Abstract
Objectives
Traditionally, facet joint injections (FJI) are performed under fluoroscopic or computed tomography (CT) guidance, mainly due to the deep anatomical location and the presence of bony landmarks. Fusion imaging technology, which couples the ultrasound scan with the corresponding CT or magnetic resonance (MR) image obtained from the diagnostic examination and reformatted in real time according to the ultrasound scanning plane, allows to combine the panoramic view and the elevated anatomical detail of MR or CT with the ease of use of ultrasound without patient exposure to ionizing radiation.
Methods
Thirty eight patients (24 females; mean age ± SD: 64 ± 9 years) received MR fusion-assisted ultrasound-guided FJI of 1 ml of a mixture of local anaesthetic and corticosteroid using a ultrasound machine (Logiq E9, GE Healthcare) equipped with a GPS-enhanced fusion imaging technology which couples real-time B-mode images with those of the previous recent diagnostic MR examination. Low-dose CT needle positioning confirmation was performed in the first 28 patients. Patients’ pain was recorded using a visual analogue scale (VAS), at baseline and at 2, 4 and 8 weeks.
Results
All fusion imaging-guided injections were performed successfully. Out of 112, 96 FJI had optimal intra-articular needle positioning (accuracy: 85.7%). Patients VAS significantly decreases after the procedure with no differences among who received CT needle positioning control and who did not receive it. No major complications were observed.
Conclusions
Ultrasound needle guidance with MR fusion assistance allows for safe and effective injection of degenerative facet joint disease.
Keywords: Facet joint, Injection, Fusion imaging, Ultrasound, Magnetic resonance
Sommario
Obiettivi
Tradizionalmente, le iniezioni delle faccette articolari (FJI) sono state eseguite sotto guida fluoroscopica o tomografia computerizzata (TC), principalmente a causa della posizione anatomica profonda e la presenza di reperi ossei. L’imaging di fusione permette di accoppiare le immagine ecografiche con quelle TC o di risonanza magnetica (RM) corrispondenti, ottenute da un precedente esame diagnostico e riformattate in tempo reale in base al piano di scansione ecografico. Questa tecnica permette di coniugare la visione panoramica e l’elevato dettaglio anatomico della RM o TC con la praticità della guida ecografica senza ulteriore esposizione del paziente a radiazioni ionizzanti.
Metodi
Trentotto pazienti (24 femmine, età media ± DS: 64 ± 9 anni) hanno ricevuto FJI guidata da fusione ecografia-RM di 1 ml di una miscela di anestetico locale e corticosteroide utilizzando una apparecchiatura ecografica (Logiq E9, GE Healthcare) dotata di tecnologia fusion. Nei primi 28 pazienti il posizionamento dell’ago è stato confermato mediante esame TC a bassa dose. Il dolore dei pazienti è stato registrato utilizzando una scala analogica visiva (VAS), al tempo 0 e dopo 2, 4 e 8 settimane.
Risultati
Tutte le iniezioni fusion guidate sono state eseguite con successo. In 96 su 112 iniezioni è stato raggiunto lo spazio intra-articolare (precisione: 85,7%). La VAS è diminuita significativamente dopo la procedura in tutti i pazienti, senza differenze tra chi ha ricevuto il controllo CT del posizionamento degli aghi e chi non lo ha ricevuto. Non sono state osservate complicanze maggiori.
Conclusioni
L’iniezione fusion ecografia-RM guidata delle faccette articolari artrosiche rappresenta una opzione terapeutica sicura ed efficace.
Introduction
Low back pain is a common condition, with high impact on general population. Up to 80% of adults experienced an episode of low back pain during lifetime [1], representing an issue in terms of morbility and costs. Although the existence of facetogenic pain had long been questioned, it is now generally accepted as a clinical entity [2] and facet joint osteoarthritis (FJOA) can be considered responsible for 15–40% of low back pain [3, 4]. Diagnostic imaging is proved to be an accurate and reliable tool for the assessment of FJ degenerative changes and its correlation with clinical symptomatology and pathological grading. [5, 6]. A correct diagnosis can be made with computed tomography (CT) or magnetic resonance (MR) demonstrating inflammation, swelling, or degenerative changes of FJ. In such cases, FJ injections (FJI) with local anesthetics or anti-inflammatory drugs can be made for analgesic and therapeutic purpose [4, 7].
Steroids FJI are widely used for the treatment of patients with low back pain [8]; to achieve the best therapeutic result, the needle tip should be placed as closer as possible to the intra-periarticular space, which is not always a simple procedure, also considering the possible morphostructural changes (i.e.: osteophytes) affecting degenerative joints [9].
A correct needle placement needs imaging guidance and can be achieved through different imaging methods [10].
