Abstract
Resistance to cancer chemotherapies leads to deadly consequences, yet current research focuses only on the roles of somatically acquired mutations in this resistance. The mutational status of the germline is also likely to play a role in the way cells respond to chemotherapy. The carrier status for the POLB rs3136797 germline mutation encoding P242R DNA polymerase beta (Pol β) is associated with poor prognosis for lung cancer, specifically in response to treatment with cisplatin. Here, it is revealed that the P242R mutation is sufficient to promote resistance to cisplatin in human cells and in mouse xenografts. Mechanistically, P242R Pol β acts as a translesion polymerase and prefers to insert the correct nucleotide opposite cisplatin intrastrand crosslinks leading to the activation of the nucleotide excision repair (NER) pathway, removal of crosslinks, and resistance to cisplatin. In contrast, wildtype (WT) Pol β preferentially inserts the incorrect nucleotide initiating mismatch repair and cell death. Importantly, in a mouse xenograft model, tumors derived from lung cancer cells expressing WT Pol β displayed a slower rate of growth when treated with cisplatin, whereas tumors expressing P242R Pol β had no response to cisplatin. Pol β is critical for mediating crosstalk in response to cisplatin and the current data strongly suggests that the status of Pol β influences cellular responses to crosslinking agents, and that Pol β is a promising biomarker to predict responses to specific chemotherapies. Finally, these results highlight that the genetic status of the germline is a critical factor in the response to cancer treatment.
Implications: Pol β has prognostic biomarker potential in the treatment of cancer with cisplatin and perhaps other intrastrand crosslinking agents.
Introduction
The faithful replication and repair of DNA is crucial to maintaining the genomic stability of cells. Because of the integral role genomic integrity has on cell survival and function, drugs that target and damage DNA are often used to kill tumor cells. Platinating agents, such as cisplatin, are commonly used chemotherapeutics that act by damaging DNA and cisplatin is the first line of therapy for many cancers including lung, testicular, ovarian, and head and neck cancers (1). After uptake of the drug into the nucleus of the cell, platinum-DNA adducts are formed (for review see (2)). The major DNA adducts of cisplatin are intrastrand crosslinks formed between two purine bases, the predominant lesion being the 1,2-d(GpG) intrastrand crosslink (Pt-GG) (3). These Pt-GG crosslinks distort the helix of the DNA and are recognized by the nucleotide excision repair (NER) machinery. NER-mediated repair is important for genomic integrity of cells because replicative polymerases cannot bypass the intrastrand crosslink, leading to blocked replication and apoptosis (4).
Treatment with cisplatin, although initially quite effective, becomes less successful as cells develop intrinsic and acquired resistance (5). There are several mechanisms leading to cisplatin resistance in cells including the bypass of the lesion by specialized polymerases (6-8). These specialized polymerases, known as translesion polymerases, avoid the replicative block of the cisplatin intrastrand adduct by catalyzing DNA synthesis using the adduct as a template, after which the replicative polymerases can resume polymerization. A number of studies have demonstrated that polymerases eta and beta (Pol η and β, respectively) are able to successfully bypass Pt-GG, and their expression has been implicated in cisplatin resistance (4,7,9,10).
Besides its platinum bypass capabilities, Pol β is the main polymerase in base excision repair (BER), the pathway involved in repairing single base damage typically resulting from alkylation or oxidation (11). Pol β is mutated in 40% of colorectal tumors (12) and some of these mutations have been found to induce cellular transformation (13-15). Besides cancer-associated variants, there are two reported germline single nucleotide polymorphisms (SNPs) in the POLB gene (16,17). One of these SNPs, encoding Pol β P242R, is present in 2.4% of the human population (16) and its expression in human cells induces cellular transformation via genomic instability (18). However, little is known about the effect of Pol β P242R on human health. DNA repair variants in the germline are also present in the tumor and may impact cancer therapies. This is of great interest because treatment with drugs that target the repair defect of the tumor personally tailor cancer therapies to the individual. Many chemotherapeutics are designed to damage DNA and the presence of DNA repair variants can modulate how effectively DNA is repaired, leading to a variety of different outcomes ranging from tumor cell kill to resistance, the latter of which can lead to more aggressive cancer. Importantly, the carrier status of the P242R Pol β germline variant can be associated with prognosis, specifically lung cancer patients carrying this variant have a worse prognosis when they are treated with cisplatin (19).
In this study, we sought to determine whether the presence of the P242R Pol β germline variant could alter cellular responses to chemotherapies. We found that expression of P242R in human cancer cells and in a mouse xenograft model, as well as its endogenous expression in human lymphoblastoid cell lines, confers resistance to cisplatin. Biochemically, P242R preferentially inserts the correct dCTP opposite the Pt-GG crosslink, allowing NER to remove, and efficiently repair the damage leading to cellular resistance. In contrast, WT Pol β preferentially inserts the incorrect nucleotide, activating the mismatch repair (MMR) pathway, ultimately leading to apoptosis. The choice of the correct versus incorrect nucleotide may coordinate crosstalk between Pol β and either NER or MMR to determine the fate of the cell. Together, our results suggest the Pol β, and specifically the germline P242R Pol β SNP, can serve as a predictor of the response to cisplatin-based therapies.
Materials and Methods
Chemicals
Cisplatin and mitomycin C (MMC) were obtained from Sigma-Aldrich. The PARP inhibitor olaparib (AZD2281) was obtained from Selleckchem.
Plasmids and Cloning
Human Pol β cDNA was cloned into the pET28a expression plasmid or into the pRVYTet retroviral vector as described previously (13,18,20). The P242R and E295K mutations were introduced using site-directed mutagenesis as described previously (13,18).
Cell Lines and Cell Culture
The GP2-293 cell line was maintained in high-glucose DMEM supplemented with 10% fetal bovine serum (FBS), 1% L-glutamine, 1% penicillin-streptomycin (P/S), 1 mM HEPES and 200 μg/ml hygromycin B (Invitrogen). A549 cells were maintained in the same media as above except 220 μg/ml hygromycin B was used for selection. The breast cancer, MCF7, cell line was maintained in RPMI-1640 medium (Invitrogen) supplemented with 10% FBS, 1% P/S and 100 μg/ml hygromycin B. The DLD1, DLD1 chromosome 2 transfer lines, HCT116 and HCT116 chromosome 3 transfer lines were gifts from Thomas Kunkel (NIEHS). DLD1 cells were maintained in DMEM/F12 medium (Invitrogen) supplemented with 10% FBS, 1% P/S, 0.4 mg/ml G418 (Invitrogen), and 50 μg/ml hygromycin B. HCT116 cells were maintained in high-glucose DMEM supplemented with 10% FBS, 1% P/S, 0.5 mg/ml G418 (Invitrogen), and 100 μg/ml hygromycin B. XP20S and XP20S+ cells complemented with XPA cDNA (XP20S(pCAH19WS)) were obtained from Kenneth Kraemer and were maintained in high glucose DMEM supplemented with 10% FBS, 1% P/S, and 100 μg/ml hygromycin B. Human lymphoblast lines were a kind gift from Kenneth Kidd (16). These cells were maintained in RPMI-1640 supplemented with 15% FBS, 1% P/S, and 1% L-glutamine. All cells were grown at 37°C in a 5% CO2 humidified incubator. A more detailed description of cell lines is included in the Supplemental Experimental Procedures. Human Pol β WT, P242R, or E295K constructs were packaged into retrovirus using the GP2-293 packaging line as previously described (18). For selection of pools, cells with the integrated construct were selected hygromycin B at the concentrations described above and expression was verified by Western blot.
