Abstract
Aims/Introduction
A large neck circumference might be an indicator of metabolic syndrome and its components, and for certain patients is more practical as an index than waist circumference. The demarcation value for neck circumference that suggests metabolic syndrome appears to vary by ethnic group. Gestational diabetes mellitus is considered a component of metabolic syndrome in pregnant women. We investigated whether neck circumference in Han Chinese women is associated with gestational diabetes mellitus in early pregnancy, and determined a predictive demarcation value.
Materials and Methods
A nested case–control study was carried out with 255 women aged 18–35 years. Gestational diabetes mellitus was diagnosed according to the criteria of the American Diabetes Association through a 2‐h, 75‐g oral glucose tolerance test.
Results
Of the total population, 41 (16%) women developed gestational diabetes mellitus by 24–28 weeks of gestation. Neck circumference at gestational week 16 positively correlated with pre‐pregnancy waist circumference, bodyweight and body mass index, and maternal age (P = 0.029) and hemoglobin A1c at gestational week 24 (P ≤ 0.001). By binary logistic regression, neck circumference was an independent predictor of gestational diabetes mellitus (odds ratio 1.840, 95% confidence interval 1.040–3.254; P = 0.036). According to the receiver operating characteristic curve, for predicting gestational diabetes mellitus the optimal demarcation for neck circumference at gestational week 16 was 35.15 cm.
Conclusions
Neck circumference is a viable tool to screen for gestational diabetes mellitus. In this population of pregnant Han Chinese women, a neck circumference of ≥35.15 cm was a predictor of gestational diabetes mellitus.
Keywords: Gestational diabetes mellitus, Metabolic syndrome, Neck circumference
Introduction
Gestational diabetes mellitus (GDM) is impaired glucose tolerance during pregnancy in women with normal glucose metabolism before pregnancy. The proportion of pregnant women with GDM is increasing worldwide, reportedly ranging from 1% to 14% in various countries, and is higher in Asian countries1. In a study carried out in New York City, susceptibility to GDM appeared to vary by ethnic group, and might not correlate with obesity2.
The reported prevalence of GDM among women in mainland China varies. A large‐scale screening in mainland China, published in 2009, applied the criteria of the American Diabetes Association and found that the prevalence of GDM in pregnant women was 4.3%3. However, in a different large study occurring from July 2011 to February 2012, the criteria of the International Association of Diabetes and Pregnancy Study Group4 were applied and the prevalence was 17.5%5.
To confirm that a pregnant woman has GDM, a standard oral glucose tolerance test (OGTT) must be administered at 24–28 weeks of gestation. Waist circumference, hip circumference and waist‐to‐hip ratio are the most commonly used factors to indicate metabolic syndrome. However, none of these might be accurate indications, as they are affected by many other factors, and can change significantly during pregnancy6.
It is well accepted that diabetes and metabolic syndrome share similar risk factors7. Neck circumference might be a better index than waist circumference or other indicators for determining metabolic syndrome or its components, and neck circumference is easily determined and has little variability8, 9, 10, 11. According to Hoebel et al.6 and others12, 13, 14, neck circumference can be a useful biomarker of risk factors in metabolic syndrome, such as insulin resistance, central obesity, blood pressure, fasting glucose levels and triglycerides. Stabe et al.12 found that neck circumference was strongly associated with insulin resistance. Other research found that the neck circumference of teenagers could indicate risk factors associated with metabolic syndrome14.
While GDM is diabetes occurring during pregnancy that is not clearly overt diabetes,15 maternal hyperglycemia in GDM is various and the metabolic disorders diverse16. Importantly, the disease is associated with other pregnancy‐associated risks, such as obesity, inflammation and hyperinsulinemia, and women with GDM are more likely to develop type 2 diabetes mellitus in the years after pregnancy17.
It is our hypothesis that a pregnant woman with a large neck circumference at gestational week 16 has a higher risk of GDM. Therefore, the present study investigated whether the neck circumference of Chinese pregnant women at the 16th week of gestation might be associated with the development of GDM in the second trimester (24–28 weeks).
