Abstract
In this article, we review the original findings from MRI and autopsy studies that demonstrated brain iron status is insufficient in individuals with restless legs syndrome (RLS). The concept of deficient brain iron status is supported by proteomic studies from cerebrospinal fluid (CSF) and from the clinical findings where intervention with iron, either dietary or intravenous, can improve RLS symptoms. Therefore, we include a section on peripheral iron status and how peripheral status may influence both the RLS symptoms and treatment strategy. Given the impact of iron in RLS, we have evaluated genetic data to determine if genes are directly involved in iron regulatory pathways. The result was negative. In fact, even the HFE mutation C282Y could not be shown to have a protective effect. Lastly, a consistent finding in conditions of low iron is increased expression of proteins in the hypoxia pathway. Although there is lack of clinical data that RLS patients are hypoxic, there are intriguing observations that environmental hypoxic conditions worsen RLS symptoms; in this chapter we review very compelling data for activation of hypoxic pathways in the brain in RLS patients. In general, the data in RLS point to a pathophysiology that involves decreased acquisition of iron by cells in the brain. Whether the decreased ability is genetically driven, activation of pathways (eg, hypoxia) that are designed to limit cellular uptake is unknown at this time; however, the data strongly support a functional rather than structural defect in RLS, suggesting that an effective treatment is possible.
Introduction
The proteins and genes in the iron pathway may be defined in broad or narrow terms. In the narrow sense, the products of iron genes are involved in iron transport, iron storage, and control of cellular iron metabolism. The latter can be outlined with a two-level scheme [1] with hepcidin controlling iron uptake from the gut and iron release into the blood on a systemic level. At the molecular level, there are iron-regulatory proteins (IRP), IRP1 and IRP2 intracellularly directing import/export, storage, and functional use of iron in response to its availability by binding to transcripts of a group of proteins that contain iron-responsive elements (IREs) in their untranslated regions (UTRs). The IRPs can bind to the IREs on the mRNA during intracellular iron deficiency. For example, an IRE at the 3’-UTR stabilizes the transcript as in case of transferrin receptor 1 (TfR1) or DMT1 (transmembraneous importer of divalent iron) while an IRE at the 5’-UTR inhibits translation as in case of ferroportin and the iron-storage protein ferritin, ALAS, the controlling enzyme of heme synthesis, or the hypoxia-inducible factor HIF2α. The relationship of iron deficiency and the HIF protein expression offers an intriguing new look at the biological underpinnings of RLS that are discussed in Section 4.
A number of players can be attached to that two-level model of iron homeostasis. These include the membranous iron exporter ferroportin that degrades in response to interaction with hepcidin, transferrin (Tf) which transports iron in plasma, and the TfR1 and TfR2. Beyond iron uptake the latter operate together with the hemochromatosis gene product (HFE) in a feedback loop controlling hepcidin expression by plasma iron [2]. These proteins and genes are reviewed herein as they relate to iron management in RLS.
1. Pre-clinical and clinical data on effects of iron deficits and iron treatments Introduction
Low brain iron despite normal peripheral iron is the best-established neurobiological abnormality in RLS. There are three fundamental scientific questions regarding iron and RLS: 1) what are the relations between low peripheral iron and RLS 2) what are the effects of increasing peripheral iron on brain iron and on RLS and 3) how does brain iron deficiency produce RLS symptoms.
1.1 Low peripheral iron and RLS
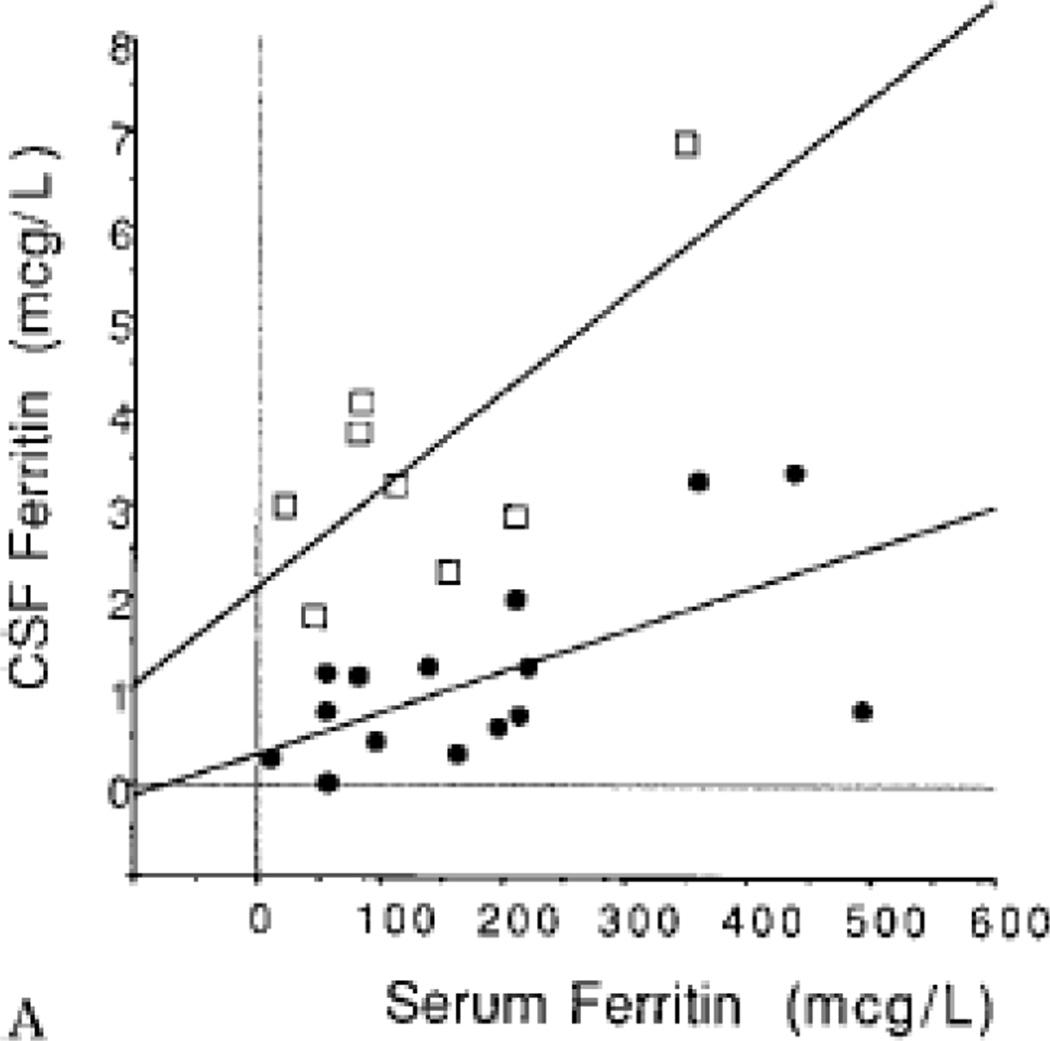
CSF and serum ferritin are significantly but poorly correlated; moreover the relation differs for RLS compared to controls, with lower CSF for a given serum ferritin for RLS shown in two separate studies and populations in Japan and the USA [3, 4]. The potential meaning of these findings are discussed in Section 2. The relation between serum and CSF ferritin for RLS indicates that very low CSF ferritin occurs with very low serum ferritin, particularly those within the diagnosis range of iron deficiency anemia (IDA) (Figure 1). Thus for those with genetic risk factors for RLS the normal serum ferritin >50 mcg/l will have variable effects on brain iron while iron deficiency levels would be expected to produce very low CSF ferritin and presumably, low brain iron. RLS prevalence, as expected, occurs aboutfive times more in IDA than in the general population [5–8]. It occurs in about 30% of patients with IDA vs. about five to seven percent of the general population [6]. However, the IDA patients with RLS do not differ from those without RLS in actual levels of peripheral iron or hemoglobin [6]. It is not the iron deficiency (ID) alone, but rather the interaction of the IDA with other systemic, presumably genetic factors that produces RLS. It appears about one third of the population carries factors making them vulnerable to RLS if they become iron deficient or conversely two thirds of the population carry genetic factors protecting them from RLS. Some of these points are explored in more detail in Section 4. In general all of the conditions that produce significant ID appear to produce about a 25 – 40% prevalence of RLS and the data from RLS with IDA indicate this is driven not by the degree of iron deficiency but rather by systemic, presumably genetic factors [6]. Trying to extrapolate these data to the general population suggests there is a subgroup of people with approximately a 33% risk of developing RLS with iron deficiency whereas the rest of the population is resistant to developing RLS even with significant ID. It can be expected that there are genetic or epigenetic factors contributing to this population difference.
Figure 1.
CSF ferritin vs serum ferritin for RLS patients (black circles) and controls (open boxes). (adapted from Earley CJ et al NEUROLOGY 2000;54:1698–1700)
1.2 Reversing the low peripheral iron to reduce brain iron and treat RLS
Iron formulation to be used
Oral iron treatment is poorly absorbed except for significant peripheral iron deficiency. IV iron offers a better method for increasing peripheral iron. Iron formulations developed for IV iron differ considerably in the rates at which they release the iron and have it taken up into macrophages [9]. Iron is released more slowly into the blood, taken up more into macrophages and thus more likely to be transported to the brain when given IV using iron dextran, ferric carboxymaltose (FCM) and iron Isomaltoside than iron sucrose or ferrous gluconate.
Preclinical data
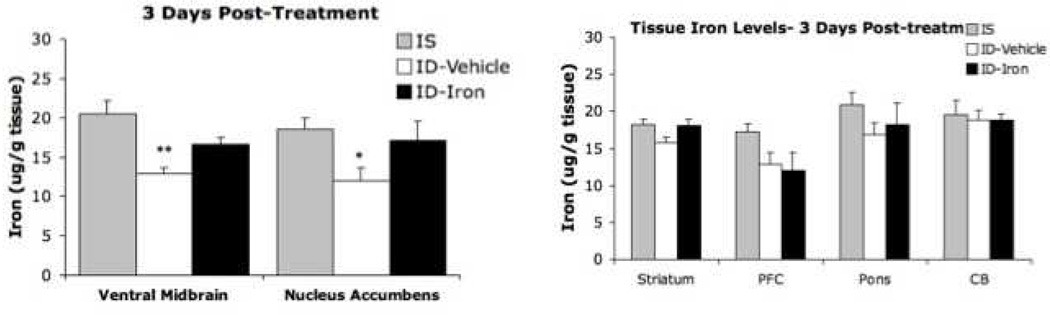
A fundamental question for Iron treatment: Does increasing peripheral iron change brain iron? This was evaluated for a strain of mice that (post-weaning) were placed on an iron-deficient diet, thus decreasing iron concentrations in the substantia nigra, as observed for RLS patients. These mice were given tail vein IV iron injections of iron Isomaltoside equivalent to 1000 mg for humans [10]. The iron deficit in the nigra was reversed without increase in other brain areas where brain iron was not low. (Figure 2) Thus large increases in peripheral iron can correct brain iron deficiency without any indication of producing brain iron overload.
