Abstract
Diabetes patients have more than double the risk of ischemic stroke compared with non‐diabetic individuals, and its neuroimaging characteristics have important clinical implications. To understand the pathophysiology of ischemic stroke in diabetes, it is important to focus not only on the stroke subtype, but also on the size and location of the occlusive vessels. Specifically, ischemic stroke in diabetes patients might be attributed to both large and small vessels, and intracranial internal carotid artery disease and small infarcts of the posterior circulation often occur. An additional feature is that asymptomatic lacunar infarctions are often seen in the basal ganglia and brain stem on brain magnetic resonance imaging. In particular, cerebral small vessel disease (SVD), including lacunar infarctions, white matter lesions and cerebral microbleeds, has been shown to be associated not only with stroke incidence, but also with the development and progression of dementia and diabetic microangiopathy. However, the pathogenesis of cerebral SVD is not fully understood. In addition, data on the association between neuroimaging findings of the cerebral SVD and diabetes are limited. Recently, the clinical importance of the link between cerebral SVD and retinal microvascular abnormalities has been a topic of considerable interest. Several clinical studies have shown that retinal microvascular abnormalities are closely related to cerebral SVD, suggesting that retinal microvascular abnormalities might be pathophysiologically linked to ischemic cerebral SVD. We review the literature relating to the pathophysiology and neuroimaging of cerebrovascular disease in diabetes, and discuss the problems based on the concept of cerebral large and small vessel disease.
Keywords: Cerebral large and small vessel disease, Diabetic retinopathy, Type 2 diabetes
Introduction
The continually increasing number of diabetes patients is a social problem worldwide, and thus prevention of the incidence and recurrence of macrovascular complications, stroke and ischemic heart disease in particular, is extremely important. Compared with non‐diabetics, ischemic stroke is two‐ to threefold more prevalent in diabetes patients1, 2, 3, 4, and a recent meta‐analysis has reported that hemorrhagic stroke is approximately 1.5‐fold more prevalent in diabetes patients than in individuals without diabetes5. However, as there are ethnic differences in hemorrhagic stroke prevalence6, diabetes is not recognized as a risk factor for hemorrhagic stroke.
Regarding the characteristics of cerebral infarctions, according to past autopsy studies7, 8, small infarctions (lacunar infarctions) in the thalamus, pons and other parts of the vertebrobasilar artery system, and intracranial internal carotid artery disease are more frequent in diabetes patients. In the acute phase, there is often early neurological deterioration and recurrence, and the prognosis is frequently poor. Generally, in diabetes, endothelial nitric oxide synthase activity and nitric oxide production decrease, leading to progression of endothelial dysfunction and impaired vasodilatation. As intracranial vessels are particularly susceptible to the effects of oxidative stress9, there is a possibility that blood–brain barrier (BBB) disruption in the intracranial carotid artery would precede the formation of atherosclerotic lesions.
The pathogenesis of cerebral small vessel disease (SVD) is not fully understood. Endothelial activation, increased BBB permeability and inflammatory processes have been implicated10, 11. In addition, a previous study has shown that hyperglycemia‐induced polyol pathway hyperactivity might play an important part in the development of diabetes atherosclerosis12. Recently, the clinical importance of the link between cerebral SVD and retinal microvascular abnormalities has been a topic of considerable interest. Hyperglycemia‐induced polyol pathway hyperactivity is considered to be one possible mechanism underlying the development of diabetic retinopathy13. As retinal microvascular abnormalities are associated with magnetic resonance imaging (MRI) markers of cerebral SVD14, 15, polyol pathway hyperactivity might play a possible pathogenic role in the development and progression of cerebral SVD in diabetes patients. In the present review, we discuss the possible mechanism underlying the development and progression of diabetes atherosclerosis, and relevant neurovascular imaging studies.
Epidemiology of Stroke in Diabetes Patients
The number of diabetes patients has been increasing worldwide, and it has become a major public health problem, with the recent International Diabetes Federation report stating that it had surpassed 400 million. When diabetes is accompanied by stroke, in many cases a caregiver is required to assist the patient in daily life because of physical disability and cognitive impairment. Compared with non‐diabetic individuals, the incidence of cerebral infarction in diabetes patients is two‐ to threefold higher1, 2, 3, 4 because of the combined effect of multiple risk factors for atherosclerosis. A recent meta‐analysis showed that diabetes raised the risk not only of cerebral infarction, but also of brain hemorrhage5. The Trial of Org 10172 in Acute Stroke Treatment classification16 that is often used in stroke‐related clinical research has cerebral large vessel disease and SVD as subtypes. When thinking about the pathophysiology of cerebral infarction in diabetes, it is important to focus on the size of the cerebral vessel that has been impaired. Diabetes is a significant risk factor for both large vessel disease and SVD17, and it is present in approximately 30% of cerebral infarction cases. An autopsy study that examined cerebral infarctions by vascular territory supply found that compared with non‐diabetics, infarctions more often occurred in the vertebrobasilar artery system of diabetes patients, in the pontine basal portion in particular7, 8.
Although few studies have examined an association between the duration of diabetes and stroke incidence in detail, it has been reported that for a disease duration of 10 years or more, the risk of developing ischemic stroke was twofold greater as compared with up to 5 years18. Compared with the recurrence rate within 2 years of the initial stroke in non‐diabetic individuals of 11.4%, the recurrence rate in diabetes patients was significantly higher at 15.2%19, and for recurrence from 5 years onwards, diabetes was the strongest predictive factor20. In contrast, glycated hemoglobin level was not associated with the risk of stroke recurrence21.
Pathogenesis of Ischemic Cerebrovascular Disorders in Diabetes
The hyperglycemic state causes cell damage by promoting advanced glycation end‐products, activating protein kinase C and through polyol pathway activation. In particular, activation of the polyol pathway consumes nicotinamide adenine dinucleotide phosphate, which reduces endothelial nitric oxide synthase activity and decreases nitric oxide production, causing endothelial dysfunction. By increasing adhesion molecule expression in the endothelium, and reducing anti‐inflammatory and vasodilatation actions, this is thought to promote atherosclerosis, leading to thrombus formation, and further to incidence and progression of cerebral infarction.
The risk factors for the development of atherosclerosis in patients with diabetes are chronic hyperglycemia, dyslipidemia, hypertension and hyperinsulinemia. These risk factors and their related abnormalities, such as decreased bioavailability of vascular nitric oxide, are well known for patients with a long duration of diabetes and older age. The involvement in atherosclerosis development of increased levels of molecular mediators, such as circulating vascular cell adhesion molecule‐1 and plasminogen activator inhibitor‐1 and tissue factor, as well as increased platelet activation, are also well known for such patients. All of this contributes to vascular dysfunction with ischemia/hypoxia22, 23, 24, 25, 26. In addition, a possible pathogenesis of diabetic complications, including microvascular disease and atherosclerosis, has been proposed on the basis of findings for hyperglycemia‐induced metabolic abnormalities, such as oxidative stress, changes in protein kinase C, glycation and the polyol pathway12, 27. Increasing evidence suggests that oxidative stress, glycation, protein kinase C activity and myoinositol metabolism have cross‐links with the polyol pathway (Figure 1).
Figure 1.
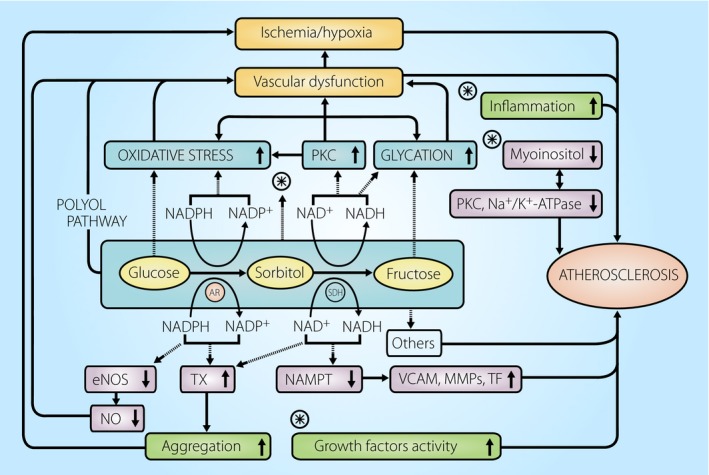
Schematic representation of the possible role of the polyol pathway in diabetes atherosclerosis. The polyol pathway consists of two steps: glucose is first reduced to sorbitol by the enzyme, aldose reductase (AR), and the resulting sorbitol is then changed to fructose by sorbitol dehydrogenase (SDH). During euglycemia, the utilization of glucose through the polyol pathway accounts for less than 3% of glucose consumption in cells. However, during hyperglycemia, total consumption of glucose through this pathway represents up to 30%137, resulting in the enhancement of glucose utilization through metabolic cascade shown. Thus, hyperglycemia‐induced polyol pathway hyperactivity might contribute to developing not only microvascular disease, but also atherosclerosis in the patients with diabetes. eNOS, endothelial nitric oxide synthase; MMP, matrix metalloproteinases; NAMPT, nicotineamide phosphoribosyl transferase; NO, nitric oxide; PKC, protein kinase C; TF, tissue factor; TX, thromboxane; VCAM, vascular cell adhesion molecule.