Methods
The Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University approved the study protocol.
Study Population and Basic Information
To undertake a nested case–control study, from October 2014 to December 2014 we assembled a cohort of 261 normal pregnant women, aged 18–35 years at gestational week 16. Each woman in the study was examined periodically during pregnancy, and information was obtained regarding anthropometry, demographic characteristics and medical history at the Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China. At recruitment, measurements of maternal neck circumference, waist circumference, body height and bodyweight were taken for all participants; pre‐pregnancy measurements were self‐reported. We followed cohort members from the entry date until baby delivery at the end of the study period (September 2015). Six women with diagnosed hypertension (pregnancy‐induced), thyroid diseases and other endocrine diseases during the subsequent screening were excluded. If a participant received a diagnosis of GDM at 24–28 weeks of gestation by OGTT, she was assigned to the GDM group (n = 41; 16%). Otherwise, she was considered part of the normal control group (n = 214).
Definition of GDM
GDM was defined as diabetes or glucose intolerance, initially recognized during pregnancy, based on the criteria for GDM of the American Diabetes Association18. To confirm if a pregnant woman had GDM, the patient underwent a 2‐h, 75‐g OGTT at 24–28 gestational weeks. Participants were considered to have GDM if one or more of the following applied regarding fasting plasma glucose levels: fasting, ≥5.1 mmol/L; 1 h, ≥10.0 mmol/L; and 2 h, ≥8.5 mmol/L.18 Participants were assigned to a GDM or normal control group, based on the aforementioned results.
Measurement
Each study participant underwent a physical examination at gestational week 16, during which participants faced the investigator while relaxing their shoulders during measurements. We recorded maternal age, gestational weeks, gravidity and parity.
All measuring instruments were calibrated before measurement. Bodyweight was measured by a digital scale to within 0.1 kg, with participants wearing only underwear. Height was measured by portable stadiometer to within 0.5 cm, with the participant barefoot. All circumference measurements were taken at the end of expiration. Waist circumference was measured to within 0.1 cm with a measuring tape circling the participant's body at the navel. Neck circumference was measured to 0.1 cm with measuring tape at the level of the upper margin of the thyroid cartilage.
Body mass index (BMI) was calculated as weight in kilograms divided by the square of the height in meters (kg/m2). Each participant's bodyweight and height were measured before pregnancy and before delivery, and thus the BMI was calculated twice.
Blood pressure (BP) was measured with a calibrated sphygmomanometer, on the right arm with the participant sitting up, after the participant had been lying down and resting for at least 5 min. The criteria for systolic blood pressure and diastolic blood pressure were the first and fifth phase of Korotkoff sounds19, respectively.
Blood samples were collected at gestational week 24, and were analyzed at the Biochemistry Laboratory of Third Hospital Affiliated to Guangzhou Medical University. Fasting blood glucose (FBG), 1‐h blood glucose and 2‐h blood glucose were determined by OGTT test20. Total cholesterol and triglycerides were measured by enzymatic calorimetric test, and hemoglobin A1c (HbA1c) by ion‐exchange chromatography. Albumin was measured using bromocresol green. Uric acid was determined by an enzymatic method. In addition, the sex and birthweight of the newborn baby of the participant were recorded at delivery.
Statistical Analysis
The Kolmogorov–Smirnov test was carried out to assess the distribution of continuous variables. To characterize the continuous variables, we used the mean and standard deviation, or median and semi‐interquartile range, according to the result of the Kolmogorov–Smirnov test. To compare the continuous variables between the GDM and control groups, Student's t‐test was used. Pearson's correlation (coefficient, r) was used to determine correlations between neck circumference and the continuous variables.
A multivariate regression analysis was carried out by assigning the neck circumference as a dependent variable. The following were independent variables: increases in systolic blood pressure, diastolic blood pressure, uric acid, albumin, FBG, HbA1c and BMI during pregnancy, and triglyceride and total cholesterol levels. A backward stepwise elimination selection procedure was used to determine and exclude independent variables that did not affect neck circumference.
