Abstract
Background
Comorbidity has a great impact on lung cancer survival. Renal function status may affect treatment decisions and drug toxicity. The survival outcome in lung cancer patients with coexisting chronic kidney disease (CKD) has not been fully evaluated. We hypothesized that CKD is an independent risk factor for mortality in patients with lung cancer.
Methods
A retrospective, propensity‐matched study of 434 patients diagnosed between June 2004 and May 2012 was conducted. CKD was defined as estimated glomerular filtration rate <60 mL/minute. Lung cancer and coexisting CKD patients were matched 1:1 to patients with lung cancer without CKD.
Results
Age, gender, smoking status, histology, and lung cancer stage were not statistically significantly different between the CKD and non‐CKD groups. Kaplan–Meier survival analysis demonstrated a median survival of 7.26 months (95% confidence interval [CI] 6.06–8.46) in the CKD group compared with 7.82 months (95% CI 6.33–9.30) in the non‐CKD group (P = 0.41). Lung cancer stage‐specific survival is not affected by CKD. Although lung cancer patients with CKD presented with an increased risk of death of 6%, this result was not statistically significant (hazard ratio 1.06, 95% CI 0.93–1.22; P = 0.41).
Conclusion
According to our limited experience, CKD is not an independent risk factor for survival in lung cancer patients. Clinicians should not be discouraged to treat lung cancer patients with CKD.
Keywords: Chronic kidney disease, lung cancer, outcome, survival
Introduction
Lung cancer is the leading cause of cancer death worldwide and is responsible for nearly 19.4% of all cancer deaths.1 Lung cancer causes more deaths than breast, colon, and pancreatic cancers combined.2 Recent cancer incidence and mortality data revealed that during 2013, 212 584 people (100 677 women) with lung cancer were diagnosed in the United States.2 Medical and technological advances have contributed to improved life expectancy for lung cancer patients. However, the aging of the population has led to a growing prevalence of patients suffering from chronic diseases and cancer. Cancer stage is usually the most important factor affecting long‐term outcome; however, comorbidities influence the care of these patients, the selection of initial treatment, and its effectiveness.
According to Na et al., patients with chronic kidney disease (CKD) have an increased risk of death of several cancers.3 However, the literature contains contradictory results of the impact of renal dysfunction on lung cancer survival.3, 4, 5 In this study, we evaluate the clinical outcomes of patients with lung cancer and coexisting CKD using a propensity‐matched study. We hypothesized that CKD is a possible adverse factor for mortality in patients with lung cancer.
Methods
All adult patients (>18 years) diagnosed with lung cancer at Chang Gung Memorial Hospital, Chiayi, from June 2004 to May 2012 were included in this retrospective study. Propensity score matching was used with a 1:1 match for patients with lung cancer and coexisting CKD to patients with lung cancer without CKD based on age, gender, smoking status, histology, and lung cancer stage. Creatinine was measured at the time of cancer work up. CKD was defined as calculated creatinine clearance (CrCl) < 60 mL/minute/1.73 m2 using the Cockcroft–Gault formula in the presence of proteinuria/hematuria or the presence of abnormal kidney imaging.6 The SOLE presence of a CrCl of <60 mL/minute/1.73 m2 was considered non‐CKD. CKD staging was conducted in accordance with the current international guidelines.7 The Cockcroft–Gault formula, the most commonly used formula to determine renal function status in the clinical care of cancer patients, has been shown to correlate with measured CrCl in multiple disease settings.8 A recent formulation to estimate renal function was developed by the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI).9 The Kidney Disease Outcomes Quality Initiative recommends using the CKD‐EPI creatinine equation to predict eGFRcreat in adults; however, its clinical use in routine practice has not been established.10
Medical records and the cancer center database at Chiayi Chang Gung Memorial Hospital were cross‐matched to determine the coexistence of lung cancer and CKD. The following clinical data were extracted from medical records: age at lung cancer diagnosis, gender, smoking history, symptom/s at presentation, creatinine clearance, lung cancer histology and stage at diagnosis, primary treatment received (all treatment modalities given within three months post diagnosis), Charlson comorbidity index (CCI), and overall survival.11 In the CCI, 19 chronic diseases are weighted according to their association with mortality. The sum of each morbidity score is added to reach a total score. The seventh edition of the tumor node metastasis (TNM) staging system for lung cancer was used for lung cancer staging.12 Clinical staging included a physical examination, chest radiography, bronchoscopy, chest computed tomography, spirometry, brain magnetic resonance imaging, bone scan, and positron emission tomography. Post‐treatment follow‐up was carried out at the outpatient clinic every three months for three years and every six months thereafter. The follow‐up examination included chest radiography, chest computed tomography and positron emission tomography. Patients with an incomplete medical record, creatinine measurement or without pathology reports were excluded. Overall survival was measured from the day of lung cancer diagnosis until the last follow‐up or at the end of 2014. Patients lost to follow‐up were contacted by telephone by the cancer center case manager; those not reachable by telephone were considered to have died if they were excluded from National Health Insurance. National Health Insurance offers universal coverage to more than 99% of the Taiwanese population. Patients are usually excluded as a result of death, missing premium payments longer than six months, emigration, or nationality change. The Health Promotion Administration, Ministry of Health and Welfare, Taiwan, release an annual death report of all cancer cases registered back to each cancer center. The institutional review board of Chang Gung Memorial Hospital approved this study.