Previous studies by our group28, 29, 30, 31, 32 and others33, 34, 35, 36, 37, 38, 39 have suggested that hyperglycemia‐induced polyol pathway hyperactivity might, in part, play an important role in the development of diabetes atherosclerosis. Recently, Tang et al.40 have observed that the combination of hyperglycemia during collagen activation leads to a positive feedback cycle of release of platelet thromboxane and enhanced platelet aggregation through polyol pathway hyperactivity. This was ameliorated by an aldose reductase (AR) inhibitor. Furthermore, a study using diabetic apo E4−/− human AR mice aortas by Vedantham et al.41 surmised that glucose flux through the polyol pathway in hyperglycemia mediates atherosclerosis in part by influencing nicotinamide phosphoribosyl transferase‐mediated nicotinamide adenine dinucleotide biosynthesis, resulting in increased expression of vascular cell adhesion molecule‐1 and tissue factor. All of their observations were improved by an AR inhibitor. The aforementioned previous studies28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, and the novel findings of Tang et al. and Vedantham et al.40, 41 strongly suggest that diabetes atherosclerosis has similarities with diabetic microangiopathy12, 27, 42, 43, 44, and might partly develop from the metabolic cascade activated through hyperglycemia‐induced polyol pathway hyperactivity, as seen in Figure 1.
Insulin resistance is also thought to be a risk factor for cerebral infarction, and it has been reported that insulin resistance was observed in approximately half of non‐diabetic individuals who had experienced transient ischemic attack or ischemic stroke45. Insulin resistance is not only related to impaired glucose tolerance, it is also considered to promote atherosclerosis by causing hypertension, dyslipidemia, reduced fibrinolytic activity and increased platelet agglutination, and promoting endothelial dysfunction46. Furthermore, insulin resistance has also been reported to be a risk factor for atherothrombotic infarction in non‐diabetic individuals47.
Diabetes and Cerebral Large Vessel Disease
Carotid artery disease
In extracranial large arteries causing cerebral infarctions, atherosclerosis frequently occurs in the bifurcation of the carotid artery and origin of the vertebral artery. As an imaging modality for evaluating atherosclerotic lesions in diabetes patients, carotid artery ultrasonography is convenient, yields much information and has high diagnostic value. The intima‐media thickness of the carotid artery is an indicator of atherosclerosis that is superior in terms of quantitativeness and reproducibility, and large‐scale observational studies have shown that it is a predictor of cardiovascular events, as well as a factor for poor outcomes48, 49. There is a strong tendency for intima‐media thickness to be greater in diabetes patients, and it is important for evaluating stenotic lesions caused by plaque. In a study that analyzed carotid artery plaque characteristics in diabetes patients and non‐diabetic individuals by high‐resolution ultrasonography, it was reported that there was significantly more echolucent plaque in the diabetes patients50. Because echolucent plaque is associated with cerebrovascular events, in recent years, evaluation of plaque characteristics using MR plaque images and computed tomography angiography has been playing an important role in this field (Figure 2a–c). Hyperintense plaque on MR T1‐weighted images is treated as unstable plaque having a lipid‐rich necrotic core or intraplaque hemorrhage. A recently published study showed that calcified plaque in type 2 diabetes patients predicted future cardiovascular events, so it will be necessary to explore further studies on an association of plaque characteristics and vascular events51.
Figure 2.
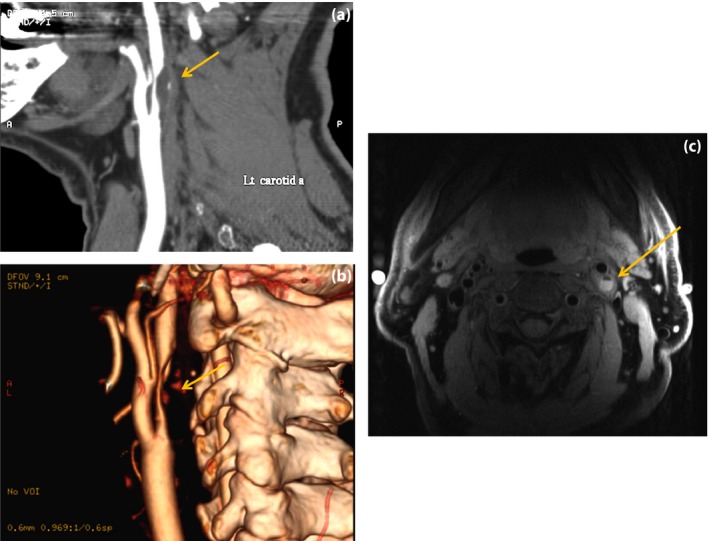
Extracranial carotid artery disease. A 75‐year‐old man with symptomatic carotid artery stenosis. (a,b) Reconstructed computed tomography angiography and 3‐D computed tomography angiography show severe stenosis of the left internal carotid artery (arrows). (c) Unstable plaque is visualized as a hyperintense signal on axial fat‐suppressed black‐blood T1‐weighted image (arrow).
In the treatment of carotid artery disease, it has been shown that some statins have a stabilizing action on carotid artery plaque52, excepting patients with symptomatic severe carotid artery stenosis and those at high risk, so optimal medical treatment combining antiplatelet agents and statin would tend to be superior to surgical treatment (carotid endarterectomy and carotid artery stenting).
Intracranial artery disease
It has been noted that ischemic stroke as a result of intracranial large artery steno‐occlusive lesions is more common in Asian populations than in Caucasian populations. Also, in a recent Chinese study, intracranial large artery stenosis (>50% stenosis) was observed in 46.6% of acute ischemic stroke53. Regarding risk factors for intracranial large artery disease, besides age and hypertension, which are the main related factors, it has been suggested that there are also associations with diabetes, insulin resistance and dyslipidemia54. In this regard, a strong association between diabetes and intracranial internal carotid artery stenosis has been shown (Figure 3). In autopsy study findings, there was an association between diabetes and both intracranial stenosis and intracranial plaque55.
Figure 3.
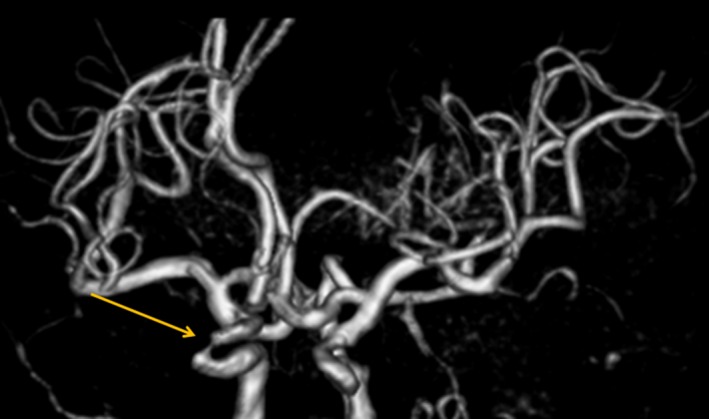
Intracranial carotid artery disease. A 66‐year‐old woman with an ipsilateral transient ischemic attack. Magnetic resonance imaging angiography volume rendering image shows severe stenosis in the right intracranial internal carotid artery.
Compared with extracranial vessels, the adventitia is thinner in internal vessels and the BBB is present. It is therefore considered that, for intracranial large arteries, BBB disruption might precede the formation of atherosclerotic lesions. It has been reported that anti‐oxidant enzymes are significantly more abundant in intracranial vessels than in extracranial vessels9, and the effect of oxidative stress is particularly remarkable in cranial vessels. Also, the decrease in endothelium‐dependent vasodilatation reactions with age is significantly greater in intracranial arteries as compared with extracranial arteries, and vasodilatation capacity is particularly susceptible to decline in diabetes patients. This is possibly a reason for the large number of intracranial internal carotid artery lesions in patients with diabetes.