Western blotting
Approximately 80-90% confluent cells were harvested by scraping with boiling SDS loading buffer (50 mM Tris pH 6.8, 100 mM DTT, 2% SDS 10% glycerol). Lysates were boiled for 5 minutes and run on a 10% SDS-PAGE gel, transferred to nitrocellulose, and probed using monoclonal mouse anti-Pol β antibody (Abcam #1831) or monoclonal HA antibody (Covance #MMS-101P). Blots were imaged using Bio Rad ChemiDoc™ XRS+ and quantified using Image Lab Software.
NCI Approved Oncology Drugs Set II Screen
MCF7 cells expressing either WT or P242R Pol β were screened using a compound library of 85 FDA-approved chemotherapeutics (NCI Approved Oncology Drugs Set II) (http://dtp.nci.nih.gov/). A more detailed description of the assay is included in the Supplemental Materials and Methods.
Clonogenic Survival
Cells were seeded at a density of 2.2 × 105 into 60 mm dishes and fresh drug-free media was given the following day. After the cells were seeded for 48 h, the cells were treated with varying concentrations of cisplatin for 24 h, MMC for 4 h, or UV-B. After treatment, cells were trypsinized, serially diluted, and replated. Cells were grown for 10-14 days before staining with 0.25% crystal violet in 80% methanol. Colonies greater than 50 cells were counted by eye.
In Vivo Tumor Xenografts
The mouse xenograft experiments were performed for a fee-for-service by the Antitumor Assessment Core Facility at Memorial Sloan-Kettering Cancer Center under an approved IACUC protocol. Briefly, human lung cancer xenografts expressing either WT or P242R Pol β were implanted by subcutaneous injection of 1x107 A549 cells with matrigel. Mice were placed into treatment groups of 5 mice per group: WT Pol β vehicle, WT Pol β cisplatin, WT Pol β MMC, P242R Pol β vehicle, P242R Pol β cisplatin, and P242R Pol β MMC. After tumors had grown to a size of 100-150 mm3, mice were treated with intraperitoneal injections of saline, 4 mg/kg cisplatin, or 5 mg/kg MMC once per week. Weights and tumor growth were monitored at least twice per week.
Pulldowns
Pulldown assays using whole cell extract prepared from Pol β−/− MEFs (14) were performed by incubating 20 μg of purified His-tagged Pol β protein to 100 μg Pol β−/− MEF extract on ice for 15 min in Ni Pulldown buffer (50 mM Tris-HCl, pH 7.6, 75 mM KCl 1 mM DTT, 10 mM imidazole, and 0.1% IGEPAL 630). Equilibrated nickel beads (Qiagen) were added and samples were rocked for 1 h at 4°C. Beads were washed 5 times with Ni Pulldown buffer, an equal volume of 2X SDS sample buffer was added, samples were boiled for 5 min, and separated by 10% SDS-PAGE. Following the transfer, membranes were probed for XPA (antibody a kind gift from Faye Rogers, Yale University).
Co-immunoprecipitations
Cells were plated at a density of 2.5 × 106 cells per 150 mm dish and allowed to attach overnight. Cells were treated with cisplatin for 24 h before harvesting in 100 μl of IP lysis buffer (50 mM Tris-HCl, pH 7.2, 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 10% glycerol, and 0.5% IGEPAL 630 supplemented with protease inhibitors). Lysates were incubated in IP lysis buffer for 30 min on ice, centrifuged at 14,000 g for 15 min, and supernatants were transferred to new tubes. Protein concentrations were determined by measuring absorbance (562 nm) after the addition of BCA protein assay reagent (Thermo-Fisher Scientific) using BSA as a reference standard. 1000 μg of lysate was incubated overnight with anti-HA antibody in 1 ml total volume. After incubation, 30 μl of equilibrated Protein A/G beads (Santa Cruz Biotechnology, Inc) were added and incubated for 3-4 hours. Beads were rinsed 3 times with IP lysis buffer, 2X SDS buffer was added and samples were boiled for 5 min before separation by SDS-PAGE. Membranes were probed with XPA and HA overnight. Bands were quantified and normalized to HA.
Whole Cell Extract Binding Assay
Cells were plated at a density of 2.5 × 106 cells per 150 mm dish. The following day cells were left untreated, treated with cisplatin or PARP inhibitor, or co-treated with cisplatin and PARP inhibitor for 24 h. After treatment, cells were trypsinized and pelleted, washed two times with ice-cold PBS, and resuspended in buffer LB (150 mM NaCl, 50 mM HEPES, pH 7.6, 1 mM MgCl2, 0.1% (v/v) Triton X-100, 1 mM DTT, 1 mM EDTA supplemented with protease inhibitors) at a concentration of 6.8 × 107 cells/ml. Lysates were incubate on ice for 10 min and cleared by centrifugation at 14,000 g for 5 min. Dynabeads® MyOne™ Streptavidin C1 beads (100 μg) were washed three times with buffer LB. 50 nM of biotinylated DNA was added to a final volume of 250 μl and incubated at 25°C for 30 min. Beads were washed three times with buffer LB before incubating with 45 μl whole cell extract for 1 h at 4°C in a total volume of 1 ml. Beads were washed three times with buffer LB, resuspended in 40 μl of 1X SDS loading buffer, boiled for 5 min, and samples were resolved by SDS-PAGE. After transfer, the blots were incubated with antibody against HA or XPA overnight at 4°C. Bands were quantified and normalized to the 10% input.
γH2AX Flow Cytometry
Cells expressing WT or P242R Pol β were plated at a density of 4 × 105 cells per 10 cm dish and were treated in the presence or absence of cisplatin for 24 h. Cells were rinsed with PBS and then replaced with fresh media and allowed to recover for 0 h and 48 h post treatment. Cells were harvested by trypsinization, washed once with 1% BSA in PBS, and pelleted. The pellet was resuspended by adding 70% ice cold ethanol dropwise while vortexing. Cells were fixed overnight at −20°C. The cells were incubated with primary phospho-γH2AX antibody (Millipore 05-636) 1:500 overnight at 4°C. Following the incubation, cells were washed twice with 1% BSA in PBS and incubated with anti-mouse secondary antibody conjugated to FITC 1:500 for 1 h at room temperature. Cells were washed twice with 1% BSA in PBS and resuspended in 400 μl PI/RNase staining buffer (BD Pharmingen). Fluorescence was analyzed by flow cytometry using the BD FACSCalibur and analyzed using FlowJo 8.8.6 software.
Annexin
Cells expressing WT or P242R Pol β were plated at a density of 1 × 105 cells per 10 cm dish and were treated in the presence or absence of cisplatin for 24 h. Cells were rinsed then with PBS and replaced with fresh media and allowed to recover for 0 h and 96 h post treatment. Media was collected, cells were washed with PBS, and harvested by centrifugation. The pellet was washed once with PBS and resuspended in 1X Annexin V binding buffer (BD Biosciences) at a concentration of ~106 cells/ml. Cells were incubated with PE Annexin V and 7-AAD according to manufacturer’s instructions and analyzed by flow cytometry using the BD FACSCalibur and analyzed using FlowJo 8.8.6 software.
Protein Expression and Purification
pET28a plasmids with human WT or P242R Pol β cDNA were transformed into Rosetta(DE3) cells (Novagen). Protein expression was induced by the addition of isopropyl β-D-thiogalactopyranoside (IPTG) (American Bioanalytical) to a final concentration of 1 mM and incubated at 37°C for two hours. Cells were harvested by centrifugation. Protein induction was verified by using 10% SDS-PAGE stained with Coommassie Blue. WT and P242R Pol β proteins were purified using fast protein liquid chromatography to >90% homogeneity based on Coomassie Blue staining of 10% SDS-PAGE gels. More details regarding the purification is available in the Supplemental Experimental Procedures.