Statistical analysis
The propensity score was calculated using logistic regression with CKD as the dependent variable. Propensity matching was used to select control patients based on several confounders simultaneously.13 A caliper width of 0.2 times the standard deviation of the propensity score without replacement was used to pair match CKD and non‐CKD patients.14
Chi‐square or Fisher's exact tests were used for categorical variables, and analysis of variance was used for numerical variables. Continuous variables were categorized using median values as the cut‐off point for risk stratification. Age was divided into groups of <75 and >75 years of age; the CCI score was divided into <9 or >9; clinical stage was separated into stage I–IIIA versus stage IIIB–IV; and treatment modality into supportive, surgical (any therapeutic surgical resection, excluding diagnostic procedure), and medical treatment groups. Overall survival was estimated using the Kaplan–Meier method and difference in survival was calculated using the log‐rank test. Cox proportional hazard analysis was used to estimate the level of significance and the relative risks with 95% confidence interval (CI). A P value of <0.05 was considered statistically significant. The clinical data was analyzed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA).
Results
Figure 1 summarizes the recruitment flow process. During the study period, 1660 lung cancer patients were diagnosed and/or treated at Chang Gung Memorial Hospital at Chiayi. One hundred and eleven patients were excluded: 55 patients had incomplete medical treatment records; 46 patients had no record of their creatinine level; and in 10 patients, lung cancer was not confirmed by our in‐house pathologist. Propensity score matching was used to match lung cancer patients with coexisting CKD (CKD group) in a 1:1 ratio to lung cancer patients without CKD (non‐CKD group).
Figure 1.
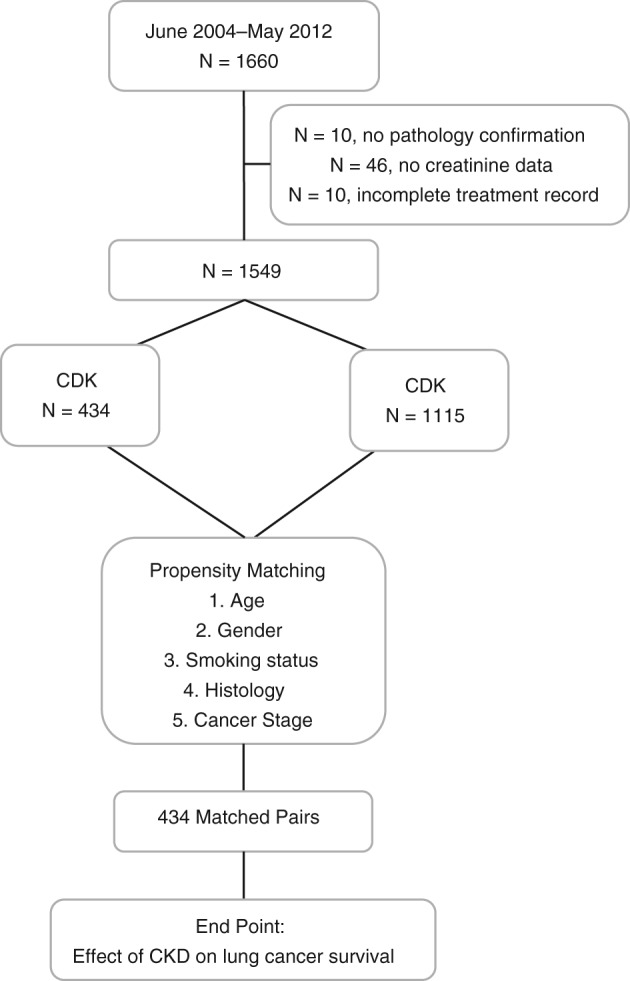
Recruitment flow process. CKD, chronic kidney disease.
