Abstract
Aims/Introduction
Insulin degludec/insulin aspart (IDegAsp) is a soluble combination of insulin degludec (70%) and insulin aspart (30%). The present exploratory trial investigated the safety of switching unit‐to‐unit from twice‐daily basal or pre‐mix insulin to twice‐daily IDegAsp in Japanese patients with type 2 diabetes.
Materials and Methods
In this 6‐week, open‐label, parallel‐group, controlled trial, 66 participants were randomized (1:1) to receive either IDegAsp or biphasic insulin aspart 30 (BIAsp 30) twice daily at the same total daily dose as pre‐trial insulin. During the trial, insulin doses were adjusted according to a pre‐specified algorithm to achieve pre‐breakfast and pre‐dinner plasma glucose of 4.4–7.2 mmol/L.
Results
No severe hypoglycemic episodes occurred. There were no statistically significant differences in rates of confirmed hypoglycemia (rate ratio IDegAsp/BIAsp 30: 0.63, 95% confidence interval: 0.31–1.30) and confirmed nocturnal hypoglycemia (rate ratio: 0.49, 95% confidence interval: 0.10–2.38) for IDegAsp vs BIAsp 30. The hypoglycemia rate for IDegAsp was constant over the 6 weeks of treatment. IDegAsp and BIAsp 30 were both safe and well tolerated. Reduction in fasting plasma glucose was statistically significantly greater for IDegAsp than for BIAsp 30 (estimated treatment difference, IDegAsp‐BIAsp 30: −1.6 mmol/L, 95% confidence interval: −2.4 to −0.8). The apparent decrease in mean postprandial plasma glucose increment (IDegAsp: 4.2–3.8 mmol/L; BIAsp 30: 4.5–2.8 mmol/L) was not statistically significantly different between treatments (estimated treatment difference: 1.0 mmol/L, 95% confidence interval: −0.1 to 2.2).
Conclusions
Switching unit‐to‐unit from basal or pre‐mix insulin to IDegAsp seems not to be associated with any concerns related to hypoglycemia or general safety in Japanese patients with type 2 diabetes.
Keywords: Insulin degludec/insulin aspart, Japanese, Type 2 diabetes
Introduction
In patients with type 2 diabetes, reduced β‐cell function and insulin resistance develop continuously. Consequently, some patients with type 2 diabetes will inevitably require insulin therapy at a certain point of disease progression in order to achieve glycated hemoglobin (HbA1c) targets1, 2. An increase in the number of patients with type 2 diabetes has recently been seen in Japan, as well as worldwide. The reason is considered to be lifestyle changes, primarily related to diet and exercise, leading to a higher prevalence of overweight and obesity, which is a high risk factor for glucose intolerance3, 4. It has been shown that glucose intolerance is associated with reduced secretion of insulin in Japanese individuals5, 6. Together with the high carbohydrate content in the Japanese diet, this can be the reason why the addition of mealtime insulin is often considered as a treatment option for Japanese patients with type 2 diabetes.
An optimal biphasic insulin product would mimic the normal physiological insulin secretion in response to a meal and at the same time provide full 24‐h basal coverage. Thus, it should consist of a rapid‐acting bolus component with short duration of action, and a basal component with a stable profile and a long duration of action. Most biphasic insulin products do not fully meet these criteria as a consequence of interference between the two components, leading to a glucose‐lowering effect from the bolus component lasting longer than required for mealtime control. Furthermore, the basal fraction of biphasic insulin products shows larger variation and shorter duration of the glucose‐lowering effect in comparison with current basal insulin analogs7.
Insulin degludec/insulin aspart (IDegAsp) is a soluble combination of long‐acting insulin degludec (IDeg; 70%) and rapid‐acting insulin aspart (IAsp; 30%)8. IDegAsp is formulated so the IDeg and IAsp components retain their individual pharmacokinetic and pharmacodynamic characteristics9. Hence, IDegAsp provides separate prandial and basal glucose‐lowering effects10, with a more distinct split of the components relative to biphasic insulin aspart 30 (BIAsp 30)11.
Based on previously published results, the pharmacological properties of IDegAsp result in clinical advantages reflecting the separate action of the IDeg and IAsp components. In a phase 3 trial in insulin‐experienced Asian patients with type 2 diabetes, including Japanese patients, IDegAsp administered twice‐daily improved glycemic control and provided greater reduction in fasting plasma glucose (FPG) levels with no statistically significant differences in rates of overall and nocturnal hypoglycemia compared with BIAsp 3012. In a global phase 3 trial in insulin‐experienced patients with type 2 diabetes, twice‐daily IDegAsp provided a similar HbA1c reduction and superior reduction in FPG compared with BIAsp 30, with statistically significantly lower end‐of‐trial insulin dose and rates of overall and nocturnal hypoglycemia13. A pooled analysis of these two phase 3 trials12, 13 provides further support of the benefits of IDegAsp compared with BIAsp 30 regarding FPG control and hypoglycemia risk14.
