Abstract
Background
This study was conducted to explore programmed cell death‐ligand‐1 (PD‐L1) expression and fibroblast growth factor receptor 1 (FGFR1) amplification in stage IIIB/IV lung squamous cell carcinoma (SQC). Correlations between PD‐L1 and FGFR1, and with clinicopathological characteristics, efficacy of platinum‐based chemotherapy, and prognosis were analyzed.
Methods
One hundred and twenty‐eight consecutive stage III/IV SQC patients were enrolled in this study from 2009 to 2014. Seventy‐eight patients received platinum‐based chemotherapy. Immunohistochemistry was used to assess PD‐L1 expression and fluorescence in situ hybridization was applied to detect FGFR1 amplification.
Results
PD‐L1 expression was detected in 61.7% (79/128) of lung SQC patients. Smokers had significantly higher PD‐L1 expression rates than non‐smokers (66.1% vs. 44.0%, P = 0.042, respectively). The objective response and disease control rates for platinum‐based chemotherapy were not significantly different between PD‐L1 negative and positive patients (43.3% vs. 36.2%, P = 0.434; 80.0% vs. 78.7% P = 0.840, respectively); however, overall survival in PD‐L1‐negative patients was significantly longer than in PD‐L1‐positive patients (41.5 vs. 19.3 months, P = 0.001). Twenty‐five percent (32/128) of patients displayed FGFR1 amplification, with a lower rate in stage III patients compared to stage IV (17.1% vs. 36.5%, P = 0.013, respectively). There was no significant difference in FGFR1 amplification levels between overall response, disease control or overall survival rates. No correlation was observed between PD‐L1 expression and FGFR1 amplification (P = 0.916).
Conclusion
PD‐L1 expression may function as a prognostic factor in Chinese stage III/IV SQC patients. FGFR1 amplification is more prevalent in late stage SQC patients but does not predict chemotherapy response. There is no apparent correlation between PD‐L1 expression and FGFR1 amplification.
Keywords: FGFR1 amplification, PD‐L1 expression, squamous lung carcinoma
Introduction
Lung cancer is the leading cause of cancer death worldwide.1 Approximately 80% of patients with lung cancer are diagnosed with non‐small cell lung cancer (NSCLC) and 30% of these have squamous cell carcinoma (SQC). Recent advances in targeted therapy have led to a major paradigm shift in clinical oncology. Molecularly targeted drugs, such as erlotinib and crizotinib, have greatly improved the clinical outcome for NSCLC patients with sensitizing epidermal growth factor receptor (EGFR) gene mutations or anaplastic lymphoma kinase (ALK) gene translocations, respectively. However, these advances have mainly concentrated on lung adenocarcinoma and not SQC. The prognosis for advanced SQC remains poor with median overall survival (OS) of 10 months.2 Therefore, new therapeutic strategies for SQC are urgently required.
In recent years, immunotherapy for multiple cancers has made rapid progress. Particularly, checkpoint inhibitors, such as anti‐programmed cell death‐1 (PD‐1)/programmed cell death‐ligand‐1 (PD‐L1) monoclonal antibodies, have brought survival benefit for advanced lung cancer patients. However, identification of high effective predictors is the key to determining the success of treatment of checkpoint inhibitors. PD‐L1 is a 40 kDa type 1 transmembrane protein speculated to play a major role in suppressing the immune system during particular events.3 PD‐L1 binds to its receptor, PD‐1, found on activated T, B, and myeloid cells, to modulate activation or inhibition.4, 5 Some previous studies have indicated that a combination of PD‐L1 and PD‐1 can raise the tyrosine phosphatase SHP‐2 through an immunoreceptor tyrosine‐based switch motif (ITSM), and then make a number of key molecules of the T cell receptor (TCR) signaling pathway dephosphorylate in order to inhibit CD4 + T/CD8 + T cell proliferation or inhibit its activity.6 In addition, activation of the PD‐1 /PD‐L1 signaling pathway can reduce the T cell immune effect within the tumor microenvironment, which mediates tumor immune escape and the acceleration of tumor growth.7 High PD‐L1 expression has been found in melanoma, renal cancer, NSCLC, and ovarian carcinoma.8, 9 However, whether PD‐L1 expression is a predictor for PD‐1/PD‐L1 inhibitors remains controversial.