Preparation of DNA Substrates
Oligonucleotides were synthesized by the W.M. Keck facility and purified by PAGE as described previously (21). More details regarding the purification are available in the Supplemental Experimental Procedures.
Gel Electrophoretic Mobility Shift Assay (EMSA)
The DNA binding constant was determined by gel electrophoretic mobility shift assay as described previously (21,22).
Platin Bypass Assay
Radiolabeled platinated and mock DNA (50 nM) and Pol β (750 nM) were combined with varying concentrations (0-2000 μM) of either the correct dCTP or incorrect dATP and 10 mM MgCl2 at 37°C. For WT and P242R Pol β, 750 nM protein was sufficient to bind 95% of DNA as was determined by EMSA. Reactions were performed on a time course from 0-120 min and were quenched by the addition of 0.5 M EDTA. The reaction products were separated on a 20% denaturing polyacrylamide gels, visualized, and quantified using a Storm 860 Phosphorimager with ImageQuant software. The concentration of extended product was plotted as a function of time and the data were fit to a single-exponential curve:
where A is the amplitude, kobs is the observed rate constant (23).
Statistics
Two-tailed t-tests and two-way analysis of variance (ANOVA) with Bonferroni’s post hoc test were used as appropriate to determine whether the mean of each cell line was different from the empty vector cells. All statistics were performed using GraphPad Prism version 5 (GraphPad Software, San Diego, CA). Data are represented as mean ± SEM.
Results
Cells expressing P242R are resistant to cisplatin
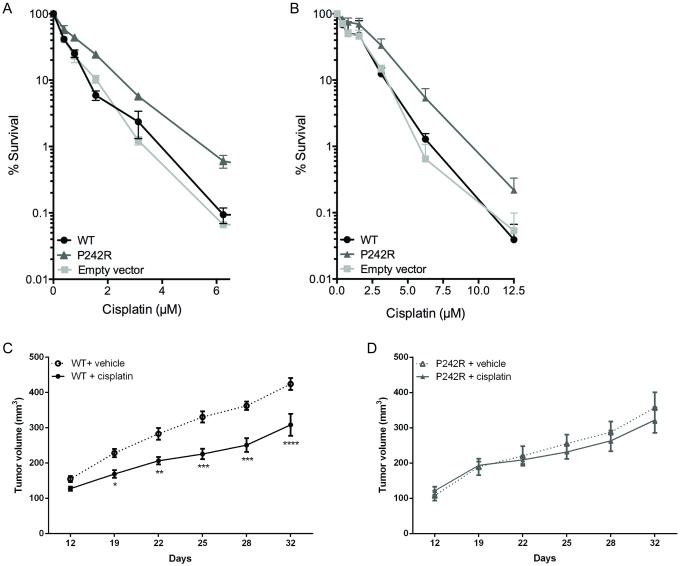
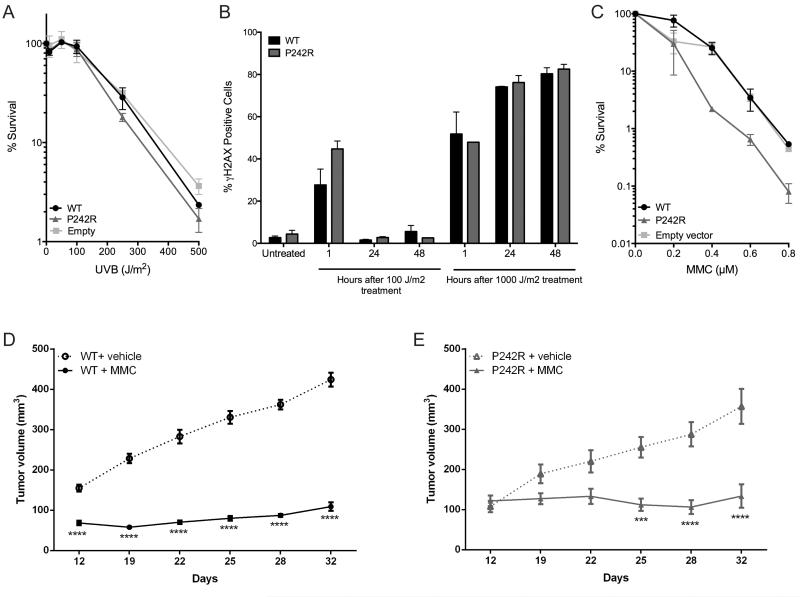
The rs3136797 POLB germline SNP encoding P242R Pol β is present in 2.4% of the population and our previous study suggests that carriers of this variant may have increased cancer susceptibility (18). We sought to determine if the presence of P242R Pol β could alter cellular responses to chemotherapies and serve as a biomarker for specific cancer therapies. We stably expressed WT or P242R Pol β in the MCF7 breast cancer cells and assessed their responses to 24 h or 72 h treatments with the 85 FDA-approved chemotherapies in the NCI Oncology Set II library using a cell viability screen. P242R-expressing cells were resistant to all platinating agents, including cisplatin, carboplatin, and oxaliplatin at one or both time points (Supplemental Table 1). Given that lung cancer patients carrying this variant have a worse prognosis when treated with cisplatin (19), we decided to focus our studies on that drug. The resistance to cisplatin was confirmed by performing dose-response curves measuring both cell viability (Figure S1) and clonogenic survival (Figure 1A) in the MCF7 cells. The cisplatin resistance exhibited by P242R-expressing cells was not cell type specific as this phenotype was also observed in the lung adenocarcinoma line, A549 (Figure 1B), nor was it due to different levels of expression of the protein in cells (Figure S2). The effect of the P242R on cisplatin even in the presence of the endogenous WT enzyme is highly suggestive that P242R acts in a dominant negative manner. This is of great importance since the P242R variant is likely to be a heterozygous mutation in individuals (16).
Figure 1. Cells expressing P242R are resistant to cisplatin in vitro and in vivo.
MCF7 (A) or A549 (B) cells expressing WT or P242R Pol β or containing empty vector were treated with varying concentrations of cisplatin (0-12.5 μM). Clonogenic survival assays were performed, and data are presented as mean ± SEM of the percent survival (n = 3-6). C-D. Mouse xenografts of A549 tumors expressing either WT (circles) or P242R (triangles) Pol β were treated by intraperitoneal injection once per week with either saline (dashed lines, open symbols) or 4 mg/kg cisplatin (solid line, closed symbols). Tumor growth measurements are presented as mean ± SEM (n=5). *, **, ***, or **** represent p<0.05, 0.01, 0.001, or 0.0001, respectively, comparing cisplatin-treated tumors to saline at each time point.
Lung cancer xenograft tumors expressing P242R Pol β displayed no response when treated with cisplatin
To examine the effect of cisplatin therapy in vivo, we implanted A549 cells expressing either WT or P242R Pol β into nude mice. When the tumors reached 100 mm2, they were treated with either vehicle (saline) or cisplatin (4 mg/kg by intraperitoneal injection) once per week. WT Pol β tumors treated with cisplatin had a significantly slower rate of growth compared to saline-treated tumors by day 12 (Figure 1C). In contrast, tumors expressing P242R Pol β remained the same size regardless of treatment with saline or cisplatin (Figure 1D). These data demonstrate that unlike WT Pol β, mouse xenografts expressing P242R Pol β do not respond to cisplatin treatment in vivo.