CT and fluoroscopy are considered the gold standard to achieve accurate guidance of the needle but expose the patient to a significant radiation dose [11–13], even considering the possibility to repeat the therapeutic treatment over time. Ultrasound suffers from poor visualization of the target due to the deep location of FJ, the frequent presence of bone spurs, and the greater difficulty of interpretation by physicians who are not familiar with the ultrasound anatomy of the spine [14]. MR imaging guidance has the advantage of guiding injection without radiation, but the procedure is cumbersome and time consuming, and requires the use of more expensive MR-compatible needles [11, 12].
Therefore, it would be desirable to fuse information from different imaging modalities, combining the panoramic views and the elevated anatomical detail of the MR or CT with the ease of use and high availability of ultrasound, possibly without patient exposure to ionizing radiation.
In recent years, fusion imaging has been described and validated in numerous articles in relation to different districts and pathologies and, in particular, in interventional and musculoskeletal radiology [15, 16].
This prospective study was performed to validate the technical feasibility of real-time MR–US fusion imaging for FJ injection. Short-term outcome of corticosteroid FJI was also evaluated.
Methods
Patients
This study was approved by our institutional board; oral and written informed consent was obtained from all patients before each procedure.
From November 2014 to August 2015, 38 patients (24 females, 14 males; mean age ± SD: 64 ± 9 years, range 57–68) with clinical diagnosis of chronic low back pain and MR signs of FJOA were included in our study.
Exclusion criteria were contraindications to the administration of local anaesthetics or steroids, uncontrolled coagulopathy, pregnancy, coexistence of neoplasm, infection or spondylitis, history of previous low back injections or surgery, absolute MRI contraindications (e.g. pacemaker excluding MRI safe ones, claustrophobia, etc.).
MR signs of FJOA were defined according to Fujiwara classification [17], a four-point MRI-based grading scale ranging from mild degenerative changes to severe OA and including the following features: narrowing of the joint space, osteophytosis of articular processes, hypertrophy of articular processes subchondral erosions and subchondral cysts. [18–20]. We selected patients from grade 1 to 3 excluding patients with severe osteoarthritis (grade 4) and without carrying out stratification of patients.
Patients underwent MR fusion-assisted ultrasound-guided FJI of 0.5 ml mepivacaine hydrochloride 2% and corticosteroid (0.5 ml of methylprednisolone acetate 40 mg/ml) using an ultrasound machine (Logiq E9, GE Healthcare, Milwaukee, WI, USA) equipped with a GPS-enhanced fusion imaging technology which couples real-time B-mode images with those of the previous recent diagnostic MR examination.
In the first 28 patients, the MR fusion-assisted ultrasound-guided FJI was performed on the CT table to perform a subsequent low-dose CT scan to assess the correct positioning of the needle tip with respect to the zygapophyseal articular joint. Other 10 patients received only FJI without CT confirmation.
Patients’ pain was recorded using a visual analogue scale (VAS), at baseline and at 2, 4 and 8 weeks.
Technique
Step1: MR acquisition
MR examination was performed using an open bore (S-SCAN 0,26T, Esaote Biomedica, Genoa, Italy) with body coil; the patients were positioned in supine position for routine spinal MR examination. The protocol included axial and sagittal SE T1, FSE T2 and STIR sequences, and a volumetric HYCE sequence with TR 10 ms, TE 5 ms, ST 1,70 mm, FOV 220 × 220 mm, Flip Angle 70°.
The MR examinations were evaluated by an experienced radiologist (P.C.) with 14 years’ experience in the field of spinal diseases and musculoskeletal interventional procedures under ultrasound guidance.
Step 2: Ultrasound equipment and setting
We used the “V-Nav” fusion imaging system (GE Healthcare, Milwaukee, WI, USA), integrated into the ultrasound machine and a low-intensity magnetic field generator docked by wire to the ultrasound machine. Two small electromagnetic position sensors connected to a position-sensing unit were attached to a 3–5 MHz convex probe through a bracket (Fig. 1a).
Fig. 1.