Diabetes and Cerebral SVD
Branch atheromatous disease and lacunar infarction
Lacunar infarctions (LIs) are attributed to disease of penetrating branches of large cerebral arteries, and the pathological mechanism is considered to mainly involve arteriosclerosis as a result of lipohyalinosis caused by hypertension56. Multiple LIs are more frequent in diabetes patients. In a 5‐year observation of type 2 diabetes patients, macroalbuminuria was the only contributing factor to the increased lacuna57. As multiple LIs are associated not only with stroke recurrence, but also cognitive decline, they have an important clinical implication. Also, it has been reported that diabetes patients with lacunar infarctions are associated with the high recurrence rate of ischemic stroke and worse clinical outcomes58. Past research based on autopsy subjects7, 8 and diagnostic imaging58, 59, 60 has also noted that posterior circulation stroke is more frequent in diabetes patients. Furthermore, with recent advances in MRI technology, it has become possible to diagnose small brainstem infarcts at the early phase of onset. The paramedian pontine artery is more directly branched at the orifice of the basilar artery, and when it becomes occluded, infarcts extending to the basal surface of the pons occur, in many cases with a poor functional prognosis (Figure 4a–c).
Figure 4.
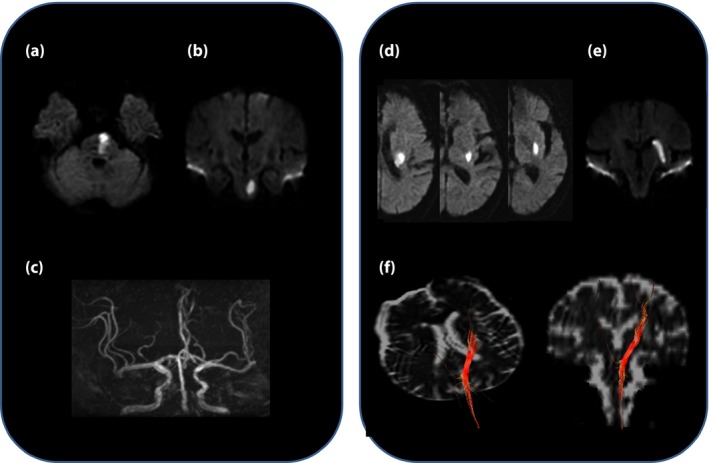
Example of infratentorial branch atheromatous lesion extending to the basal surface of the pons on diffusion‐weighted images. (a) Axial image. (b) Coronal image. (c) Magnetic resonance imaging angiography shows mild stenosis of the basilar artery. (d,e) Example of supratentorial branch atheromatous lesion in the left lenticulostriate artery territory on diffusion‐weighted images. (d) Axial image. (e) Coronal image. A 73‐year‐old man presented progressive motor deficits on day 2 after symptom onset. (f) The infarct is located in the posterior segment of the lenticulostriate artery territory, and the corticospinal tracts (red cables) are shown to cross the lenticulostriate artery territory in the posterosuperior segment on diffusion tensor imaging.
Caplan et al.61 proposed the term ‘branch atheromatous disease’, having as its cause microatheroma at the orifice of the penetrating artery. It has been noted that in diabetes, there are relatively many infarcts of this type that occur in the paramedian pontine artery area, and neurological deterioration is likely to progress in the acute phase62. In contrast, for penetrating artery infarcts including branch atheromatous disease in the region of the lenticulostriate artery, which branches from the middle cerebral artery (Figure 4d,e), it was reported that there was no significant association between diabetes and early neurological deterioration, whereas albuminuria was an independently related factor63. In this regard, using diffusion‐tensor imaging (Figure 4f), a study aiming to predict neurological deterioration by evaluating the location of the corticospinal tract has been carried out64. In recent years, the ability to visualize plaque lesions by high‐resolution MRI in penetrating branches of the basilar and middle cerebral arteries has been receiving attention65, 66, 67. Regarding treatment of branch atheromatous disease, in the acute phase, many patients have resistance to drugs, and although small‐scale research suggests that administration of cilostazol, a drug with endothelial protective and vasodilatory actions, in addition to anticoagulants (argatroban), free radical scavengers (edaravon) and statins is efficacious, there is no established optimal medical therapy at present. However, in Asian populations, as the risk of intracranial hemorrhage increases with the use of antithrombotic drugs, it is important to consider hemorrhagic risk when choosing agents for medical therapy.
Silent brain infarctions, white matter lesions and cerebral microbleeds
In elderly diabetes patients without a history of stroke, silent brain infarctions (SBIs), white matter lesions (WMLs) and cerebral microbleeds (CMBs) are often observed on brain MRI (Figure 5a–c). These lesions are MRI expressions of SVD, and have an important clinical significance because of the association of their progression with stroke incidence10. A recent systematic review and meta‐analysis showed that SBI is associated with a twofold increased risk of future stroke68. Apart from age, hypertension is the most widely accepted risk factor for SBI; however, whether diabetes is also a risk factor for SBI remains unclear69, 70. Indeed, the results of large‐scale observational studies have been inconsistent with the relationship between diabetes and the incidence of SBI71, 72, 73, 74. Also, neuroimaging findings for diabetes patients have found associations of diabetes with LIs and brain atrophy, but there is no unified view regarding an association with SBIs and WMLs (Table 1)74, 75, 76, 77, 78, 79, 80, 81, 82. The pathogenesis of cerebral SVD is not fully understood. Cerebral SVD is considered to be caused by an increased permeability of the BBB, leading to development of SBIs, WMLs and CMBs11, 83, 84. A previous small study showed that type 2 diabetes patients showed increased BBB permeability associated with neuroimaging features using MRI with intravenous gadolinium‐diethylene triamine pentaacetic acid85. However, it is not clear whether BBB permeability readily increases in diabetes. Regarding the possible mechanism for the development of WMLs, recent pathological research suggests that there is first a decline in vessel integrity and then an increase in BBB permeability as a result of endothelial dysfunction86. With recent advances in MRI techniques, changes in brain microstructure obtained in assessments by diffusion tensor imaging have become an early marker for WMLs in type 2 diabetes patients, and it has been suggested that this marker has higher sensitivity than classical MRI markers of SVD87, 88. Inflammatory processes are also involved in the pathogenesis of cerebral SVD. Endothelial dysfunction is a possible causal factor, and circulating markers of endothelial activation and inflammation are elevated in patients with SVD89, 90, 91. Circulating levels of endothelial and inflammatory markers are elevated in people with type 2 diabetes compared with non‐diabetic population78, 92. We previously reported associations between levels of soluble intercellular adhesion molecule‐1, a marker of vascular endothelial dysfunction, and progression of SBI and WMLs in type 2 diabetes patients93, 94. Furthermore, previous studies showed that higher levels of soluble intercellular adhesion molecule‐1 and high‐sensitivity C‐reactive protein were associated with the risk of future stroke in type 2 diabetes patients95. Vascular endothelial dysfunction easily progresses in diabetes patients, and at the level of small and microvessels in the brain, associations with incidence and progression of SVD are also possible, as a result of microcirculation and vasodilatation disorders.
Figure 5.
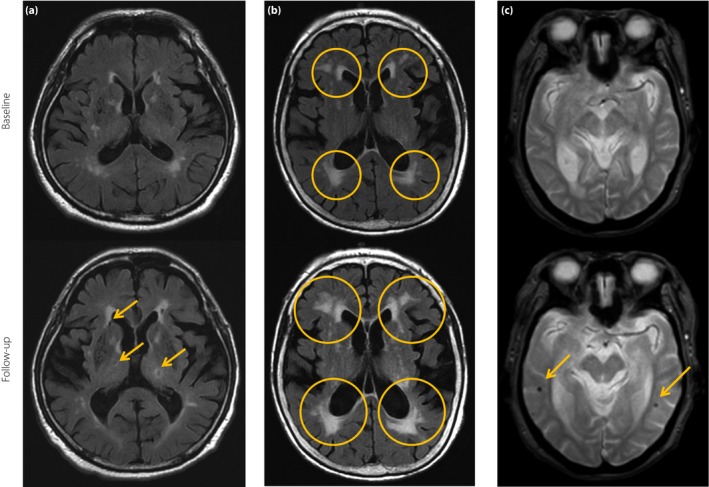
Magnetic resonance imaging expressions of cerebral small vessel disease. (a) New lacunes (arrows) in the basal ganglia and lateral ventricular anterior horn have appeared on 8‐year follow‐up fluid‐attenuated inversion recovery images. (b) Periventricular white matter lesions (open circle) extend into deep white matter over 6‐year follow up. (c) Gradient‐recalled echo T2*‐weighted magnetic resonance imaging of patients who had developed new microbleeds without cardiovascular events over 3‐year follow up. Arrows indicate new microbleeds on the follow‐up scan.