When switching from a current insulin product to a new insulin product, the potential risk of hypoglycemia in the period immediately after the switch could be a point of concern. In the present phase 2 trial, we investigated the safety related to a unit‐to‐unit switch from twice‐daily basal or pre‐mix insulin therapy to twice‐daily IDegAsp treatment in Japanese patients with type 2 diabetes, with specific focus on the incidence of hypoglycemia. The current exploratory trial also formed the basis for proceeding with therapeutic confirmatory trials in Japanese patients in the IDegAsp clinical development program.
Materials and Methods
Trial design and participants
The present trial was a 6‐week, open‐label, randomized, parallel‐group trial carried out in Japan (8 sites) between January 2009 and June 2009 (clinicaltrials.gov identifier: NCT00842361). The trial was carried out in accordance with the Declaration of Helsinki15, and the Ministry of Health and Welfare Ordinance on Good Clinical Practice16, and was approved by appropriate local institutional review boards. All participants gave written informed consent before any trial‐related activities.
Eligible participants were Japanese men and women with type 2 diabetes aged ≥20 years, with HbA1c levels <10.0% and body mass index (BMI) <30.0 kg/m2, and who had been treated with twice‐daily insulin for ≥12 weeks using the same basal insulin (except insulin glargine) or pre‐mix insulin (except BIAsp 30) throughout this period. Patients were excluded if they received a current total insulin dose of >100 U/day, if they had been treated with oral antidiabetic drugs (OADs) within the past 12 weeks, if they had any clinically significant disease or disorder (renal or hepatic impairment, serious heart disease, uncontrolled hypertension, cancer or medical history of cancer, proliferative retinopathy or maculopathy requiring treatment, or history of recurrent severe hypoglycemia or hypoglycemia unawareness) or if they were pregnant or breastfeeding.
Trial procedures
Randomization to either IDegAsp or BIAsp 30 treatment (1:1) was carried out using a remote registration system (BELLSYSTEM24 Inc., Tokyo, Japan). Randomization was stratified by pre‐trial insulin treatment (pre‐mix human insulin, pre‐mix insulin analog and basal insulin). IDegAsp (70% IDeg and 30% IAsp, 100 U/mL; NovoPen® 300; Novo Nordisk A/S, Bagsværd, Denmark) or BIAsp 30 (NovoMix® 30, 100 U/mL; FlexPen®, Novo Nordisk A/S) was taken twice daily (before breakfast and before dinner) by subcutaneous injection for 6 weeks. For each participant, the total daily starting dose in units was the same as the pre‐trial total daily insulin dose, and the daily dose was divided into two approximately equal doses as judged by the investigator.
During the 6‐week treatment period, insulin doses were adjusted weekly at telephone or site visits based on pre‐breakfast and pre‐dinner self‐measured plasma glucose (SMPG) values with a target of 4.4–7.2 mmol/L (80–129 mg/dL). The breakfast insulin dose was adjusted based on the pre‐dinner SMPG, whereas the dinner insulin dose was adjusted based on the pre‐breakfast SMPG. As a guide to the investigator, the corresponding insulin dose was to increase by 1 U or 2 U in the case of pre‐breakfast or pre‐dinner SMPG values of 7.2–8.8 mmol/L (130–159 mg/dL) or ≥8.9 mmol/L (≥160 mg/dL), respectively, whereas the corresponding insulin dose was to decrease by 1 U in the case of pre‐breakfast or pre‐dinner SMPG values <4.4 mmol/L (<80 mg/dL). Symptoms of hypoglycemia or hyperglycemia, previous responses to dose adjustments and other indicators of glycemic control were also taken into consideration during dose adjustment.
Assessments
FPG was assessed at baseline, 2 weeks and 6 weeks, and measured at a central laboratory (SRL Inc., Tokyo, Japan). A nine‐point SMPG profile (before and 120 min after the start of each meal, at bedtime, 03.00 h and pre‐breakfast the next day) was recorded at baseline and at the end of treatment. SMPG was measured by participants on capillary whole blood using supplied glucose meters and test strips providing plasma calibrated glucose values: Glutest AceR®, Glutest PRO R® (both Sanwa‐Kagaku, Nagoya, Japan), Glucocard Diameter or Glucocard Diameter α (both Arkray KDK Corp, Kyoto, Japan).
The main safety assessment was hypoglycemia, classified as severe (if the participant was not able to treat him/herself), confirmed non‐severe (non‐severe and verified by a plasma glucose [PG] measurement of <3.1 mmol/L [<56 mg/dL]) or symptoms only (no PG measurement or PG ≥3.1 mmol/L [≥56 mg/dL]). Nocturnal hypoglycemia was defined as an episode occurring between 23.00 h and 05.59 h (both inclusive). Additional safety measures included other adverse events, hematology and biochemistry laboratory tests carried out by a central laboratory (SRL Inc.), bodyweight, blood pressure, physical examination, and electrocardiogram.