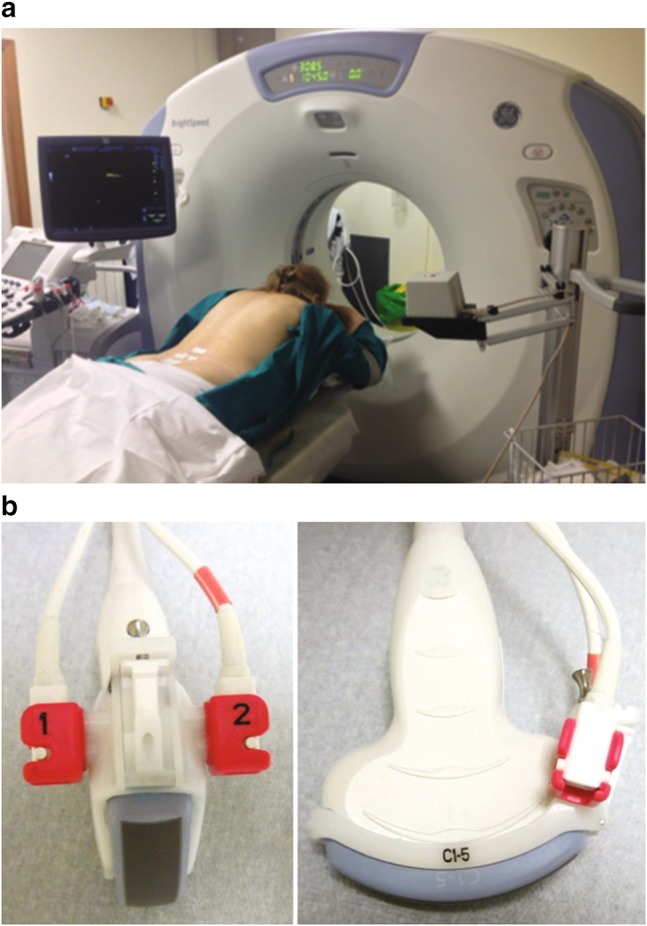
Standard ultrasound probe with bracket fitting the position sensor unity (a). Patient in prone position on the CT bed ready to perform the fusion imaging MR–US infiltration and the post-procedure low-dose CT scan. The low-field magnetic generator is visible in the foreground (b)
MR images were loaded using standard DICOM format on the US machine through CD-ROM device.
VNav software allowed us to choose the sequences to be merged with the ultrasound image, before and after calibration of the images. The software allows to upload multiple scans at the same time, provided they have the same spatial orientation. For this reason, we have developed an MRI protocol providing for adequate scans in the transverse axis [16].
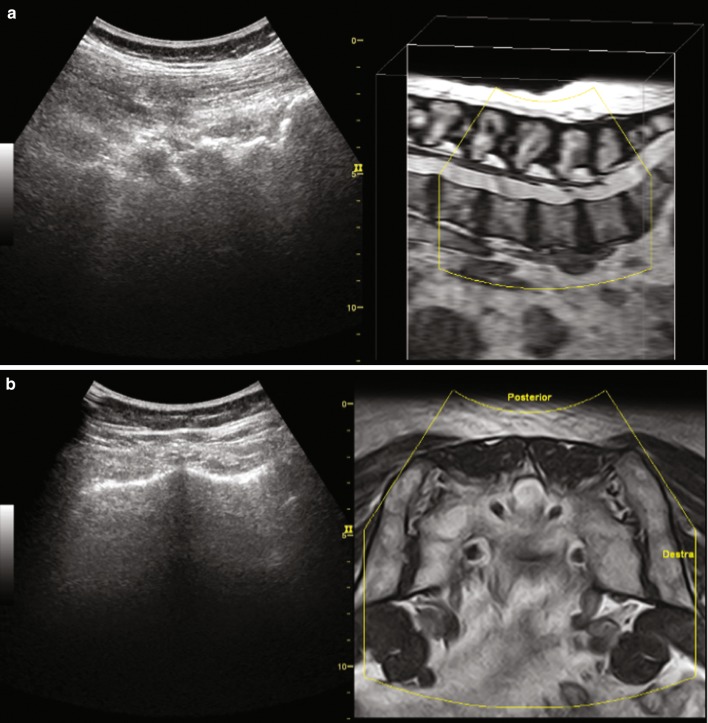
For correct superimposition of ultrasound and MR images, we defined body landmarks with both imaging techniques such as bony contours of: spinous process of the second, third, fourth and fifth lumbar vertebra, posterior superior iliac spine, first posterior sacral foramen, and the SI joint. We take care to choose body markers on small reproducible landmarks like the border of the foramina or bony eminencies of the spinous process for an accurate identification of the landmarks with both imaging techniques (Fig. 2).
Fig. 2.
MR–US fusion imaging setting procedure, showing the MR images in axial (a) and sagittal (b) orientation before alignment confirmation. You can appreciate the spinous process both on US and MR image
Image calibration occurs through two steps. In the first step, it is mandatory to find an ultrasound scan consistent with the orientation of the resonance; we placed the probe at the level of the spinous process of L5, upright and with axial orientation, after checking on MR images if there were supernumerary vertebrae or partial/complete spinal fusions.
When the MR scanning plane corresponds to the ultrasound scanning plane and the operator provides the confirmation input, the software connects the MR image to the ultrasound, allowing a synchronous motion, consistent with movements and rotations of the probe.
The second step involves calibrating end of anatomical landmarks of the two imaging methods. We have found it convenient to locate the spinous process of the lower vertebra of the joint complex to infiltrate. Now, the images are synchronized and image superimposition can be activated check the accuracy of the calibration.
Once the operator has identified the spinous process, he has to position the cursor over it and confirm the first “accuracy point”. The screen will display an icon at the selected point, at the same time both on the MR and US images, which will follow the target thanks to the virtual volume provided by the magnetic field and recorded by position sensors.