NER is required for P242R-mediated cisplatin resistance
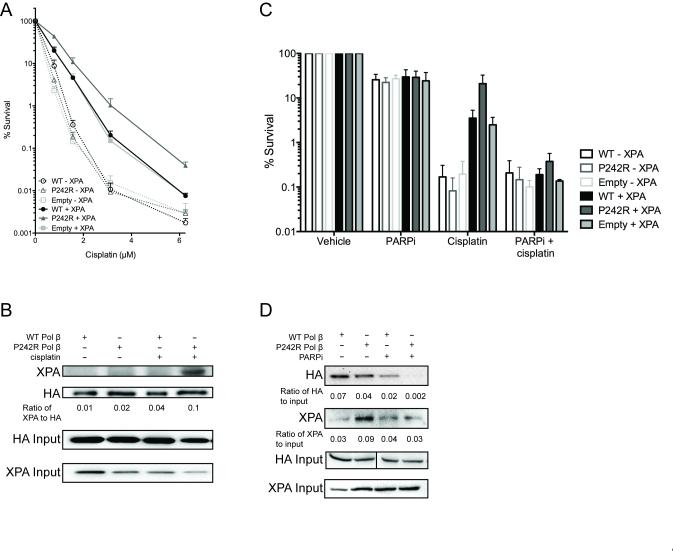
Next, we set out to elucidate the mechanism of cisplatin resistance conferred by P242R Pol β. NER is the primary pathway that repairs intrastrand crosslinks induced by cisplatin (24). We used an NER-deficient cell line, XP20S, derived from an individual with xeroderma pigmentosum group A that harbors a mutated XPA gene, resulting in decreased NER capacity (25). We expressed WT and P242R in XP20S cells (−XPA) and in XP20S cells complemented with functional XPA (+XPA) to test whether an intact NER pathway was required for P242R Pol β to confer cisplatin resistance. In the cell lines complemented with the XPA gene, P242R-expressing cells are resistant to cisplatin (Figure 2A, solid lines; filled triangles). In the absence of functional XPA protein in the XPA deficient XP20S cells, (dashed lines; open symbols), all cells are sensitive to cisplatin due to the loss of NER function. However, within the NER-deficient background there is no difference between WT and P242R expressing cells suggesting that in the absence of functional NER, P242R are no longer resistant to cisplatin (Figure 2A, dashed lines; open symbols). Together, these data indicate that P242R-mediated resistance to cisplatin requires an intact NER pathway.
Figure 2. NER is required for P242R-mediated cisplatin resistance.
A. XP20S cells deficient in XPA (−XPA; dashed lines, open symbols) or complemented with XPA (+XPA; solid lines, filled symbols) expressing WT, P242R, or empty vector were treated with various concentrations of cisplatin (0-6.25 μM). Clonogenic survival assays were performed and data are presented as mean ± SEM of the percent survival (n = 3). B. Whole cell extracts from A549 cells expressing either HA-tagged WT or P242R Pol β were immunoprecipated with anti-HA antibody as described in Experimental Procedures. Samples were separated by SDS-PAGE and the membrane was probed with antibodies against XPA or HA. The ratio of XPA to HA was quantified and shown under the blot. Co-immunoprecipitations were performed 3 times. C. XP20S deficient in XPA (−XPA; open bars) or complemented with XPA (+XPA; solid bars) expressing WT, P242R, or empty vector were treated with 1.6 μM cisplatin in the presence or absence of 10 μM PARPi (olaparib; AZD2281). Clonogenic survival assays were performed and data are presented as mean ± SEM of the percent survival (n = 3). D. A whole cell extract binding assay was performed with biotinylated Pt-GG DNA and lysates from A549 cells expressing either HA-tagged WT or P242R Pol β as described in Experimental Procedures. Westerns were performed and membranes were probed with anti-HA or anti-XPA antibodies. The ratios of HA or XPA to 10% HA input were quantified and are shown under the blots (n=3). XPA input is also shown.
The activation of P242R Pol β and XPA to cisplatin crosslinks is dependent on PARP1
To further interrogate how NER is linked to P242R-mediated cisplatin resistance, we first performed pulldowns with whole cell extracts from Pol β−/− MEFs and purified WT or P242R Pol β proteins to test whether Pol β interacts with XPA in vitro. As shown in Figure S3, both WT and P242R Pol β physically interact with XPA at similar levels. Next, we queried whether this interaction was physiological and occurred in cells, and if it was affected by treatment with cisplatin. We performed co-immunoprecipitations in A549 cells expressing either WT or P242R Pol β treated in the presence or absence of cisplatin. There was little detectable XPA interacting with WT or P242R Pol β in the absence of cisplatin treatment (Figure 2B, lanes 1 and 2). Strikingly, there was a strong interaction between P242R and XPA in cisplatin-treated cells (lane 4), but not in WT-expressing cells (lane 3). This observation suggests that the interaction between the P242R Pol β germline variant protein and XPA, but not WT Pol β and XPA, was dependent on cisplatin treatment. This interaction may activate the NER machinery to then repair cisplatin damage.
We next addressed how XPA binds the cisplatin intrastrand crosslink in P242R Pol β cells after treatment with cisplatin. PARP1 activity is required for the association of between XPA and PARP1, and for the recruitment of other NER proteins to the site of UV-induced DNA damage (26-28). We treated XP20S and the XPA complemented XP20S cells with the PARP inhibitor olaparib (AZD2281) concurrently with cisplatin to determine if PARP1 mediated the NER-dependent cisplatin resistance observed in P242R-expressing cells. Treatment with olaparib alone had only a modest effect on overall cell survival. NER-proficient cells (+XPA) expressing P242R and co-treated with olaparib and cisplatin were no longer resistant to cisplatin and had similar cell survival as the NER-deficient cells (Figure 2C). In contrast, treatment with olaparib and cisplatin did not differ from treatment with cisplatin alone in the XPA deficient cells (Figure 2C). We next queried whether PARP1 activity helped direct P242R Pol β to the Pt-GG crosslinks. We performed whole cell extract binding assays with biotin-labeled Pt-GG crosslinked DNA using extracts from A549 cells expressing either WT or P242R Pol β. Cells were treated with olaparib and the extracts were isolated and incubated with streptavidin beads bound to the biotinylated DNA substrate. Western analysis of HA-tagged Pol β revealed that although similar amounts of WT and P242R Pol β were bound to the DNA with the Pt-GG crosslink, there was much less P242R bound compared to WT in the presence of olaparib (Figure 2D). In addition, three times more XPA protein was bound to the DNA in lysates prepared from P242R-expressing cells compared to WT-expressing cells, and this interaction was abrogated in cells treated with olabarib (Figure 2D). Together, these data demonstrate a requirement for catalytically active PARP1 for P242R Pol β and XPA to bind the DNA at sites of cisplatin induced intrastrand crosslinks, leading to P242R-mediated cisplatin resistance.
P242R expressing cells have enhanced repair of cisplatin damage
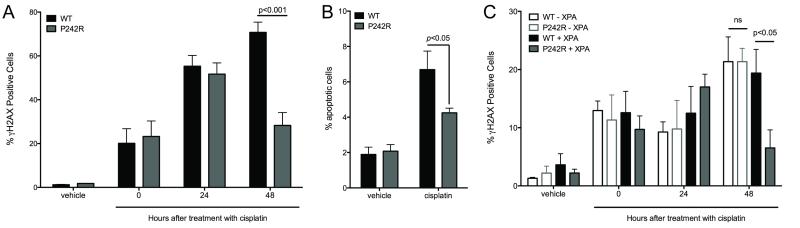
Our results suggest that P242R Pol β is recruited to the Pt-GG adduct, along with XPA, to facilitate its repair. We next measured the levels of γH2AX that were induced upon treatment of the cells with cisplatin, as an indicator of the presence of DNA breaks. After treatment with cisplatin, equivalent numbers of WT and P242R-expressing cells stained with γH2AX. However, after 48 hours of recovery, the levels of γH2AX-positive P242R cells were significantly decreased compared to WT cells, indicating that WT- but not P242R-expressing cells accumulate DNA breaks (Figure 3A) upon treatment with cisplatin. Further, the inefficient repair of cisplatin-induced DNA breaks leads to a statistically significant increase in apoptosis in the WT cells (Figure 3B). The repair in the P242R-expressing cells is mediated by NER since we do not observe a decrease in γH2AX positive cells in a NER-deficient background (Figure 3C; open bars).