Demography
There was no statistically significant difference in age, gender, smoking status, histology, or lung cancer stage between the CKD and non‐CKD groups. The median creatinine level was 1.37 mg/dL for the CKD group compared with 0.84 mg/dL for the non‐CKD group (P < 0.001). The median CrCl in the CKD group was 48.17 mL/minute compared with 82.14 mL/minute in the non‐CKD group (P < 0.001). Twenty patients in the CKD group received renal replacement therapy with hemodialysis prior to the diagnosis of lung cancer. The patients’ demographic characteristics are presented in Table 1. The median CCI score was 8 for the CKD group compared with 9 for the non‐CKD group (P < 0.001). Distribution of the CCI is presented in Table 2.
Table 1.
Demographic characteristics of patients with CKD and non‐CKD
| Characteristics | Non‐CKD N (%) | CKD N (%) | P |
|---|---|---|---|
| Number of patients | 434 | 434 | |
| Age (median + SD, years) | 75 ± 8.22 | 75 ± 9.44 | 0.45 |
| Gender | 0.76 | ||
| Male | 313 (72.1) | 318 (73.3) | |
| Female | 121 (27.9) | 116 (26.7) | |
| Current and past smoker | 221 (50.9) | 229 (52.88) | 0.63 |
| Creatinine (median + SD in mg/dL) | 0.84 ± 0.18 | 1.37 ± 1.82 | <0.001 |
| Creatinine clearance (median + SD in mL/minute) | 82.14 ± 21.04 | 48.17 ± 14.70 | <0.001 |
| Comorbidity score (median + SD) | 8 ± 2.05 | 9 ± 2.52 | <0.001 |
| Histology | 0.32 | ||
| NSCLC | 371 (85.5) | 382 (88) | |
| Adenocarcinoma | 117 (40.8 | 173 (39.9) | |
| Adenosquamous | 16 (3.7) | 14 (3.2) | |
| Squamous | 110 (25.3) | 113 (26) | |
| NSCLC | 59 (13.6) | 67 (15.4) | |
| LC | 4 (0.9) | 6 (1.4) | |
| Other | 5 (1.2) | 9 (2.1) | |
| SCLC | 63 (14.5) | 52 (12) | |
| Stage | 0.32 | ||
| I–IIIA | 68 (15.7) | 80 (18.4) | |
| IIIB–IV | 366 (84.3) | 354 (81.6) | |
| Treatment modality | 0.12 | ||
| Supportive care | 103 (23.7) | 141 (32.5) | |
| Medical treatment | 292 (67.3) | 252 (58.1) | |
| CT | 140 (32.3) | 114 (26.3) | |
| RT | 39 (9) | 25 (5.8) | |
| CT + RT | 62 (14.3) | 53 (12.2) | |
| CT + TKI | 8 (1.8) | 8 (1.8) | |
| RT + TKI | 9 (2.1) | 13 (3) | |
| TKI | 34 (7.8) | 39 (9) | |
| Surgical treatment | 39 (9.0) | 41 (9.4) | |
| OP | 21 (4.8) | 20 (4.6) | |
| OP + CT | 15 (3.5) | 21 (4.8) | |
| OP + RT | 1 (0.2) | 0 | |
| OP + CT + RT | 2 (0.5) | 0 |
CKD, chronic kidney disease; CT, chemotherapy; LC, large cell carcinoma; NSCLC, non‐small cell lung cancer; OP, surgical resection; RT, radiotherapy; SCLC, small cell lung cancer; SD, standard deviation; TKI, tyrosine kinase inhibitor.
Table 2.