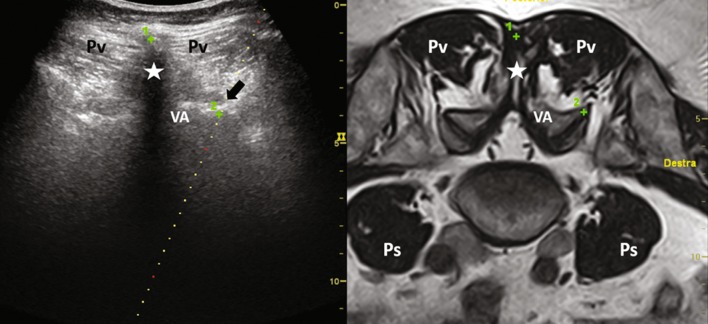
This allows the operator to have a clear view of the target (the facet joint) even on ultrasound image, otherwise almost invisible (Fig. 3).
Fig. 3.
L5–S1 level-fused images. Green crosses represent accuracy points set by operator on the MR image and automatically replicated on the US image. Pv paravertebral muscles, Star spinous process, VA vertebral arch, Ps psoas muscles
Step 3: fusion imaging injection
For the purposes of the study, we placed the patient prone on the CT bed during the infiltrative procedure.
In our protocol, we treated four FJ per patient, at L4–L5 and L5–S1 level, which are those with the highest prevalence of OA alterations, even though MR examinations showed slight degenerative changes also at other lumbar levels [21, 22].
The ultrasound-guided procedure begins with the selection of the angle of the biopsy guide and placement of biopsy trace on the target. At this point, we can proceed with the introduction of the needles, starting from the right L5–S1 FJ, then moving cranially to the right L4–L5 FJ. Now we have to rotate by 180° the probe to have the biopsy line guide on the left side, and so we have to repeat the sequence by checking in real time the actual correspondence of MR images with the US images.
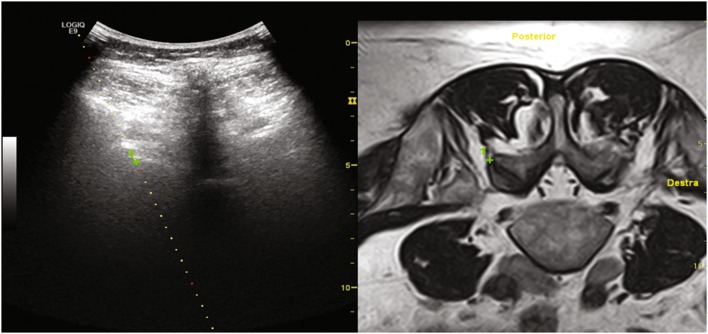
FJI was performed with 20G needles, 90 mm in length, with a solution of 0.5 ml methylprednisolone acetate 40 mg/ml (Depo-medrol, Pfizer, Italy), diluted with mepivacaine hydrochloride 2% 0.5 ml (Fig. 4).
Fig. 4.
Biopsy guide is positioned over the green cross target allowing a precise needle placement
Step 4: low-dose CT scan
CT scan for needle placement confirmation was performed on 28 patients (19 females), with a low-dose CT protocol (thickness 2.5 mm; pitch 1.675: 1; 120 kV; 65 mA; ASIR 60%).
The distance of the tip of the needle from the facet joint space was measured on the CT scan after the introduction of the four needles on the selected facet joints. We recorded the distance of 112 tips with respect to the outermost joint space, in the three planes of space and in the oblique maximal radius, obtained by rebuilding oblique planes with a dedicated workstation. In these cases, we have performed the infiltrative ultrasound-guided procedure directly on the CT bed (Fig. 1b).
In addition, the time needed to perform the whole procedure (comprehensive of MR data import, MR–ultrasound image fusion, 4 joints injection) was calculated for every therapeutic session. Data obtained in patients in whom the needle was correctly placed were compared to those in whom the needle was not correctly placed using the U Mann-Whitney test. VAS changes over time were assessed using an ANOVA multiple measure model. A P-value lower than 0.05 was considered as statistically significant.
Results
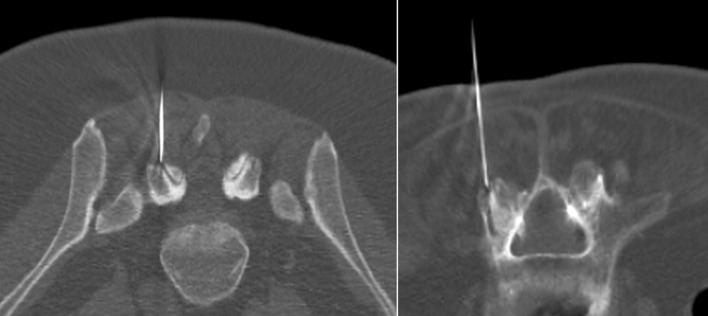
All fusion imaging-guided injections were performed successfully. 96 on 112 FJ injections resulted in the facet joint intra-articular space (accuracy: 85.7%), (Fig. 5). In the other 4 patients, the mean distance of the tip of the needle from the zygapophyseal articular border was: 3.6 ± 3.3 mm (mean ± SD; range 1.1–8.9 mm).