In spite of the slow research progress of targeted therapy against SQC, some progress has been made in recent years in preclinical and early clinical studies, suggesting fibroblast growth factor receptor 1 (FGFR1) as a potential driver gene of lung SQC.10 FGFR1 is a receptor tyrosine kinase whose ligands are specific members of the fibroblast growth factor family. FGFR1 regulates key cell behaviors, such as cell proliferation, differentiation, migration, and survival.11 Weiss et al. first identified frequent and focal FGFR1 amplification in lung SQC (n = 155), which had an approximately 20% positive rate, but not in other subtypes of lung cancer.12, 13 Treatment with 100 mg/kg twice a day of FGFR1 inhibitor resulted in tumor shrinkage in a mice xenograft model.12 Therefore, FGFR1 is considered a driver gene of lung SQC. Clinical trials involving targeted FGFR1 and other related pathways for SQC are ongoing.
It is important to investigate the correlation between FGFR1 amplification and PD‐L1 expression and to establish a foundation for the combination of PD‐1/PD‐L1 inhibitors with FGFR1 individualized targeted treatment for SQC patients. The aim of this study was to analyze the expression of PD‐L1 and amplification of the FGFR1 gene in advanced SQC tissues and to explore their relationship and effects on the efficacy of gemcitabine plus platinum and survival time, thereby laying the foundation for a future therapeutic strategy which combines an anti‐PD1/PD‐L1 monoclonal antibody with an FGFR1 inhibitor for advanced SQC.
Methods
The patients enrolled in the study were histologically or cytologically confirmed to have lung SQC and received chemotherapy at the Beijing Cancer Hospital between May 2009 and May 2014. Paraffin specimens of 128 cases were archived, including 119 men and nine women. The median age of the study cohort was 60 years (range: 36–78). According to World Health Organization standard classification and the 2009 Union for International Cancer Control Tumor Node Metastasis staging system, 75 cases exhibited poorly differentiated carcinoma, 40 cases undifferentiated carcinoma, and 13 cases high differentiation. There were 77 cases in stage III and 51 in stage IV and 120 cases of metastasis to local lymph nodes.
Complete follow‐up data was available for all 128 patients. Overall survival was defined as the interval from the pathological date to the last follow‐up (November 1, 2014) or the date of death. As of the last follow‐up, 71 patients had died.
Immunohistochemistry for programmed cell death‐ligand‐1 (PD‐L1) expression
Immunohistochemistry (IHC) was used to demonstrate the presence and location of PD‐L1 in the lung tissue samples. Dried five‐micron slides with formalin‐fixed and paraffin‐embedded tissue were prepared. Antigens were retrieved from tissue samples using combined citrate buffer PH 6 and incubating at 100°C for 15 minutes. Slides were then incubated overnight at 4°C with 7 μg/mL polyclonal rabbit anti‐PD‐L1 antibody (ab58810, Abcam, Cambridge, UK). A two‐step polymer‐horseradish peroxidase method (GT Vision, Beijing, China) was used for detection and 3,3′‐diaminobenzidine‐tetrahydrochloride staining. No staining was observed for negative controls, which were incubated with phosphate buffered saline instead of polyclonal rabbit anti‐PD‐L1 antibody.
Programmed cell death‐ligand‐1 positive expression was calculated according to the immunoreactive score (IRS) using a visual grading system based on the extent of staining by percentage of positive tumor cells (graded on a scale of 0–4: 0 < 5%, 1 = 5–25%, 2 = 26–50%, 3 = 51–75%, 4 > 75%) and the intensity of staining (graded on a scale of 1–3: 1 = weak, 2 = moderate, 3 = strong staining). The IRS = the percentage of positive tumor cells × the staining intensity, ranging from 0 (no positive tumor cells) to 12 (>75% of tumor cells with intense staining). IRS ≥3 served as the cut‐off value for dividing the expression of proteins into positive and negative. Different investigators and a pathologist independently evaluated IHC staining.