Statistical analysis
The primary objective of the trial was to investigate the safety of switching unit‐to‐unit from twice‐daily basal or pre‐mix insulin therapy to twice‐daily IDegAsp treatment, with specific focus on hypoglycemia. Short‐term glucose control was included as a secondary objective. The sample size for this exploratory trial was small, and not based on statistical considerations. This should be taken into account when interpreting treatment differences and results of statistical analyses.
Safety evaluations were based on data from all randomized participants who received at least one dose of trial product (safety analysis set). The overall number of hypoglycemic episodes was analyzed and compared between treatments using a generalized linear model based on a negative binomial distribution. The model included the treatment group and pre‐trial insulin as fixed factors, and observation time as an offset variable. Rates of hypoglycemic episodes occurring each week as well as over a 24‐h period were calculated as the total number of hypoglycemic episodes divided by the overall exposure for all participants in the given time‐period. Rates of hypoglycemic episodes per week as well as over a 24‐h period were not analyzed statistically because of the small sample size.
Efficacy evaluations were based on data from all randomized and exposed participants with at least one time‐point of FPG or nine‐point SMPG profile after the start of treatment (full analysis set). The following end‐points were derived from the nine‐point SMPG profiles: PG at each time‐point, mean PG and mean postprandial PG increment for the three main meals together. The change in these end‐points and in FPG from baseline to end of treatment was analyzed using an analysis of variance (anova) model with treatment group and pre‐trial insulin as fixed factors, and baseline value as a covariate. The same statistical model was used to analyze change in insulin dose from baseline to the end of treatment.
Results
Patient disposition and baseline characteristics
Of the 69 patients screened, 66 patients were randomized to receive IDegAsp or BIAsp 30. One patient randomized to BIAsp 30 was not exposed, as the patient required treatment with a systemic corticosteroid (prohibited concomitant medication) before initiation of trial product treatment. Thus, 33 patients were exposed to IDegAsp, and 32 patients were exposed to BIAsp 30. One patient in each treatment group withdrew during the first week of treatment, thus a total of 32 patients in the IDegAsp group and 31 patients in the BIAsp 30 group completed the trial (Figure 1).
Figure 1.
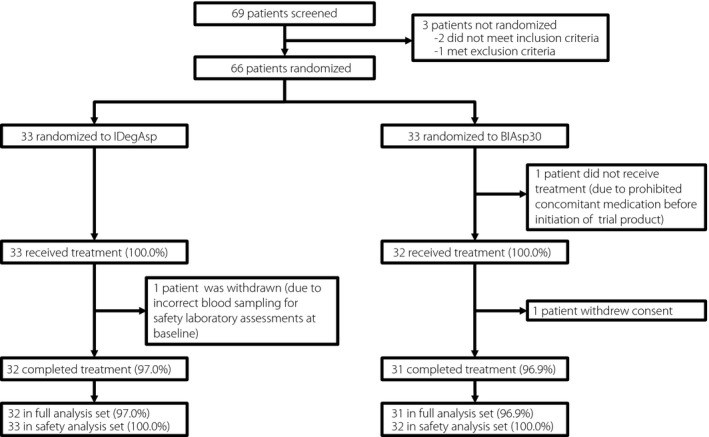
Trial flow diagram. The full analysis set included all randomized and exposed participants with at least one time‐point of fasting plasma glucose or nine‐point self‐measured plasma glucose profile after start of treatment. The safety analysis set included all randomized participants who received at least one dose of the trial product. BIAsp 30, biphasic insulin aspart 30; IDegAsp, insulin degludec/insulin aspart.
Demography and baseline characteristics are shown in Table 1. The fraction of male participants was higher in the IDegAsp group (72.7%) than in the BIAsp 30 group (56.3%), but as the absolute numbers were low, the difference was considered minor. Apart from that, demography and baseline characteristics were comparable between IDegAsp and BIAsp 30 groups. Most of the participants used either pre‐mix human insulin (~62%) or pre‐mix insulin analog (~25%) before the trial, and the insulin dose split between breakfast and dinner was close to 50%/50% in both groups.
Table 1.