Figure 2.
Tissue iron concentration at 3 days after tail vein injection of 1000 mg iron isomlatoside or vehicle only compared to animals without any iron deficiency. Note the increased peripheral iron corrects the iron deficiency in the ventral mid-brain and nucleus accumbens without increasing iron in the brain regions without iron deficiency. Iron overload in the brain does not seem to occur. (from Unger, et al, Neuroscience (2013) 246c:179-85)
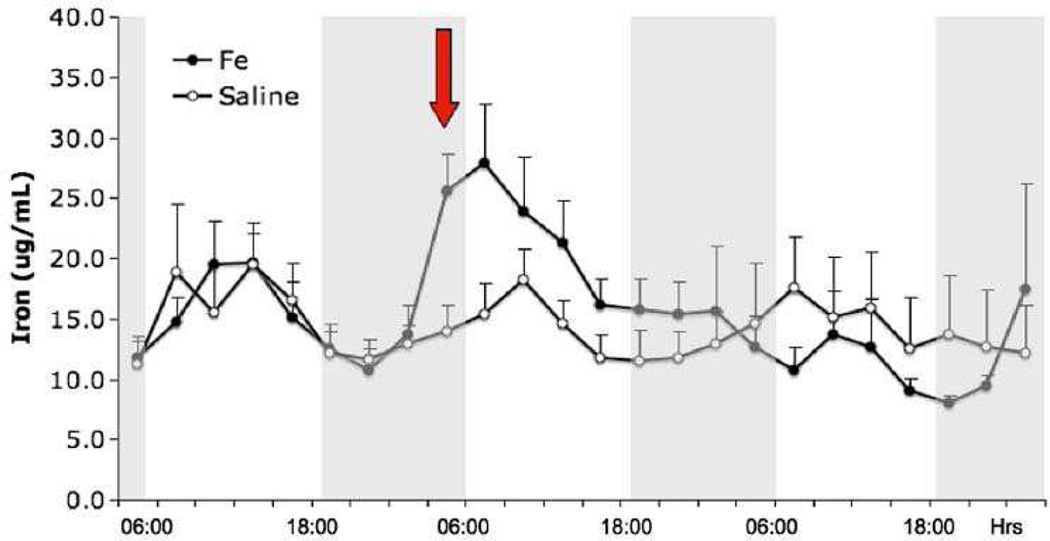
These mice showed a circadian rhythm for extra-cellular striatal iron, with these particular iron levels increasing in the inactive period. Moreover, the iron in the extra-cellular space rapidly increased after the injection and then decreased over two days to normal levels (Figure 3). As noted in Figure 3, however, iron increased in critical regions, persisting for three days; further data showed the increase continued for at least 10 days [10].
Figure 3. Striatal microdialysis assessment of extra-cellular non-transferrin bound iron in mice.
This figure describes the average iron content from ventral midbrain (VMB) for eight mice with iron isomaltoside 1000 and nine with saline injections.
Bars indicate standard errors. Red arrow is the first measure after the injection, which was about 3 h before this measure. Dark periods (18:00–06:00) are indicated by shaded background. Marks at the bottom correspond with Microdialysis samples taken every 3 h starting at 24 h before injection. (from Unger, et al, Neuroscience (2013) 246c:179-85)
Clinical data
Patients with RLS from IDA when treated with 1,000 mg IV of Iron dextran had a 76% positive response [11]. The 34% who did not respond showed persistent anemia (Hgb<12.5 g/dl), indicating possible insufficient doses of IV iron. Patients with RLS (despite having normal peripheral iron levels) were first treated with IV iron dextran by Nordlander in 1952 [12] and more recently in four separate open-label studies, two to general RLS patients [13, 14], one to pediatric RLS [15] and a third to refractory RLS patients [16]. FCM, 1000 mg given IV to RLS patients in a double-blinded, placebo-controlled trial showed significantly reduced RLS symptoms. All of these studies showed significant benefit for ≥ six-weeks in about 40–60% of patients and for > four months for about 20 –30% of patients. Two studies treating RLS with about 1000 mg of IV iron sucrose failed to show statistically significant benefit, as might be expected given its limited uptake in macrophages [9, 13, 17].
1.3 Iron dopamine relation
This is covered in more detail in another chapter on dopamine in RLS, but the preclinical data deserve special note here. Adding iron to the ventral midbrain (VMB) by an indwelling cannula produce an increased extra-cellular dopamine in the striatum [18]. It has now been well established that RLS occurs with increased striatal dopamine [19–22]. Adding iron to the striatum had no effect on its dopamine levels. Thus, the increases in iron seen in VMB iron with IV iron treatment would be expected to produce the changes in striatal dopamine seen in RLS.
2. Pathophysiology of RLS
One of the key observations from autopsy studies on RLS brain tissue is that the cause of RLS is not rooted in pathologies associated with classical neurodegenerative processes [23]. For example, there is no noticeable loss of tyrosine hydroxylase neurons in the substantia nigra or presence of inclusion bodies as reported in Parkinson’s Disease [23]. As a result of the autopsy studies, we have postulated that RLS is a functional disorder of iron acquisition by the brain. The data supporting this position are reviewed in this section. The original focus on the substantia nigra in RLS was driven by the clinical evidence that RLS patients were responsive to dopaminergic agents (discussed in this issue) and also by neuroimaging studies that reported an apparent decrease in iron content in the substantia nigra [24]. Subsequently, analyses on brain tissue from RLS patients revealed significant decrease in iron content in the substantia nigra as well as changes in expression profiles of iron management proteins that were consistent with decreased iron content. There was one exception to the latter statement regarding expression profile of the iron management proteins in the SN, a decrease rather than increase in the TfR expression. This apparent discrepancy in the otherwise overwhelming evidence for iron deficiency in the brain in RLS potentially indicated the cause of iron deficiency, namely, misregulation of the TfR on tyrosine hydroxylase neurons in RLS. This latter observation on the TfR expression led to the studies on the activation of hypoxic pathways in the RLS brain (see section 3). As a result of the autopsy studies, we concluded that RLS is a functional disorder of iron acquisition by the brain. Although most studies have focused on the SN in RLS, additional studies have shown that there is a decrease in myelin expression in the RLS brain [25], which is consistent with reports of hypomyelination in animal models and human studies of iron deficiency and also consistent with the idea of a more wide-spread iron insufficiency in RLS brains. The finding of myelin insufficiency in RLS may be relevant to the increased incidence of RLS in patients with multiple sclerosis [26].
As mentioned earlier, examination of protein expression patterns in the CSF are also consistent with brain iron insufficiency and these findings also suggest the inadequate brain iron extends beyond the SN. For example, Tf, the iron transport protein is elevated in the CSF of RLS patients and ferritin levels are decreased [3, 4]. This expression pattern for Tf and ferritin when seen in the serum is considered an indicator of systemic iron deficiency. Elevated CSF Tf levels also were observed in a developmental non-human primate model of iron deficiency, despite supplementation with iron and resolution of hematological iron deficiency [27]. The persistence of elevated Tf in CSF despite repletion of systemic iron brings up the possibility that a set point for iron status in the brain was altered during development that may render the brain sensitive to iron challenges into adulthood. One noteworthy observation has been the decrease in pro-hepcidin in the CSF of RLS patients compared to control. Hepcidin is crucial for stopping the export of iron from cells. However, it is not known how the expression of pro-hepcidin is related to the functionally active hepcidin protein. There have been technical challenges for measuring hepcidin in CSF that have precluded revisiting this study. However, if hepcidin activity is decreased in the CSF in RLS patients it could indicate that in RLS the brain lacks the ability to maintain normal iron levels. This idea is consistent with data showing increased iron in the CSF of RLS patients compared to non-RLS controls [3]. It is also consistent with our reports that lymphocytes from RLS patients have increased iron export [28].
Lastly, studies involving changes in startle reflex (both visual and auditory) in RLS patients versus non-RLS patients argue for brain iron insufficiency in RLS patients because the response in RLS is similar to that seen in iron deficiency models [29, 30]. These studies also support the concept that brain iron sufficiency extends beyond the nigro-striatal pathway.
One of the outcomes of the studies on RLS brain tissue was the hypothesis and subsequent paradigm shifting observation that the blood-brain-barrier (BBB) is not just a passive conduit for transport of nutrients but a site of regulation for nutrient uptake into the brain [31]. This was a novel concept primarily based on two critical pieces of data: First, because of the high density of mitochondria in the endothelial cells forming the brain microvasculature and comprising the BBB, it was clear that there was a significant need for iron in these cells to meet their oxidative metabolic requirements. Second, the ferritin levels in the brain microvasculature indicate that these cells stored iron [32]. Both pieces of data made it clear that iron was not simply passed through the endothelial cells to the brain parenchyma but rather released to and stored within the endothelial cells. These observations raised two fundamental questions: 1) can iron that is stored in endothelial cells of the brain be released to the brain parenchyma and 2) what is the protein profile expression of the iron management proteins in the microvasculature from RLS patients.
The first question was evaluated in a cell culture model of the BBB using bovine brain endothelial cells. The initial study demonstrated that iron transport across the BBB cells is influenced by the iron status of the cells. These data suggested there is direct transport of iron across endothelial cells but that transport is influenced by the iron status of the endothelial cells. This finding suggested that the expression of TfRs on the endothelial cells are down-regulated following iron-loading (an event only possible if the iron levels of the endothelial cells had increased [33]). In a second experiment in which the endothelial cells were first loaded with iron, the amount of iron released from the endothelial cells was increased by apo-Tf (iron poor Tf), media from iron deficient astrocytes and by CSF from iron deficient monkeys [31]. Iron release from the endothelial cells was decreased by hepcidin or from media of iron loaded astrocytes. These data indicate that there are signals from the brain that inform the endothelial cells of the BBB regarding brain iron status. These signals may be disrupted in the RLS brains. Protein profiles in the CSF, beyond the standard iron management proteins are altered in RLS patients [34].