Fluorescence in situ hybridization for fibroblast growth factor receptor 1 (FGFR1) amplification
We performed FGFR1‐specific fluorescence in situ hybridization (FISH) on tissue slides using FISH probes that hybridized to the 8p12–8p11.23 region, Spectrum Orange (red), and to the centromere region of chromosome eight using a fluorophore (ZytoLight SPEC FGFR1/CEN 8 Dual Color Probe, ZytoVision GmbH, Munich, Germany) following the manufacturers’ instructions. Two experienced investigators interpreted the FISH results.
According to previous criteria, at least 60 nuclei were evaluated per sample. The FGFR1/CEP8 ratio, the average number of FGFR1 signals per tumor‐cell nucleus, and the percentage of tumor cells containing FGFR1 signals were used to define FGFR1 amplification. FGFR1 amplification and high polysomy were defined based on previous studies. In accordance to original research regarding the definition of high and low levels of FGFR1 amplification types, 100 cells were analyzed in each case. High‐level amplification was defined by an FGFR1/CEN8 ratio ≥2.0, an average number of FGFR1 signals per tumor cell nucleus ≥6, or the percentage of tumor cells containing ≥15 FGFR1 signals or large clusters ≥10%. Low‐level amplification was defined by ≥5 FGFR1 signals in ≥50% of tumor cells. Two independent pathologists blinded to all clinical data performed FGFR1 FISH analyses.
Statistical analysis
Survival curves were estimated using the Kaplan–Meier method and differences between the groups were compared using the log‐rank test. Cox proportional hazard models were used for multivariate analysis to assess the variables, including degree of differentiation, lymph node metastasis, PD‐L1 expression, FGFR1 amplification, and efficacy of treatment with gemcitabine plus cisplatinum (GP) therapy, and to predict the hazard rates for progression‐free survival (PFS) and OS. The statistical significance level was defined as P < 0.05. All statistical analyses were performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). All P values were two‐sided.
Results
Expression of PD‐L1 in advanced squamous cell carcinoma (SQC) and the correlation with clinicopathologic characteristics
Our study involved tissue samples from 128 patients with advanced SQC. The patients’ characteristics are shown in Table 1.
Table 1.
PD ‐L1 expression and clinicopathologic features
| Feature | Cases | PD‐L1 | ||
|---|---|---|---|---|
| − | + | P | ||
| Gender | ||||
| Male | 119 | 41 (34.5%) | 78 (65.5%) | − |
| Female | 9 | 7 (77.8%) | 2 (22.2%) | |
| Smoking status | ||||
| Ever or current | 103 | 34 (33.0%) | 69 (67.0%) | 0.039 |
| Never | 25 | 14 (56.0%) | 11 (44.0%) | |
| Age (years) | ||||
| ≥60 | 64 | 23 (35.9%) | 41 (64.1%) | 0.635 |
| <60 | 64 | 25 (39.0%) | 39 (61.0%) | |
| Degree of differentiation | ||||
| I + II | 53 | 17 (32.1%) | 36 (67.9%) | 0.107 |
| III | 75 | 31 (41.3%) | 44 (58.7%) | |
| Lymph node metastasis | ||||
| Yes | 120 | 43 (35.8%) | 77 (64.2%) | 0.066 |
| No | 8 | 5 (62.5%) | 3 (37.5%) | |
| Clinical stage | ||||
| III | 77 | 28 (36.4%) | 49 (63.6%) | 0.927 |
| IV | 51 | 20 (39.2%) | 31 (60.8%) | |
PD‐L1, programmed cell death‐ligand‐1.
Based on the cut‐off value of IRS > =3 for PD‐L1 positivity, 79 cases (61.7%) of lung SQC were PD‐L1 positive. PD‐L1 expression was mostly detected on cell membrane and/or the cytoplasm of tumor cells and/or lymphocytes. Brownish yellow to brown particles or clumps were observed within the cells (Fig 1).
Figure 1.
