Non‐alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus are two common health problems worldwide. Globally, it has been estimated that one‐quarter of the adult population in the world currently suffers from NAFLD1. In Asia, probably attributed to the obesity epidemic, the prevalence of NAFLD was similar, or even slightly higher, when compared with that in the Western population (27% in Asia vs 24.1% in North America and 23.7% in Europe), and the incidence was approximately 52 per 1,000 person‐years1. Similarly, there are more than 380 million people with diabetes worldwide, and the International Diabetes Federation estimates that this will rise to almost 592 million within a generation. Importantly, individuals with NAFLD are often comorbid with type 2 diabetes, or vice versa. Type 2 diabetes is present in almost one‐quarter of patients with NAFLD and in almost half of those with non‐alcoholic steatohepatitis (NASH), a critical stage in the spectrum of NAFLD. In contrast, NAFLD is found in as many as 75% of patients with type 2 diabetes.1 Strikingly, these two conditions can interact with each other and cause a significant negative health impact. The presence of NAFLD increases the risk of all‐cause mortality among patients with type 2 diabetes, whereas type 2 diabetes increases the risk of advanced fibrosis by threefold, doubles the risk of hepatocellular carcinoma, and independently predicts the overall and liver mortality in NAFLD. However, given the large scale of the problem and the projected considerable healthcare burden, multiple limitations and unmet needs in the management of NAFLD in type 2 diabetes patients remain to be addressed.
First, despite this strong and proven bidirectional relationship between NAFLD and type 2 diabetes, specific guidelines are still lacking on whom, when and how to screen for NAFLD among patients with type 2 diabetes. Universal screening for NAFLD in type 2 diabetes patients is not advocated in the current practice guidelines for NAFLD management in the USA, partly related to the issue of cost‐effectiveness. NAFLD is defined as the presence of more than 5% of hepatic steatosis. Commonly used imaging techniques, such as hepatic ultrasound, are insensitive to mild hepatic steatosis (<30%). In contrast, more sensitive tools, such as magnetic resonance spectroscopy, are however, limited by cost and availability. In addition, as NAFLD spans a spectrum from simple hepatic steatosis to NASH, liver cirrhosis and hepatocellular carcinoma, universal screening for NAFLD in type 2 diabetes patients could result in the inclusion of a proportion of patients who might remain as simple hepatic steatosis with minimal risk of progression.
Second, although type 2 diabetes is well known to be a risk factor for NAFLD progression, monitoring of disease progression is challenging. Measuring alanine aminotransferase (ALT) levels is an insensitive method of disease monitoring, as ALT fluctuates within the spectrum of NAFLD1. Liver biopsy used to be the gold standard for evaluating the different stages of NAFLD. However, this is limited by its invasive nature, sampling errors and complication rates. Indeed, given the large number of type 2 diabetes patients suffering from NAFLD, it is both impractical and technically difficult to follow them up individually for their hepatic progression using serial liver biopsies. The advent of transient elastography has rendered a non‐invasive monitoring of both hepatic steatosis and fibrosis feasible, while utilizing estimates from the controlled attenuation parameter and liver stiffness measurements, respectively. Currently, however, it is still not possible to differentiate NASH from simple hepatic steatosis using non‐invasive and commercially available tools, including transient elastography.
Over the years, there has been extensive research looking for sensitive and well‐validated biomarkers for NASH. Some are quite promising, such as circulating cytokeratin‐18 fragment, a hepatocyte apoptotic marker, as well as several adipokines or obesity‐related protein markers that have also been investigated as emerging NASH biomarkers, partly because obesity serves as a common risk factor for NAFLD and type 2 diabetes2. Our observation of a protective role of adiponectin in NASH, published in the Journal of Clinical Investigation in 20033 has been extensively replicated. We also found, in a study published in Journal of Hepatology in 20134, that the expression of adipocyte fatty acid‐binding protein, an adipokine involved in the trafficking of lipids, was elevated in the Kupffer cells of mice with NASH, and treatment with a small molecule of adipocyte fatty acid‐binding protein inhibitor could protect obese mice from NASH. In addition, along with our Australian collaborators, we found that circulating adipocyte fatty acid‐binding protein levels positively correlated with the degree of inflammation and fibrosis in NAFLD. In humans, serum levels of fibroblast growth factor 21 (FGF21), an emerging metabolic regulator closely related to various obesity‐related conditions, were elevated in patients with biopsy‐proven NASH compared with that of healthy controls. Whether these circulating proteins could be usefully employed as NASH biomarkers, especially in type 2 diabetes patients, remains to be confirmed in large diabetic cohorts. Nevertheless, these findings have opened up opportunities for better understanding of the complex pathogenic mechanisms of NASH, which undoubtedly, has become a major complication of type 2 diabetes.