Demography and baseline characteristics
| IDegAsp (n = 33) | BIAsp 30 (n = 32) | |
|---|---|---|
| Sex, n (%) | ||
| Male | 24 (72.7) | 18 (56.3) |
| Female | 9 (27.3) | 14 (43.8) |
| Age (years) | 64.3 (8.4) | 64.7 (11.2) |
| Bodyweight (kg) | 61.2 (9.9) | 57.3 (7.9) |
| BMI (kg/m2) | 23.2 (2.9) | 22.9 (2.3) |
| Duration of diabetes (years) | 18.3 (9.1) | 16.3 (8.7) |
| HbA1c (%) | 7.4 (0.9) | 7.4 (0.8) |
| FPG (mmol/L) (mg/dL) |
8.0 (2.0) 144.6 (36.4) |
7.8 (2.1) 140.8 (38.6) |
| Pre‐trial antidiabetic regimen, n (%) | ||
| Pre‐mix human insulin | 20 (60.6) | 20 (62.5) |
| Pre‐mix insulin analog | 8 (24.2) | 8 (25.0) |
| Basal insulin† | 5 (15.2) | 4 (12.5) |
| Pre‐trial insulin dose (U) | ||
| Before breakfast | 11.8 (5.2) | 12.4 (5.0) |
| Before dinner | 10.5 (4.8) | 9.8 (4.3) |
| Total daily dose | 22.2 (9.1) | 22.1 (8.3) |
Data are mean (standard deviation) based on the safety analysis set unless otherwise stated. †Long‐acting insulin analog or intermediate‐acting insulin. BIAsp 30, biphasic insulin aspart 30; BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; IDegAsp, insulin degludec/insulin aspart; U, units.
Insulin doses during the trial
The change from baseline to the end of treatment in mean insulin dose before breakfast (11.4–12.7 U for IDegAsp, and 12.3–13.3 U for BIAsp 30) was not statistically significantly different between treatment groups (estimated treatment difference in change from baseline, IDegAsp‐BIAsp 30: 0.4 U, 95% confidence interval [CI]: −1.0 to 1.7). The change in mean insulin dose before dinner from baseline to the end of treatment (10.5–10.7 U for IDegAsp, and 9.9–11.8 U for BIAsp 30) was statistically significantly less for IDegAsp compared with BIAsp 30 (estimated treatment difference, IDegAsp‐BIAsp 30: −1.8 U, 95% CI: −3.1 to −0.5). The change from baseline to the end of treatment in mean total daily insulin dose (21.9–23.4 U for IDegAsp, and 22.1–25.1 U for BIAsp 30) did not differ statistically significantly between treatments (estimated treatment difference, IDegAsp‐BIAsp 30: −1.4 U, 95% CI: −3.7 to 0.8).
Hypoglycemia and other safety results
Frequency and analysis of hypoglycemic episodes are presented in Table 2. No participants in the present trial experienced severe hypoglycemia. Approximately 60% of participants experienced confirmed non‐severe hypoglycemia (57.6% for IDegAsp and 59.4% for BIAsp 30). There was no statistically significant difference between treatment groups in the rate of confirmed non‐severe hypoglycemia (IDegAsp: 14 episodes/patient/year; BIAsp 30: 22 episodes/patient/year; rate ratio, IDegAsp/BIAsp 30: 0.63, 95% CI: 0.31–1.30). Approximately 12% of participants experienced nocturnal confirmed non‐severe hypoglycemia during this trial. There was no statistically significant difference between treatment groups in the rate of nocturnal confirmed non‐severe hypoglycemia (IDegAsp: 1 episode/patient/year; BIAsp 30: 2 episodes/patient/year; rate ratio, IDegAsp/BIAsp 30: 0.49, 95% CI: 0.10–2.38).
Table 2.
Frequency and analysis of hypoglycemic episodes
| IDegAsp | BIAsp 30 | Rate ratio (95% CI) IDegAsp/BIAsp 30 | |||||
|---|---|---|---|---|---|---|---|
| n (%) | E | Rate | n (%) | E | Rate | ||
| Exposed | 33 (100.0) | 32 (100.0) | |||||
| Hypoglycemic episodes | |||||||
| Severe | 0 (0.0) | 0 | 0.00 | 0 (0.0) | 0 | 0.00 | ND |
| Confirmed non‐severe | 19 (57.6) | 55 | 13.63 | 19 (59.4) | 86 | 21.83 | 0.63 (0.31–1.30) |
| Nocturnal hypoglycemic episodes | |||||||
| Severe | 0 (0.0) | 0 | 0.00 | 0 (0.0) | 0 | 0.00 | ND |
| Confirmed non‐severe | 4 (12.1) | 4 | 0.99 | 4 (12.5) | 8 | 2.03 | 0.49 (0.10–2.38) |
Rate: The number of hypoglycemic episodes per year of exposure. Severe hypoglycemic episodes: The patient was not able to treat him/herself. Confirmed non‐severe hypoglycemic episodes: Non‐severe and verified by a plasma glucose measurement of <3.1 mmol/L (<56 mg/dL). Nocturnal hypoglycemic episodes: Onset between 23.00 h and 05.59 h (inclusive). BIAsp 30, biphasic insulin aspart 30; CI, confidence interval; E, number of events; IDegAsp, insulin degludec/insulin aspart; ND, not done.