Distribution of Charlson comorbidities in CKD and non‐CKD
| Charlson Comorbidity Index | Non‐CKD N (%) | CKD N (%) |
|---|---|---|
| Myocardial infarction (+) | 30 (6.9) | 43 (9.9) |
| Congestive heart failure (+) | 42 (9.7) | 40 (9.2) |
| Peripheral vascular disease (+) | 44 (10.1) | 43 (9.9) |
| Cerebrovascular disease (+) | 80 (18.4) | 68 (15.7) |
| Dementia (+) | 27 (6.2) | 15 (3.5) |
| Chronic pulmonary disease (+) | 230 (53) | 184 (42.4) |
| Connective tissue disease (+) | 8 (1.8) | 7 (1.6) |
| Peptic ulcer disease (+) | 167 (38.5) | 156 (36) |
| Diabetes without end‐organ damage (+) | 73 (16.8) | 8 (1.8) |
| Hemiplegia (+) | 28 (6.5) | 10 (2.3) |
| Diabetes with end‐organ damage (+) | 103 (23.7) | 133 (30.6) |
| Any non‐metastatic solid tumor (+) | 89 (20.5) | 144 (33.2) |
| Leukemia (+) | 0 | 1 (0.2) |
| Lymphoma (+) | 1 (0.2) | 0 |
| Moderate to severe liver disease (+) | 62 (14.3) | 33 (7.6) |
| Metastatic solid tumor (+) | 363 (83.6) | 296 (68.2) |
CKD, chronic kidney disease.
Treatment
The proportion of patients that received supportive treatment was much higher in the CKD compared to the non‐CKD group (32% vs. 23.7%). The proportion of patients that received medical treatment was much higher in the non‐CKD compared with the CKD group (67.3% vs. 58.1%). The proportion of patients that received surgical treatment was similar for both groups (Table 1).
Chronic kidney disease (CKD) versus non‐CKD survival
Kaplan–Meier survival analysis demonstrated a median survival of 7.26 months (95% CI 6.06–8.46) in the CKD group compared with 7.82 months (95% CI 6.33–9.30) in the non‐CKD group (P = 0.41). Although lung cancer patients with CKD had an increased risk of death of 6%, this was not statistically significant (hazard ratio [HR] 1.06, 95% CI 0.93–1.22; P = 0.41) (Fig. 2). Survival duration in the CKD group did not differ significantly from the non‐CKD group according to age, gender, smoking status, CCI score, histology, stage, or treatment (Table 3). Cancer survival for stage I–IIIA and stage IIIB and IV patients according to the severity of CKD on presentation was not significantly different. The median survival for lung cancer stage I‐IIIA was 35.84 months in the non‐CKD group, 24.80 months in CKD stage 3–4, and 29.01 months in CKD stage 5 with hemodialysis, respectively (P = 0.26). The median survival for lung cancer stage IIIB‐IV was 6.67 months in the non‐CKD group, 6.01 months for CKD stage 3–4, 3.08 months for CKD stage 5, and 2.50 months for CKD stage 5 with hemodialysis, respectively (P = 0.68) (Table 4). Subgroup analysis of stage IV lung cancer revealed a median survival of 5.19 months in CKD patients (95% CI 3.92–6.46) and 6.34 months in non‐CKD patients (95% CI 5.33–7.35; P = 0.21) (Table 4).
Figure 2.
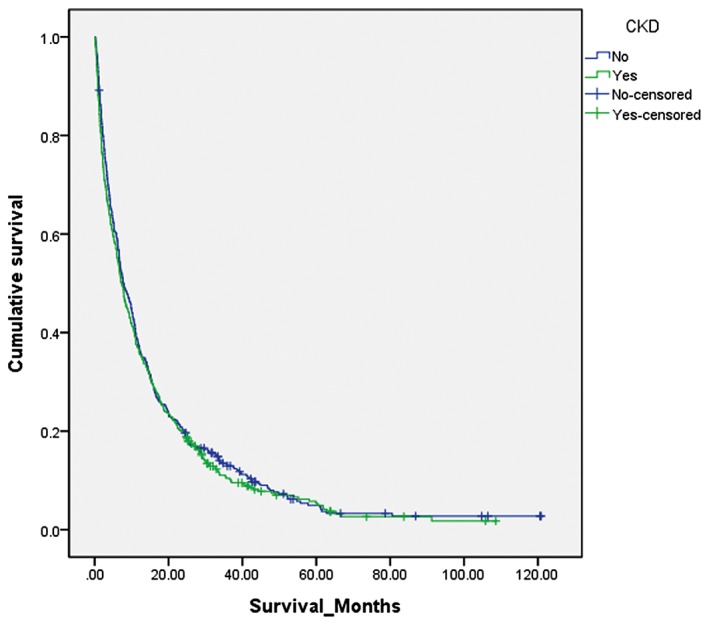
Kaplan–Meier survival curve for lung cancer patients with and without chronic kidney disease (CKD). The median survival was 7.82 months (95% confidence interval 6.33–9.30) in the non‐CKD group compared with 7.26 (95% confidence interval 6.06–8.46) in the CKD group. Log rank test: P = 0.41.
