Metheny NA, Stewart BJ, Smith L, Yan H, Diebold M, Clouse RE. PH and concentration of bilirubin in feeding tube aspirates as predictors of tube placement. Nurs Res. 1999;48:189–197. doi: 10.1097/00006199-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Christen S, Hess T. Genügt eine klinische Lagekontrolle nasogastraler Sonden? Dtsch Med Wschr. 1996;121:1119–1122. doi: 10.1055/s-2008-1043115. [DOI] [PubMed] [Google Scholar]
- 17.Galbois A, et al. Colorimetric capnography, a new procedure to ensure correct feeding tube placement in the intensive care unit: an evaluation of a local protocol. J Crit Care. 2011;26:411–414. doi: 10.1016/j.jcrc.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Metheny NA, McSweeney CT, Wehrle MA, Wiersema L. Effectivness of the auscultatory method in predicting feeding tube location. Nurs Res. 1990;39(5):262–267. doi: 10.1097/00006199-199009000-00002. [DOI] [PubMed] [Google Scholar]
- 19.National Patient Safety Agency (Patienty Safety Alert 2011) Reducing the harm caused by misplaced nasogastric feeding tubes in adults, children and infants (online). Available from: http://www.nrls.npsa.nhs.uk/alerts/?entryd45=129640. Accessed 10 Mar 2011
- 20.Stroud M, Duncan H, Nightingale J. Guidelines for enteral feeding in adult hospital patients. Gut. 2003;52(Suppl VII):vii–vii12. doi: 10.1136/gut.52.suppl_7.vii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law RL, Pullybank AM, Eve Ieigh M, Stack N. Avoiding never events: improving nasogastric intubation practice and standards. Clin Radiol. 2013;68:239–244. doi: 10.1016/j.crad.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SJ. Confirming nasogastric feeding tube position versus the need to feed. Intensive Crit Care Nurs. 2013;29:59–69. doi: 10.1016/j.iccn.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Law R. Radiographers, “never events” and the nasogastric tube. Radiography. 2014;20:1–92. doi: 10.1016/j.radi.2013.09.003. [DOI] [Google Scholar]
- 24.Royal College of Radiologists. Chest X-ray confirmation of nasogastric tube placement: radiographer responsibility. http://www.rcr.ac.uk/audittemplate.aspx?PageID=1020&AuditTemplateID=258. Accessed 6 Jan 2014
- 25.Haas LEM, Tjan DHT, van Zaten ARH. “Nutrothorax” due to misplacement of a nasogastric feeding tube. Neth J Med. 2006;64(10):385–386. [PubMed] [Google Scholar]
- 26.Nguyen L, Lewiss RE, Drew J, Saul TA. Novel approach to confirming nasogastric tube placement in the ED. Am J Emerg Med. 2012;30:1662.e5–1667.e7. doi: 10.1016/j.ajem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Socorro CR, Marin J, Ruiz-Santana S, Santana S, Manzano JL. Bedside sonographic-guided versus blind nasoenteric feeding tube placement in critically ill patients. Crit Care Med. 1996;24:160–1694. doi: 10.1097/00003246-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Lock G, Reng CM, Köllinger M, Rogler G, Schölmerich J, Schlottmann K. Sonographische Kontrolle von Magensonden bei Intensivpatienten. Intensivmed. 2003;40:693–697. doi: 10.1007/s00390-003-0417-9. [DOI] [Google Scholar]
- 29.Vigneau C, Baudel J, Guidet B, Offenstadt G, Maury E. Sonography as an alternative to radiography for nasogastric feeding tube location. Intensive Care Med. 2005;31:1570–1572. doi: 10.1007/s00134-005-2791-1. [DOI] [PubMed] [Google Scholar]
- 30.Gok F, Kilicaslan A, Yosunkaya A. Ultrasound-guided nasogastric feeding tube placement in critical care patients. Nutr Clin Pract. 2015;30(2):257–260. doi: 10.1177/0884533614567714. [DOI] [PubMed] [Google Scholar]
- 31.Chenaitia H, Brun P, Querellou E, Leyral J, Bessereau J, Aimè C, Bouaziz R, Georges A, Lousi F. Ultrasound to confirm gastric tube placement in prehospital management. Resuscitation. 2012;83:447–451. doi: 10.1016/j.resuscitation.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 32.Kim HM, So BH, Jeong WJ, Choi SM, Park KN. The effectiveness of ultrasonography in verifying the placement of a nasogastric tube in patients with low consciousness at an emergency centre. Scand J Trauma Resusc Emerg Med. 2012;20:38. doi: 10.1186/1757-7241-20-38. [DOI] [PMC free article] [PubMed] [Google Scholar]