The observed rate of all hypoglycemic episodes appeared to be comparable for IDegAsp and BIAsp 30 during each week of treatment (Figure S1a). Based on evaluation of onset of all hypoglycemic episodes over a 24‐h period, the observed rate of all hypoglycemic episodes appeared to be lower for IDegAsp than for BIAsp 30 in the time‐periods 10.00–16.00 h and 18.00–24.00 h, while lower for BIAsp 30 than for IDegAsp in the time interval 06.00–08.00 h. It should, however, be noted that no statistical analysis was carried out to confirm these findings because of the small sample size and the exploratory nature of the trial. There were no apparent differences between IDegAsp and BIAsp 30 in the rate of all hypoglycemic episodes for any of the other time periods (Figure S1b). The frequency of hypoglycemia by pre‐trial insulin is shown in Table S1.
There was one serious adverse event reported in this trial. This was an event of thermal burn occurring in the IDegAsp group. The event was moderate in intensity, assessed by the investigator to be unlikely related to treatment, and there was no change to the dose of trial product.
Additional safety results did not differ between treatment groups. There were no reports of severe adverse events, and the majority of adverse events were mild and assessed unlikely to be related to the trial product. A summary of adverse events by pre‐trial insulin is shown in Table S2. There were no clinically relevant changes in bodyweight, blood pressure, physical examination, electrocardiogram and laboratory measurements, and no injection site reactions were reported.
Glycemic control
A reduction in FPG during the 6 weeks of treatment was observed in the IDegAsp group, whereas a minor increase was observed in the BIAsp 30 group (Figure 2a). The change in FPG from baseline to 6 weeks was statistically significantly different between IDegAsp and BIAsp 30 (estimated treatment difference, IDegAsp‐BIAsp 30: −1.6 mmol/L, 95% CI −2.4 to −0.8).
Figure 2.
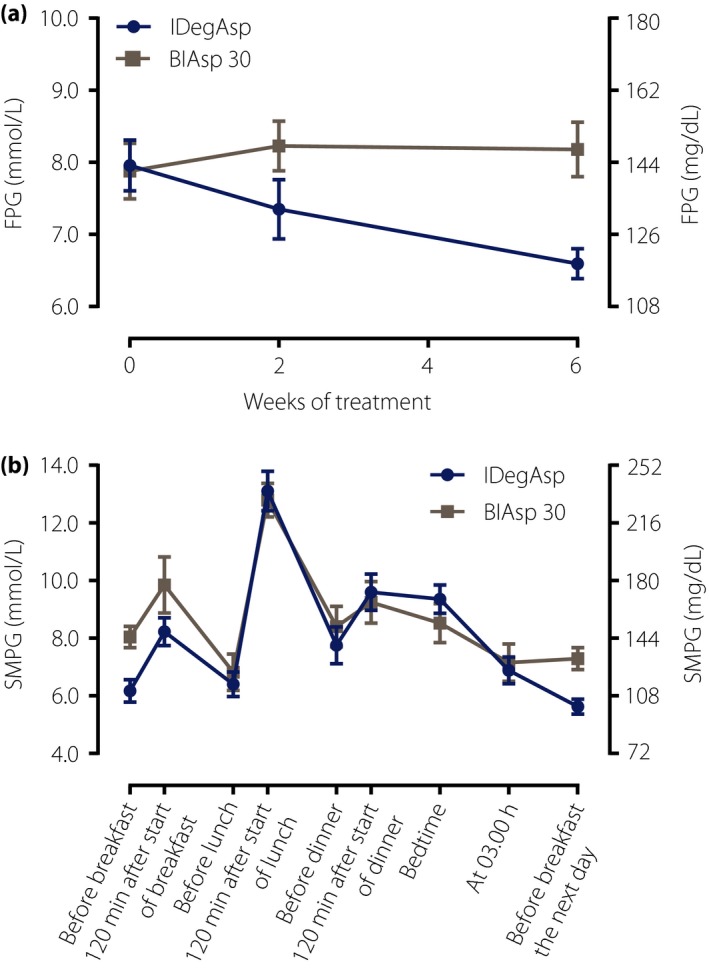
(a) Mean fasting plasma glucose over time, and (b) nine‐point self‐measured plasma glucose at 6 weeks for insulin degludec/insulin aspart (IDegAsp; circles) and biphasic insulin aspart 30 (BIAsp 30; squares). Data are mean ± standard error of the mean. FPG, fasting plasma glucose; SMPG, self‐measured plasma glucose.