Figure 3. Cisplatin-induced damage is repaired more efficiently in P242R-expressing cells.
A-B. A549 cells expressing WT or P242R were treated with 6.25 μM cisplatin for 24 h and allowed to recover for 0-96 h. Double-strand breaks (A) or apoptotic cells (B) were measured by staining with γH2AX antibody or Annexin V, respectively using flow cytometry. C. XP20S cells deficient in XPA (−XPA; open bars) or complemented with XPA (+XPA; filled bars) expressing WT or P242R were treated with 1.6 μM cisplatin and allowed to recover for 0-48 h. Double-strand breaks were measured by staining with γH2AX antibody using flow cytometry. All data are presented as mean ± SEM (n = 3-4). Two-way ANOVA with Bonferroni’s post hoc test was performed to determine significance.
Mismatch repair is required for WT sensitivity to cisplatin
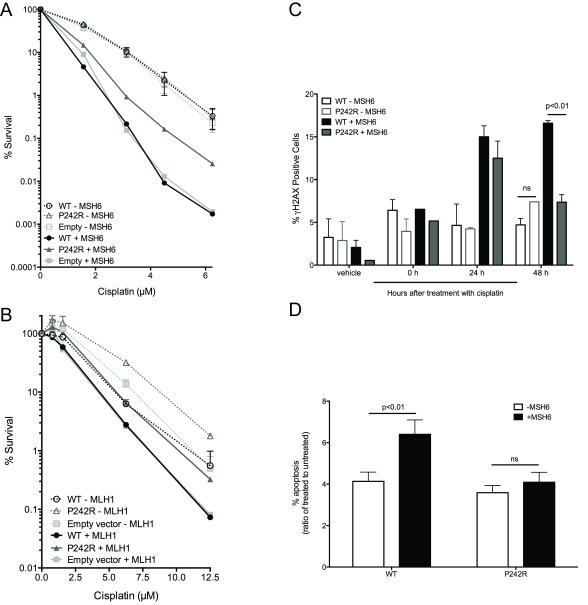
One mechanism utilized by cells treated with cisplatin to undergo apoptosis is through MMR-dependent futile cycling (29). The MutSα heterodimer consisting of MSH2 and MSH6 recognizes and binds to intrastrand crosslinks formed by cisplatin at low levels (30,31). However, there is enhanced binding when the incorrect base is inserted across from, or adjacent to the crosslink (31,32). Despite the ability for MutSα to bind to the bulky adduct, MMR cannot repair the DNA, and after another round of replication and binding by MutSα, the unrepaired cell dies (29). We interrogated whether the apoptosis observed in WT cells was dependent on MMR. To do this, we expressed WT and P242R in MSH6-deficient cells (DLD1) (−MSH6) and DLD1 cells complemented with chromosome 2, restoring MMR function (+MSH6) or in MLH1-deficient cells (HCT116) and HCT116 cells complemented with chromosome 3, restoring MMR function. It is important to note that in the absence of functional MMR, either through the loss of MSH6 or MLH1, all cells were resistant to cisplatin regardless of Pol β. In DLD1 cells complemented with MSH6, P242R expression led to cisplatin resistance (Figure 4A, solid lines; filled symbols). However, in the absence of MSH6, these cells were no longer resistant (Figure 4A, dashed lines; open symbols). This was not the case in MLH1-deficient HCT116 cells. P242R expression renders cells resistant to cisplatin regardless of functional MLH1 (Figure 4B). Therefore our results suggest that recognition of the Pt-GG crosslink by MSH6 is impaired by P242R. Thus, the enhanced repair by P242R and the apoptosis observed in WT cells is dependent on MMR, specifically MSH6, since these effects are abrogated in MSH6-deficient cells. (Figure 4C-D).
Figure 4. Mismatch repair is required for cisplatin-induced apoptosis in WT cells.
A. DLD1 cells deficient in MSH6 (−MSH6; dashed lines, open symbols) or complemented with MSH6 (+MSH6; solid lines, filled symbols) expressing WT or P242R Pol β or empty vector, were treated with various concentrations of cisplatin (0-6.25 μM). B. HCT116 cells deficient in MLH1 (−MLH1; dashed lines, open symbols) or complemented with MLH1 (+MLH1; solid lines, filled symbols) were treated with various concentrations of cisplatin (0-12.5 μM). A-B. Clonogenic survival assays were performed. C-D. DLD1 cells deficient in MSH6 (−MSH6; open bars) or complemented with MSH6 (+MSH6; filled bars) were treated with 6.25 μM cisplatin and allowed to recover for 0-48 h (C) or 0 and 96 h (D). C. Double-strand breaks were measured by staining with γH2AX antibody using flow cytometry. D. Apoptotic cells were measured by staining with Annexin V antibody. Data are presented as the ratio of apoptotic cells in cisplatin-treated cells vs untreated cells for each cell line. All data are presented as mean ± SEM (n = 3). Two-way ANOVA with Bonferroni’s post hoc test (C) or two-tailed t-tests (D) were performed to determine significance.
P242R bypasses Pt-GG crosslinks more faithfully than WT Pol β
Although the main role of Pol β is gap-filling synthesis in BER, Chaney and colleagues (8,10) have shown that Pol β can act as a translesion polymerase that bypasses Pt-GG crosslinks. Since P242R possesses a reduced catalytic efficiency during BER in vitro (18), we tested its efficiency of bypassing Pt-GG crosslinks. Although WT and P242R bind the damaged DNA with similar affinities, P242R is 3.5 fold less efficient at incorporating the correct dCTP across from the Pt-GG crosslink (Table 1). There is no difference in the catalytic rate between WT and P242R for undamaged DNA suggesting that the slow P242R rate is specific to the Pt-GG adduct. Importantly, our kinetic studies revealed that the WT Pol β protein is 19-fold more efficient at inserting the incorrect dATP compared to P242R. Most strikingly, the WT protein prefers the incorrect over the correct nucleotide, as demonstrated by the 2-fold faster rate of incorporation of the incorrect nucleotide. These data support the hypothesis that error-prone translesion synthesis, in this case performed by WT Pol β, produces a mismatched substrate recognized by the MSH6 complex that results in futile cycling, leading to the production of DNA breaks and apoptosis (29).
Table 1.
Kinetic data for WT and P242R Pol β
| Pol β | Amino Acid 242 | Fold (P/R) | |||
|---|---|---|---|---|---|
| P | R | ||||
| DNA Substrate |
GG | dCTPkobs (s−1) | 0.17±0.01 | 0.11±0.01 | 1.5 |
| G^G | dCTP kobs (s−1) | 0.0045±0.0009 | 0.0013±0.0003 | 3.5 | |
| dATP kobs (s−1) | 0.0097±0.0001 | 0.0005±0.0001 | 19.4 | ||
| KD(DNA) (nM) | 39.6±12.0 | 36.1 ±8.2 | 1.1 | ||
To determine if the resistance mediated by P242R is dependent on the insertion of the correct nucleotide and not due to the slower catalytic activity of the polymerase, we tested the effect of E295K Pol β on the cellular response to cisplatin. E295K is a cancer-associated variant of Pol β that has no polymerase activity (13). Clonogenic survival assays in cells expressing this variant, as well as the P242R/E295K double mutant are no different from WT suggesting that polymerase activity is required for a Pol β mutant protein to confer resistance to cisplatin (Figure S4).