To evaluate the association between risk factors for GDM and neck circumference, we used logistic regression analysis to obtain the odds ratio (OR) and 95% confidence interval (CI). The receiver operating characteristic curves were generated to evaluate the efficiency of neck circumference for indicating GDM, by calculating the area under the curve and the 95% CI.
We determined the optimal sex‐specific neck circumference cut‐off points with regard to GDM by using the Youden index, defined as: sensitivity + specificity – 1. Statistical analyses were carried out using SPSS software (version 20.0; SPSS, Chicago, IL, USA). Test levels for significance were defined by a P‐value <0.05.
Results
The study sample consisted of 255 pregnant women with a mean age of 29.1 ± 3.7 years (Table 1). GDM was identified in 41 (16%) of the pregnant women. All of the tested variables were significantly different between the GDM and normal groups, except for height, increased weight and BMI after pregnancy, systolic blood pressure, diastolic blood pressure, uric acid, albumin, and total cholesterol. The mean age, weight and BMI before pregnancy, and neck and waist circumferences were significantly higher in the women with GDM than in the normal group. Mean FBG, 1‐h blood glucose, 2‐h blood glucose, HbA1c and triglyceride levels were also different, with higher values for women with GDM.
Table 1.
Characteristics of the gestational diabetes mellitus and control groups†
| Normal | GDM | P | ||
|---|---|---|---|---|
| Participants (n) | 214 | 41 | ||
| Age (years) | 28.7 ± 3.7 | 31.0 ± 3.0 | <0.001 | |
| Height (m) | 1.60 ± 0.05 | 1.60 ± 0.04 | 0.719 | |
| Weight (kg) | Pre‐pregnancy | 53.3 ± 9.1 | 59.1 ± 17.1 | 0.004 |
| Increase during pregnancy | 15.4 ± 7.8 | 16.0 ± 15.3 | 0.940 | |
| BMI (kg/m2) | Pre‐pregnancy | 21.0 ± 2.9 | 23.3 ± 4.4 | 0.003 |
| Increase during pregnancy | 6.4 ± 3.9 | 5.7 ± 4.0 | 0.330 | |
| Body circumference (cm) | Neck | 33.89 ± 2.04 | 35.20 ± 2.56 | 0.003 |
| Waist | 97.95 ± 6.25 | 103.16 ± 8.00 | <0.001 | |
| Blood pressure (mmHg) | SBP | 117.7 ± 9.9 | 117.8 ± 11.1 | 0.971 |
| DBP | 73.4 ± 7.5 | 73.0 ± 8.3 | 0.762 | |
| FBG (mmol/L) | Fasting | 4.18 ± 0.44 | 4.86 ± 0.81 | <0.001 |
| 1 h | 7.37 ± 1.30 | 10.29 ± 1.82 | <0.001 | |
| 2 h | 6.41 ± 1.12 | 8.95 ± 1.81 | <0.001 | |
| Blood tests‡ | Triglyceride (mmol/L) | 1.91 ± 0.92 | 2.47 ± 1.10 | 0.001 |
| Total cholesterol (mmol/L) | 7.20 ± 21.17 | 5.51 ± 1.23 | 0.610 | |
| HbA1c (%) | 5.15 ± 0.36 | 5.81 ± 0.54 | <0.001 | |
| Uric acid (μmol/L) | 326.52 ± 81.45 | 340.41 ± 87.18 | 0.324 | |
| Albumin (μmol/L) | 36.66 ± 21.38 | 34.26 ± 2.45 | 0.475 |
†Each study participant underwent a physical examination at gestational week 16, during which participants faced the investigator while relaxing their shoulders during measurements. ‡Blood samples were collected at gestational week 24, and were analyzed at the Biochemistry Laboratory of Third Hospital Affiliated of Guangzhou Medical University, Guangzhou, China. BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; GDM, gestational diabetes mellitus; HbA1c, hemoglobin A1c; SBP, systolic blood pressure.