The mean nine‐point SMPG profiles for IDegAsp and BIAsp 30 at 6 weeks of treatment are shown in Figure 2b. The change from baseline to 6 weeks of treatment was analyzed for each time‐point of the nine‐point SMPG profile. There were statistically significant differences in favor of IDegAsp vs BIAsp 30 both before breakfast (estimated treatment difference, IDegAsp‐BIAsp 30: −1.7 mmol/L, 95% CI: −2.8 to −0.6) and before breakfast the next day (estimated treatment difference, IDegAsp‐BIAsp 30: −1.6 mmol/L, 95% CI: −2.5 to −0.7). For all other time‐points, the change in PG from baseline to 6 weeks was not statistically significantly different between IDegAsp and BIAsp 30.
From baseline to 6 weeks, an apparent decrease in mean PG was observed based on nine‐point SMPG profiles (9.1 to 8.1 mmol/L for IDegAsp, and 9.0 to 8.5 mmol/L for BIAsp 30), and the change from baseline did not differ statistically significantly between treatment groups (estimated treatment difference in change from baseline, IDegAsp‐BIAsp 30: −0.4 mmol/L, 95% CI: −1.3 to 0.5). Likewise, the apparent decrease from baseline to 6 weeks in mean postprandial PG increment based on nine‐point SMPG profiles (4.2 to 3.8 mmol/L for IDegAsp, and 4.5 to 2.8 mmol/L for BIAsp 30) did not differ statistically significantly between treatment groups (estimated treatment difference in change from baseline, IDegAsp‐BIAsp 30: 1.0 mmol/L, 95% CI: −0.1 to 2.2).
Discussion
In the current phase 2 trial, we explored the safety of switching unit‐to‐unit from twice‐daily basal or pre‐mix insulin therapy to twice‐daily IDegAsp treatment in Japanese patients with type 2 diabetes. Overall rates of confirmed hypoglycemia and nocturnal confirmed hypoglycemia across the 6 weeks of treatment were not statistically significantly different between IDegAsp and BIAsp 30. No participants experienced severe hypoglycemia. There were no apparent differences between IDegAsp and BIAsp 30 with respect to adverse events and standard safety assessments. IDegAsp, as compared with BIAsp 30, was associated with improved glycemic control in terms of a statistically significant reduction in FPG and pre‐breakfast SMPG levels after 6 weeks of treatment, whereas the apparent reduction in postprandial PG increments did not differ statistically significantly between IDegAsp and BIAsp 30. Change in total daily insulin dose during the trial was not statistically significantly different between IDegAsp and BIAsp 30.
The present trial included a focused investigation of the safety associated with switching unit‐to‐unit from twice‐daily basal or pre‐mix insulin therapy to twice‐daily IDegAsp, with particular attention to hypoglycemic events. Hypoglycemia might occur more frequently at the point of switchover and in the period immediately after, and could therefore be a point of concern when patients switch from a current to a new insulin product. Thus, the results of the present trial are reassuring, indicating that the rate of all hypoglycemic episodes for IDegAsp was constant over the first 6 weeks of treatment, and that patients appeared to experience comparable rates of all hypoglycemic episodes for IDegAsp vs BIAsp 30 during each of the first 6 weeks of treatment after switching from their current insulin. There was no obvious indication that results on hypoglycemia or adverse events were dependent on pre‐trial insulin. However, it is important to emphasize that such subgroup summaries in a relatively small study should be interpreted with great caution.
In a study in Asian patients with type 2 diabetes, including Japanese patients, there were no statistically significant differences between IDegAsp and BIAsp 30 in confirmed hypoglycemia (rate ratio IDegAsp/BIAsp 30 of 1.00) and nocturnal confirmed hypoglycemia (rate ratio of 0.67)12. In contrast, global studies of patients with type 2 diabetes have shown statistically significantly lower rates of confirmed hypoglycemia (rate ratios of 0.68 and 0.42)13, 17, and nocturnal confirmed hypoglycemia (rate ratios of 0.27 and 0.33)13 for IDegAsp compared with BIAsp 30. In the current study, estimated hypoglycemia rate ratios for IDegAsp vs BIAsp 30 were in between the aforementioned studies, and with no statistically significant treatment differences (Table 2). The observed discrepancies between studies could be due to the different populations, but might also reflect that the sample size determination of each individual study did not target the analysis of hypoglycemia. When including two of the studies12, 13 in a pooled analysis, thereby increasing the sample size, rates of confirmed hypoglycemia and nocturnal confirmed hypoglycemia were statistically significantly lower by 19 and 57%, respectively, for IDegAsp vs BIAsp 3014. Risk of hypoglycemia is a substantial point of concern when treating patients with diabetes. In a survey carried out globally among insulin‐treated patients with diabetes, also including Japan, approximately 70–80% of physicians reported that their treatment aggressiveness is limited by the risk of hypoglycemia, that management of both efficacy and safety is difficult to accomplish at the same time, and that aggressive treatment of diabetes would be facilitated if their hypoglycemia concern was alleviated18.