Table 1.
Cross‐sectional and longitudinal relationship between type 2 diabetes and magnetic resonance imaging measures
| Author, reference (year of publication) | Study design | Mean follow‐up period (years) | Participants (n) | Mean age (years) | Mean diabetes duration (years) | Outcome measures results | P‐value | Adjustment variables |
|---|---|---|---|---|---|---|---|---|
|
Manschot et al.75
(2006) |
Cross‐sectional | – |
T2DM n = 113 Control n = 51 |
66.1 65.1 |
8.8 – |
PWML (Scheltens scale 0–12) DWML (Scheltens scale 0–36) Silent brain infarction (SBI) Cerebral atrophy |
P = 0.13 P = 0.02 P = 0.06 P < 0.001 vs control |
NA |
|
van Harten et al.76
(2007) |
Cross‐sectional | – |
T2DM with HT n = 44 T2DM without HT n = 44 Control n = 4 4 |
73.5 73.4 73.1 |
11.9 16.5 – |
PVH (Scheltens score 0–6) DWML (Scheltens score 0–24) Lacunar infarction Global atrophy |
NS P < 0.05 NS NS vs control |
NA |
|
Jongen et al.77
(2007) |
Cross‐sectional | – |
T2DM n = 99 Control n = 46 |
65.9 65.0 |
8.7 – |
Total brain volume*
–21.8 (–34.2 to –.4) WML volume*, † 0.45(0.04–0.86) |
P < 0.01 P < 0 .05 |
Age, sex, intracranial volume and education level |
|
Umemura et al.78
(2008) |
Cross‐sectional | – |
T2DM n = 130 Control n = 130 |
59.6 58.4 |
11.9 – |
SBI OR (95%CI) =1.22 (0.70–2.14) |
NS | NA |
|
De Bresser et al.79
(2010) |
Longitudinal | 4 |
T2DM n = 55 Control n = 28 |
65.9 64.2 |
9.5 – |
Total brain volume**
–0.18 (–0.49 to 0.13) WMH volume** 0.02 (–0.09 to 0.12 |
NS NS |
Age and sex |
|
Van Elderen et al.80
(2010) |
Longitudinal | 3 |
DM n = 89 Control n = 438 |
74.7 75.0 |
NA |
Total brain atrophy (%)***
1.57 (0.26) vs 0.96 (0.10) Total WMH volume (cc)*** 1.78 (0.29) vs 2.21(0.17) Infarction*** 0.40 (1.23) vs 0.19 (0.68) |
P < 0.01 NS P = 0.07 |
Age and sex |
|
Espeland et al.81
(2013) |
Cross‐sectional Longitudinal |
– 4.7 |
T2DM n = 58 Control n = 640 |
77.8 78.1 |
NA |
Total brain atrophy –3.02 cc (diff) Gray matter volume –3.02 cc (diff) WML volume 2.41 cc (diff) |
P = 0.11 P = 0.22 P = 0.66 |
Age, clinic site, WHI treatment, time from WHI enrollment, time between scans and baseline volume |
|
Moran et al.82
(2013) |
Cross‐sectional | – |
T2DM n = 350 Control n = 363 |
67.8 72.1 |
7.0 – |
Gray matter volume**
–3.1 (–18.7 to –7.6) Total hippocampal volume** –0.88 (–1.01 to –0.75) WML volume** 0.59 (–0.54 to 1.71) Infarction 0.62 (0.21–1.04)** Microbleed –0.25 (–0.97 to 0.46)** |
P < 0.001 P < 0.001 NS P = 0.001 NS |
Age, sex and total intracranial volume Age and sex |
*Data are mean (95% confidence interval). **Data are β (95% confidence interval). ***Data are mean (standard error). †White matter lesions (WML) volumes are analyzed as log‐transformed values. DM, diabetes mellitus; DWML, deep white matter lesion; NA, not applicable; NS, not significant; PVH, periventricular hyperintensity; PWML, periventricular white matter lesion; SBI; silent brain infarction; T2DM; type 2 diabetes mellitus; WMH, white matter hyperintensity.
CMBs are also a manifestation of cerebral SVD on brain MRI, and have attracted considerable attention. CMBs are visualized as small, round, well‐defined foci of low signal intensity on T2*‐weighted MRI. It has been suggested that CMBs are a useful imaging marker for pathological damage to small vessels from hypertension or cerebral amyloid angiopathy96. Histologically, CMBs represent hemosiderin, likely from leakage through cerebral small vessels, contained within surrounding macrophages in the brain parenchyma97. Age and hypertension have been strictly associated with CMBs. However, the relationship between diabetes and the development of CMBs is still unclear.
Impact of Cerebral SVD and Brain Atrophy on Cognitive Impairment in Diabetes
It has been known for some time that diabetes is associated not only with the risk of vascular dementia, but also with Alzheimer's disease (AD)98. Regarding associations of diabetes and AD, it has been reported that there was significant atrophy in the medial temporal lobe, including the hippocampus and amygdala, in diabetes patients as compared with non‐diabetic individuals99, and an association between medial temporal lobe atrophy and insulin resistance was also shown100. Recently, computer‐aided voxel‐based morphometry has been applied to detect early brain atrophic changes. Matsuda et al.101, 102 developed a computer‐assisted analysis using voxel‐based morphometry for diagnosing AD at an early phase. Atrophy in the medial temporal lobe might be semiquantitatively assessed using free software for this procedure, called vozel‐based specific regional analysis system for analysis system for Alzheimer's disease (VSRAD) (Eisai Co., Ltd, Tokyo, Japan), and it is used as a diagnostic tool for the early diagnosis of AD101, 102. It has been reported that, as evaluated by VSRAD, internal hippocampal atrophy was stronger in degree in diabetes patients than in non‐diabetic individuals, and that there was an association of such atrophy and cognitive dysfunction103, 104.
It has also been shown that elderly people with diabetes develop extensive vascular pathology, which alone or together with AD‐type pathology, particularly in apo E4 carriers, results in an increased risk of clinical dementia105. Although significant associations of severity and progression of brain atrophy with cognitive decline have been reported in diabetes patients75, 80, 82, 106, 107, as there might also be an influence from WMLs, the ability to evaluate brain microstructure and damage to vessel integrity from MRI is important to understanding the pathophysiology of the disease in the early phase.
SVD is associated with dementia, and it has been noted that progression of SBIs and WMLs is associated with cognitive decline, in particular frontal lobe dysfunction94, 108, 109. A previous longitudinal follow‐up study showed that the rate of brain atrophy in patients with SVD is approximately twice compared with age‐matched control subjects110. Although the mechanism of brain atrophy in SVD is not fully understood, endothelial and inflammatory biomarkers would be associated with neuroimaging markers of brain atrophy. Regarding associations between biomarkers and brain atrophy, an association of high levels of sICAM‐1 and vasodilatation impairment in the brain has been reported in type 2 diabetes patients, and it has been suggested that changes in vasoregulation might be related to brain atrophy111. In community‐based cross‐sectional analysis, circulating inflammatory markers (interleukin‐6, osteoprotegerin and tumor necrosis factor‐α) were significantly associated with total brain volume112. Also, higher interleukin‐6 levels were associated with MRI markers of brain atrophy including white matter hyperintensities volume, lower gray matter and hippocampal volumes in community‐dwelling participants113. Furthermore, a previous study suggested that albuminuria, a maker of chronic kidney disease, was associated with increased white matter hyperintensity volume114. A recent study has shown that albuminuria is associated with the severity and progression of hippocampal atrophy in elderly type 2 diabetes patients104, 115.
In the future, irrespective of the presence or absence of diabetes, it will be necessary to elucidate mechanisms for associations between biomarkers of chronic kidney disease and cardiac failure with brain atrophy, as well as with atrophy of the hippocampus and amygdala. Regarding prevention, as it remains unclear whether the administration of drugs with vascular endothelial protective effects, such as statins and antihypertensives (angiotensin receptor blockers, etc.), and agents with neuroprotective effects (dipeptidyl peptidase‐4 inhibitors, etc.) will reduce the incidence and progression of SVD or the progression of brain atrophy, it will be necessary to explore their associations in prospective research.