Finally, managing NAFLD in type 2 diabetes is further challenged by the limitation of treatment strategies. To our knowledge, other than lifestyle modification, there is currently no licensed or approved pharmacological therapy for the treatment of NAFLD. Lifestyle modification is easier said than done. Patients who have initiated lifestyle intervention with dietary modification and weight reduction often have difficulties in maintaining their efforts. In recent years, however, new agents for the management of NAFLD in type 2 diabetes have emerged (Figure 1). Farnesoid X nuclear receptor agonist, for instance, is a novel class of agents that was shown in a multicenter, randomized, placebo‐controlled trial published in 2015 to show benefits in patients with type 2 diabetes and NASH. Activation of farnesoid X nuclear receptor inhibits hepatic de novo lipogenesis, hepatic gluconeogenesis and glycogenolysis5. In the farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis trial, which comprised more than 50% of participants with type 2 diabetes (142 out of 283 participants), obeticholic acid improved the histological features of NASH after 72 weeks of treatment.
Figure 1.
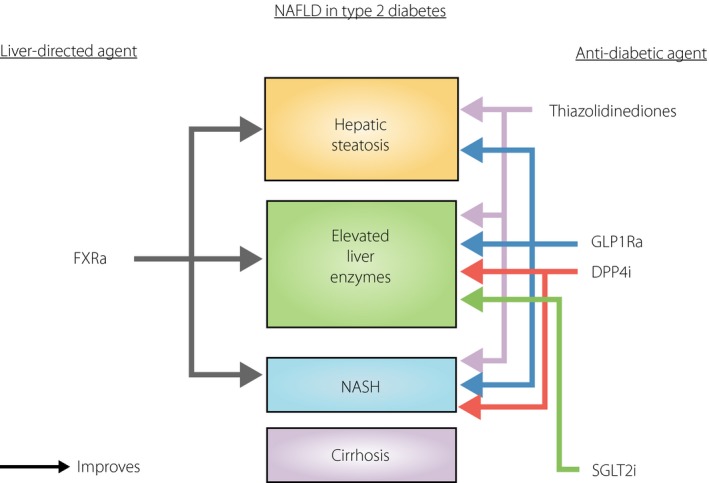
Potentially useful pharmacotherapy for management of non‐alcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes. DPP4i, dipeptidyl peptidase‐4 inhibitors; FXRa, farnesoid X receptor agonists; GLP1Ra, glucagon‐like peptide receptor agonists; NASH, non‐alcoholic steatohepatitis; SGLT2i, sodium–glucose cotransporter 2 inhibitors.