P242R does not participate in canonical NER
Since P242R cells are resistant to cisplatin and cisplatin-induced adducts are primarily repaired by NER, we asked whether P242R played a direct role in NER. To test this hypothesis, we exposed A549 cells to UV-B light, which produces NER-specific lesions, and analyzed cell survival and DNA breaks by clonogenic survival and γH2AX flow cytometry, respectively. We observed no differences in cell survival or DNA damage in P242R-compared to WT-expressing cells (Figure 5A-B) indicating that the response to cisplatin is specific to the type of damage induced in the cells and that P242R does not participate directly in NER. Since cisplatin treatment is the standard of care for many cancers, and individuals with the P242R SNP may not respond well, we sought to identify a chemotherapeutic to which P242R-expressing cells were sensitive. The interstrand crosslinkers mitomycin C (MMC) and cyclophosphamide were identified in our high-throughput screen as being cytotoxic in P242R-expressing cells compared to WT (Table S1). We confirmed the sensitivity to MMC by performing clonogenic survival assays in the A549 cell line (Figure 5C). In addition, in vivo xenograft model using tumors derived from either WT or P242R-expressing cells are both extremely sensitive to MMC (Figure 5D-E).
Figure 5. P242R-mediated resistance to cisplatin is lesion-specific.
A-B. A549 cells expressing WT or P242R Pol β or empty vector were treated with varying doses of UVB and allowed to recover for up to 48 h. A. Clonogenic survival assays were performed, and data are presented as mean ± SEM of the percent survival (n = 3). B. Double-strand breaks were measured by staining with γH2AX antibody using flow cytometry. C. Cells were treated various concentrations of mitomycin C (MMC) and clonogenic survival assays were performed. Data are presented as mean ± SEM of the percent survival (n = 3). D-E. Mouse xenografts of A549 tumors expressing either WT (circles) or P242R (triangles) Pol β were treated by intraperitoneal injection once per week with either saline (dashed lines, open symbols) or 5 mg/kg MMC (solid line, closed symbols). Tumor growth measurements are presented as mean ± SEM (n=5). *** or **** represents p< 0.001 or 0.0001, respectively, comparing MMC-treated tumors to saline at each time point.
P242R may serve as a biomarker for cisplatin resistance
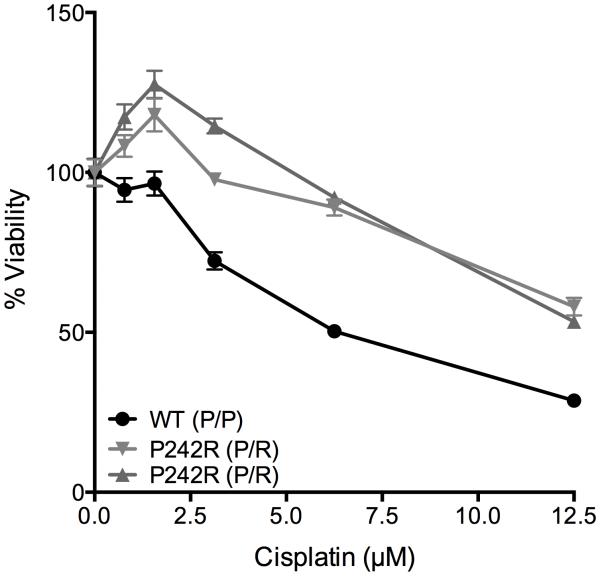
Although there is limited epidemiological data regarding carriers of the P242R variant, there is evidence that lung cancer patients harboring this variant have a worse overall prognosis, as well as a lack of response to platin-based therapies (19). To test whether P242R status could be a predictor of cisplatin resistance, we treated lymphoblastoid cell lines that were derived from individuals who were either homozygous (WT; P/P) or heterozygous for arginine (P242R; P/R) with cisplatin and measured the cell viability. Surprisingly, we found that both individual-derived cell lines that were heterozygous for P242R were resistant to cisplatin compared to the WT cell line (Figure 6). These data strongly suggest the P242R status of an individual can serve as a biomarker to predict an individual’s response to cisplatin treatment.
Figure 6. Heterozygosity for P242R confers resistance to cisplatin in population-derived cells.
Human lymphoblastoid cells derived from individuals with the genotype P/P (WT) or P/R (heterozygous for P242R) at residue 242 were plated at a density of 20,000 cells/well in a 96 well plate. Cells were treated with various concentrations (0-12.5 μM) of cisplatin for 24 h and cell viability was measured using CellTiter-Glo®. Data are presented as mean ± SEM of the percent viability (n = 3).
Discussion
In the new era of personalized medicine, it is critical to determine if mutations in the germline or in tumors impact cancer progression and/or responses to chemotherapy. Our previous work showed that the presence of the POLB rs3136797 germline SNP encoding P242R Pol β may lead to carcinogenesis and increase an individual’s risk for cancer (18). Herein, we have demonstrated that both breast, lung, and colon cancer cells, as well as an in vivo mouse xenograft model using tumors derived from cells expressing the P242R germline variant are resistant to treatment with cisplatin compared to WT-expressing cells. These results are recapitulated in lymphoblastoid cell lines derived from individuals that are heterozygous for P242R. Pol β substrate choice underlies the mechanistic basis of the cellular response to cisplatin and determines the fate of the cells. Together, our data suggest that Pol β may serve as a prognostic marker in the treatment of cancer with cisplatin and perhaps other intrastrand crosslinking agents.
Pol β is known to function in the base excision repair pathway, which removes oxidative and alkylation damage (11,33). In addition, Pol β has been shown to bypass bulky crosslinks induced by cisplatin (4,10). Cisplatin is the standard of care for many cancers (1), but the cells often develop resistance (5). Moreover, it has been shown that individuals who carry the P242R germline SNP have decreased overall survival when treated with platinum-based therapies (19). Although, there are several proposed mechanisms for acquired cisplatin resistance, our data suggest that P242R Pol β is able to successfully bypass Pt-GG crosslinks (4,10) in order to avoid the replicative block of the cisplatin intrastrand adduct, thereby promoting repair of the damage. Our biochemical kinetic studies revealed that P242R Pol β inserts the correct nucleotide opposite the intrastrand crosslink over 2.5-fold more efficiently than the incorrect nucleotides, whereas WT Pol β inserts the incorrect nucleotide 2-fold more efficiently than correct. Ultimately, WT Pol β exhibits a 19-fold preference for insertion of incorrect dATP opposite the intrastrand crosslink. Polymerase activity is required for the resistance since the catalytically inactive E295K Pol β variant is not resistant to cisplatin. We postulate that the correct nucleotide incorporation by P242R provides the proper DNA substrate for the NER pathway and by incorporating the correct nucleotide, P242R is facilitating the initiation of NER, as shown in the model in Figure 7.
Figure 7. Model for P242R-medicated cisplatin resistance.
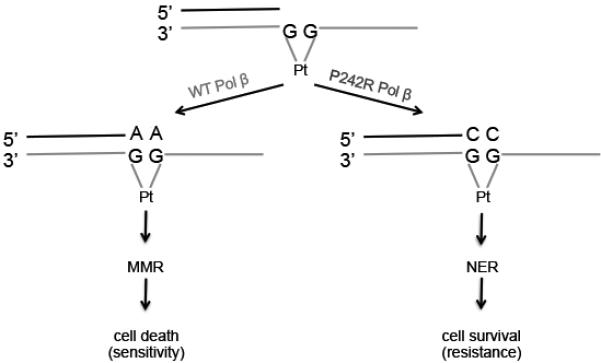
After DNA is damaged by cisplatin, and intrastrand crosslink is formed. If WT Pol β bypasses the lesion, it preferentially inserts the incorrect A opposite the crosslink. This mismatch is recognized by the mismatch repair pathway (MMR), but cannot be repaired leading to cell death. In contrast, if the intrastrand crosslink is bypassed by the P242R Pol β variant, the correct nucleotide, C, is inserted across the lesion. This is now recognized by the nucleotide excision repair pathway (NER), which properly excises and repairs the DNA, resulting in cell survival.