Interaction between Cerebral and Retinal Microvascular Abnormalities in Diabetes
Association of hyperglycemia and polyol pathway hyperactivity with diabetes atherosclerosis
Cerebral and retinal small vessels have similar vascular structure (end small arteries that have no anastomoses), and the BBB is structurally and functionally similar to the blood–retinal barrier116. The retinal vascular bed can be visualized directly and non‐invasively using retinal photography, and retinal microvascular abnormalities (arteriovenous nicking, focal arteriolar narrowing, microaneurysms and microhemorrhages) can be serially evaluated117. Several clinical studies have shown that retinal microvascular abnormalities are closely related to cerebral SVD, including LIs, WMLs and CMBs, suggesting that retinal microvascular abnormalities are an imaging marker for cerebral microvascular disease14, 15, 118. In addition, previous population‐based studies suggest that retinal microvascular abnormalities are also associated with decline in cognitive performance, such as executive function and processing speed119. These studies further reinforce the idea that retinal microvascular abnormalities might be pathophysiologically linked to ischemic cerebral SVD.
In fact, there are several studies suggesting an association between diabetic retinopathy and lacunar stroke120, 121, 122 or cognitive impairment123, 124 in patients with diabetes. As a potential mechanism of lacunar stroke in patients with diabetes, they hypothesize that similar changes, as well as those in the blood–retina barrier, might induce breakdown of the BBB with subsequent damage to the walls of the small vessels and perivascular edema120, 121. In addition, other findings strongly support an association between retinal microvascular signs and lacunar stroke122. Furthermore, as some cognitive dysfunction in patients with diabetes is associated with vascular impairment, breakdown of the BBB might play an important role in the development of cognitive dysfunction123, 124.
The retinal tissue is protected from the bloodstream by a tight barrier. It consists of an anterior blood–retina barrier component towards the retinal circulation and a posterior blood–retina barrier component towards the choroidal circulation. The anatomical bases of the posterior and anterior blood–retina barriers are tight junctions between the pigment epithelial cells, and between the retinal vascular endothelial cells, respectively125. Müller cells are also involved in the maintenance of the blood–retina barrier. Clinically, breakdown of the blood–retina barrier in patients with diabetes can be observed as leakage of intravenously administered fluorescein. Furthermore, it is well known that the loss of pericytes located outside of the endothelial cells of the microvascular wall in the retinal tissue initiates the abnormality of morphological detection in the early stage of diabetic retinopathy126, 127. The interaction between pericytes and the endothelial cells plays a crucial role in maintaining the structural and functional integrity of the retinal vascular walls. The failure of this integrity induced by hyperglycemia might contribute to breakdown of the blood–retina barrier, and the subsequent development of diabetic retinopathy. Although an exact mechanism for the disruption of the blood–retina barrier in patients with diabetes has not been established, one of the possibilities is that hyperglycemia‐induced polyol pathway hyperactivity might partially play an important role13, 42, 44, 128, 129, 130, 131, 132, 133. Indeed, studies on human retinal tissues134, as well as those on animal retinal tissues135, 136, have shown the presence of AR, a key enzyme of the polyol pathway, in blood vessels, pigment epithelial cells and Müller cells. Based on all of this evidence, besides the proposed mechanism for blood–retina barrier disruption mentioned above, acceleration of the polyol pathway hyperactivity‐induced metabolic cascade (Figure 1) might be partly involved in lacunar stroke or cognitive impairment in patients with diabetes. As retinal microvascular abnormalities are associated with neuroimaging markers of cerebral SVD (Figure 6), polyol pathway hyperactivity might be a possible mechanism for the development and progression of cerebral SVD in diabetes patients.
Figure 6.
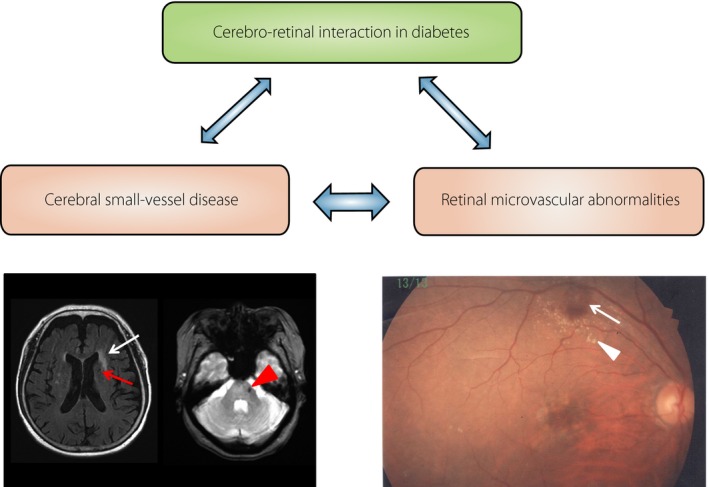
Cerebro–retinal interaction in diabetes. A 76‐year‐old woman with simple diabetic retinopathy. (a) Magnetic resonance imaging expressions of cerebral small vessel disease including silent brain infarction (red arrow), white matter lesion (white arrow) and microbleed (arrow head). (b) Retinal photograph of diabetic retinopathy signs showing microaneurysm and retinal hemorrhages (arrow), and hard exudates (arrow head).
Conclusion and Future Perspective
Diabetes promotes atherosclerosis and raises the risk of stroke, in particular that of ischemic stroke. As vasodilatation impairment as a result of endothelial dysfunction is an important factor in diabetes, it will be necessary to develop endothelium‐targeted therapeutic strategies in the future. In particular, as prevention of SVD progression not only leads to preventing stroke incidence, but also dementia, comprehensive treatment is desirable from the early stage of diabetes. Collaboration between diabetologists and neurologists would help achieve such therapeutic strategies.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2017; 8: 134–148
References
- 1. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham Study. JAMA 1979; 241: 2035–2038. [DOI] [PubMed] [Google Scholar]
- 2. Abbott RD, Donahue RP, MacMahon S, et al Diabetes and the risk of stroke: the Honolulu Heart Program. JAMA 1987; 257: 949–952. [PubMed] [Google Scholar]
- 3. Davis PH, Dambrosia JM, Schoenberg BS, et al Risk factors for ischemic stroke: a prospective study in Rochester, Minnesota. Ann Neurol 1987; 22: 319–327. [DOI] [PubMed] [Google Scholar]
- 4. Mankovsky BN, Ziegler D. Stroke in patients with diabetes vmellitus. Diabetes Metab Res Rev 2004; 20: 268–287. [DOI] [PubMed] [Google Scholar]
- 5. The Emerging Risk Factors Collaboration . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet 2010; 375: 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Asch CJ, Luitse MJ, Rinkel GJ, et al Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta‐analysis. Lancet Neurol 2010; 9: 167–176. [DOI] [PubMed] [Google Scholar]
- 7. Aronson SM. Intracranial vascular lesions in patients with diabetes mellitus. J Neuropathol Exp Neurol 1973; 32: 183–196. [DOI] [PubMed] [Google Scholar]
- 8. Kameyama M, Fushimi H, Udaka F. Diabetes mellitus and cerebral vascular disease. Diabetes Res Clin Pract 1994; 24(Suppl): S205–S208. [DOI] [PubMed] [Google Scholar]
- 9. D'Armiento FP, Bianchi A, de Nigris F, et al Age‐related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke 2001; 32: 2472–2480. [DOI] [PubMed] [Google Scholar]
- 10. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 11. Wardlaw JM. Blood‐brain barrier and cerebral small vessel disease. J Neurol Sci 2010; 299: 66–71. [DOI] [PubMed] [Google Scholar]
- 12. Hotta N. New concepts and insights on pathogenesis and treatment of diabetic complications: polyol pathway and its inhibition. Nagoya J Med Sci 1997; 60: 89–100. [PubMed] [Google Scholar]
- 13. Hotta N, Kakuta H, Koh N, et al Aldose reductase inhibitor can improve diabetic retinopathy in experimental and clinical studies. Diabetes 1988; 37: 123A. [Google Scholar]
- 14. Qiu C, Cotch MF, Sigurdsson S, et al Retinal and cerebral microvascular signs and diabetes: the age, gene/environment susceptibility‐Reykjavik study. Diabetes 2008; 57: 1645–1650. [DOI] [PubMed] [Google Scholar]