The question of iron management protein expression profiles in RLS at the blood-brain interface was addressed by obtaining brain microvasculature and choroid plexus from RLS brain tissue harvested at autopsy. The microvasculature was selected from the motor cortex. In the choroid plexus, iron levels were dramatically decreased in the RLS tissue as was ferritin. TfR was elevated in RLS. This is a profile consistent with iron deficiency in these cells, yet ferroportin (the iron export protein) was elevated. The iron levels in CSF were elevated in RLS patients [3], which would be consistent with elevated ferroportin in choroid plexus. The data suggest that iron is being passed into the CSF from the choroid plexus at rates higher than normal. But it is not known if the iron is getting into the brain or passing from the CSF into the systemic circulation. It is reported that CSF and brain extracellular space are in a dynamic equilibrium [31]. If so, perhaps elevated iron in the CSF is sending the wrong signal to the BBB that brain iron status is normal in RLS. But if CSF iron status is normal or even elevated in RLS, why don’t the cells in the brain take up the iron from the extracellular space? The data would suggest that the iron in the CSF is not getting access to the brain. The bulk of the iron getting to cells in the brain would then be coming from across the BBB. In RLS, Tf and the TfR and H-ferritin were all decreased in the endothelial cells of the RLS patients [35]. This would suggest decreased uptake and storage of iron within the cells of the BBB and would be consistent with elevated iron in the extracellular space in RLS; this raises the possibility that the problem with neuronal acquisition of iron in the RLS lies within the cells of the brain and perhaps the neurons. One additional consideration, however, is that the neuronal iron acquisition in RLS is normal but mishandled once it enters the cell. It has been reported that mitochondrial ferritin and the number of mitochondria are both increased in the neuromelanin containing cells in the substantia nigra in RLS compared to control [36]. Overexpression of mitochondrial ferritin is known to induce cytosolic iron deficiency which would be consistent with the decreased ferritin reported in the neuromelanin cells in RLS tissue [37]. One of the known physiological events that cause an increase in mitochondria is hypoxia [38]. Thus it is possible that the activation of hypoxic pathways increases mitochondria, which alters the energy metabolism of these cells while the increased mitochondrial ferritin within these cells further adds to cytosolic iron deficiency. The evidence for activation of hypoxic pathways in RLS is discussed below in section 3.
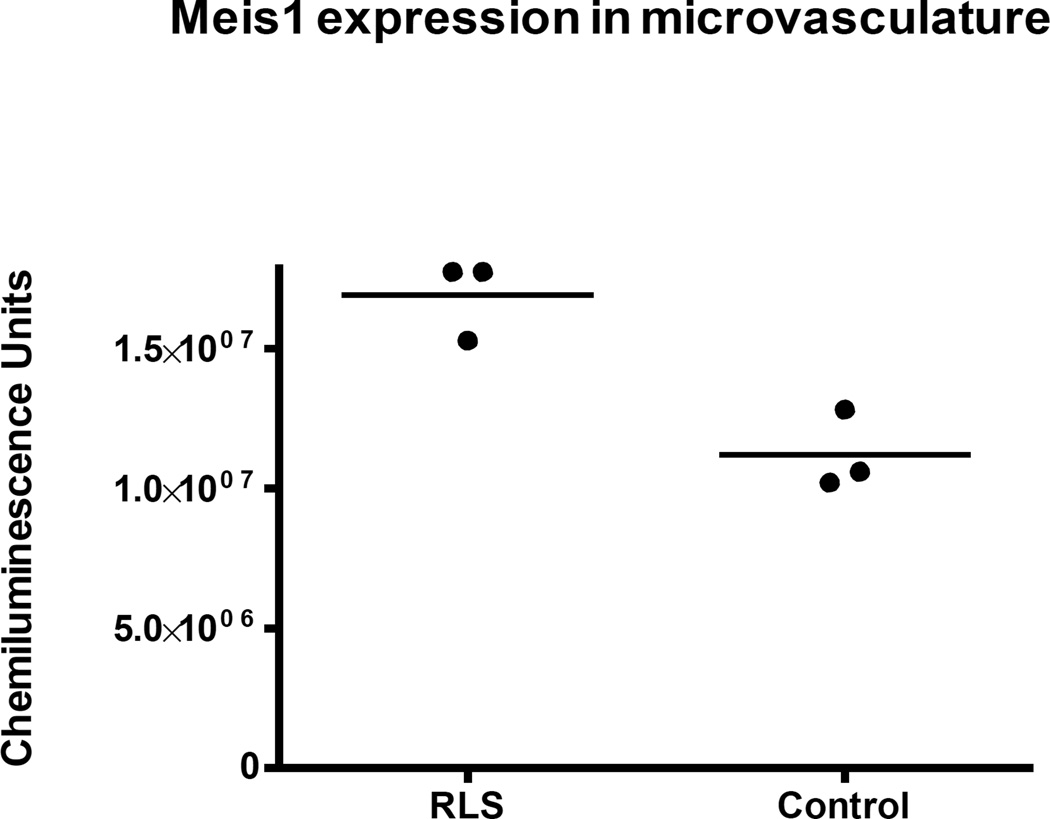
Lastly, to consider proteins which are directly related to risk genes in RLS (this issue), a preliminary analysis of MEIS1 expression was undertaken in the brain microvasculature of RLS tissue compared to control (Figure 3). MEIS1 protein levels were surprisingly detected in the brain microvasculature suggesting an undiscovered function for this protein. Moreover, the MEIS1 levels were elevated in the microvasculature isolated from RLS brain tissue. In a cell culture model of the BBB (Figure 4), MEIS1 expression could be increased by treatment with an iron chelator and decreased by iron loading (Figure 5; data provided by Padma Ponnuru). These findings support the concept of brain iron deficiency in RLS in the BBB and suggest a novel role for MEIS1 in the BBB that should be explored.
Figure 4. MEIS1 is elevated in microvasculature isolated from RLS brains compared to control.
These data on 3 samples of RLS and control brain tissue establish that MEIS1 is expressed in brain microvasculature. In combination with the data in Figure 5, the elevation of MEIS1 in RLS is consistent with the concept of brain iron deficiency in RLS. The elevation of MEIS1 is RLS microvasculature is also consistent with activation of hypoxic pathways.
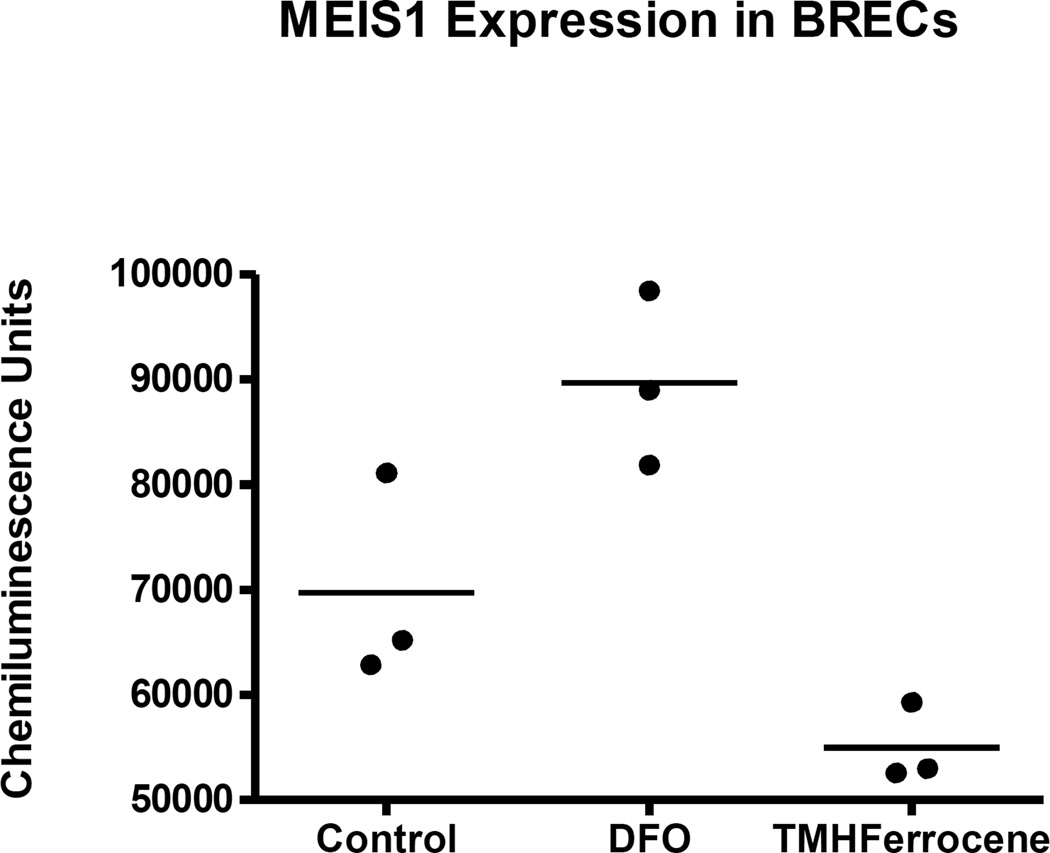
Figure 5. Iron status regulates MEIS1 expression in BREC cultures.
These data on 3 sets of BREC cultures demonstrates that iron-deficient BREC cultures have an increased MEIS1 expression while iron-loaded BREC cultures have decreased MEIS1 expression compared to the control cultures.
3. Does inadequate brain iron have consequences that may impact beyond the DA system
Iron is an important co-factor in dopamine biosynthesis and is intricately involved in the regulation of dopamine levels in the brain. It is also well known that iron acts as a co-factor in the regulation of the hypoxia inducible factor (HIF) pathway and that iron deficiency inhibits hydroxylation of hypoxia inducible factor-1α (HIF-1α) by the prolyl hydroxylase, which lead to stabilization of HIF-1α and activation of the HIF pathway. The subsequent upregulation of the hypoxia response pathway leads to upregulation of proteins that are involved in angiogenesis, erythropoiesis, cell survival and proliferation, and glucose and iron metabolism.