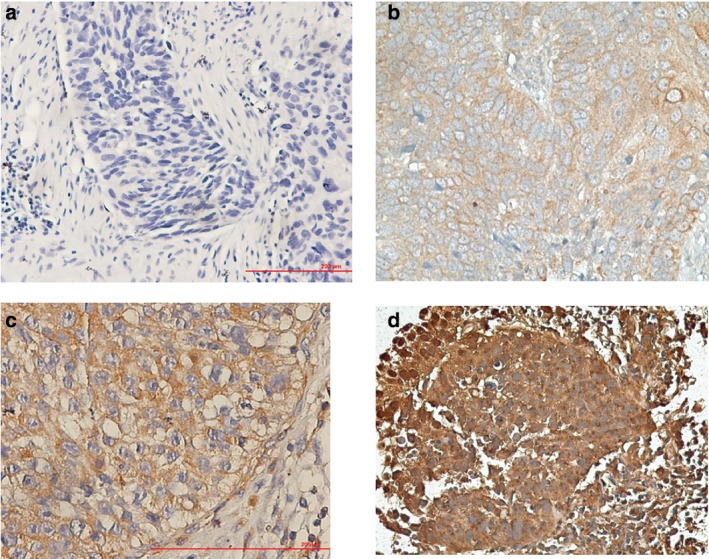
Different level programmed cell death‐ligand‐1 expression according to immunoreactive score: (a) negative, (b) low, (c) moderate, and (d) high expression.
We investigated whether there was a potential relationship between PD‐L1 expression and the patients’ clinicopathologic parameters. We found that PD‐L1 expression did not correlate with patient age, degree of differentiation, clinical stage, or lymph node metastasis. There were significantly higher expression rates in smokers than in non‐smokers (66.0% vs. 44%, P = 0.042, respectively; Table 1).
The association of PD‐L1 expression, as a dependent variable, with age and differentiation and stage and smoking history was also evaluated by logistic regression analysis to take into consideration the reciprocal effects of the covariates investigated. PD‐L1 expression was independently associated with smoking status (odds ratio 2.64).
Amplification of FGFR1 and the correlation with clinicopathologic characteristics in advanced SQC
According to FISH results, 32 of the 128 patients (25.0%) displayed FGFR1 amplification. Of these, 28 patients showed low levels and four patients high levels of FGFR1 amplification (Fig 2). The rates of FGFR1 amplification were significantly higher in stage IV than in stage III patients (65.5% vs. 22.2%, P = 0.013; Table 2).
Figure 2.
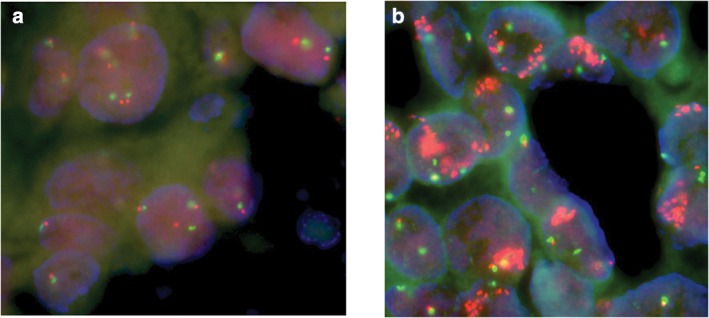
(a) Fluorescence in situ hybridization (FISH) of a lung squamous cancer sample with two copies of CEP7 (red) and fibroblast growth factor receptor 1 (FGFR1, green). (b) FISH showing a ratio of ≥2 between FGFR1 (green) and CEP7 (red) signals, resembling a high level of FGFR1 gene amplification.
Table 2.