- 15. Cheung N, Mosley T, Islam A, et al Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain 2010; 133: 1987–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adams HP, Bendixen BH, Kappelle J, et al Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 17. Karapanayiotides TH, Piechowski‐Jozwiak B, van Melle G, et al Stroke patterns, etiology, and prognosis in patients with diabetes mellitus. Neurology 2004; 62: 1558–1562. [DOI] [PubMed] [Google Scholar]
- 18. Banerjee C, Moon YP, Paik MC, et al Duration of diabetes and risk of ischemic stroke: the Northern Manhattan Study. Stroke 2012; 43: 1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hier DB, Foulkes MA, Swiontoniowski M, et al Stroke recurrence within 2 years after ischemic infarction. Stroke 1991; 22: 155–161. [DOI] [PubMed] [Google Scholar]
- 20. Hankey GJ. Long‐term outcome after ischaemic stroke/transient ischaemic attack. Cerebrovasc Dis 2003; 16(Suppl 1): 14–19. [DOI] [PubMed] [Google Scholar]
- 21. Alter M, Lai SM, Friday G, et al Stroke recurrence in diabetics. Dose control of blood glucose reduce risk? Stroke 1997; 28: 1153–1157. [DOI] [PubMed] [Google Scholar]
- 22. Kawamura T, Umemura T, Kanai A, et al The incidence and characteristics of silent cerebral infarction in elderly diabetic patients: association with serum‐soluble adhesion molecules. Diabetologia 1998; 41: 911–917. [DOI] [PubMed] [Google Scholar]
- 23. Feener EP, Dzau VJ. Pathogenesis of cardiovascular disease in diabetes In: Kahn GR, Weir GC, King GL, Jacobson AM, Moses AC, Smith RJ. (eds). Joslin's Diabetes Mellitus. 4th edn Philadelphia: Lippincott Williams&Wilkins, 2005; 867–884. [Google Scholar]
- 24. Rask‐Madsan C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab 2013; 17: 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sena CM, Pereira AM, Seica R. Endothelial dysfunction‐A major mediator of diabetic vascular disease. Biochem Biophys Acta 2013; 1832: 2216–2231. [DOI] [PubMed] [Google Scholar]
- 26. Liu Q, Wang S, Cai L. Diabetic cardiomyopathy and its mechanisms: role of oxidative stress and damage. J Diabetes Investig 2014; 5: 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oates PJ. Aldose reductase, still a compelling target for diabetic neuropathy. Current Drug Target 2008; 9: 14–36. [DOI] [PubMed] [Google Scholar]
- 28. Sakakibara F, Hotta N, Koh N, et al Effects of high glucose concentrations and epalrestat on sorbitol and myo‐inositol metabolism in cultured rabbit aortic smooth muscle cells. Diabetes 1993; 42: 1594–1600. [DOI] [PubMed] [Google Scholar]
- 29. Hara T, Nakamura J, Koh N, et al An aldose reductase inhibitor, TAT, reduces ADP‐induced platelet hyperaggregation in streptozotocin‐induced diabetic rats with neuropathy. J Lab Clin Med 1995; 126: 541–547. [PubMed] [Google Scholar]
- 30. Kasuya Y, Nakamura J, Hamada Y, et al An aldose reductase inhibitor prevents the glucose‐induced increase in PDGF‐β receptor in cultured rat aortic smooth muscle cells. Biochem Biophys Res Commun 1999; 261: 853–898. [DOI] [PubMed] [Google Scholar]
- 31. Kasuya Y, Ito M, Nakamura J, et al An aldose reductase inhibitor prevents the intimal thickening in coronary arteries of galactose‐fed beagle dogs. Diabetologia 1999; 42: 1404–1409. [DOI] [PubMed] [Google Scholar]
- 32. Nakamura J, Kasuya Y, Hamada Y, et al Glucose‐induced hyperproliferation of cultured rat aortic smooth muscle cells through polyol pathway hyperactivity. Diabetologia 2001; 44: 480–487. [DOI] [PubMed] [Google Scholar]
- 33. Vikramedithyan RK, Hu Y, Noh HL, et al Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest 2005; 115: 2434–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watarai A, Nakashima E, Naruse K, et al Aldose reductase gene is associated with diabetic macroangiopathy in Japanese type 2 diabetic patients. Diabet Med 2006; 23: 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lo AC, Cheung AK, Hung VK, et al Deletion of aldose reductase leads to protection against cerebral ischemic injury. J Cereb Blood Flow Metab 2007; 27: 1496–1509. [DOI] [PubMed] [Google Scholar]
- 36. Gleissner CA, Sanders JM, Nadler J, et al Upregulation of aldose reductase during foam cell formation as possible link among diabetes, hyperlipidemia, and atherosclerosis. Arterioscler Thromb Vasc Biol 2008; 28: 1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeung CM, Lo CA, Cheung AK, et al More severe type 2 diabetes‐associated ischemic stroke injury is alleviated in aldose reductase‐deficient mice. J Neurosci Res 2010; 88: 2026–2034. [DOI] [PubMed] [Google Scholar]
- 38. Tang WH, Cheng WT, Kravtsov GM, et al Cardiac contractile dysfunction during acute hyperglycemia due to impairment of SERCA by polyol pathway‐mediated oxidative stress. Am J Physiol Cell Physiol 2010; 299: C643–C653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vedantham S, Noh HL, Ananthakrishnan R, et al Human aldose reductase expression accelerates atherosclerosis in diabetic apolipoprotein E −/− mice. Arterioscler Thromb Vasc Biol 2011; 31: 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tang WH, Stitham J, Gleim S, et al Glucose and collagen regulate human platelet activity through aldose reductase induction of thromboxane. J Clin Invest 2011; 121: 4462–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vedantham S, Thiagarajan D, Ananthakrishnan R, et al Aldose reductase drives hyperacetylation of Egr‐1 in hyperglycemia and consequent upregulation of proinflammatory and prothrombotic signals. Diabetes 2014; 63: 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hotta N, Akanuma Y, Kawamori R, et al Long‐term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3‐year, multicenter, comparative aldose reductase inhibitor‐diabetes complications trial. Diabetes Care 2006; 29: 1538–1544. [DOI] [PubMed] [Google Scholar]
- 43. Hotta N. Is there a place for inhibition of transforming growth factor‐β and the polyol pathway in therapy for diabetic retinopathy ? J Diabetes Investig 2010; 1: 134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hotta N, Kawamori R, Fukuda M, et al Long‐term clinical effects of epalerestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med 2012; 29: 1529–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kernan WN, Inzucchi SE, Viscoli CM, et al Impaired insulin sensitivity among nondiabetic patients with a recent TIA or ischemic stroke. Neurology 2003; 60: 1447–1451. [DOI] [PubMed] [Google Scholar]
- 46. Kernan WN, Inzucchi SE, Viscoli CM, et al Insulin resistance and risk for stroke. Neurology 2002; 59: 809–815. [DOI] [PubMed] [Google Scholar]
- 47. Shinozaki K, Naritomi H, Shimizu T, et al Role of insulin resistance associated with compensatory hyperinsulinemia in ischemic stroke. Stroke 1996; 27: 37–43. [DOI] [PubMed] [Google Scholar]
- 48. O'Leary DH, Polak JF, Kronmal RA, et al Carotid–artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med 1999; 340: 14–22. [DOI] [PubMed] [Google Scholar]
- 49. Tsivgoilis G, Vemmos K, Papamichael C, et al Common carotid artery intima‐media thickness and the risk of stroke recurrence. Stroke 2006; 37: 1913–1916. [DOI] [PubMed] [Google Scholar]
- 50. Östling G, Hedblad B, Berglund G, et al Increased echolucency of carotid plaques in patients with type 2 diabetes. Stroke 2007; 38: 2074–2078. [DOI] [PubMed] [Google Scholar]
- 51. de Kreutzenberg SV, Fadini GP, Guzzinati S, et al Carotid plaque calcification predicts future cardiovascular events in type 2 diabetes. Diabetes Care 2015; 38: 1937–1944. [DOI] [PubMed] [Google Scholar]
- 52. Tang TY, Howarth SP, Miller SR, et al The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study: evaluation using ultrasmall superparamagnetic iron oxide‐enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol 2009; 53: 2039–2050. [DOI] [PubMed] [Google Scholar]
- 53. Wang Y, Zhao X, Liu L, et al Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014; 45: 663–669. [DOI] [PubMed] [Google Scholar]
- 54. Turan TN, Makki AA, Tsappidi S, et al Risk factors associated with severity and location of intracranial arterial stenosis. Stroke 2010; 41: 1636–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mazighi M, Labreuche J, Gongora‐Rivera F, et al Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke 2008; 39: 1142–1147. [DOI] [PubMed] [Google Scholar]