PARP1 catalyzes the transfer of poly ADP-ribose (PAR) (PARylation) subunits from NAD+ molecules to target proteins (34). The posttranslational modification of PARylation has been implicated in several cellular pathways including DNA repair and apoptosis. Although initially characterized for its role in BER (35), PARP1 is also a key player in NER (26-28). XPA is a core NER protein that recognizes and binds to damaged DNA (36). In response to UV-induced DNA damage, XPA is PARylated by PARP1, and this modification is required for XPA to associate with chromatin and to be recruited to sites of UV-induced DNA damage (26,27). In our studies, XPA specifically interacts with P242R Pol β, but not WT Pol β, in cells treated with cisplatin. In addition, PARP1 activity is required for this interaction and for XPA and P242R to associate with cisplatin intrastrand crosslinks on the DNA. In fact, P242R-mediated cisplatin resistance is reversed in cells treated with olaparib. Together, this suggests that PARylation of XPA, and perhaps P242R Pol β, mediates the cellular response to cisplatin treatment and is necessary for the resistant phenotype. The co-treatment of cisplatin and olaparib in cells is of clinical relevance and provides a potential personalized therapeutic approach to individuals who carry the P242R germline SNP and would otherwise not respond well to platin-based chemotherapies.
Our high-throughput screen using the NCI Oncology Set II library of 85 FDA-approved chemotherapies provided us with the identities of drugs that are cytotoxic to P242R-expressing compared to WT cells. Among these, the interstrand crosslinking (ICL) agent MMC was identified (Table S1) and MMC sensitivity in P242R-expressing cells was confirmed by clonogenic survival as well as in mouse xenografts. The precise mechanism of how P242R-expressing cells are sensitive to an interstrand crosslinker is unresolved.
Another mechanism of acquired cisplatin resistance in cells is enhanced NER activity in cells (37). It is unlikely that this is the case as our data demonstrate that the expression of P242R has no effect on UV-induced survival or DNA damage. This strongly suggests that P242R Pol β does not have a direct role in the NER pathway. Furthermore, although expression levels of Pol β in various cancers have been linked to resistance to cisplatin (38-41), others have demonstrated the overexpression of Pol β increases the translesion synthesis activity of the enzyme, but not NER activity (40).
The MMR pathway has been shown to function downstream of BER in cisplatin sensitivity (32). Our data suggest that the preference that WT Pol β has for incorporating the incorrect nucleotide opposite the Pt-GG crosslink allows the MutSα heterodimer to recognize the lesion leading to futile cycling and apoptosis, as shown in the model in Figure 7. When this heterodimer isn’t formed in MSH6-deficient cells, this apoptotic pathway is prevented in WT-expressing cells. The insertion of the incorrect base opposite the adduct triggers this MutSα binding. This is supported by our data with E295K Pol β showing that although its sensitivity to cisplatin is comparable to WT, the apoptosis observed is independent of MMR (Figure S4C).
Because DNA repair is critical for repairing endogenous DNA damage and maintaining the genomic integrity of the cell, DNA remains a major target of chemotherapeutics. Since functional DNA repair pathways influence the efficacy of drugs, the genes in these pathways have become attractive targets of chemotherapies (for review see (42)). Genetically, there are SNPs in genes involved in repair pathways that can increase the risk of cancer and alter drug responses. Functional analysis into the role of the P242R germline SNP in POLB has generated novel mechanistic insights into the role this variant and Pol β in general has regarding cancer therapies. To the best of our knowledge, this is the first study that demonstrates that the fidelity of Pol β initiates crosstalk between other DNA repair pathways. This work has the high potential to shift current clinical practice in this era of personalized medicine.
Supplementary Material
Acknowledgements
We thank Dr. Ryan Jensen for helpful comments. We thank the Developmental Therapeutics Program of the National Cancer Institute for the NCI Approved Oncology Drugs Set II collection used in this work.
This work was supported by NIH grant ES019179 to J.B.S and by grants P30 CA08748 and U54 OD020355‐01 (E.d.S.).
Footnotes
The authors have declared no conflicts of interest exist.
References
- 1.Weiss RB, Christian MC. New cisplatin analogues in development. A review. Drugs. 1993;46(3):360–77. doi: 10.2165/00003495-199346030-00003. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4(4):307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 3.Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–66. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann JS, Pillaire MJ, Maga G, Podust V, Hubscher U, Villani G. DNA polymerase beta bypasses in vitro a single d(GpG)-cisplatin adduct placed on codon 13 of the HRAS gene. Proc Natl Acad Sci U S A. 1995;92(12):5356–60. doi: 10.1073/pnas.92.12.5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001;478(1-2):23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 6.Mamenta EL, Poma EE, Kaufmann WK, Delmastro DA, Grady HL, Chaney SG. Enhanced replicative bypass of platinum-DNA adducts in cisplatin-resistant human ovarian carcinoma cell lines. Cancer Res. 1994;54(13):3500–5. [PubMed] [Google Scholar]
- 7.Vaisman A, Masutani C, Hanaoka F, Chaney SG. Efficient translesion replication past oxaliplatin and cisplatin GpG adducts by human DNA polymerase eta. Biochemistry. 2000;39(16):4575–80. doi: 10.1021/bi000130k. [DOI] [PubMed] [Google Scholar]
- 8.Bassett E, Vaisman A, Havener JM, Masutani C, Hanaoka F, Chaney SG. Efficiency of extension of mismatched primer termini across from cisplatin and oxaliplatin adducts by human DNA polymerases beta and eta in vitro. Biochemistry. 2003;42(48):14197–206. doi: 10.1021/bi035359p. [DOI] [PubMed] [Google Scholar]
- 9.Albertella MR, Green CM, Lehmann AR, O'Connor MJ. A role for polymerase eta in the cellular tolerance to cisplatin-induced damage. Cancer Res. 2005;65(21):9799–806. doi: 10.1158/0008-5472.CAN-05-1095. [DOI] [PubMed] [Google Scholar]
- 10.Vaisman A, Chaney SG. The efficiency and fidelity of translesion synthesis past cisplatin and oxaliplatin GpG adducts by human DNA polymerase beta. J Biol Chem. 2000;275(17):13017–25. doi: 10.1074/jbc.275.17.13017. [DOI] [PubMed] [Google Scholar]
- 11.Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet. 2004;38:445–76. doi: 10.1146/annurev.genet.38.072902.092448. [DOI] [PubMed] [Google Scholar]