Fig. 5.
Post-needle insertion CT scan showed a precise needle tip position relative to intra-articular space
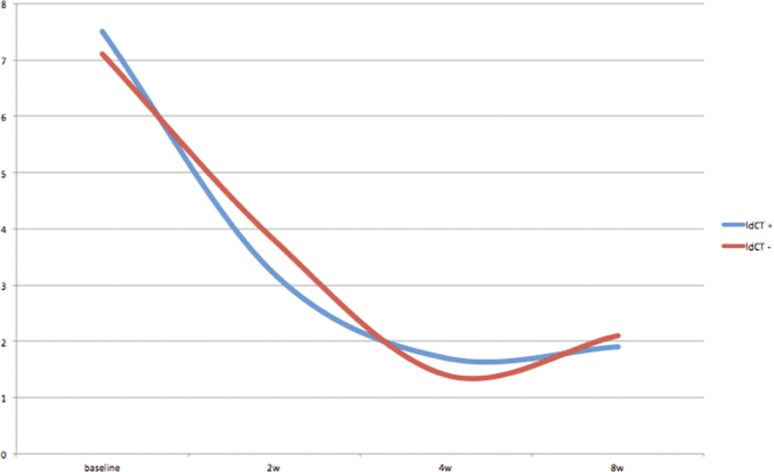
The results of the group of patients underwent a low-dose CT scan after the procedure and of the group which did not undergo a low-dose CT scan are reported in Table 1 and graphically represented in Fig. 6. The overall VAS data showed no statistically significant differences between the two groups (p = 0.765). VAS score modified significantly overtime both in patients and controls (p < 0.001).
Table 1.
VAS scale data. VAS values at different time points are expressed in mean value ± standard deviation (SD)
| Low-dose CT needle postioning control (mean ± SD) | No needle control (mean ± SD) | |
|---|---|---|
| Baseline | 7.3 ± 1.7 | 7.1 ± 1.1 |
| 2 weeks | 3.2 ± 1.5 | 3.8 ± 0.7 |
| 4 weeks | 1.7 ± 0.9 | 1.4 ± 1.3 |
| 8 weeks | 2.0 ± 1.2 | 2.3 ± 1.6 |
Fig. 6.
Chart showing pain reduction using VAS scale with no significant difference between post procedure CT-scan group (blue line) and only MR–US fusion guidance group (red line)
No significant peri-procedural complication occurred. Twelve FJI in 10 patients caused a mild subcutaneous haematoma along the path of the needle, spontaneously resolved in few days without long-term complications.
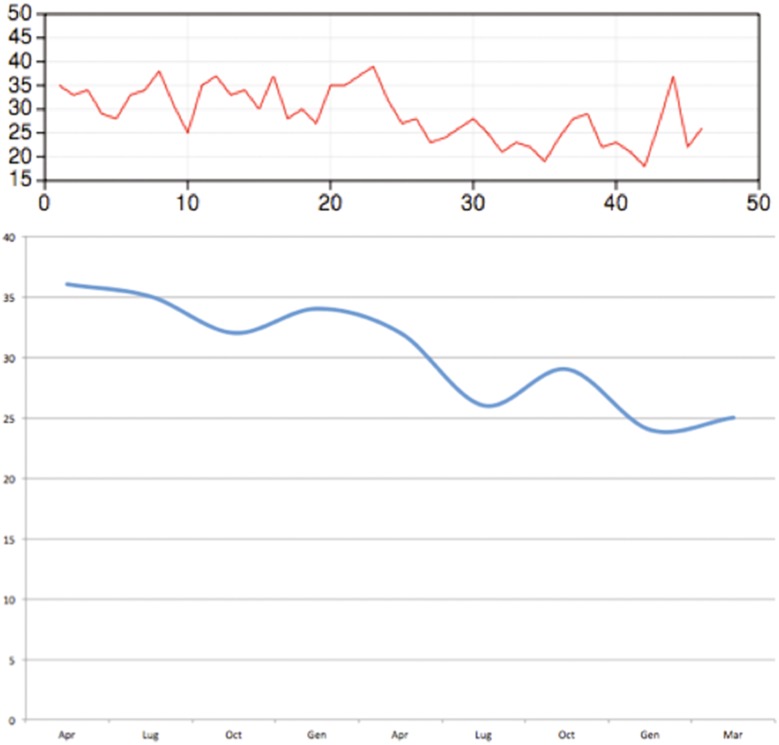
Mean procedure time was 28 min. It decreased from April 2014 to August 2015 from 36 to 25 min, in connection with the learning curve of the procedure (Fig. 7).
Fig. 7.