Neck circumference was significantly positively associated with the following factors: waist circumference, weight before pregnancy, BMI before pregnancy, HbA1c and age (Table 2). However, neck circumference was uncorrelated with FBG, 1‐h glucose, 2‐h glucose and triglyceride levels (Table 2).
Table 2.
Correlation between neck circumference and gestational diabetes mellitus risk factors
| Neck circumference | ||
|---|---|---|
| r | P | |
| Weight before pregnancy† | 0.567 | <0.001 |
| Waist circumference† | 0.488 | <0.001 |
| BMI before pregnancy† | 0.470 | <0.001 |
| HbA1c† | 0.215 | 0.001 |
| Age (years)† | 0.137 | 0.029 |
| Triglyceride‡ | 0.122 | 0.052 |
| 1‐h glucose‡ | 0.079 | 0.211 |
| 2‐h glucose‡ | 0.075 | 0.232 |
| FBG‡ | 0.074 | 0.236 |
†A significant positive association between neck circumference and the indicated risk factor. ‡An insignificant association between neck circumference and the indicated risk factor. BMI, body mass index; FBG, fasting blood glucose; GDM, gestational diabetes mellitus; HbA1c, hemoglobin A1c.
When adjusted for age, the analysis showed that neck circumference was significantly and positively associated with the following factors: waist circumference, weight before pregnancy and BMI before pregnancy (Table 3). However, neck circumference was uncorrelated with HbA1c, FBG, 1‐h glucose, 2‐h glucose and triglyceride levels.
Table 3.
Correlation between neck circumference and gestational diabetes mellitus risk factors (adjusted for age)
| Neck circumference | ||
|---|---|---|
| r | P | |
| Weight before pregnancy† | 0.688 | <0.001 |
| BMI before pregnancy† | 0.588 | <0.001 |
| Waist circumference† | 0.475 | <0.001 |
| HbA1c‡ | 0.193 | 0.002 |
| Triglyceride‡ | 0.106 | 0.092 |
| 1‐h glucose‡ | 0.054 | 0.394 |
| FBG‡ | 0.052 | 0.414 |
| 2‐h glucose‡ | 0.051 | 0.421 |
†A significant positive association between neck circumference and the indicated risk factor. ‡An insignificant association between neck circumference and the indicated risk factor. BMI, body mass index; FBG, fasting blood glucose; GDM, gestational diabetes mellitus; HbA1c, hemoglobin A1c.
The binary logistic regression analysis (backward stepwise elimination method) showed that, considering the one dependent variable GDM, the following were independent variables for GDM: FBG, 1‐h glucose, 2‐h glucose, HbA1c, waist circumference and neck circumference (Table 4).
Table 4.
Logistic regression analysis of dichotomous variables and gestational diabetes mellitus
| Independent variable | OR | 95% CI | P |
|---|---|---|---|
| HbA1c | 66.194 | 7.473–586.329 | <0.001 |
| FPG | 4.706 | 1.002–22.108 | 0.050 |
| 2‐h glucose | 4.683 | 1.661–13.201 | 0.004 |
| 1‐h glucose | 3.701 | 1.531–8.941 | 0.004 |
| Neck circumference | 1.840 | 1.040–3.254 | 0.036 |
| Waist circumference | 1.216 | 1.010–1.464 | 0.039 |
CI, confidence interval; FBG, fasting blood glucose; GDM, gestational diabetes mellitus; HbA1c, hemoglobin A1c; OR, odds ratio.
Regarding the receiver operating characteristic curve analysis for GDM (Figure 1), neck circumference is shown as the area under the curve, which was 0.653 (95% CI 0.552–0.755). Waist circumference was 0.700 (95% CI 0.607–0.793); weight before pregnancy 0.651 (95% CI 0.556–0.747); BMI before pregnancy 0.650 (95% CI 0.554–0.746); BMI during pregnancy 0.369 (95% CI 0.280–0.459); and weight during pregnancy 0.389 (95% CI 0.298–0.479).