3.1 Normoxic activation of hypoxic pathways is seen in RLS autopsy samples
Patients with primary RLS display no symptoms of hypoxia, such as shortness of breath or cyanosis. Nonetheless, studies, have consistently indicated cellular hypoxic pathway activation in RLS [39]. Several normoxic triggers of hypoxia response pathway activation, such as nitric oxide (NO), adenosine, and iron chelation have been described [40, 41]. There was a report in which individuals with RLS demonstrate allelic similarities on chromosome 12q (where the nNOS gene is located) [42], but these data have not been reproduced in specific studies [43] or GWAS analyses. The potential role of nitric oxide alterations in the pathophysiology of RLS is noteworthy, with respect to peripheral microcirculation. A recent pilot study demonstrated reduced daytime tibialis anterior intramuscular blood flow in female patients with restless legs syndrome as compared to their age-matched controls [44]. Anderson et al [45] also demonstrated impaired microcirculation in RLS patients as compared to controls. Gulyani et al reported that a human neuroblastoma cell line treated with the iron chelator DFO and an iron-deficient mouse model have increases in Adenosine A2A receptors [46]. This local metabolic cellular stress (iron deficiency) could also induce an increase in adenosine release which could be involved in the normoxic activation of the HIF pathway. Numerous studies support the finding of increased HIF-1α (or HIF-2α) in many RLS tissues, including brain, microvasculature and peripheral blood lymphocytes [39]. Increased VEGF levels have also been reported in RLS/WED muscle cells, indicating HIF pathway activation in RLS/WED [47]. There is also an upregulation of neuronal nitric oxide synthase (nNOS) and nitrotyrosine in brain tissue of RLS patients [39]. Nitric oxide is a readily diffusible molecule that can affect the permeability of brain microvasculature and intracellular HIF activation. nNOS has been proposed as the major source of NO in cerebral regulation of blood flow [48]. NO has also been demonstrated to be a potent regulator of the dopamine transporter (DAT) [49].
Moreover, we have determined protein profiles in the CSF of RLS patients. In addition to the changes in iron management proteins discussed in Section 2, a number of protein concentrations were changed and many of these such as Vitamin D binding protein, Prostaglandin D2 synthase, cystatin C, β-hemoglobin could be tied to activation of hypoxic pathways [50–53].
3.2 Activation of hypoxic pathways can contribute to observations and help explain some of the mismatches in expected iron homeostatic pathways in RLS
A number of unexpected observations regarding iron homeostasis have been detected in the brains of patients with RLS and many of these alterations are also observed under hypoxia pathway or nitric oxide pathway activation. Total (IRP) activity, IRP1 activity and IRP1 protein levels are decreased in RLS as compared to controls [54] and this finding has also been observed in HepG2 cells under conditions of hypoxia [55]. H-ferritin is down regulated in substantia nigra, choroid plexus and microvasculature of patients with RLS [35, 54] and has also been reported to be reduced in human K562 and B6 fibroblast cells upon exposure to endogenously expressed nitric oxide [56, 57] and exogenously sources of nitric oxide in human B6 fibroblasts [57]. TfR was reported to be down regulated in the substantia nigra and microvasculature [35, 54] which can occur under conditions of hypoxia through miRNA210 [58].
Further evidence for activation of the hypoxia response pathway is observed in the BBBs of patients with RLS. In microvasculature preparations, elevations of HIF-2α as well as vascular endothelial growth factor (VEGF) have been reported [39].
There is mounting evidence suggesting a correlation between decreased oxygen status and Restless Legs Syndrome (RLS). Epidemiologic studies have demonstrated a higher prevalence of RLS in patients residing in higher altitudes [59, 60]. RLS prevalence has been reported to be increased in patients with pulmonary disease, including chronic obstructive pulmonary disease (COPD) [61] and obstructive sleep apnea (OSA) [62]. In addition to the epidemiological studies, physiological studies on patients with RLS have demonstrated altered skeletal muscle morphology, suggesting significantly lower predicted maximal oxygen uptake [63], as well as decreased oxygen partial pressure in the legs of RLS patients [64]. A recent pilot study (unpublished data) where patients with RLS and their age-matched controls were exposed to hypoxia for 10 minutes and they showed trends for alterations in femoral artery blood flow, heart rate and minute ventilation that further supported hypoxia pathway activation (Figures 6–8).
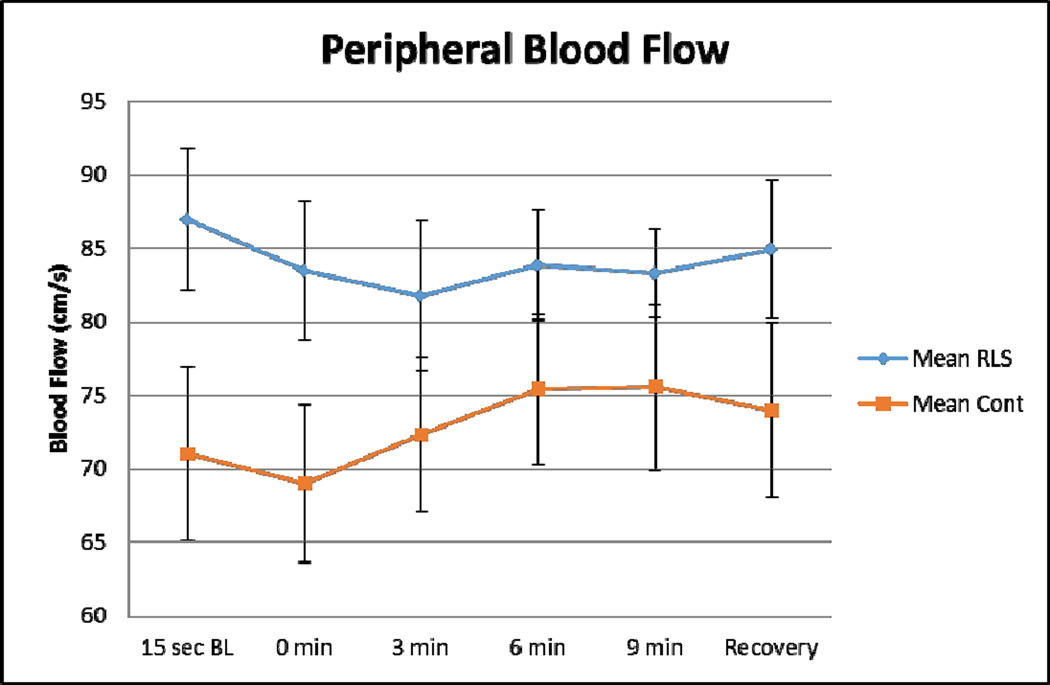
Figure 6. Alterations in Femoral Artery Blood Flow.
This figure demonstrates the femoral artery blood flow in RLS (blue) and control (orange) subjects. Error bars are standard error of the mean. n=18 (9 RLS; 9 age- and gender-matched controls). Baseline femoral artery blood flow is increased 22.5% in RLS subjects. RLS subjects demonstrate only a 1.7% increase in blood flow following exposure to hypoxia, whereas control subjects demonstrate a 7.2% increase in blood flow following hypoxia. (unpublished data)
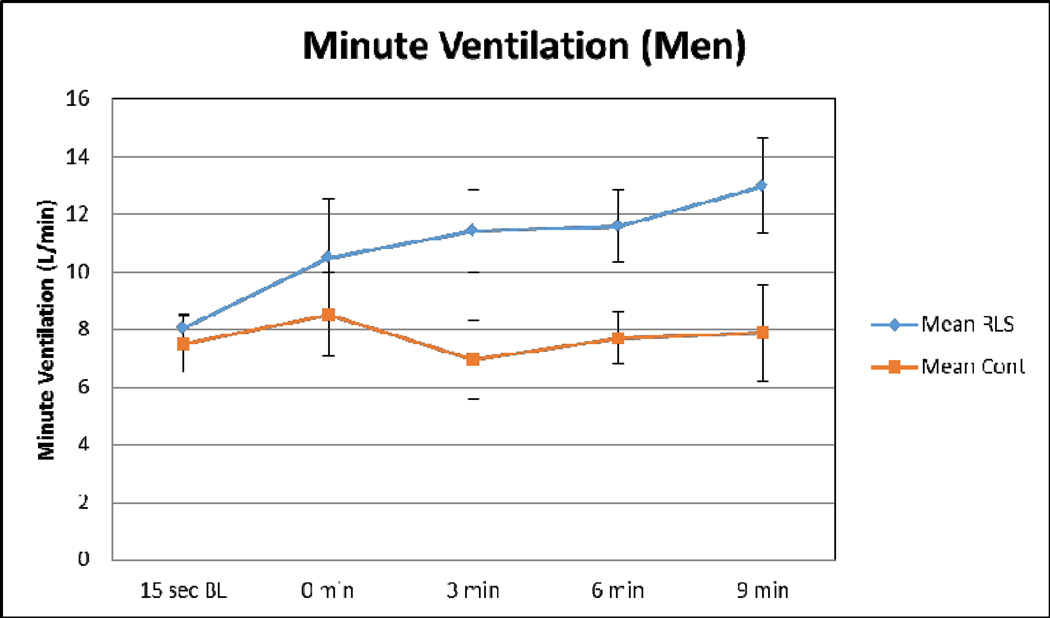
Figure 8. Alterations in Minute Ventilation.
This figure demonstrates the minute ventilation measurements in RLS (blue) and control (orange) subjects. Error bars are standard error of the mean. n=14 (7 RLS; 7 age-matched controls). Male RLS subjects had a slightly elevated minute ventilation rate at baseline. Male RLS subjects had a 65% increase in minute ventilation with hypoxic insult.(unpublished data)
4. Given the impact of iron in RLS are there genetic mutations in iron genes in RLS?
The number of gene products involved in iron regulation goes far beyond those discussed in the introduction and also includes miRNAs [65]. Moreover, various relations between iron and lipid metabolism have been identified recently by several lines of evidence, eg, by GWAS [66] on serum iron parameters, by the association of CSF ferritin with CSF apolipoprotein E and independently with the APOE ε4 genotype [67], or by the identification of gentisic acid (2,5-DHBA) as a siderophore for intracellular iron transport [68, 69].
Looking at iron genes in a wider sense (ie, at genes whose products interact with iron in one way or another), increases the number even more. Iron genes particularly relate to oxygen and mitochondrial energy metabolism, since iron is involved in enzymatic redox reactions and electron transport. In the citric acid cycle step catalyzed by the iron-containing 2-oxoglutarate dependent dioxygenases (2- OGDOs) the oxidation of 2-oxoglutarate may be combined with the hydroxylation or demethylation of other substrates [70, 71]. There are more than 60 2-OGDOs operating in various domains including connective tissue maturation by collagen hydroxylases, epigenetic regulation by DNA or histone demethylases, and reaction to hypoxia or iron-deficiency by HIFα hydroxylation (see below), leading back to the iron genes in the narrow sense. The number of iron genes is growing steadily. Recently, for instance, the chaperone PCBP2 has been found to form an iron-transport pipeline with DMT1, possibly being only a component of a postulated Fe-channeling metabolon [72].