Although the long‐term efficacy and safety data of farnesoid X nuclear receptor agonists are eagerly awaited, the presence of NAFLD in patients with type 2 diabetes might well influence the choice of antidiabetic agents. Indeed, there are emerging data of NAFLD protection among various existing agents in the growing list of antidiabetic armamentarium. (Figure 1) Pioglitazone, the only thiazolidinedione currently available on the market, was shown in a proof‐of‐concept study published by Beifort et al. in the New England Journal of Medicine in 20066 to improve steatosis, ballooning and inflammation among patients with impaired glucose tolerance or type 2 diabetes. Thiazolidinediones are peroxisome proliferator activated receptor gamma agonists that possess potent insulin‐sensitizing properties. They mediate NAFLD protection through increasing adiponectin levels, resulting in a reduction in free fatty acid influx, increased fatty acid oxidation and decreased inflammation. In contrast, the incretin‐based therapies, which comprise oral dipeptidyl peptidase‐4 inhibitors and injectable glucagon‐like peptide‐1 (GLP1) receptor agonists, have also been suggested as effective treatment options of NAFLD in a recent meta‐analysis7. However, the primary outcome used was solely the reduction in serum ALT levels, and among the incretin‐based therapies, only sitagliptin and liraglutide have been extensively studied in humans. Nevertheless, as dipeptidyl peptidase‐4 expression is found in hepatic stellate cells, it has been postulated that dipeptidyl peptidase‐4 inhibitors might attenuate the activation of hepatic stellate cells, and decrease the production of hepatic transforming growth factor‐beta 1. With regard to GLP1 receptor agonists, although the presence of GLP1 receptors on human hepatocytes remains controversial, it is possible that GLP1 receptor agonists can mediate NAFLD protection through mechanisms other than an improvement in glycemic control and considerable weight loss. In animal studies, liraglutide has been shown to increase FGF21, FGF receptor messenger ribonucleic acid and protein expression. In fact, in diet‐induced obese mice, chronic treatment with recombinant FGF21 also improved hepatic steatosis through the inhibition of hepatic lipogenesis2, although the use of recombinant FGF21 analogs to treat NAFLD in human subjects with type 2 diabetes remains to be investigated2. More recently, the sodium–glucose cotransporter 2 inhibitors, the first oral hypoglycemic agent to show cardiovascular benefits, have been shown to be another antidiabetic drug that can be of benefit in NAFLD. Sodium–glucose cotransporter 2 inhibitors improved hepatic steatosis in mice models, through a decrease in de novo lipogenesis and possibly an increase in fatty acid oxidation8. Furthermore, the use of ipragliflozin also improved liver dysfunction in terms of ALT levels, independent of weight changes, among 25 subjects with coexisting type 2 diabetes and ultrasound‐proven hepatic steatosis8.
Opportunities follow challenges. This has been well demonstrated over the past decade in the management of NAFLD in type 2 diabetes patients. Clearly, there exists a gap between scientific evidence and current clinical practice, as well as multiple unmet needs, from diagnostics to therapeutics. Nevertheless, we can foresee that the tremendous efforts of both clinicians and scientists, striving to turn such challenges into opportunities of medical advancement, will bring in new insights and breakthroughs, to overcome this emerging complication of diabetes.
Disclosure
The authors declare no conflict of interest.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, et al Global Epidemiology of Non‐Alcoholic Fatty Liver Disease‐Meta‐Analytic Assessment of Prevalence, Incidence and Outcomes. Hepatology 2015. doi:10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2. Hui E, Xu A, Bo Yang H, et al Obesity as the common soil of non‐alcoholic fatty liver disease and diabetes: role of adipokines. J Diabetes Investig 2013; 4: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu A, Wang Y, Keshaw H, et al The fat‐derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Investig 2003; 112: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoo RL, Lee IP, Zhou M, et al Pharmacological inhibition of adipocyte fatty acid binding protein alleviates both acute liver injury and non‐alcoholic steatohepatitis in mice. J Hepatol 2013; 58: 358–364. [DOI] [PubMed] [Google Scholar]
- 5. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, et al Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015; 385: 956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belfort R, Harrison SA, Brown K, et al A placebo‐controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006; 355: 2297–2307. [DOI] [PubMed] [Google Scholar]
- 7. Carbone LJ, Angus PW, Yeomans ND. Incretin‐based therapies for the treatment of non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. J Gastroenterol Hepatol 2016; 31: 23–31. [DOI] [PubMed] [Google Scholar]
- 8. Komiya C, Tsuchiya K, Shiba K, et al Ipragliflozin Improves Hepatic Steatosis in Obese Mice and Liver Dysfunction in Type 2 Diabetic Patients Irrespective of Body Weight Reduction. PLoS ONE 2016; 11: e0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]