FGFR amplification and clinicopathologic features
| Feature | Cases | Amplification of FGFR1 | ||
|---|---|---|---|---|
| (−) | (+) | P | ||
| Gender | ||||
| Male | 119 | 88 (73.9%) | 31 (26.1%) | 0.318 |
| Female | 9 | 8 (88.9%) | 1 (11.1%) | |
| Smoking status | ||||
| Ever or current | 103 | 77 (74.8%) | 26 (25.2%) | 0.898 |
| Never | 25 | 19 (76.0%) | 6 (24.0%) | |
| Ages (years) | ||||
| ≥60 | 64 | 51 (79.7%) | 13 (20.3%) | 0.221 |
| <60 | 64 | 45 (70.3%) | 19 (29.7%) | |
| Degree of differentiation | ||||
| I + II | 53 | 41 (77.4%) | 12 (22.6%) | 0.757 |
| III | 75 | 55 (73.3%) | 20 (26.7%) | |
| Metastasis of lymph node | ||||
| Yes | 120 | 90 (75.0%) | 30 (25.0%) | 0.842 |
| No | 8 | 6 (75.0%) | 2 (25.0%) | |
| Clinical stage | ||||
| III | 77 | 63 (81.8%) | 14 (18.2%) | 0.013 |
| IV | 51 | 32 (62.7%) | 19 (37.3%) | |
FGFR, fibroblast growth factor receptor.
Other clinical/pathologic parameters, such as gender, smoking status, and degree of differentiation, were not associated with FGFR1 amplification.
Relationship between FGFR1 amplification and PD‐LI expression
Among 79 patients with PD‐L1 positive expression, FGFR1 positive and negative rates were 25.3% (20/79) and 74.7% (59/79), respectively. In the patients with PD‐L1 negative expression, FGFR1 positive and negative rates were 24.5% (12/49) and 75.5% (37/49), respectively. No correlation between PD‐L1 expression and FGFR1 amplification was observed (P = 0.916).
Influence of two molecular parameters on response to gemcitabine plus cisplatinum chemotherapy
Gemcitabine plus cisplatin was the most commonly utilized first‐line chemotherapy regimen for advanced lung SQC in China. Among 128 cases of advanced lung SQC, 77 patients were treated with two to six cycles of GP as first‐line treatment. The objective response rate (ORR), including partial and complete responses, was 39.0% (30/77) and the disease control rate (DCR) was 79.2% (61/78). Of these 77 cases, patients with PD‐L1 positive expression presented an ORR of 36.2% (17/47) and DCR of 78.7.7% (37/47; P = 0.840), while those with negative PD‐L1 expression presented an ORR of 43.3% (13/30) and DCR of 80.0% (24/30; P = 0.434) after GP regimen treatment. No significant difference in response to GP regimen was found between FGFR1‐positive and FGFR1‐negative patients (ORR 40.9% vs. 38.2%, P = 0.663; DCR 81.8% vs. 78.2%, P = 0.659, respectively).
Association between two molecular parameters with survival
We also analyzed the potential implication of PD‐L1 expression in predicting clinical outcome. The median OS of these 128 patients was 32.01 months (range: 8.1–67.5). The OS in patients with PD‐L1 negative was significantly longer than in patients with PD‐L1 positive expression (41.5 [95% confidence interval CI 35.3–54.5] vs. 19.3 months [95% CI, 14.1–24.5], P = 0.001 by log‐rank test; Fig 3a). No difference was obtained when we stratified the data by FGFR1 status (32.7 vs. 28.4 months, P = 0.628 by log‐rank test; Fig 3b).
Figure 3.
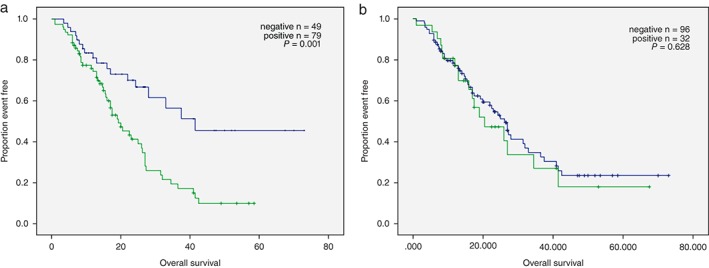
Overall survival curves for the 128 patients by (a) programmed cell death‐ligand‐1 expression and (b) fibroblast growth factor receptor 1 amplification.
In multivariate Cox regression analysis where the smoking status, degree of differentiation, clinical stage, and lymph node metastasis were included as covariates together with PD‐L1 expression, PD‐L1 expression (OR 2.38, 95% CI 1.35—4.17; P = 0.003) was an independent predictor of OS.