Figure 1.
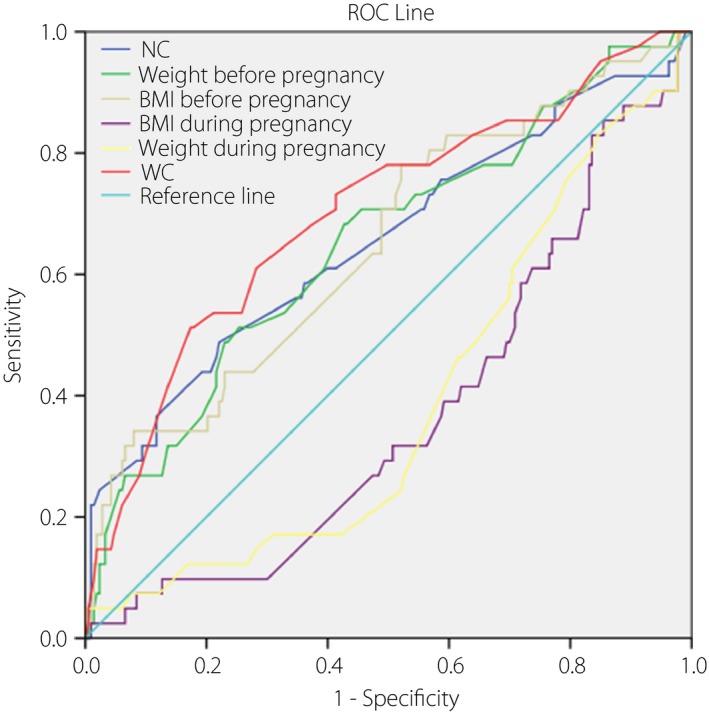
Receiver operating characteristic (ROC) curve of neck circumference (NC), waist circumference (WC), weight and body mass index (BMI) in gestational diabetes mellitus. Neck circumference is shown as the area under the curve, which was 0.653 (95% confidence interval [CI] 0.552–0.755). Waist circumference was 0.700 (95% CI 0.607–0.793); weight before pregnancy 0.651 (95% CI 0.556–0.747); BMI before pregnancy 0.650 (95% CI 0.554–0.746); BMI during pregnancy 0.369 (95% CI 0.280–0.459); and weight during pregnancy 0.389 (95% CI 0.298–0.479). Among the pregnant women, the optimal cut‐off point for neck circumference for indicating gestational diabetes mellitus was 35.15 cm, with the sensitivity of 0.488 and specificity of 0.779.
Among these pregnant women, the optimal cut‐off point for neck circumference for indicating GDM was 35.15 cm, with a sensitivity of 0.488 and specificity of 0.779.
Discussion
The present population‐based prospective study of Chinese women investigated whether neck circumference might predict GDM in early pregnancy, with the greater aim of enabling interventions to reduce the incidence and consequences of GDM. The study comprised 255 gestational women, aged 18–35 years. Using a nested control study design, we found that neck circumference of pregnant women measured at gestational week 16 could predict an increased risk of GDM.
Among non‐pregnant fertile women, the major indicators of metabolic syndrome are bodyweight and BMI, and in the present study these factors, present before pregnancy, were confirmed to be significantly associated with GDM. We also found that both triglyceride levels (tested at gestational week 24) and waist circumference (gestational week 16) were positively associated with GDM.
It is well known that insulin resistance is a risk factor for high blood glucose levels in metabolic syndrome21. In pregnant women, insulin resistance can lead to GDM, and thus GDM might be related to metabolic syndrome. Waist circumference and hip circumference are frequently used to show a risk of metabolic syndrome in women who are not pregnant22. However, with the increased uterine volume that occurs during pregnancy, waist circumference and hip circumference will change, so that neither is appropriate as an indicator. Neck circumference has previously been associated with metabolic syndrome23, and thus central obesity as well. Neck circumference was also found to be a useful tool to identify insulin resistance and metabolic syndrome in teenagers14. In the present study, neck circumference was less strongly associated with GDM than BMI or waist circumference (Figure 1). However, neck circumference is positively associated with risks of central obesity24 and type 2 diabetes25, 26. We still considered that neck circumference was a reliable and independent anthropometric index to predict GDM; during pregnancy, neck circumference does not change notably with gestational age, and can be easily measured by both examiners and the woman.