The statistically significantly greater reductions in FPG and in pre‐breakfast SMPG levels for IDegAsp vs BIAsp 30 observed in the present study are in accordance with results of a trial in Asian patients with type 2 diabetes, including Japanese patients12, as well as with findings from global studies in patients with type 2 diabetes13, 17. It might seem contradictory that FPG and pre‐breakfast SMPG levels were significantly reduced with IDegAsp compared with BIAsp 30 when the titration target was the same for the two treatment groups. However, this might be explained by the differences in pharmacodynamic properties between IDegAsp and BIAsp 30. Thus, as a result of the shorter duration of action of BIAsp 30, titration of the dinner dose based on pre‐breakfast SMPG expectedly led to a greater dinner BIAsp 30 dose. Still, the dose increase with BIAsp 30 might have been somewhat limited by the ‘shoulder’ effect of BIAsp 30 in the late evening hours. The findings overall show the full 24‐h basal coverage provided by IDegAsp compared with BIAsp 30. This is in accordance with the long duration of action of the IDeg component in IDegAsp compared with the protaminated fraction of IAsp in BIAsp 3010. Along these lines, it is important to note that the rate of nocturnal hypoglycemia in the present study was generally low with IDegAsp. Thus, the statistically significantly greater reduction in FPG for IDegAsp than for BIAsp 30 was achieved concurrently with a low rate of nocturnal hypoglycemia. These combined benefits of IDegAsp are likely due to the long action, the flat and stable glucose‐lowering effect profile, and the low within‐subject day‐to‐day variability of the IDeg component19, 20.
Being an exploratory study, the current trial inherently carried some limitations, in particular the limited sample size, which implied that the results should be regarded as exploratory and hypothesis generating. Because of the exploratory nature of the current study, the applied titration guidance was conservative (pre‐breakfast and pre‐dinner SMPG target 4.4–7.2 mmol/L) in comparison with the titration procedure used in the subsequent phase 3 trial in Asian patients (pre‐breakfast and pre‐dinner SMPG target 4.0–4.9 mmol/L)12. In light of this, it is reassuring that the subsequent larger phase 3 trial has confirmed the results of the present trial12. Another limitation was the short treatment and observation period, implying that change in HbA1c levels during the trial could not be used as an efficacy end‐point, thus glycemic control in the present trial was based on FPG and SMPG assessment. Furthermore, the trial was open‐label because of the different appearance of the IDegAsp and BIAsp 30 formulations. Consequently, the possibility of underlying reporting bias could not be completely excluded, as it is likely that investigators are more cautious, and that patients are more alert when using a new treatment. Finally, we cannot exclude potential underestimation of nocturnal hypoglycemia, as hypoglycemia reporting was based on PG self‐measurement rather than continuous glucose monitoring. This should, however, have affected the treatment arms similarly.
In conclusion, in the present study, switching unit‐to‐unit from a basal insulin or pre‐mix insulin to IDegAsp twice daily was not associated with any safety concerns in Japanese patients with type 2 diabetes. IDegAsp appears to be a safe and effective option for insulin treatment in Japanese patients with type 2 diabetes. The findings of the current exploratory trial have been confirmed in larger trials with Japanese patients.
Disclosure
Yukiko Onishi has received clinical research grants from AbbVie, Astellas, Boehringer‐Ingelheim, Bristol‐Myers Squibb, GlaxoSmithKline, Novo Nordisk, Daiichi‐Sankyo, Sanwa Kagaku Kenkyusho, Shionogi and Taisho, and has received lecture honoraria from Astellas, AstraZeneca, Boehringer‐Ingelheim, Eli Lilly, Kyowa Hakko Kirin, MSD, Novo Nordisk, Sanofi and Sumitomo Dainippon Pharma. Kenichi Yamada has received clinical research grants from Daiichi‐Sankyo, GlaxoSmithKline and Sumitomo Dainippon Pharma. Jeppe Zacho is an employee of and owns stock in Novo Nordisk. Jan Ekelund is an employee of Novo Nordisk. Yasuhiko Iwamoto has received clinical research grants from AbbVie, Astellas, Boehringer‐Ingelheim, Bristol‐Myers Squibb, GlaxoSmithKline, Novo Nordisk, Daiichi‐Sankyo, Sanwa Kagaku Kenkyusho, Shionogi and Taisho.
Supporting information
Figure S1| Rate of all hypoglycemic episodes by week of treatment and by time of day for insulin degludec/insulin aspart and biphasic insulin aspart 30.
Table S1| Frequency of hypoglycemic episodes according to pre‐trial insulin.
Table S2| Frequency of adverse events according to pre‐trial insulin.