Learning curve of MR–US fusion-guided FJ injection. In one year procedure, time decreased from 36 to 25 min
Discussion
Low back pain has multifactorial origin; however, it has been shown that at least in some situations FJOA is a significant underlying cause, independent from health factors and disc height narrowing. [6]. Several radiological classifications of facet joints degeneration have been proposed; among these the most reliable is the Grogan classification, which is based on CT findings and ranges from uniform thick cartilage and thin cortical bone to complete absence of cartilage with marginal osteophytes and dense cortical bone greater than half the facet joint [20].
Although MR imaging is still not usually considered to be equivalent to CT for the evaluation of facet joints [23], it is the most common evaluation performed for the assessment of low back pain in clinical practice. In this setting, we used the MRI-based Fujiwara classification for the pre-treatment grading of FJOA.
FJI is an established method for the treatment of deep low back pain caused by FJOA but palpation-guided injections are inaccurate in up to 50% of cases [16, 24].
CT and fluoroscopy are the most common methods for image guidance, providing excellent visualization of anatomical landmark and therefore enabling highly accurate needle placement, but require the use of ionizing radiation, which is the serious drawback, especially in serial therapeutic procedures [25–27].
MR-guided facet joint injection therapy is a valid alternative, able to avoid radiation exposure to patients and personnel, but high cost, increased timing of the procedure and the need for MR-compatible needles have limited the clinical development of this practice [15, 16, 28, 29].
Fusion imaging is a technique that allows to synchronize and superimpose images from different modalities to assess simultaneously the area of interest through the fields of view of each technique; specifically, it provides CT or MR cross-sectional multiplanar images that correspond to the acquired real-time US images, and all images can be displayed simultaneously and in real time according to the angle of the US transducer.
Our results confirm the existing literature about the effectiveness of steroid injections for FJOA. Moreover, we found no significant differences in pain reduction between the group that carried out the post-needle insertion CT scan and the group that did not conduct any post-infiltration control. This finding confirms the technical feasibility of FJI under fusion imaging guidance and reinforces our confidence regarding the effectiveness of the FJI in terms of pain reduction, although this finding was not the primary purpose of the study.
Some authors indicate that the complete accuracy of needle placement may not be essential for satisfactory outcome [11, 12]. This statement could be true when dealing with corticosteroids and ozone that gets acceptable therapeutic effects even if you do not reach precisely the joint space due to the good spread of these substances on the periarticular space. However, a similar concept cannot be applied when hyaluronic acid is used, as these substances explicate their action only when injected directly in the joint space [30]. Thanks to its high tolerability and low side effects, the use of hyaluronic acid has increased in the local treatment of FJOA and could be suggested in patients with contraindication to corticosteroids [31, 32].
The slight but significant increase in the VAS value reported by patients after 8 weeks is indicative of the only palliative role of the treatment and, consequently, the need to repeat the infiltration over time and also to allow a good compliance to physiotherapy. The lack of ionizing radiation in the guide introduction needle is, therefore, not negligible.
In this study, we used an in-plane approach to the probe for needle insertion; this made the process simple and immediate, with a constant display of the virtual target.
In situations where exuberant osteophytosis makes this approach impossible injections can be performed with an out-of-plane approach. This approach is complex and it is more difficult to reach the target because it is not possible to have a constant visualization of the needle tip.
The fusion system allows the use of so-called virtual tracking, a system based on a position and direction sensor mounted over the needle base that generates on the ultrasound screen a track of the current position of the tip, the direction and the position that the tip will have when it reaches the ultrasound scan plane [33].
This system has been tested in other fields and its development could enable the routine use of approaches that are currently not viable [34, 35].
One of the main limitations of our study was the use of diagnostic MR scans acquired with an open bore low-field system, with patients lying in the supine position. The choice has depended on the will to recreate the most probable clinical routine scenario, in which patients perform MRI for diagnostic purposes, with no expectation of any interventional procedure at the lumbar spine or at sacrum-iliac joint. However, any kind of dataset in DICOM format could be used for fusion purposes. The patient position in which MRI was performed (supine) does not correspond, therefore, to the position in which it was carried out the infiltration (prone), and then the vertebrae of the lumbar spine will have slightly different position among them. This has been offset by the real-time calibration corrections and the use of multiple bony landmarks at each vertebra before the introduction of the needle. It is possible to speculate that the execution of MR examinations in the prone position facilitates and makes faster the calibration procedure. However this will not add any significant advantage in fusion procedure accuracy, also with the disadvantage of having to carry a dedicated examination.
A crucial point is to keep perfectly still the magnetic field generator (transmitter), and so you have to place it in such a way as not to obstruct the interventional procedures. A displacement of the generator, even minimal, entails the need to recalibrate the entire procedure.
Conclusion
In conclusion, MR and ultrasound image fusion-guided needle insertion resulted a valuable procedure, which allows for a quick, safe and effective injection of degenerative facet joint disease. It can be of specific benefit in younger patients with chronic lower back pain, in whom repeated injections are necessary over time using a single MR data set with the aim to avoid radiation exposure and reduce time of procedures. Further, this technique can be easily implemented in other applications where spinal needles are used even if a relatively short learning curve is needed to get confidence with the procedure.