In 2011 [73] a set of 111 iron management genes [1, 74, 75] were tested for association with RLS in a sample of 922 cases and 1526 controls with age and sex as covariates. Vice versa, the six RLS genes known at that time were tested for associations with serum iron parameters in a population sample of 3447 individuals; both analyses had negative results. Notably, this included MEIS1, which has been linked to thalamic ferritin expression [76] and BTBD9 (has been assumed to be associated with serum ferritin) [77]. The latter assumption also could not be reproduced in a recent meta-analysis on 5 × 104 individuals (courtesy of B. Benyamin, Queensland, Australia). In fact, while the RLS risk allele in BTBD9 seemed to decrease serum ferritin [78], homozygous knockout of Btbd9 produced mice with RLS-like phenotype but increased serum iron and normal striatal iron [79] adding further questions to the assumed relation between BTBD9 and RLS. Of course, these GWAS results analyses mentioned here do not exclude tissue-specific associations that are not apparent in relation to serum parameters. Moreover, they do not exclude the possibility that more recently discovered iron genes are associated with RLS.
4.1 Does the HFE gene variant protect in RLS?
The analysis referred to above [73] also included the common HFE variant C282Y (rs1800652, minor allele frequency 5%) which is the leading cause of hemochromatosis. Although C282Y is the variant with the largest single influence on the variance of serum ferritin (explaining 0.5% of the variance of log(ferritin)) [66], it was not found (not even nominally) to be associated with RLS. The same result (p = 0.57) was found when the analysis was repeated with a 10× larger sample size (11519 cases, 69783 controls, courtesy of J. Winkelmann, München, Germany).
4.1.1 If no, then what does this suggest?
The analysis for association between HFE C282Y and RLS can be regarded as a Mendelian randomization study on serum ferritin as a causal factor in RLS. The negative result is consistent with several previous studies on ferritin and RLS, as well as the association of RLS with ferritin appears to depend on cases where iron deficiency is so severe that it has functional consequences ie, anemia) [6, 83] while the association of HFE C282Y with iron-deficient anemia appears to be smaller than expected [84]. Moreover, it cannot be excluded that the HFE C282Y variant has an effect on RLS disposition that is antagonistic to its effect on serum ferritin, which would impair the overall association of this HFE variant with RLS.
4.2 Do we know the incidence of RLS in people with HFE gene variants
Anecdotally we know people with HC have RLS
RLS patients with the HFE C282Y mutation have been observed. Barton et al [85] reported on a homozygous male patient with a serum ferritin concentration of 658 ng/ml. Shaughnessy et al [86] diagnosed RLS in 10 of 61 patients with hemochromatosis due to HFE mutation (mainly C282Y homozygosity) who received therapeutic phlebotomy. Five of them had RLS before already which deteriorated in three but improved in one after treatment was started. Haba-Rubio et al [87] reported two unrelated patients homozygous for C282Y who needed phlebotomy. The female patient had RLS before, the male patient developed RLS while on a phlebotomy regime. In both, brain MRI showed reduced iron levels in substantia nigra, red nucleus, and pallidum although serum ferritin was elevated or normal, respectively, indicating that HFE C282Y is not a reliable predictor of elevated brain iron.
4.3 Epigenetics and iron in RLS
4.3.1 Would activation of hypoxic pathways alter epigenetics?
As mentioned in Section 3, there is upregulation of hypoxic pathways in the brain in autopsy samples from RLS patients. Hypoxia inflicts epigenetic changes at the chromatin level [70, 71]. Many of these changes are mediated by hypoxia-inducible transcription factor (HIF) acting on the expression of chromatin-modifying enzymes [88–91] but direct effects of oxygen tension on histone-modifying demethylase activities have also been observed [92, 93]. Hypoxia-sensing of the HIF pathway involves oxygen-dependent HIF hydroxylases (prolyl hydroxylases, PHD1–3, and factor inhibiting HIF, FIH) that modify the HIFα subunit and thus induce its degradation [94, 95].
HIF hydroxylases also appear to be entry points for the action of nitric oxide (NO). Under normoxic conditions PHD activity was found to be inhibited by NO [96]. In contrast under hypoxia, NO reduces HIFα levels due to induction of PHD2 expression [97]. The HIF hydroxylases themselves as well as a large group of epigenetically modifying enzymes belong to the 2-OGDO class of enzymes (see above). The latter includes the histone demethylases KDM6A, -B, and -C which specifically remove dimethyl and trimethyl residues from lysine 27 of histone 3 (K27H3me2/3) and thus have a chromatin opening and gene activating effect. They are of special interest in neurobiology due to their key roles in pre- and postnatal differentiation of neurons including dopaminergic neurons [98–101]. Haploinsufficiency of KDM6A is a cause of mental retardation in Kabuki syndrome (MIM 147920). Interestingly, the preponderance of open chromatin marks in Kabuki syndrome appears to be amenable to inhibitors of histone deacetylation [102] such as β-hydroxybutyrate generated by ketogenic diet [103].
Being dependent on iron for their enzymatic activity, 2-OGODs may act as sensors not only of hypoxia but also of iron deficiency. The HIF PDHs are sensitive to iron chelators and activated by iron chaperones [104]; thus iron seems to exert epigenetic influence via the hypoxia pathway as indicated above. Since the affinity of iron to the active site of PHD is very high, the physiological response to iron deficiency probably requires protein turnover [70]. By that route, of course, iron could potentially influence the activity of any epigenetically relevant 2-OGOD directly. Their turnover rates would then attain differential relevance.
4.4 Neurodegenerative Diseases and RLS
What is the prevalence of RLS in diseases such as AD, PD and ALS that are thought to involve excess brain iron?
Assuming that RLS relates to brain iron deficiency, it is of interest to know the prevalence of RLS in patients with neurodegenerative disorders that are thought to involve aberrant brain iron homeostasis. In Alzheimer’s disease (AD) about four precent of the patients also had RLS [105] which appears to be somewhat less frequent than in the general population. Periodic leg movements in sleep (PLMS) have been found to be significantly more frequent in patients with Parkinsonism (PD) not receiving levodopa than in controls, AD patients, or PD patients receiving levodopa [106]. Concerning amyotrophic lateral sclerosis (ALS) RLS was substantially more frequent in patients than in controls (25% vs. 8%) [107]. A similar high rate (19%) of RLS in ALS patients was observed in another European population [108]. If RLS is supposed to relate to neuronal iron deficiency and low serum ferritin, these findings need to be reconciled with the results on increased intracellular iron of substantia nigra neurons in PD [109] and on increased serum ferritin in ALS patients [110, 111]. Of course, in the neurodegenerative process, serum ferritin levels are likely reflective of the inflammatory status associated with the disease and activation of the microglia in general. Because there is no evidence of inflammation or microglial activation in RLS, the relationship between serum ferritin in neurodegenerative diseases and RLS is very likely a different process.
4.5 Prion disease changes brain iron status
There is considerable evidence that for the major neurodegenerative diseases AD, PD, and ALS, the pathogenesis involves prion-like seeding, propagation, and pathological aggregation of proteins such as β-amyloid (Aβ) and tau in AD, α-synuclein in PD, and a number of RNA-binding proteins like in ALS [112–115]. The pathogenicity of these protein aggregates may be due to their specific toxicity or to the deficiency of the proteins’ normal functions such as intracellular liquid-like compartment generation [116].
The prion protein PrPc appears to have a role in iron uptake across membranes by its function as a ferrireductase [117] analogous to dcytb and steap3. In knockout mice the iron content of major systemic organs, hematopoietic cells, and the brain is reduced [118]. Conversion of PrPc into the infectious scrapie molecule PrPsc likely interferes with that function. Moreover, PrPsc aggregates with ferritin thus sequestering iron into a non-accessible form. As a consequence, there is evidence of neuronal iron deficiency in PrPsc-induced neurodegeneration [119]. The Connor group examined RLS autopsy material for the presence of prions, but none were detected (unpublished observations). Incidentally, amyloid precursor protein (APP) also is involved in cellular iron-metabolism. It stabilizes the iron exporter ferroportin [120] and shows reduced expression in response to intracellular iron-deficiency due to an IRE in the 5’-UTR of its mRNA [121]. The cleavage of APP has also recently been shown to be regulated by nitric oxide levels [122]. The iron-associated neurodegeneration in PD which does not involve Aβ plaque formation has been related to nitric oxide mimicking iron-deficiency and inappropriately suppressing APP translation [109]. The increased expression of nitric oxide in RLS could impact the expression of APP and through stabilization of ferroportin contribute to intracellular iron deficiency in RLS.
Summary conclusions
Murine striatal brain iron has a significant circadian rhythm, and when nigra iron is low it can be increased by peripheral IV iron injections without producing iron overload in other brain regions. It is presumed this may apply to humans.
Increasing VMB iron increases dopamine in the striatum in animals, matching the increased dopamine seen with RLS.
It appears that about one third of the general population carries the genetic or systemic factors making them susceptible to RLS when the peripheral iron is reduced. Presumably the reduced peripheral iron results in reduced nigra and probably thalamic iron that are associated with RLS, although this critical concept has not been adequately evaluated.
IV iron treatment reverses RLS for some patients for several months, but not for all. It is unclear if this is a dose issue or if this reflects either difference in iron transport to the brain or in the role of iron for RLS.
Some patients with IDA and RLS may have a persisting RLS suggesting that iron deficiency may produce in some patients long lasting changes not corrected when the ID is resolved. Perhaps the iron transport to the brain is altered, but this remains to be studied.
Finally, IV iron that has a slow release, such as FCM, needs to be evaluated in appropriate large clinical trials to determine if it provides a safe and effective treatment for RLS.
RLS brains have lower than normal levels of iron and the iron insufficiency is not limited to the nigro-striatal pathway.
There are alterations in iron management protein profiles in the brain microvasculature in RLS patients that suggests the iron insufficiency in the RLS brains begins at the BBB with altered iron acquisition.
MEIS1, a RLS risk gene, is expressed at the protein level in the BBB and is elevated in RLS tissue. This elevation can be induced by iron deficiency supporting the concept that the brain is iron deficient in RLS.
Future studies regarding brain iron acquisition are key to understanding why some RLS patients respond to IV iron status and some don’t.