Discussion
This study investigated the status of PD‐L1 expression and FGFR1 amplification in stage IIIB/IV SQC, their relationships each other, and their influence on clinicopathological characteristics, chemotherapy efficacy, and prognosis. The results showed PD‐L1 expression and FGFR1 amplification rates of 65.5 and 25.0% in advanced SQC, respectively. PD‐L1 expression was higher in men and smokers than in women and non‐smokers. PD‐L1 expression and FGFR1 amplification might be irrelevant. Neither PD‐L1 nor FGFR1 was associated with response to gemcitabine‐based chemotherapy. PD‐L1 could act as a prognostic factor in Chinese stage III/IV SQC patients.
Most of the previously reported studies of lung cancer have focused on PD‐L1 expression in NSCLC patients. Several studies have demonstrated that PD‐L1 overexpression is related to lymph node metastasis, clinical staging, and survival in NSCLC patients.14, 15 However, contrary results, in which no relationship was found between PD‐L1 expression and clinicopathological characteristics, including gender, smoking status, histological type, and TNM stage, have also been reported.16 Our study confirmed that PD‐L1 expression in SQC patients was 62.5% and gender, smoking status, and OS were related to PD‐L1 expression in patients with advanced SQC. Smoking causes chronic inflammation of bronchial mucosa, which may promote immunogenicity.17 Therefore, smoking‐related malignant tumors, including lung SQC often possess higher PD‐1/PD‐L1 expression, which may be why checkpoint inhibitors could be more effective in smokers.
Some studies have confirmed that an increase in PD‐L1 expression on the tumor cell surface of cells in the microenvironment can combine with PD‐1 on activated T cells. This combination negatively regulates signal transduction, which can lead to the apoptosis of tumor antigen specific T cells, thus inhibiting the immune response.3 Studies have shown that PD‐L1 expression in NSCLC patients may be related to immunotherapy efficacy. A phase I clinical trial confirmed ORRs of anti PD‐1 monoclonal antibody in PD‐L1 positive and negative patients of 36% and 0%, respectively.18 Consequently, the positive rate of PD‐L1 is thought to be a potential biological target for anti PD‐1/PD‐L1 antibody therapy. However, other studies have found no significant correlation between PD‐L1 and ORR.
Increasing evidence has revealed that PD‐L1 is upregulated in multiple tumors, including lung cancer, by activation of key oncogenic pathways, such as the phosphoinositide 3‐kinase‐protein kinase B and RAS‐Raf‐mitogen‐activated protein kinase pathways.19, 20 FGFR1 is the most common molecular aberrance for SQC with an approximate 20% positive rate. Our study yielded the same result. Agents targeted to FGFR1, such as AZD4547 and nintedanib, have presented good safety and definite efficacy in this subtype of NSCLC.21, 22 Along with developments in immunotherapy and targeted therapy for SQC, a great challenge faced is how to combine these two kinds of treatments to improve patient prognosis. While exploration of the association between PD‐L1 expression and FGFR1 amplification may lay the foundation for a reasonable combination of immunotherapy with targeted treatment for SQC, our results showed no correlation between PD‐L1 expression and FGFR1 amplification. This might imply that FGFR1 amplification did not increase the immunogenicity of SQC. These factors should be considered in future for a combined strategy of checkpoint inhibitors and FGFR pathway inhibitors in advanced SQC.
The results of previous studies involving the association of FGFR1 and survival have been inconsistent. Some studies have found that FGFR1 amplification is associated with poor clinical outcomes in other cancers.12, 23 Weiss et al. reported a trend toward inferior survival among those with FGFR1 amplification; however, Heist et al. conducted a study of 226 Caucasian patients and implied that there was no correlation between FGFR1 amplification and prognosis.12, 23 Our results and those of previous studies demonstrated no significant difference in OS by FGFR1 amplification status. These experiments revealed that there is no evidence to indicate that FGFRl amplification is a prognostic factor for lung SQC.