In the present study, neck circumference was significantly associated with GDM, and we conclude that measuring neck circumference might be a novel and effective method for identifying GDM.
In the present study, we found that the optimal neck circumference demarcation for predicting GDM in pregnant women was of 35.15 cm. However, in a study carried out in Brazil24, the optimal neck circumference demarcation for women as a predictor of metabolic syndrome and insulin resistance was much higher, at >36 cm27. Furthermore, a greater neck circumference for predicting metabolic syndrome was shown in Turkey23. It appears that the optimal neck circumference for predicting metabolic syndrome, just as waist circumference, differs among ethnic groups, possibly as a result of differences in body size28. Therefore, it is likely that the optimal cut‐off value for neck circumference to predict GDM needs to be determined specifically for each ethnic group.
In conclusion, neck circumference might serve as an independent predictor of risk of GDM in the Han Chinese population, and could be an effective method for identifying this disease.
This is the first study to evaluate a correlation between neck circumference and GDM. However, the conclusion would be more persuasive if more samples were included in this study. As all the participants in the study were Han Chinese, the conclusion might be limited to this ethnic group.
The present research used a nested case–control design to evaluate an association between neck circumference and GDM in Chinese women during pregnancy. It showed that pregnant women with a neck circumference of ≥35.15 cm at gestational week 16 had a greater chance of developing GDM than did women with a neck circumference below this value. We suggest that women measure and record their neck circumference throughout pregnancy to access the risk of GDM and adjust their diet accordingly. We also conclude that neck circumference might be a simple and effective means for the clinical prediction of a risk of GDM.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We greatly acknowledge the biochemistry laboratory of Third Hospital Affiliated to Guangzhou Medical University for their technical help. We also thank Athena Ren and Guoyue Yuan for their invaluable comments during discussions. This study was supported by the National Natural Science Foundation of China (no. 81270868 to X. X.).
J Diabetes Investig 2017; 8: 168–173
References
- 1. American Diabetes Association . Gestational diabetes mellitus. Diabetes Care 2004; 27: S88–S90. [DOI] [PubMed] [Google Scholar]
- 2. Savitz DA, Janevic TM, Engel SM, et al Ethnicity and gestational diabetes in New York City, 1995‐2003. BJOG 2008; 115: 969–978. [DOI] [PubMed] [Google Scholar]
- 3. Yang H, Wei Y, Gao X, et al Risk factors for gestational diabetes mellitus in Chinese women—a prospective study of 16 286 pregnant women in China. Diabet Med 2009; 26: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 4. Metzger BE, Gabbe SG, Persson B, et al International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: comment to the International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu WW, Yang HX, Wei YM, et al Evaluation of the value of plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes Care 2013; 36: 586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnold TJ, Onyewu C, Hurtado ME, et al Neck and waist circumference biomarkers of cardiovascular risk in a cohort of predominantly African‐American College students: a preliminary study. J Acad Nutr Diet 2014; 114: 107–116 PMID:2405110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grundy SM Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol, 2006; 47: 1093–100. [DOI] [PubMed] [Google Scholar]