Table 3.
Kaplan‐Meier survival analysis for non‐CKD and CKD group
| Non‐CKD | CKD | ||||
|---|---|---|---|---|---|
| Median (months) | 95% CI | Median (months) | 95% CI | P | |
| Age | 0.58 | ||||
| <75 | 10.28 | 8.11–12.46 | 9.80 | 7.34–12.24 | 0.36 |
| >75 | 6.34 | 4.65–8.03 | 5.39 | 3.94–6.84 | 0.92 |
| Gender | 0.52 | ||||
| Female | 10.87 | 7.39–14.36 | 7.26 | 4.14–10.38 | 0.34 |
| Male | 6.90 | 5.98–7.82 | 7.10 | 5.83–8.37 | 0.86 |
| Smoking history | 0.44 | ||||
| No | 9.30 | 7.01–11.59 | 7.79 | 6.11–9.46 | 0.60 |
| Yes | 7.46 | 5.86–9.06 | 7.10 | 5.42–8.77 | 0.57 |
| Comorbidity score | 0.76 | ||||
| <9 | 8.67 | 6.49–10.86 | 9.99 | 7.74–12.24 | 0.59 |
| >9 | 7.52 | 5.99–9.06 | 6.11 | 4.76–7.46 | 0.36 |
| Stage | 0.25 | ||||
| Stage I–IIIA | 35.84 | 28.71–42.97 | 24.80 | 16.77–32.84 | 0.14 |
| Stage IIIB–IV | 6.67 | 5.52–7.82 | 5.98 | 4.78–7.17 | 0.52 |
| Histology | 0.39 | ||||
| NSCLC | 9.62 | 7.94–11.31 | 7.79 | 6.39–9.19 | 0.20 |
| SCLC | 4.24 | 3.33–5.15 | 4.66 | 0.52–8.80 | 0.35 |
| Treatment | 0.71 | ||||
| Supportive treatment | 3.05 | 2.29–3.82 | 2.40 | 1.56–3.24 | 0.42 |
| Medical treatment | 8.25 | 6.60–9.89 | 8.70 | 7.11–10.30 | 0.75 |
| Surgical treatment | 52.14 | 30.66–73.61 | 41.72 | 19.55–63.90 | 0.75 |
CI, confidence interval; CKD, chronic kidney disease; NSCLC, non‐small cell lung cancer; SCLC, small cell lung cancer.
Table 4.
Cancer stage survival according to the presence or not of chronic kidney disease and stage of chronic kidney disease
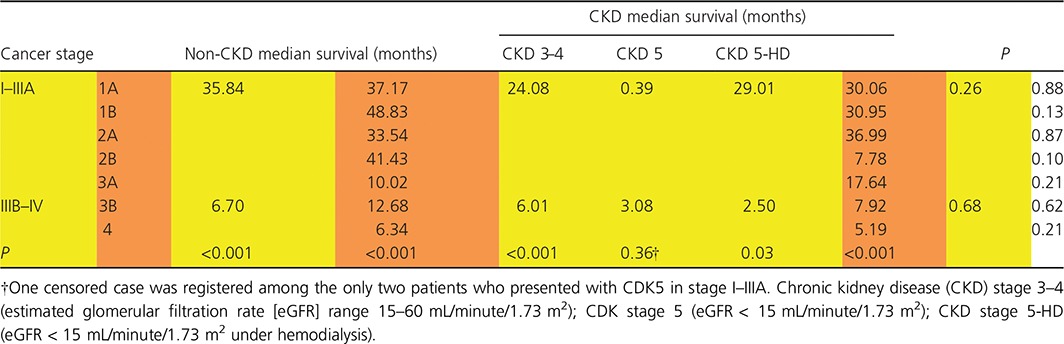
In the adjusted Cox proportional hazard analysis model for CKD patients, stage IIIB–IV (HR 1.93, 95% CI 1.38–2.70; P < 0.001) and supportive treatment (HR 1.98, 95% CI 1.60–2.46; P < 0.001) presented an increased risk of death, while surgical treatment (HR 0.45, 95% CI 0.28–0.74; P < 0.001) was associated with a decreased risk of death. The effect of age, CCI score, and histology did not reach statistical significance (Table 5).