In conclusion, PD‐L1 expression in advanced lung SQC was an independent predictor of poor prognosis in Chinese stage III/IV SQC patients. As PD‐L1 expression suppresses T‐cell function and promotes tumor cell immune escape, blocking the PD‐L1 PD‐1/signal pathway could be a new strategy for the treatment of lung SQC, as the PD‐1 inhibitor could bind PD‐1 and restore T cell function. Our results show that there is no apparent correlation between PD‐L1 expression and FGFR1 amplification, a fact that may be helpful to explore the combined therapeutic strategy of anti‐PD‐1/Pd‐L1 and FGFR1 inhibitors in the future.
Disclosure
No authors report any conflict of interest.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 2. Zheng YW, Li RM, Zhang XW, Ren XB. Current adoptive immunotherapy in non‐small cell lung cancer and potential influence of therapy outcome. Cancer Invest 2013; 31: 197–205. [DOI] [PubMed] [Google Scholar]
- 3. Postmus PE, Brambilla E, Chansky K et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007; 2: 706–14. [DOI] [PubMed] [Google Scholar]
- 4. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Francisco LM, Salinas VH, Brown KE et al. PD‐L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206: 3015–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yokosuka T, Takamatsu M, Kobayashi‐Imanishi W et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 2012; 209: 1201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Velcheti V, Schalper KA, Carvajal DE et al. Programmed death ligand‐1 expression in non‐small cell lung cancer. Lab Invest 2014; 94: 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spranger S, Spaapen RM, Zha Y et al. Up‐regulation of PD‐L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5 (200): 200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cancer Genome Atlas Research Network . Comprehensive genomic characterization of squamous cell lung cancers. (Published erratum appears in Nature 2012; 491: 288) Nature 2012; 489: 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perez‐Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: Molecular subtypes and therapeutic opportunities. Clin Cancer Res 2012; 18: 2443–51. [DOI] [PubMed] [Google Scholar]
- 12. Weiss J, Sos ML, Seidel D et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2010; 2: 62ra93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dutt A, Ramos AH, Hammerman PS et al. Inhibitor‐sensitive FGFR1 amplification in human non‐small cell lung cancer. PLoS One 2011; 6 (6): e20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen YB, Mu CY, Huang JA. Clinical significance of programmed death‐1 ligand‐1 expression in patients with non‐small cell lung cancer: A 5‐year‐follow‐up study. Tumori 2012; 98: 751–5. [DOI] [PubMed] [Google Scholar]
- 15. Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD‐L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011; 28: 682–8. [DOI] [PubMed] [Google Scholar]
- 16. Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka‐Akita H, Nishimura M. B7‐H1 expression on non‐small cell lung cancer cells and its relationship with tumor‐infiltrating lymphocytes and their PD‐1 expression. Clin Cancer Res 2004; 10: 5094–100. [DOI] [PubMed] [Google Scholar]
- 17. Hatsukami DK, Jorenby DE, Gonzales D et al. Immunogenicity and smoking‐cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther 2011; 89: 392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ota K, Azuma K, Kawahara A et al. Induction of PD‐L1 expression by the EML4‐ALK Oncoprotein and downstream signaling pathways in non‐small cell lung cancer. Clin Cancer Res 2015; 21: 4014–21. [DOI] [PubMed] [Google Scholar]
- 20. Atefi M, Avramis E, Lassen A et al. Effects of MAPK and PI3K pathways on PD‐L1 expression in melanoma. Clin Cancer Res 2014; 20: 3446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang J, Zhang L, Su X et al. Translating the therapeutic potential of AZD4547 in FGFR1‐amplified non‐small cell lung cancer through the use of patient‐derived tumor xenograft models. (Published erratum appears in Clin Cancer Res 2013; 19: 3714) Clin Cancer Res 2012; 18: 6658–67. [DOI] [PubMed] [Google Scholar]
- 22. Noonan S, Man Wong K, Jimeno A. Nintedanib, a novel triple angiokinase inhibitor for the treatment of non‐small cell lung cancer. Drugs Today (Barc) 2015; 51: 357–66. [DOI] [PubMed] [Google Scholar]
- 23. Heist RS, Mino‐Kenudson M, Sequist LV et al. FGFR1 amplification in squamous cell carcinoma of the lung. J Thorac Oncol 2012; 7: 1775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