- 56. Fisher CM. Lacunes: small, deep cerebral infarcts. Neurology 1965; 15: 774–784. [DOI] [PubMed] [Google Scholar]
- 57. Inoue T, Fushimi H, Yamada Y, et al The changes of lacunar state during a 5‐year period in NIDDM. Diabetes Res Clin Pract 1998; 42: 155–160. [DOI] [PubMed] [Google Scholar]
- 58. Palacio S, McClure LA, Benavente OR, et al Lacunar strokes in patients with diabetes mellitus: risk factors, infarct location, and prognosis: the secondary prevention of small subcortical strokes study. Stroke 2014; 45: 2689–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ichikawa H, Kuriki A, Kinno R, et al Occurrence and clinicotopographical correlates of brainstem infarction in patients with diabetes mellitus. J Stroke Cerebrovasc Dis 2012; 21: 890–897. [DOI] [PubMed] [Google Scholar]
- 60. Iwase M, Yamamoto M, Yoshinari M, et al Stroke topography in diabetic and nondiabetic patients by magnetic resonance imaging. Diabetes Res Clin Pract 1998; 42: 109–116. [DOI] [PubMed] [Google Scholar]
- 61. Caplan LR. Intracranial branch atheromatous disease: A neglected, understudied, and underused concept. Neurology 1989; 39: 1246–1250. [DOI] [PubMed] [Google Scholar]
- 62. Yamamoto Y, Ohara T, Hamanaka M, et al Characteristics of intracranial branch atheromatous disease and its association with progressive motor deficits. J Neurol Sci 2011; 304: 78–82. [DOI] [PubMed] [Google Scholar]
- 63. Umemura T, Senda J, Fukami Y, et al Impact of albuminuria on early neurological deterioration and lesion volume expansion in lenticulostriate small infarcts. Stroke 2014; 45: 587–590. [DOI] [PubMed] [Google Scholar]
- 64. Konishi J, Yamada K, Kizu O, et al MR tractography for the evaluation of functional recovery from lenticulostriate infarcts. Neurology 2005; 64: 108–113. [DOI] [PubMed] [Google Scholar]
- 65. Klein IF, Lavallée PC, Mazighi M, et al Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a High‐Resolution MRI Study. Stroke 2010; 41: 1405–1409. [DOI] [PubMed] [Google Scholar]
- 66. Yoon Y, Lee DH, Kang DW, et al Single subcortical infarction and atherosclerotic plaques in the middle cerebral artery high‐resolution magnetic resonance imaging findings. Stroke 2013; 44: 2462–2467. [DOI] [PubMed] [Google Scholar]
- 67. Leng X, Wong KS, Liebeskind DS. Evaluating intracranial atherosclerosis rather than intracranial stenosis. Stroke 2014; 45: 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gupta A, Giambrone AE, Gialdini G, et al Silent brain infarction and risk of future stroke: a systematic review and meta‐analysis. Stroke 2016; 47: 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007; 6: 611–619. [DOI] [PubMed] [Google Scholar]
- 70. Fanning JP, Wesley AJ, Wong AA, et al Emerging spectra of silent brain infarction. Stroke 2014; 45: 3461–3471. [DOI] [PubMed] [Google Scholar]
- 71. Kobayashi S, Okada K, Koide H, et al Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke 1997; 28: 1932–1939. [DOI] [PubMed] [Google Scholar]
- 72. Vermeer SE, Koudstaal PJ, Oudkerk M, et al Prevalence and risk factors of silent brain infarcts in the population‐based Rotterdam Scan Study. Stroke 2002; 33: 21–25. [DOI] [PubMed] [Google Scholar]
- 73. Gouw AA, van der Flier WM, Fazekas F, et al Progression of white matter hyperintensities and incidence of new lacunes over a 3‐year period: the Leukoaraiosis and Disability Study. Stroke 2008; 39: 1414–1420. [DOI] [PubMed] [Google Scholar]
- 74. van Harten B, de Leeuw FE, Weinstein HC, et al Brain imaging in patients with diabetes. Diabetes Care 2006; 29: 2539–2548. [DOI] [PubMed] [Google Scholar]
- 75. Manschot SM, Brands AM, van der Grond J, et al Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 2006; 55: 1106–1113. [DOI] [PubMed] [Google Scholar]
- 76. van Harten B, Oosterman JM, van Loon BJ, et al Brain lesions on MRI in elderly patients with type 2 diabetes mellitus. Eur Neurol 2007; 57: 70–74. [DOI] [PubMed] [Google Scholar]
- 77. Jongen C, van der Grond J, Kappelle LJ, et al Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia 2007; 50: 1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Umemura T, Kawamura T, Sakakibara T, et al Association of soluble adhesion molecule and C‐reactive protein levels with silent brain infarction in patients with and without type 2 diabetes. Curr Neurovasc Res 2008; 5: 106–111. [DOI] [PubMed] [Google Scholar]
- 79. de Bresser J, Tiehuis AM, van den Berg E, et al Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 2010; 33: 1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. van Elderen SG, de Roos A, de Craen AJ, et al Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3‐year follow‐up. Neurology 2010; 75: 997–1002. [DOI] [PubMed] [Google Scholar]
- 81. Espeland MA, Bryan RN, Goveas JS, et al Influence of type 2 diabetes on brain volumes and changes in brain volumes. Diabetes Care 2013; 36: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moran C, Phan TG, Chen J, et al Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 2013; 36: 4036–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wardlaw JM, Sandercock PA, Dennis MS, et al Is breakdown of the blood‐brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003; 34: 806–812. [DOI] [PubMed] [Google Scholar]
- 84. Topakian R, Barrick TR, Howe FA, et al Blood‐brain barrier permeability is increased in normal‐apperaing white matter in patients with stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry 2010; 81: 192–197. [DOI] [PubMed] [Google Scholar]
- 85. Starr JM, Wardlaw JM, Ferguson K, et al Increased blood‐brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2003; 74: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Young VG, Halliday GM, Kril J. Neuropathologic correlates of white matter hyperintensities. Neurology 2008; 71: 804–811. [DOI] [PubMed] [Google Scholar]
- 87. Reijmer YD, Brundel M, deBresser J , et al Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care 2013; 36: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Biessels GJ, Reijmer YD. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes 2014; 63: 2244–2252. [DOI] [PubMed] [Google Scholar]
- 89. van Dijk EJ, Prins ND, Vermeer SE, et al C‐reactive protein and cerebral small‐vessel disease: the Rotterdam Scan Study. Circulation 2005; 112: 900–905. [DOI] [PubMed] [Google Scholar]
- 90. Wright CB, Moon Y, Paik M, et al Inflammatory biomarkers of vascular risk as correlates of leukoaraiosis. Stroke 2009; 40: 3466–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rouhl RP, Damoiseaux JG, Lodder J, et al Vascular inflammation in cerebral small vessel disease. Neurobiol Aging 2012; 33: 1800–1806. [DOI] [PubMed] [Google Scholar]
- 92. Song Y, Manson JE, Tinker L, et al Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes 2007; 56: 1898–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kawamura T, Umemura T, Kanai A, et al Soluble adhesion molecules and C‐reactive protein in the progression of silent cerebral infarction in patients with type 2 diabetes mellitus. Metabolism 2006; 55: 461–466. [DOI] [PubMed] [Google Scholar]
- 94. Umemura T, Kawamura T, Umegaki H, et al Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small‐vessel disease and cognitive impairment: a 6‐year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry 2011; 82: 1186–1194. [DOI] [PubMed] [Google Scholar]
- 95. Kanai A, Kawamura T, Umemura T, et al Association between future events of brain infarction and soluble levels of intercellular adhesion molecule‐1 and C‐reactive protein in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2008; 82: 157–164. [DOI] [PubMed] [Google Scholar]
- 96. Vernooij MW, van der Lugt A, Ikram MA, et al Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology 2008; 70: 1208–1214. [DOI] [PubMed] [Google Scholar]