- 12.Donigan KA, Sun KW, Nemec AA, Murphy DL, Cong X, Northrup V, et al. Human POLB gene is mutated in high percentage of colorectal tumors. J Biol Chem. 2012;287(28):23830–9. doi: 10.1074/jbc.M111.324947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang T, Dalal S, Chikova A, DiMaio D, Sweasy JB. The E295K DNA polymerase beta gastric cancer-associated variant interferes with base excision repair and induces cellular transformation. Mol Cell Biol. 2007;27(15):5587–96. doi: 10.1128/MCB.01883-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemec AA, Donigan KA, Murphy DL, Jaeger J, Sweasy JB. Colon cancer-associated DNA polymerase beta variant induces genomic instability and cellular transformation. J Biol Chem. 2012;287(28):23840–9. doi: 10.1074/jbc.M112.362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemec AA, Murphy DL, Donigan KA, Sweasy JB. The S229L colon tumor-associated variant of DNA polymerase beta induces cellular transformation as a result of decreased polymerization efficiency. J Biol Chem. 2014;289(20):13708–16. doi: 10.1074/jbc.M114.550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamtich J, Speed WC, Straka E, Kidd JR, Sweasy JB, Kidd KK. Population-specific variation in haplotype composition and heterozygosity at the POLB locus. DNA Repair (Amst) 2009;8(5):579–84. doi: 10.1016/j.dnarep.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohrenweiser HW, Wilson DM, 3rd, Jones IM. Challenges and complexities in estimating both the functional impact and the disease risk associated with the extensive genetic variation in human DNA repair genes. Mutat Res. 2003;526(1-2):93–125. doi: 10.1016/s0027-5107(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 18.Yamtich J, Nemec AA, Keh A, Sweasy JB. A germline polymorphism of DNA polymerase Beta induces genomic instability and cellular transformation. PLoS Genet. 2012;8(11):e1003052. doi: 10.1371/journal.pgen.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matakidou A, el Galta R, Webb EL, Rudd MF, Bridle H, Consortium G, et al. Genetic variation in the DNA repair genes is predictive of outcome in lung cancer. Hum Mol Genet. 2007;16(19):2333–40. doi: 10.1093/hmg/ddm190. [DOI] [PubMed] [Google Scholar]
- 20.Lang T, Maitra M, Starcevic D, Li SX, Sweasy JB. A DNA polymerase beta mutant from colon cancer cells induces mutations. Proc Natl Acad Sci U S A. 2004;101(16):6074–9. doi: 10.1073/pnas.0308571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy DL, Kosa J, Jaeger J, Sweasy JB. The Asp285 variant of DNA polymerase beta extends mispaired primer termini via increased nucleotide binding. Biochemistry. 2008;47(31):8048–57. doi: 10.1021/bi702104y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalal S, Chikova A, Jaeger J, Sweasy JB. The Leu22Pro tumor-associated variant of DNA polymerase beta is dRP lyase deficient. Nucleic Acids Res. 2008;36(2):411–22. doi: 10.1093/nar/gkm1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamtich J, Starcevic D, Lauper J, Smith E, Shi I, Rangarajan S, et al. Hinge residue I174 is critical for proper dNTP selection by DNA polymerase beta. Biochemistry. 2010;49(11):2326–34. doi: 10.1021/bi901735a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck DJ, Brubaker RR. Effect of cis-platinum(II)diamminodichloride on wild type and deoxyribonucleic acid repair deficient mutants of Escherichia coli. J Bacteriol. 1973;116(3):1247–52. doi: 10.1128/jb.116.3.1247-1252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy DD, Saijo M, Tanaka K, Kraemer KH. Expression of a transfected DNA repair gene (XPA) in xeroderma pigmentosum group A cells restores normal DNA repair and mutagenesis of UV-treated plasmids. Carcinogenesis. 1995;16(7):1557–63. doi: 10.1093/carcin/16.7.1557. [DOI] [PubMed] [Google Scholar]
- 26.King BS, Cooper KL, Liu KJ, Hudson LG. Poly(ADP-ribose) contributes to an association between poly(ADP-ribose) polymerase-1 and xeroderma pigmentosum complementation group A in nucleotide excision repair. J Biol Chem. 2012;287(47):39824–33. doi: 10.1074/jbc.M112.393504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robu M, Shah RG, Petitclerc N, Brind'Amour J, Kandan-Kulangara F, Shah GM. Role of poly(ADP-ribose) polymerase-1 in the removal of UV-induced DNA lesions by nucleotide excision repair. Proc Natl Acad Sci U S A. 2013;110(5):1658–63. doi: 10.1073/pnas.1209507110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flohr C, Burkle A, Radicella JP, Epe B. Poly(ADP-ribosyl)ation accelerates DNA repair in a pathway dependent on Cockayne syndrome B protein. Nucleic Acids Res. 2003;31(18):5332–7. doi: 10.1093/nar/gkg715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaisman A, Varchenko M, Umar A, Kunkel TA, Risinger JI, Barrett JC, et al. The role of hMLH1, hMSH3, and hMSH6 defects in cisplatin and oxaliplatin resistance: correlation with replicative bypass of platinum-DNA adducts. Cancer Res. 1998;58(16):3579–85. [PubMed] [Google Scholar]
- 30.Duckett DR, Drummond JT, Murchie AI, Reardon JT, Sancar A, Lilley DM, et al. Human MutSalpha recognizes damaged DNA base pairs containing O6-methylguanine, O4-methylthymine, or the cisplatin-d(GpG) adduct. Proc Natl Acad Sci U S A. 1996;93(13):6443–7. doi: 10.1073/pnas.93.13.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada M, O'Regan E, Brown R, Karran P. Selective recognition of a cisplatin-DNA adduct by human mismatch repair proteins. Nucleic Acids Res. 1997;25(3):491–6. doi: 10.1093/nar/25.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kothandapani A, Sawant A, Dangeti VS, Sobol RW, Patrick SM. Epistatic role of base excision repair and mismatch repair pathways in mediating cisplatin cytotoxicity. Nucleic Acids Res. 2013;41(15):7332–43. doi: 10.1093/nar/gkt479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362(6422):709–15. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 34.Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. BioEssays : news and reviews in molecular, cellular and developmental biology. 2004;26(8):882–93. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 35.Dantzer F, de La Rubia G, Menissier-De Murcia J, Hostomsky Z, de Murcia G, Schreiber V. Base excision repair is impaired in mammalian cells lacking Poly(ADP-ribose) polymerase-1. Biochemistry. 2000;39(25):7559–69. doi: 10.1021/bi0003442. [DOI] [PubMed] [Google Scholar]
- 36.Robins P, Jones CJ, Biggerstaff M, Lindahl T, Wood RD. Complementation of DNA repair in xeroderma pigmentosum group A cell extracts by a protein with affinity for damaged DNA. The EMBO journal. 1991;10(12):3913–21. doi: 10.1002/j.1460-2075.1991.tb04961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Earley JN, Turchi JJ. Interrogation of nucleotide excision repair capacity: impact on platinum-based cancer therapy. Antioxid Redox Signal. 2011;14(12):2465–77. doi: 10.1089/ars.2010.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canitrot Y, Cazaux C, Frechet M, Bouayadi K, Lesca C, Salles B, et al. Overexpression of DNA polymerase beta in cell results in a mutator phenotype and a decreased sensitivity to anticancer drugs. Proc Natl Acad Sci U S A. 1998;95(21):12586–90. doi: 10.1073/pnas.95.21.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raaphorst GP, Cybulski SE, Sobol R, Ng CE. The response of human breast tumour cell lines with altered polymerase beta levels to cisplatin and radiation. Anticancer Res. 2001;21(3B):2079–83. [PubMed] [Google Scholar]
- 40.Bergoglio V, Canitrot Y, Hogarth L, Minto L, Howell SB, Cazaux C, et al. Enhanced expression and activity of DNA polymerase beta in human ovarian tumor cells: impact on sensitivity towards antitumor agents. Oncogene. 2001;20(43):6181–7. doi: 10.1038/sj.onc.1204743. [DOI] [PubMed] [Google Scholar]
- 41.Kothandapani A, Dangeti VS, Brown AR, Banze LA, Wang XH, Sobol RW, et al. Novel role of base excision repair in mediating cisplatin cytotoxicity. J Biol Chem. 2011;286(16):14564–74. doi: 10.1074/jbc.M111.225375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481(7381):287–94. doi: 10.1038/nature10760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.