RLS brains and brain microvasculature demonstrate a protein profile of hypoxia pathway activation.
RLS patients demonstrate measureable alterations in peripheral hypoxia markers in lymphocytes and physiological parameters such as femoral artery blood flow, heart rate and minute ventilation.
RLS patients demonstrate alterations in their CSF proteomic profile in proteins that is consistent with iron deficiency, hypoxia activation and dopamine dysregulation.
The overall association between serum ferritin and RLS is inconsistent but probably non-linear so that very low serum ferritin appears to be a relevant risk factor for RLS.
As of yet, GWAS data neither have indicated an association of iron genes with RLS nor an association of RLS genes with serum iron parameters.
The role of iron in RLS may relate to a gene-environment interaction, possibly involving epigenetic regulation.
Recommended Future Directions
Despite significant progress and clearly establishing a role for iron insufficiency in the brain in RLS, multiple areas of investigation of significant clinical importance remain. A key finding in the preclinical studies has been the circadian rhythm for brain iron. This finding highlights that almost nothing is known about the efflux mechanism for iron from the brain, which has tremendous relevance to RLS.
The observation that some but not all RLS patients respond to IV iron provides a key opportunity to interrogate biomarkers and genetics that may likely predict responders versus non-responders. The same is true for treatment refractory RLS versus RLS that is more responsive to medical management in general. Similarly study of the differences in response to iron deficiency anemia and response to IV iron treatment A key question from the data indicating hypoxia activation in RLS brains (and perhaps the periphery) is whether this observation identifies a novel pathway for therapeutic targeting.
More preclinical studies are necessary to determine the functions of proteins associated with RLS risk genes. For example, the observation reported in this chapter for MEIS1 expression in microvasculature and that MEIS1 is iron responsive suggest novel research directions.
The effects of iron in treatment of RLS deserve further scientific study since non-efficient treatment and potential adverse effects must be avoided. The appropriate role of IV iron for RLS treatment should be better defined by clinical and animal studies.
Supplementary Material
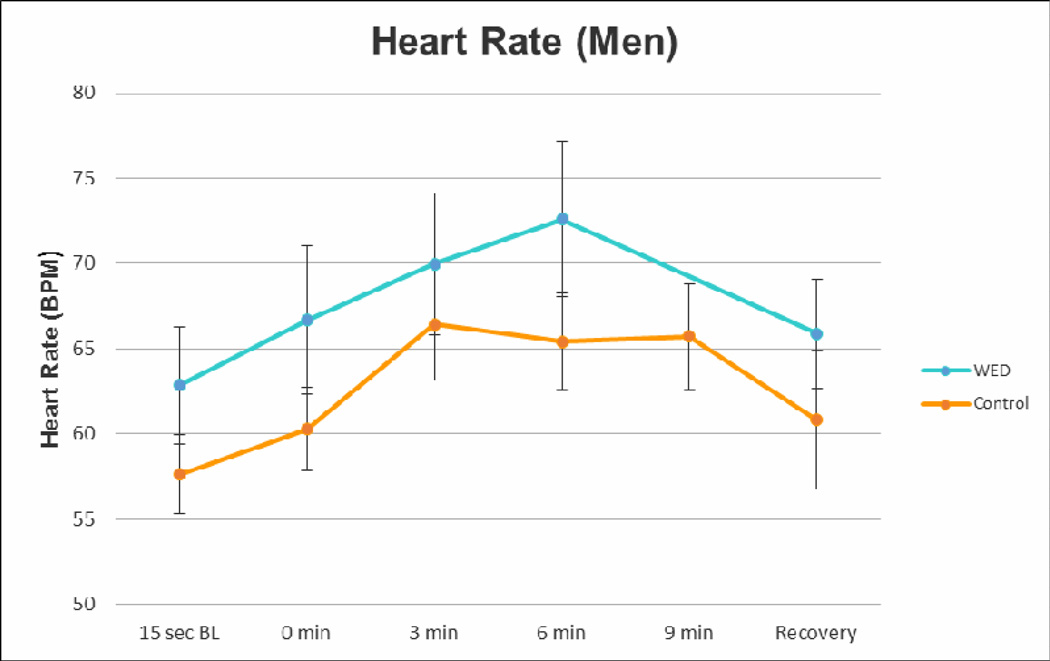
Figure 7. Alterations in Heart Rate.
This figure demonstrates the heart rate measurements in RLS (blue) and control (orange) subjects. As shown in this figure, a trend towards increased baseline heart rate is present in RLS subjects as compared to their age-matched controls. Error bars are standard error of the mean. n=14 (7 RLS; 7 age-matched controls) (unpublished data).
Highlights.
RLS brains have lower than normal levels of iron and the iron insufficiency is not limited to the nigro-striatal pathway.
About 1/3rd of the population carries the genetic or systemic factors making them susceptible to RLS when the peripheral iron is reduced.
There is activation of hypoxic pathways in RLS brains and peripheral tissues.
Studies on acquisition of brain iron in RLS led to paradigm shifting studies on regulation of iron uptake at the blood-brain-barrier.
Acknowledgments
The work presented in this chapter has been supported in part by the National Institutes of Health and by the Restless Legs Syndrome Foundation. The autopsy material that was studied and critically important to understanding the biological basis of RLS came from the RLS foundation brain bank. The authors are profoundly grateful to the patients and families who made the decision to donate brain tissue for study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hentze MW, et al. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142(1):24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Muckenthaler MU. How mutant HFE causes hereditary hemochromatosis. Blood. 2014;124(8):1212–1213. doi: 10.1182/blood-2014-07-581744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Earley CJ, et al. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54(8):1698–1700. doi: 10.1212/wnl.54.8.1698. [DOI] [PubMed] [Google Scholar]
- 4.Mizuno S, et al. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14(1):43–47. doi: 10.1111/j.1365-2869.2004.00403.x. [DOI] [PubMed] [Google Scholar]
- 5.Akyol A, et al. Iron deficiency anemia and restless legs syndrome: is there an electrophysiological abnormality? Clin Neurol Neurosurg. 2003;106(1):23–27. doi: 10.1016/j.clineuro.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Allen RP, et al. The prevalence and impact of restless legs syndrome on patients with iron deficiency anemia. Am J Hematol. 2013;88(4):261–264. doi: 10.1002/ajh.23397. [DOI] [PubMed] [Google Scholar]
- 7.Rangarajan S, D'Souza GA. Restless legs syndrome in Indian patients having iron deficiency anemia in a tertiary care hospital. Sleep Med. 2007;8(3):247–251. doi: 10.1016/j.sleep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Aspenstroem G. [Pica and Restless Legs in Iron Deficiency] Sven Lakartidn. 1964;61:1174–1177. [PubMed] [Google Scholar]
- 9.Connor JR, et al. Comparative evaluation of nephrotoxicity and management by macrophages of intravenous pharmaceutical iron formulations. PLoS One. 2015;10(5):e0125272. doi: 10.1371/journal.pone.0125272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unger EL, et al. Effects of IV iron isomaltoside-1000 treatment on regional brain iron status in an iron-deficient animal. Neuroscience. 2013;246:179–185. doi: 10.1016/j.neuroscience.2013.04.049. [DOI] [PubMed] [Google Scholar]
- 11.Mehmood T, et al. Response to intravenous iron in patients with iron deficiency anemia (IDA) and restless leg syndrome (Willis-Ekbom disease) Sleep Med. 2015;15(12):1473–1476. doi: 10.1016/j.sleep.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Nordlander NB. Therapy in restless legs. Acta Med Scand. 1953;145(6):453–457. [PubMed] [Google Scholar]
- 13.Earley CJ, et al. A randomized, double-blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med. 2009;10(2):206–211. doi: 10.1016/j.sleep.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho YW, Allen RP, Earley CJ. Lower molecular weight intravenous iron dextran for restless legs syndrome. Sleep Med. 2013;14(3):274–277. doi: 10.1016/j.sleep.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Grim K, et al. Treatment of childhood-onset restless legs syndrome and periodic limb movement disorder using intravenous iron sucrose. Sleep Med. 2013;14(11):1100–1104. doi: 10.1016/j.sleep.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Ondo WG. Intravenous iron dextran for severe refractory restless legs syndrome. Sleep Med. 2010;11(5):494–496. doi: 10.1016/j.sleep.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Grote L, et al. A randomized, double-blind, placebo controlled, multi-center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Mov Disord. 2009;24(10):1445–1452. doi: 10.1002/mds.22562. [DOI] [PubMed] [Google Scholar]
- 18.Unger EL, et al. Low brain iron effects and reversibility on striatal dopamine dynamics. Exp Neurol. 2014;261:462–468. doi: 10.1016/j.expneurol.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen RP, et al. Abnormally increased CSF 3-Ortho-methyldopa (3-OMD) in untreated restless legs syndrome (RLS) patients indicates more severe disease and possibly abnormally increased dopamine synthesis. Sleep Med. 2009;10(1):123–128. doi: 10.1016/j.sleep.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor JR, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132(Pt 9):2403–2412. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earley CJ, et al. Altered brain iron homeostasis and dopaminergic function in Restless Legs Syndrome (Willis-Ekbom Disease) Sleep Med. 2014;15(11):1288–1301. doi: 10.1016/j.sleep.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Earley CJ, et al. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep. 2013;36(1):51–57. doi: 10.5665/sleep.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connor JR, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61(3):304–309. doi: 10.1212/01.wnl.0000078887.16593.12. [DOI] [PubMed] [Google Scholar]
- 24.Allen RP, et al. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56(2):263–265. doi: 10.1212/wnl.56.2.263. [DOI] [PubMed] [Google Scholar]
- 25.Chang Y, et al. Altered white matter integrity in primary restless legs syndrome patients: diffusion tensor imaging study. Neurol Res. 2014;36(8):769–774. doi: 10.1179/1743132814Y.0000000336. [DOI] [PubMed] [Google Scholar]
- 26.Sieminski M, Losy J, Partinen M. Restless legs syndrome in multiple sclerosis. Sleep Med Rev. 2015;22:15–22. doi: 10.1016/j.smrv.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Geguchadze RN, et al. CSF proteomic analysis reveals persistent iron deficiency-induced alterations in non-human primate infants. J Neurochem. 2008;105(1):127–136. doi: 10.1111/j.1471-4159.2007.05113.x. [DOI] [PubMed] [Google Scholar]
- 28.Earley CJ, et al. Altered iron metabolism in lymphocytes from subjects with restless legs syndrome. Sleep. 2008;31(6):847–852. doi: 10.1093/sleep/31.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frauscher B, et al. Auditory startle reaction is disinhibited in idiopathic restless legs syndrome. Sleep. 2007;30(4):489–493. doi: 10.1093/sleep/30.4.489. [DOI] [PubMed] [Google Scholar]