Table 5.
Adjusted Cox proportional hazard analysis for CKD group
| CKD group | |||
|---|---|---|---|
| Adjusted hazard ratio | 95% CI | P | |
| Age | 0.08 | ||
| <75 | Ref | ||
| >75 | 1.19 | 0.98–1.46 | |
| Comorbidity score | 0.53 | ||
| <9 | Ref | ||
| >9 | 1.07 | 0.87–1.32 | |
| Stage | <0.001 | ||
| Stage I–IIIA | Ref | ||
| Stage IIIB–IV | 1.93 | 1.38–2.70 | |
| Histology | 0.12 | ||
| NSCLC | Ref | ||
| SCLC | 1.27 | 0.94–1.73 | |
| Treatment | <0.001 | ||
| Supportive | 1.98 | 1.60–2.46 | |
| Medical | 1 Ref | ||
| Surgical | 0.45 | 0.28–0.74 | |
CI, confidence interval; CKD, chronic kidney disease; NSCLC, non‐small cell lung cancer; SCLC, small cell lung cancer.
Discussion
Chronic kidney disease is a common clinical condition in the elderly population. It is estimated that 44% of individuals aged 65 years or older have CKD.15 The reported incidence of coexisting lung cancer and CKD is around 13%.4, 5 In this report, the incidence of lung cancer with coexisting chronic renal disease was 28.01% (434/1549). This higher result could be related to the high incidence and prevalence of CKD under hemodialysis in southern Taiwan (513/million and 3297/million, respectively).16 The 1988–1994 and 1999–2004 National Health and Nutrition Examination Surveys revealed that the prevalence of CKD had increased from 5.4% to 7.7%, respectively.17 As the global population ages, the incidence of lung cancer with coexisting CKD is expected to rise.
Lung cancer is a disease that mostly affects elderly patients. The median age at diagnosis in our study participants (CKD group 75 ± 9.44 years, non‐CKD 75 ± 8.21) is consistent with the SEER Cancer Statistics Review, 1975–2013, which reported the age at diagnosis of 70 years.18 We used the median age (75 years) to evaluate the effect of age on survival. The younger group (<75) had better survival duration than the older group (>75) (median 10.28 vs. 6.34 months in the non‐CKD group and 9.79 vs. 5.39 in the CKD group; P < 0.001). The inferior survival rate for older patients may be related to the following factors: less protocol‐specified treatment because of intolerance of side effects, either no treatment or only supportive treatment available, and patients are ineligible for surgical resection. In the same age group, the difference between the CKD and non‐CKD groups was not significant (P = 0.58). The survival duration of older patients after radical treatment did not differ significantly from the younger patients.19
Using SEER and Medicare records of early stage lung cancer patients, Wisnivesky et al. found that women had better lung cancer specific, overall, and relative survival than men in all treatment groups.20 Sagerup et al. found that regardless of stage, age, period of diagnosis, and selected histological subgroups, women had better survival rates than men.21 Our data analysis revealed different outcomes: the non‐CKD group demonstrated better survival for women (18.3 months, 95% CI 14.51–22.09) compared with men (15.13 months, 95% CI 12.66–13.32; P = 0.048). In the CKD group, the gender differences were not significant: 16.08 months (95% CI 12.13–20.03) for women and 14.72 (95% CI 12.27–17.16) for men (P = 0.43). This discrepancy warrants further investigation.