- 97. Greenberg SM, Vernooij MW, Cordonnier C, et al Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009; 8: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Biessels GJ, Staekenborg S, Brunner E, et al Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006; 5: 64–74. [DOI] [PubMed] [Google Scholar]
- 99. den Heijer T, Vermeer SE, van Dijk EJ, et al Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 2003; 46: 1604–1610. [DOI] [PubMed] [Google Scholar]
- 100. Willette AA, Xu G, Johnson SC, et al Insulin resistance, brain atrophy, and cognitive performance in late middle‐aged adults. Diabetes Care 2013; 36: 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hirata Y, Matsuda H, Nemoto K, et al Voxel‐based morphometry to discriminate early Alzheimer's disease from controls. Neurosci Lett 2005; 382: 269–274. [DOI] [PubMed] [Google Scholar]
- 102. Matsuda H, Mizumura S, Nemoto K, et al Automatic voxel‐based morphometry of structural MRI by SPM8 plus diffeomorphic anatomic registration through exponentiated lie algebra improves the diagnosis of probable Alzheimer Disease. AJNR Am J Neuroradiol 2012; 33: 1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hayashi K, Kurioka S, Yamaguchi T, et al Association of cognitive dysfunction with hippocampal atrophy in elderly Japanese people with type 2 diabetes. Diabetes Res Clin Pract 2011; 94: 180–185. [DOI] [PubMed] [Google Scholar]
- 104. Kawamura T, Umemura T, Imamine R, et al Factors associated with brain atrophy estimated with automatic voxel‐based morphometry of structural magnetic resonance images in elderly diabetic patients: impact of albuminuria on hippocampal atrophy. J Diabetes Metab 2015; 6: 491. [Google Scholar]
- 105. Ahtiluoto S, Polvikoski T, Peltonen M, et al Diabetes, Alzheimer disease, and vascular dementia: a population‐based neuropathologic study. Neurology 2010; 75: 1195–1202. [DOI] [PubMed] [Google Scholar]
- 106. Reijmer YD, van den Berg E, de Bresser J, et al Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes Metab Res Rev 2011; 27: 195–202. [DOI] [PubMed] [Google Scholar]
- 107. Roberts RO, Knopman DS, Prybelski SA, et al Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 2014; 82: 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kawamura T, Umemura T, Hotta N. Cognitive impairment in diabetic patients: can diabetic control prevent cognitive decline? J Dibetes Investig 2012; 3: 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Imamine R, Kawamura T, Umemura T, et al Dose cerebral small vessel disease predict future decline of cognitive function in elderly people with type 2 diabetes? Diabetes Res Clin Pract 2011; 94: 91–99. [DOI] [PubMed] [Google Scholar]
- 110. Nitkunan A, Lanfranconi S, Charlton RA, et al Brain atrophy and cerebral small vessel disease: a prospective follow‐up study. Stroke 2011; 42: 133–138. [DOI] [PubMed] [Google Scholar]
- 111. Novak V, Zhao P, Manor B, et al Adhesion molecules, altered vasoreactivity, and brain atrophy in type 2 diabetes. Diabetes Care 2011; 34: 2438–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jefferson AL, Massaro JM, Wolf PA, et al Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology 2007; 68: 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Satizabal CL, Zhu YC, Mazoyer B, et al Circulating IL‐6 and CRP are associated with MRI findings in the elderly: the 3C‐Dijon Study. Neurology 2012; 78: 720–727. [DOI] [PubMed] [Google Scholar]
- 114. Weiner DE, Bartolomei K, Scott T, et al Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis 2009; 53: 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kawamura T, Umemura T, Umegaki H, et al Factors associated with changes in brain atrophy during a three‐year observation in elderly diabetic patients: effect of renal impairment on hippocampal atrophy. Dement Geriatr Cogn Dis Extra 2016; 6: 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Baker ML, Hand PJ, Wang JJ, et al Retinal signs and stroke: revisiting the link between eye and brain. Stroke 2008; 39: 1371–1379. [DOI] [PubMed] [Google Scholar]
- 117. Doubal FN, MacGillivray TJ, Hokke PE, et al Differences in retinal vessels support a distinct vasculopathy causing lacunar stroke. Neurology 2009; 72: 1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Yatsuya H, Folsom AR, Wong TY, et al Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke 2010; 41: 1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Qiu C, Cotch MF, Sigurdsson S, et al Cerebral microbleeds, retinopathy, and dementia: the AGES‐Reykjavik Study. Neurology 2010; 75: 2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wardlaw JM, Doubal F, Armitage P, et al Lacunar stroke is associated with diffuse blood‐brain barrier dysfunction. Ann Neurol 2009; 65: 194–202. [DOI] [PubMed] [Google Scholar]
- 121. Doubal FN, Hokke PE, Wardlaw JM. Retinal microvascular abnormalities and stroke: a systematic review. J Neurol Neurosurg Psychiatry 2009; 80: 158–165. [DOI] [PubMed] [Google Scholar]
- 122. Lindley RI, Wang JJ, Wong MC, et al Retinal microvasculature in acute lacunar stroke: a cross‐sectional study. Lancet Neurol 2009; 8: 628–634. [DOI] [PubMed] [Google Scholar]
- 123. Crosby‐Nwaobi RR, Sivaprasad S, Amiel S, et al The relationship between diabetic retinopathy and cognitive impairment. Diabetes Care 2013; 36: 3177–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kawamura T, Umemura T, Hotta N. Curious relationship between cognitive impairment and diabetic retinopathy. J Diabetes Investig 2015; 6: 21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bek T. Histopathology and pathophysiology of diabetic retinopathy In: van Paul Bijsterveld O. (ed). Diabetic Retinopathy. London: Martin Dunitz, 2000; 169–187. [Google Scholar]
- 126. Cogan DG, Kuwabara T. The mural cell in perspective. Arch Ophthalmol 1967; 78: 133–139. [DOI] [PubMed] [Google Scholar]
- 127. Chazan BL, Kuwabara T, Balodimos MC. The nature of conjunctival microaneurysms: clinical and electron microscopic. Diabetes 1968; 17: 303. [PubMed] [Google Scholar]
- 128. Robison WG Jr, Kador PF, Kinoshita JH. Retinal capillaries: basement membrane thickening by galactosemia prevented with aldose reductase inhibitor. Science 1983; 221: 1177–1179. [DOI] [PubMed] [Google Scholar]
- 129. Engerman RL, Kern TS. Experimental galactosemia produces diabetic‐like retinopathy. Diabetes 1984; 33: 97–100. [DOI] [PubMed] [Google Scholar]
- 130. Robison WG Jr, Nagata M, Laver N, et al Diabetic‐like retinopathy in rats prevented with an aldose reductase inhibitor. Invest Ophthalmol Vis Sci 1989; 30: 2285–2292. [PubMed] [Google Scholar]
- 131. Vinores SA, Campochiano PA. Prevention or moderation of some ultrastructual changes in the RPE and retina of galactosemic rats by aldose reductase inhinitor. Exp Eye Res 1989; 49: 495–510. [DOI] [PubMed] [Google Scholar]
- 132. Hotta N, Koh N, Sakakibara F, et al An aldose reductase inhibitor, TAT, prevents electroretinographic abnormalities and ADP‐induced hyperaggregability in streptozotocin‐induced diabetic rats. Eur J Clin Invest 1995; 25: 948–954. [DOI] [PubMed] [Google Scholar]
- 133. Naruse K, Nakamura J, Hamada Y, et al Aldose reductase inhibition prevents glucose‐induced apoptosis in cultured bovine retinal microvascular pericytes. Exp Eye Res 2000; 71: 309–315. [DOI] [PubMed] [Google Scholar]
- 134. Akagi Y, Yajima Y, Kador PF, et al Localization of aldose reductase in the human eye. Diabetes 1984; 33: 562–566. [DOI] [PubMed] [Google Scholar]
- 135. Ludvigson MA, Sorenson RI. Immunohistochemical localization of aldose reductase:II. Rat eye and kidney. Diabetes 1980; 29: 450–459. [DOI] [PubMed] [Google Scholar]
- 136. Chakrabarti S, Sima AAF, Nakajima T, et al Aldose reductase in the BB rat: isolation, immunological identification and localization in the retina and peripheral nerve. Diabetologia 1987; 30: 244–251. [DOI] [PubMed] [Google Scholar]
- 137. Chung SS, Ho EC, Lam KS, et al Contribution of polyol pathway to diabetes‐induced oxidative stress. J Am Soc Nephrol 2003; 14(Suppl 3): S233–S236. [DOI] [PubMed] [Google Scholar]