- 30.Unger EL, et al. Acoustic startle response is disrupted in iron-deficient rats. Pharmacol Biochem Behav. 2006;84(2):378–384. doi: 10.1016/j.pbb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Simpson IA, et al. A novel model for brain iron uptake: introducing the concept of regulation. J Cereb Blood Flow Metab. 2015;35(1):48–57. doi: 10.1038/jcbfm.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burdo JR, et al. Regulation of the profile of iron-management proteins in brain microvasculature. J Cereb Blood Flow Metab. 2004;24(1):67–74. doi: 10.1097/01.WCB.0000095800.98378.03. [DOI] [PubMed] [Google Scholar]
- 33.Burdo JR, et al. Mechanisms and regulation of transferrin and iron transport in a model blood-brain barrier system. Neuroscience. 2003;121(4):883–890. doi: 10.1016/s0306-4522(03)00590-6. [DOI] [PubMed] [Google Scholar]
- 34.Patton SM, et al. Proteomic analysis of the cerebrospinal fluid of patients with restless legs syndrome/Willis-Ekbom disease. Fluids Barriers CNS. 2013;10(1):20. doi: 10.1186/2045-8118-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connor JR, et al. Profile of altered brain iron acquisition in restless legs syndrome. Brain. 2011;134(Pt 4):959–668. doi: 10.1093/brain/awr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder AM, et al. Mitochondrial ferritin in the substantia nigra in restless legs syndrome. J Neuropathol Exp Neurol. 2009;68(11):1193–1199. doi: 10.1097/NEN.0b013e3181bdc44f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corsi B, et al. Human mitochondrial ferritin expressed in HeLa cells incorporates iron and affects cellular iron metabolism. J Biol Chem. 2002;277(25):22430–22437. doi: 10.1074/jbc.M105372200. [DOI] [PubMed] [Google Scholar]
- 38.Gutsaeva DR, et al. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci. 2008;28(9):2015–2024. doi: 10.1523/JNEUROSCI.5654-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patton SM, et al. Hypoxia-inducible factor pathway activation in restless legs syndrome patients. Eur J Neurol. 2011;18(11):1329–1335. doi: 10.1111/j.1468-1331.2011.03397.x. [DOI] [PubMed] [Google Scholar]
- 40.De Ponti C, et al. Adenosine A2a receptor-mediated, normoxic induction of HIF-1 through PKC and PI-3K-dependent pathways in macrophages. J Leukoc Biol. 2007;82(2):392–402. doi: 10.1189/jlb.0107060. [DOI] [PubMed] [Google Scholar]
- 41.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Winkelmann J, et al. Evidence for further genetic locus heterogeneity and confirmation of RLS-1 in restless legs syndrome. Mov Disord. 2006;21(1):28–33. doi: 10.1002/mds.20627. [DOI] [PubMed] [Google Scholar]
- 43.Jimenez-Jimenez FJ, et al. Neuronal nitric oxide synthase (nNOS, NOS1) rs693534 and rs7977109 variants and risk for restless legs syndrome. J Neural Transm (Vienna) 2015;122(6):819–823. doi: 10.1007/s00702-014-1322-z. [DOI] [PubMed] [Google Scholar]
- 44.Oskarsson E, Wahlin-Larsson B, Ulfberg J. Reduced daytime intramuscular blood flow in patients with restless legs syndrome/Willis-Ekbom disease. Psychiatry Clin Neurosci. 2014;68(8):640–643. doi: 10.1111/pcn.12170. [DOI] [PubMed] [Google Scholar]
- 45.Anderson KN, Di Maria C, Allen J. Novel assessment of microvascular changes in idiopathic restless legs syndrome (Willis-Ekbom disease) J Sleep Res. 2013;22(3):315–321. doi: 10.1111/jsr.12025. [DOI] [PubMed] [Google Scholar]
- 46.Gulyani S, et al. Diminished iron concentrations increase adenosine A(2A) receptor levels in mouse striatum and cultured human neuroblastoma cells. Exp Neurol. 2009;215(2):236–242. doi: 10.1016/j.expneurol.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wahlin-Larsson B, et al. The expression of vascular endothelial growth factor in skeletal muscle of patients with sleep disorders. Muscle Nerve. 2009;40(4):556–561. doi: 10.1002/mus.21357. [DOI] [PubMed] [Google Scholar]
- 48.Bauser-Heaton HD, Bohlen HG. Cerebral microvascular dilation during hypotension and decreased oxygen tension: a role for nNOS. Am J Physiol Heart Circ Physiol. 2007;293(4):H2193–H2201. doi: 10.1152/ajpheart.00190.2007. [DOI] [PubMed] [Google Scholar]
- 49.Volz TJ, Schenk JO. L-arginine increases dopamine transporter activity in rat striatum via a nitric oxide synthase-dependent mechanism. Synapse. 2004;54(3):173–182. doi: 10.1002/syn.20075. [DOI] [PubMed] [Google Scholar]
- 50.Jordan W, et al. Biochemical markers of cerebrovascular injury in sleep apnoea syndrome. Eur Respir J. 2002;20(1):158–164. doi: 10.1183/09031936.02.00862001. [DOI] [PubMed] [Google Scholar]
- 51.Ahmad Y, et al. An insight into the changes in human plasma proteome on adaptation to hypobaric hypoxia. PLoS One. 2013;8(7):e67548. doi: 10.1371/journal.pone.0067548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Wu D, Vinters HV. Hypoxia and reoxygenation of brain microvascular smooth muscle cells in vitro: cellular responses and expression of cerebral amyloid angiopathy-associated proteins. Apmis. 2002;110(5):423–434. doi: 10.1034/j.1600-0463.2002.100509.x. [DOI] [PubMed] [Google Scholar]
- 53.Gou X, et al. Whole-genome sequencing of six dog breeds from continuous altitudes reveals adaptation to high-altitude hypoxia. Genome Res. 2014;24(8):1308–1315. doi: 10.1101/gr.171876.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Connor JR, et al. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology. 2004;62(9):1563–1567. doi: 10.1212/01.wnl.0000123251.60485.ac. [DOI] [PubMed] [Google Scholar]
- 55.Cheng CM, et al. Iron Regulatory Protein 1 Suppresses Hypoxia-Induced Iron Uptake Proteins Expression and Decreases Iron Levels in HepG2 Cells. J Cell Biochem. 2015;116(9):1919–1931. doi: 10.1002/jcb.25147. [DOI] [PubMed] [Google Scholar]
- 56.Domachowske JB, et al. Nitric oxide alters the expression of gamma-globin, H-ferritin, and transferrin receptor in human K562 cells at the posttranscriptional level. Blood. 1996;88(8):2980–2988. [PubMed] [Google Scholar]
- 57.Pantopoulos K, Hentze MW. Nitric oxide signaling to iron-regulatory protein: direct control of ferritin mRNA translation and transferrin receptor mRNA stability in transfected fibroblasts. Proc Natl Acad Sci U S A. 1995;92(5):1267–1271. doi: 10.1073/pnas.92.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshioka Y, et al. Micromanaging Iron Homeostasis: hypoxia-inducible micro-RNA-210 suppresses iron homeostasis-related proteins. J Biol Chem. 2012;287(41):34110–34119. doi: 10.1074/jbc.M112.356717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sevim S, et al. Unexpectedly low prevalence and unusual characteristics of RLS in Mersin, Turkey. Neurology. 2003;61(11):1562–1569. doi: 10.1212/01.wnl.0000096173.91554.b7. [DOI] [PubMed] [Google Scholar]
- 60.Castillo PR, et al. Prevalence of restless legs syndrome among native South Americans residing in coastal and mountainous areas. Mayo Clin Proc. 2006;81(10):1345–1347. doi: 10.4065/81.10.1345. [DOI] [PubMed] [Google Scholar]
- 61.Lo Coco D, et al. Increased frequency of restless legs syndrome in chronic obstructive pulmonary disease patients. Sleep Med. 2009;10(5):572–576. doi: 10.1016/j.sleep.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 62.Kapsimalis F, Kryger M. Sleep breathing disorders in the U.S. female population. J Womens Health (Larchmt) 2009;18(8):1211–1219. doi: 10.1089/jwh.2008.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larsson BW, et al. Skeletal muscle morphology in patients with restless legs syndrome. Eur Neurol. 2007;58(3):133–137. doi: 10.1159/000104712. [DOI] [PubMed] [Google Scholar]
- 64.Salminen AV, Rimpila V, Polo O. Peripheral hypoxia in restless legs syndrome (Willis-Ekbom disease) Neurology. 2014;82(21):1856–1861. doi: 10.1212/WNL.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 65.Silva B, Faustino P. An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochim Biophys Acta. 2015;1852(7):1347–1359. doi: 10.1016/j.bbadis.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 66.Benyamin B, et al. Novel loci affecting iron homeostasis and their effects in individuals at risk for hemochromatosis. Nat Commun. 2014;5:4926. doi: 10.1038/ncomms5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ayton S, Faux NG, Bush AI. Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE. Nat Commun. 2015;6:6760. doi: 10.1038/ncomms7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Devireddy LR, et al. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141(6):1006–1017. doi: 10.1016/j.cell.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ananth S, et al. Regulation of the cholesterol efflux transporters ABCA1 and ABCG1 in retina in hemochromatosis and by the endogenous siderophore 2,5-dihydroxybenzoic acid. Biochim Biophys Acta. 2014;1842(4):603–612. doi: 10.1016/j.bbadis.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karuppagounder SS, et al. Metabolism and epigenetics in the nervous system: Creating cellular fitness and resistance to neuronal death in neurological conditions via modulation of oxygen-, iron-, and 2-oxoglutarate-dependent dioxygenases. Brain Res. 2015;1628(Pt B):273–287. doi: 10.1016/j.brainres.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hancock RL, et al. Epigenetic regulation by histone demethylases in hypoxia. Epigenomics. 2015;7(5):791–811. doi: 10.2217/epi.15.24. [DOI] [PubMed] [Google Scholar]
- 72.Lane DJ, Richardson DR. Chaperone turns gatekeeper: PCBP2 and DMT1 form an iron-transport pipeline. Biochem J. 2014;462(1):e1–e3. doi: 10.1042/BJ20140720. [DOI] [PubMed] [Google Scholar]
- 73.Oexle K, et al. Dilution of candidates: the case of iron-related genes in restless legs syndrome. Eur J Hum Genet. 2013;21(4):410–414. doi: 10.1038/ejhg.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Constantine CC, et al. SNP selection for genes of iron metabolism in a study of genetic modifiers of hemochromatosis. BMC Med Genet. 2008;9:18. doi: 10.1186/1471-2350-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oexle K, et al. Novel association to the proprotein convertase PCSK7 gene locus revealed by analysing soluble transferrin receptor (sTfR) levels. Hum Mol Genet. 2011;20(5):1042–1047. doi: 10.1093/hmg/ddq538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Catoire H, et al. Restless legs syndrome-associated MEIS1 risk variant influences iron homeostasis. Ann Neurol. 2011;70(1):170–175. doi: 10.1002/ana.22435. [DOI] [PubMed] [Google Scholar]