Non‐small cell lung cancer is responsible for nearly 80% of lung cancers, and neuroendocrine tumors account for approximately 20% (nearly 14% by small‐cell lung cancer).22 In our study, the proportion of NSCLC and small‐cell lung cancer in the CKD and non‐CKD groups were similar. This pattern of distribution is similar to a previous report of lung cancer patients with associated CKD.5 Lung cancer is usually recognized late in the disease course. The proportion of patients in our study with stage IIIB or IV at presentation was similar between the groups. Cancer stage‐specific survival according to the presence or absence of CKD was not statistically significantly different between the groups. We evaluated the effect of CKD in patients with stage IV lung cancer and found that median survival rates did not differ significantly: 6.34 months (95% CI 5.33–7.35) for the non‐CKD group compared with 5.19 (95% CI 3.92–6.46) for the CKD group (P = 0.21). Further survival analysis according to the different stages of renal impairment (non‐CKD, CKD 3, CDK 4, CKD 5, and CKD 5‐under renal replacement therapy) in patients with lung cancer stage I–IIIA and stage IIIB–IV was not statistically significantly different. However, survival rates did differ significantly between the CDK and non‐CDK patients according to cancer stage (Table 4). According to the treatment modality for these patients, the proportion of patients receiving supportive treatment is much higher in the CKD compared with the non‐CKD group (32.5% vs. 23.7%). The median survival in CKD patients was 2.40 months for supportive care, 8.70 months for medical treatment, and 41.75 months for surgical treatment (P < 0.001). Surgical resection is usually recommended for early lung cancer stages. Although surgical treatment is the treatment modality with the best chance of cure, the comorbidity and physical condition of patients with CKD could render those early stage patients medically inoperable.
Comorbidity has a significant influence on the treatment selection and survival of cancer patients. A recent article by Iachina et al., evaluated the impact of the individual component of the CCI on lung cancer survival.23 In their report, cardiovascular disease, diabetes, cerebrovascular disorders, and chronic obstructive pulmonary disease have a significant impact on the survival of NSCLC patients.23 However, as renal disease and other comorbidities were grouped together, the independent effect of CKD was not evaluated.23 Marcus et al. found that higher comorbidity severity was associated with higher lung cancer‐specific mortality, and higher CCI score determines an increased risk of lung cancer‐specific mortality.24 Because every patient in the CKD group in this study had at least one type of cancer and renal impairment, the total number of comorbidities was not used. In our cohort, each of the independent morbidity scores was added for a total score and stratified according to the median score. In the CKD group, the median survival for patients with a CCI > 9 was 6.11 months compared with 9.99 months in patients with CCI < 9 (P = 0.002). However, in the non‐CKD group, the difference was not significant, with survival rates of 7.52 months for CCI > 9 compared with 8.67 months for CCI < 9 (P = 0.09). The CCI score HR in the adjusted model was 1.07 (95% CI 0.87–1.32; P = 0.53). Although the CCI score did not reach statistical significance, we believe that the number and severity of comorbidities influenced treatment selection in these patients.
Lung cancer is a deadly disease with a five‐year survival rate of only 17.7%.18 Moderate renal dysfunction (estimated glomerular filtration rate <60 mL/minute) is associated with an increased overall mortality rate of 12% for several types of cancer, but not lung cancer, independent of other known risk factors.3 Similar survival rates between CKD and non‐CKD lung cancer patients were reported by Patel et al. in a small retrospective report (n = 107), in which all patients with CrCl <90 mL/minute (mean CrCl of 71 mL/minute) were included.5 We believe that the renal function in this group of patients was too good to be categorized as CKD. Our results revealed median survival rates in patients with CKD of 7.26 months and without CKD of 7.82 months (P = 0.41). Although lung cancer patients with CKD presented with an increased risk of death of 6%, this result was not statistically significant (HR 1.06, 95% CI 0.93–1.22, P = 0.41) (Fig. 2). CKD was not an independent predictor for lung cancer survival. In the adjusted model for the CDK group, Cox proportional hazard analysis revealed that the risk of death increases almost two‐fold for patients with stage IIIB–IV (HR 1.93, 95% CI 1.38–2.70; P < 0.001). With medical treatment as the reference, patients receiving palliative treatment have a nearly two‐fold increased risk of death (HR 1.98, 95% CI 1.60–2.46), while in those receiving surgical treatment the likelihood of death decreases by 55% (P < 0.001).
There are several limitations to our study that need to be addressed. The retrospective design, lack of standardization and overlapping of treatment, and the relatively small number of patients included may have an influence on the survival outcomes.