Compliance with ethical standard
Conflict of interest
The authors have no conflict of interest to disclose.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. The present study was approved by the Institutional review board.
Informed consent
Informed patients’ consent was obtained for the present study.
References
- 1.Andersson GB. Epidemiology of low back pain. Acta Orthop Scand Suppl. 1998;281:28–31. doi: 10.1080/17453674.1998.11744790. [DOI] [PubMed] [Google Scholar]
- 2.Van Kleef M, Vanelderen P, Cohen SP, Lataster A, Van Zundert J, Mekhail N. Pain originating from the lumbar facet joints. Pain Pract. 2010;10(5):459–469. doi: 10.1111/j.1533-2500.2010.00393.x. [DOI] [PubMed] [Google Scholar]
- 3.Lewinnek GE, Warfield CA. Facet joint degeneration as a cause of low back pain. Clin Orthop Relat Res. 1986;213:216–222. [PubMed] [Google Scholar]
- 4.Boswell MV, Colson JD, Sehgal N, Dunbar EE, Epter R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician. 2007;10(1):229–253. [PubMed] [Google Scholar]
- 5.Zhou X, Liu Y, Zhou S, Fu XX, Yu XL, Fu CL, Zhang B, Dai M. The correlation between radiographic and pathologic grading of lumbar facet joint degeneration. BMC Med Imaging. 2016;29:16–27. doi: 10.1186/s12880-016-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suri P, Hunter DJ, Rainville J, Guermazi A, Katz JN. Presence and extent of severe facet joint osteoarthritis are associated with back pain in older adults. Osteoarthr Cartil. 2013;21(9):1199–1206. doi: 10.1016/j.joca.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manchikanti L, Pampati V, Rivera J, Fellows B, Beyer C, Damron K. Role of facet joints in chronic low back pain in the elderly: a controlled comparative prevalence study. Pain Pract. 2001;1:332–337. doi: 10.1046/j.1533-2500.2001.01034.x. [DOI] [PubMed] [Google Scholar]
- 8.Gorbach C, Schmid MR, Elfering A, Hodler J, Boos N. Therapeutic efficacy of facet joint blocks. AJR Am J Roentgenol. 2006;186:1228–1233. doi: 10.2214/AJR.04.1042. [DOI] [PubMed] [Google Scholar]
- 9.Orlandi D, Corazza A, Silvestri E, Serafini G, Savarino EV, Garlaschi G, Mauri G, Cimmino MA, Sconfienza LM. Ultrasound-guided procedures around the wrist and hand: how to do. Eur J Radiol. 2014;83(7):1231–1238. doi: 10.1016/j.ejrad.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Chaturvedi A, Chaturvedi S, Sivasankar R. Image guided lumbar facet joint infiltration in non radicular low back pain. Indian J Radiol Imaging. 2009;19:29–34. doi: 10.4103/0971-3026.44522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch MC, Taylor JF. Facet joint injection for low back pain. A clinical study. J Bone Joint Surg Br. 1986;68:138–141. doi: 10.1302/0301-620X.68B1.2934398. [DOI] [PubMed] [Google Scholar]
- 12.Boswell MV, Colson JD, Sehgal N, Dunbar EE, Epter R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician. 2007;10(1):229–253. [PubMed] [Google Scholar]
- 13.Silbergleit R, Mehta BA, Sanders WP, Talati SJ. Imaging-guided injection techniques with fluoroscopy and CT for spinal pain management. Radiographics. 2001;21(4):927–942. doi: 10.1148/radiographics.21.4.g01jl15927. [DOI] [PubMed] [Google Scholar]
- 14.Chen ECS, Mousavi P, Gill S, Fichtinger G, Abolmaesumi P. Ultrasound guided spine needle insertion. Proc of SPIE. 2010;7625:381–388. [Google Scholar]
- 15.Fritz J, Clasen S, Boss A, Thomas C, König CW, Claussen CD, Pereira PL. Real-time MR fluoroscopy-navigated lumbar facet joint injections: feasibility and technical properties. Eur Radiol. 2008;18(7):1513–1518. doi: 10.1007/s00330-008-0890-4. [DOI] [PubMed] [Google Scholar]
- 16.Freyhardt P, Hartwig T, De Bucourt M, Maurer M, Renz D, Gebauer B, Hamm B, Teichgräber UK, Streitparth F. MR-guided facet joint injection therapy using an open 1.0-T MRI system: an outcome study. Eur Radiol. 2013;23:3296–3303. doi: 10.1007/s00330-013-2940-9. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara A, Tamai K, Yamato M, An HS, Yoshida H, Saotome K, Kurihashi A. The relationship between facet joint osteoarthritis and disc degeneration of the lumbar spine: an MRI study. Eur Spine J. 1999;8:396–401. doi: 10.1007/s005860050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ewertsen C, Săftoiu A, Gruionu LG, Karstrup S, Nielsen MB. Real-time image fusion involving diagnostic ultrasound. AJR. 2013;200(3):249–255. doi: 10.2214/AJR.12.8904. [DOI] [PubMed] [Google Scholar]