- 77.Stefansson H, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357(7):639–647. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 78.Sorensen E, et al. A genetic risk factor for low serum ferritin levels in Danish blood donors. Transfusion. 2012;52(12):2585–2589. doi: 10.1111/j.1537-2995.2012.03629.x. [DOI] [PubMed] [Google Scholar]
- 79.DeAndrade MP, et al. Motor restlessness, sleep disturbances, thermal sensory alterations and elevated serum iron levels in Btbd9 mutant mice. Hum Mol Genet. 2012;21(18):3984–3992. doi: 10.1093/hmg/dds221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berger K, et al. Iron metabolism and the risk of restless legs syndrome in an elderly general population--the MEMO-Study. J Neurol. 2002;249(9):1195–1199. doi: 10.1007/s00415-002-0805-2. [DOI] [PubMed] [Google Scholar]
- 81.Hogl B, et al. Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology. 2005;64(11):1920–1924. doi: 10.1212/01.WNL.0000163996.64461.A3. [DOI] [PubMed] [Google Scholar]
- 82.Kim KW, et al. Prevalence, comorbidities and risk factors of restless legs syndrome in the Korean elderly population - results from the Korean Longitudinal Study on Health and Aging. J Sleep Res. 2010;19(1 Pt 1):87–92. doi: 10.1111/j.1365-2869.2009.00739.x. [DOI] [PubMed] [Google Scholar]
- 83.Richards KC, et al. Diagnostic accuracy of behavioral, activity, ferritin, and clinical indicators of restless legs syndrome. Sleep. 2015;38(3):371–380. doi: 10.5665/sleep.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beutler E, et al. Haematological effects of the C282Y HFE mutation in homozygous and heterozygous states among subjects of northern and southern European ancestry. Br J Haematol. 2003;120(5):887–893. doi: 10.1046/j.1365-2141.2003.04215.x. [DOI] [PubMed] [Google Scholar]
- 85.Barton JC, Wooten VD, Acton RT. Hemochromatosis and iron therapy of Restless Legs Syndrome. Sleep Med. 2001;2(3):249–251. doi: 10.1016/s1389-9457(01)00081-8. [DOI] [PubMed] [Google Scholar]
- 86.Shaughnessy P, Lee J, O'Keeffe ST. Restless legs syndrome in patients with hereditary hemochromatosis. Neurology. 2005;64(12):2158. doi: 10.1212/01.WNL.0000165954.42289.03. [DOI] [PubMed] [Google Scholar]
- 87.Haba-Rubio J, et al. Restless legs syndrome and low brain iron levels in patients with haemochromatosis. J Neurol Neurosurg Psychiatry. 2005;76(7):1009–1010. doi: 10.1136/jnnp.2003.030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pollard PJ, et al. Regulation of Jumonji-domain-containing histone demethylases by hypoxia-inducible factor (HIF)-1alpha. Biochem J. 2008;416(3):387–394. doi: 10.1042/BJ20081238. [DOI] [PubMed] [Google Scholar]
- 89.Wellmann S, et al. Hypoxia upregulates the histone demethylase JMJD1A via HIF-1. Biochem Biophys Res Commun. 2008;372(4):892–897. doi: 10.1016/j.bbrc.2008.05.150. [DOI] [PubMed] [Google Scholar]
- 90.Xia X, et al. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci U S A. 2009;106(11):4260–4265. doi: 10.1073/pnas.0810067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee HY, et al. HIF-1-dependent induction of Jumonji domain-containing protein (JMJD) 3 under hypoxic conditions. Mol Cells. 2014;37(1):43–50. doi: 10.14348/molcells.2014.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cascella B, Mirica LM. Kinetic analysis of iron-dependent histone demethylases: alpha-ketoglutarate substrate inhibition and potential relevance to the regulation of histone demethylation in cancer cells. Biochemistry. 2012;51(44):8699–8701. doi: 10.1021/bi3012466. [DOI] [PubMed] [Google Scholar]
- 93.Sanchez-Fernandez EM, et al. Investigations on the oxygen dependence of a 2-oxoglutarate histone demethylase. Biochem J. 2013;449(2):491–496. doi: 10.1042/BJ20121155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 95.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 96.Metzen E, et al. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14(8):3470–3481. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berchner-Pfannschmidt U, et al. Nitric oxide modulates oxygen sensing by hypoxia-inducible factor 1-dependent induction of prolyl hydroxylase 2. J Biol Chem. 2007;282(3):1788–1796. doi: 10.1074/jbc.M607065200. [DOI] [PubMed] [Google Scholar]
- 98.Jepsen K, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450(7168):415–419. doi: 10.1038/nature06270. [DOI] [PubMed] [Google Scholar]
- 99.Shahhoseini M, et al. Retinoic acid dependent histone 3 demethylation of the clustered HOX genes during neural differentiation of human embryonic stem cells. Biochem Cell Biol. 2013;91(2):116–122. doi: 10.1139/bcb-2012-0049. [DOI] [PubMed] [Google Scholar]
- 100.Park DH, et al. Activation of neuronal gene expression by the JMJD3 demethylase is required for postnatal and adult brain neurogenesis. Cell Rep. 2014;8(5):1290–1299. doi: 10.1016/j.celrep.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He XB, et al. Vitamin C facilitates dopamine neuron differentiation in fetal midbrain through TET1-and JMJD3-dependent epigenetic control manner. Stem Cells. 2015;33(4):1320–1332. doi: 10.1002/stem.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bjornsson HT, et al. Histone deacetylase inhibition rescues structural and functional brain deficits in a mouse model of Kabuki syndrome. Sci Transl Med. 2014;6(256):256ra135. doi: 10.1126/scitranslmed.3009278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Benjamin JS, et al. The ketogenic diet rescues defects of hippocampal neurogenesis in a mouse model of Kabuki syndrome. Ann Meeting of Am Soc Hum Genet. 2015 [Google Scholar]
- 104.Nandal A, et al. Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metab. 2011;14(5):647–657. doi: 10.1016/j.cmet.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Talarico G, et al. Restless legs syndrome in a group of patients with Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2013;28(2):165–170. doi: 10.1177/1533317512470208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bliwise DL, et al. Periodic leg movements in sleep in elderly patients with Parkinsonism and Alzheimer's disease. Eur J Neurol. 2012;19(6):918–923. doi: 10.1111/j.1468-1331.2012.03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lo Coco D, Piccoli F, La Bella V. Restless legs syndrome in patients with amyotrophic lateral sclerosis. Mov Disord. 2010;25(15):2658–2661. doi: 10.1002/mds.23261. [DOI] [PubMed] [Google Scholar]
- 108.Limousin N, et al. The high frequency of restless legs syndrome in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011;12(4):303–306. doi: 10.3109/17482968.2011.557736. [DOI] [PubMed] [Google Scholar]
- 109.Ayton S, et al. Parkinson's disease iron deposition caused by nitric oxide-induced loss of beta-amyloid precursor protein. J Neurosci. 2015;35(8):3591–3597. doi: 10.1523/JNEUROSCI.3439-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goodall EF, Haque MS, Morrison KE. Increased serum ferritin levels in amyotrophic lateral sclerosis (ALS) patients. J Neurol. 2008;255(11):1652–1656. doi: 10.1007/s00415-008-0945-0. [DOI] [PubMed] [Google Scholar]
- 111.Su XW, et al. Serum ferritin is elevated in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(1–2):102–107. doi: 10.3109/21678421.2014.984723. [DOI] [PubMed] [Google Scholar]
- 112.Couthouis J, et al. Evaluating the role of the FUS/TLS-related gene EWSR1 in amyotrophic lateral sclerosis. Hum Mol Genet. 2012;21(13):2899–2911. doi: 10.1093/hmg/dds116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim HJ, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495(7442):467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goedert M NEURODEGENERATION. Alzheimer's and Parkinson's diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science. 2015;349(6248):1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- 115.Jaunmuktane Z, et al. Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature. 2015;525(7568):247–250. doi: 10.1038/nature15369. [DOI] [PubMed] [Google Scholar]
- 116.Patel A, et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell. 2015;162(5):1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 117.Singh A, et al. Prion protein regulates iron transport by functioning as a ferrireductase. J Alzheimers Dis. 2013;35(3):541–552. doi: 10.3233/JAD-130218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singh A, et al. Prion protein (PrP) knock-out mice show altered iron metabolism: a functional role for PrP in iron uptake and transport. PLoS One. 2009;4(7):e6115. doi: 10.1371/journal.pone.0006115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh N. The role of iron in prion disease and other neurodegenerative diseases. PLoS Pathog. 2014;10(9):e1004335. doi: 10.1371/journal.ppat.1004335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCarthy RC, Park YH, Kosman DJ. sAPP modulates iron efflux from brain microvascular endothelial cells by stabilizing the ferrous iron exporter ferroportin. EMBO Rep. 2014;15(7):809–815. doi: 10.15252/embr.201338064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rogers JT, et al. An iron-responsive element type II in the 5'-untranslated region of the Alzheimer's amyloid precursor protein transcript. J Biol Chem. 2002;277(47):45518–45528. doi: 10.1074/jbc.M207435200. [DOI] [PubMed] [Google Scholar]
- 122.Cai ZX, et al. Double-Edged Roles of Nitric Oxide Signaling on APP Processing and Amyloid-beta Production In Vitro: Preliminary Evidence from Sodium Nitroprusside. Neurotox Res. 2016;29(1):21–34. doi: 10.1007/s12640-015-9564-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.