- 8. Zhou JY, Hui G, Ming‐fan Z, et al Neck circumference as an independent predictive contributor to cardio‐metabolic syndrome. Cardiovasc Diabetol 2013; 12: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang GR, Yuan SY, Fu HJ, et al Neck circumference positively related with central obesity, overweight, and metabolic syndrome in chinese subjects with type 2 diabetes: Beijing Community Diabetes Study 4. Diabetes Care 2010; 33: 2465–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ben‐Noun L, Laor A. Relationship between changes in neck circumference and changes in blood pressure. Am J Hypertens 2004; 17: 409–414. [DOI] [PubMed] [Google Scholar]
- 11. Yan Q, Sun D, Li X, et al Neck circumference is a valuable tool for identifying metabolic syndrome and obesity in Chinese elder subjects: a community‐based study. Diabetes Metab Res Rev 2014; 30: 69–76. [DOI] [PubMed] [Google Scholar]
- 12. Hoebel S, Malan L, de Ridder JH. Determining cut‐off values for neck circumference as a measure of the metabolic syndrome amongst a South African cohort: the SABPA study. Endocrine 2012; 42: 335–342. [DOI] [PubMed] [Google Scholar]
- 13. Stabe C, Vasques AC, Lima MM, et al Neck circumference as a simple tool for identifying the metabolic syndrome and insulin resistance: results from the Brazilian Metabolic Syndrome Study. Clin Endocrinol 2013; 78: 874–881. [DOI] [PubMed] [Google Scholar]
- 14. Silva CDCD, Zambon MP, Vasques AC, et al Neck circumference as a new anthropometric indicator for prediction of insulin resistance and components of metabolic syndrome in adolescents: Brazilian Metabolic Syndrome Study. Rev Paul Pediatr 2014; 32: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association . Standards of medical care in diabetes—2014. Diabetes Care 2014; 37(Suppl 1): 14–80 [DOI] [PubMed] [Google Scholar]
- 16. Landon MB, Gabbe SG. Gestational diabetes mellitus. Obstet Gynecol 2011; 118: 1379–1393. [DOI] [PubMed] [Google Scholar]
- 17. Damm P. Future risk of diabetes in mother and child after gestational diabetes mellitus. Int J Gynecol Obstet 2009; 104: 25–6. [DOI] [PubMed] [Google Scholar]
- 18. Association AD . Standards of medical care in diabetes–2011. Diabetes Care 2011; 34: 11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ogedegbe G, Pickering T. Principles and techniques of blood pressure measurement. Cardiol Clin 2010; 28: 571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderwald C, Tura A, Winhofer Y, et al Glucose absorption in gestational diabetes mellitus during an oral glucose tolerance test. Diabetes Care 2011; 34: 1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Catalano PM. Trying to understand gestational diabetes. Diabet Med 2014; 31: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hara K, Matsushita Y, Horikoshi M, et al A proposal for the cutoff point of waist circumference for the diagnosis of metabolic syndrome in the Japanese population. Diabetes Care 2006; 29: 1123–1124. [DOI] [PubMed] [Google Scholar]
- 23. Onat A, Hergenç G, Yüksel H, et al Neck circumference as a measure of central obesity: associations with metabolic syndrome and obstructive sleep apnea syndrome beyond waist circumference. Clin Nutr 2009; 28: 46–51. [DOI] [PubMed] [Google Scholar]
- 24. LaBerge RC, Vaccani JP, Gow RM, et al Inter‐ and intra‐rater reliability of neck circumference measurements in children. Pediatr Pulmonol 2009; 44: 64–69. [DOI] [PubMed] [Google Scholar]
- 25. Preis SR, Pencina MJ, D'Aqostino RB Sr, et al Neck circumference and the development of cardiovascular disease risk factors in the Framingham heart study. Diabetes Care 2013; 36: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Preis SR, Massaro JM, Hoffmann U, et al Neck circumference as a novel measure of cardiometaboolic risk: the Framingham Heart study. J Clin Endocirnol Metab 2010; 95: 3701–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang GR, Yuan SY, Fu HJ, et al Neck circumference positively related with central obesity, overweight and metabolic syndrome in Chinese subjects with type 2 diabetes: Beijing Community Diabetes Study 4. Diabetes Care 2010; 33: 2464–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zimmet P, Magliano D, Matsuzawa Y, et al The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb 2005; 12: 295–300. [DOI] [PubMed] [Google Scholar]