Acknowledgments
This study was funded by Novo Nordisk. Medical writing support was provided by Carsten Roepstorff, Larix A/S, Copenhagen, Denmark, funded by Novo Nordisk. Additional participating investigators: Fuminobu Okuguchi, Okuguchi Clinic of Internal Medicine, Miyagi; Hiroshi Ohashi, Oyama East Clinic, Tochigi; Takeshi Osonoi, Nakakinen Clinic, Ibaraki; Nobuyuki Sato, Social Insurance Kamata General Hospital, Tokyo; Akira Yamauchi, Suruga Clinic, Shizuoka; and Shuji Nakamura, Iryo Houjin Shadan Kowakai Heiwadai Hospital, Miyazaki.
J Diabetes Investig 2017; 8: 210–217
References
- 1. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014; 383: 1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Home P, Riddle M, Cefalu WT, et al Insulin therapy in people with type 2 diabetes: opportunities and challenges? Diabetes Care 2014; 37: 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chan JC, Malik V, Jia W, et al Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009; 301: 2129–2140. [DOI] [PubMed] [Google Scholar]
- 4. Kawamori R. Diabetes trends in Japan. Diabetes Metab Res Rev 2002; 18: 9–13. [DOI] [PubMed] [Google Scholar]
- 5. Heianza Y, Arase Y, Fujihara K, et al High normal HbA(1c) levels were associated with impaired insulin secretion without escalating insulin resistance in Japanese individuals: the Toranomon Hospital Health Management Center Study 8 (TOPICS 8). Diabet Med 2012; 29: 1285–1290. [DOI] [PubMed] [Google Scholar]
- 6. Onishi Y, Hayashi T, Sato KK, et al Fasting tests of insulin secretion and sensitivity predict future prediabetes in Japanese with normal glucose tolerance. J Diabetes Investig 2010; 1: 191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans M, Schumm‐Draeger PM, Vora J, et al A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes Obes Metab 2011; 13: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalra S. Insulin degludec aspart: the first co‐formulation of insulin analogues. Diabetes Ther 2014; 5: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Havelund S, Ribel U, Hubálek F, et al Investigation of the physico‐chemical properties that enable co‐formulation of basal insulin degludec with fast‐acting insulin aspart. Pharm Res 2015; 32: 2250–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heise T, Nosek L, Roepstorff C, et al Distinct prandial and basal glucose‐lowering effects of insulin degludec/insulin aspart (IDegAsp) at steady state in subjects with type 1 diabetes mellitus. Diabetes Ther 2014; 5: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heise T, Nosek L, Klein O, et al Insulin degludec/insulin aspart produces a dose‐proportional glucose‐lowering effect in subjects with type 1 diabetes mellitus. Diabetes Obes Metab 2015; 17: 659–664. [DOI] [PubMed] [Google Scholar]
- 12. Kaneko S, Chow F, Choi DS, et al Insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Asian patients with type 2 diabetes inadequately controlled on basal or pre‐/self‐mixed insulin: a 26‐week, randomised, treat‐to‐target trial. Diabetes Res Clin Pract 2015; 107: 139–147. [DOI] [PubMed] [Google Scholar]
- 13. Fulcher GR, Christiansen JS, Bantwal G, et al Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin‐treated type 2 diabetes: a phase 3a, randomized, treat‐to‐target trial. Diabetes Care 2014; 37: 2084–2090. [DOI] [PubMed] [Google Scholar]
- 14. Christiansen JS, Niskanen L, Rasmussen S, et al Lower rates of hypoglycemia during maintenance treatment with IDegAsp versus BIAsp 30: a combined analysis of two phase 3a studies in type 2 diabetes. J Diabetes 2016; 8: 720–728. [DOI] [PubMed] [Google Scholar]
- 15. World Medical Association . Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. 59th WMA General Assembly, Seoul, October 2008.
- 16. Ministry of Health and Welfare Ordinance on Good Clinical Practice (MHW Ordinance No. 28). March 27, 1997. Available from: http://www.pmda.go.jp/files/000152996.pdf. Accessed August 30, 2016.
- 17. Niskanen L, Leiter LA, Franek E, et al Comparison of a soluble co‐formulation of insulin degludec/insulin aspart vs biphasic insulin aspart 30 in type 2 diabetes: a randomised trial. Eur J Endocrinol 2012; 167: 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peyrot M, Barnett AH, Meneghini LF, et al Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012; 29: 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heise T, Nosek L, Bøttcher SG, et al Ultra‐long‐acting insulin degludec has a flat and stable glucose‐lowering effect in type 2 diabetes. Diabetes Obes Metab 2012; 14: 944–950. [DOI] [PubMed] [Google Scholar]
- 20. Heise T, Hermanski L, Nosek L, et al Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady‐state conditions in type 1 diabetes. Diabetes Obes Metab 2012; 14: 859–864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1| Rate of all hypoglycemic episodes by week of treatment and by time of day for insulin degludec/insulin aspart and biphasic insulin aspart 30.
Table S1| Frequency of hypoglycemic episodes according to pre‐trial insulin.
Table S2| Frequency of adverse events according to pre‐trial insulin.