- 19.Wong-On M, Til-Pérez L, Balius R. Evaluation of MRI-US fusion technology in sports-related musculoskeletal injuries. Adv Ther. 2015;32(6):580–594. doi: 10.1007/s12325-015-0217-1. [DOI] [PubMed] [Google Scholar]
- 20.Grogan J, Nowicki BH, Schmidt TA, Haughton VM. Lumbar facet joint tropism does not accelerate degeneration of the facet joints. Am J Neuroradiol. 1997;18:1325–1329. [PMC free article] [PubMed] [Google Scholar]
- 21.Gellhorn AC, Katz JN, Suri P. Osteoarthritis of the spine: the facet joints. Nat Rev Rheumatol. 2013;9(4):216–224. doi: 10.1038/nrrheum.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalichman L, Li L, Kim DH, Guermazi A, Berkin V, O’Donnell CJ, Hoffmann U, Cole R, Hunter DJ. Facet joint osteoarthritis and low back pain in the community-based population. Spine. 2008;33(23):2560–2565. doi: 10.1097/BRS.0b013e318184ef95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weishaupt D, Zanetti M, Boos N, Hodler MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal Radiol. 1999;28(4):215–219. doi: 10.1007/s002560050503. [DOI] [PubMed] [Google Scholar]
- 24.Cluff R, Mehio AK, Cohen SP, Chang Y, Sang CN, Stojanovic MP. The technical aspects of epidural steroid injections: a national survey. Anesth Analg. 2002;95:403–408. doi: 10.1097/00000539-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich KM, Nemec S, Peloschek P, Pinker K, Weber M, Trattnig S. The prevalence of lumbar facet joint edema in patients with low back pain. Skelet Radiol. 2007;36:755–760. doi: 10.1007/s00256-007-0293-7. [DOI] [PubMed] [Google Scholar]
- 26.Gallucci M, Limbucci N, Paonessa A, Splendiani A. Degenerative disease of the spine. Neuroimaging Clin N Am. 2007;17:87–103. doi: 10.1016/j.nic.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Nawfel RD, Judy PF, Silverman SG, Hooton S, Tuncali K, Adams DF. Patient and personnel exposure during CT fluoroscopy-guided interventional procedures. Radiology. 2000;216(1):180–184. doi: 10.1148/radiology.216.1.r00jl39180. [DOI] [PubMed] [Google Scholar]
- 28.Fritz J, Pereira PL. MR-guided pain therapy: principles and clinical applications. Rofo. 2007;179:914–924. doi: 10.1055/s-2007-963200. [DOI] [PubMed] [Google Scholar]
- 29.Fritz J, Henes JC, Thomas C, Clasen S, Fenchel M, Claussen CD, Lewin JS, Pereira PL. Diagnostic and interventional MRI of the sacroiliac joints using a 1.5-T open-bore magnet: a one-stop-shopping approach. AJR. 2008;191(6):1717–1724. doi: 10.2214/AJR.08.1075. [DOI] [PubMed] [Google Scholar]
- 30.Orlandi D, Corazza A, Fabbro E, Ferrero G, Sabino G, Serafini G, Silvestri E, Sconfienza LM. Ultrasound-guided percutaneous injection to treat de Quervain’s disease using three different techniques: a randomized controlled trial. Eur Radiol. 2014;25(5):1512–1519. doi: 10.1007/s00330-014-3515-0. [DOI] [PubMed] [Google Scholar]
- 31.Colen S, Haverkamp D, Mulier M, van den Bekerom MP. Hyaluronic acid for the treatment of osteoarthritis in all joints except the knee: what is the current evidence? BioDrugs. 2012;26(2):101–112. doi: 10.2165/11630830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.DePalma MJ, Ketchum JM, Queler ED, Trussell BS. Prospective pilot study of painful lumbar facet joint arthropathy after intra-articular injection of hylan G-F 20. PMR. 2009;1(10):908–915. doi: 10.1016/j.pmrj.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Turtulici G, Orlandi D, Corazza A, Sartoris R, Derchi LE, Silvestri E, Baek JH. Percutaneous radiofrequency ablation of benign thyroid nodules assisted by a virtual needle tracking system. Ultrasound Med Biol. 2014;40(7):1447–1452. doi: 10.1016/j.ultrasmedbio.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Mauri G, Solbiati L. Virtual navigation and fusion imaging in percutaneous ablations in the neck. Ultrasound Med Biol. 2015;41(3):898. doi: 10.1016/j.ultrasmedbio.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Orlandi D, Turtulici G. Reply regarding Virtual navigation and fusion imaging in percutaneous ablations in the neck. Ultrasound Med Biol. 2015;41(3):899. doi: 10.1016/j.ultrasmedbio.2014.11.013. [DOI] [PubMed] [Google Scholar]