In our limited experience of Taiwanese patients, CKD is not an independent risk factor for lung cancer survival. Lung cancer stage and the treatment provided are the major determinants of survival. Patients with good physical performance should be aggressively treated to achieve a reasonable outcome.
Disclosure
No authors report any conflict of interest.
Acknowledgment
The authors thank all members of the Cancer Center, Chang Gung Memorial Hospital at Chiayi for their invaluable help.
Reference
- 1. World Health Organization, International Agency for Research on Cancer . GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Lung Cancer. [Cited 12 Jan 2017.] Available from URL: globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 2. U.S. Cancer Statistics Working Group . United States Cancer Statistics: 1999–2013 Incidence and Mortality Data. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, Atlanta, GA: 2016. [Cited 12 Jan 2017.] Available from URL: www.cdc.gov/uscs. [Google Scholar]
- 3. Na SY, Sung JY, Chang JH et al. Chronic kidney disease in cancer patients: An independent predictor of cancer‐specific mortality. Am J Nephrol 2011; 33: 121–30. [DOI] [PubMed] [Google Scholar]
- 4. Tammemagi CM, Neslund‐Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer 2003; 103: 792–802. [DOI] [PubMed] [Google Scholar]
- 5. Patel P, Henry LL, Ganti AK, Potti A. Clinical course of lung cancer in patients with chronic kidney disease. Lung Cancer 2004; 43: 297–300. [DOI] [PubMed] [Google Scholar]
- 6. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 7. Kidney Disease: Improving Global Outcome (KDIGO) Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013; 3: 1–50. [DOI] [PubMed] [Google Scholar]
- 8. Kutluk Cenik B, Sun H, Gerber DE. Impact of renal function on treatment options and outcomes in advanced non‐small cell lung cancer. Lung Cancer 2013; 80: 326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. (Published erratum appears in Ann Intern Med 2011; 155: 408.) Ann Intern Med 2009; 150: 604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inker LA, Astor BC, Fox CH et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014; 63: 713–35. [DOI] [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 12. Goldstraw P, Crowley J, Chansky K et al. The IASLC Lung Cancer Staging Project: Proposal for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007; 2: 706–14. [DOI] [PubMed] [Google Scholar]
- 13. Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997; 127 (8 Pt 2): 757–63. [DOI] [PubMed] [Google Scholar]
- 14. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10: 150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevens LA, Li S, Wang C et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: Results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 2010; 55: (3 Suppl. 2)S23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. 2014 Annual Report on Kidney Disease in Taiwan National Health Research Institutes, Department of Health, Executive Yuan, Taiwan. [Cited 12 Jan 2017.] Available from URL: http://www.tsn.org.tw.
- 17. Coresh J, Selvin E, Stevens LA et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–47. [DOI] [PubMed] [Google Scholar]
- 18. Howlader N, Noone AM, Krapcho M et al. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute, Bethesda, MD: 2016. [Cited 12 Jan 2017.] Available from URL: http://seer.cancer.gov/csr/1975_2013/. [Google Scholar]
- 19. Palma DA, Tyldesley S, Sheehan F et al. Stage I non‐small cell lung cancer (NSCLC) in patients aged 75 years and older: Does age determine survival after radical treatment? J Thorac Oncol 2010; 5: 818–24. [DOI] [PubMed] [Google Scholar]
- 20. Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: Do tumors behave differently in elderly women? J Clin Oncol 2007; 25: 1705–12. [DOI] [PubMed] [Google Scholar]
- 21. Sagerup CM, Småstuen M, Johannesen TB, Helland A, Brustugun OT. Sex‐specific trends in lung cancer incidence and survival: A population study of 40,118 cases. Thorax 2011; 66: 301–7. [DOI] [PubMed] [Google Scholar]
- 22. National Comprehensive Cancer Network . Small Cell Lung Cancer NCCN Practice Guidelines in Oncology, Version 1 2015. [DOI] [PubMed]
- 23. Iachina M, Jakobsen E, Møller H et al. The effect of different comorbidities on survival of non‐small cells lung cancer patients. Lung 2015; 193: 291–7. [DOI] [PubMed] [Google Scholar]
- 24. Marcus MW, Chen Y, Duffy SW, Field JK. Impact of comorbidity on lung cancer mortality – a report from the Liverpool Lung Project. Oncol Lett 2015; 9: 1902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


