Abstract
Aims/Introduction
Dipeptidyl peptidase‐4 inhibitors are used for treatment of patients with type 2 diabetes. In addition to glycemic control, these agents showed beneficial effects on lipid metabolism in clinical trials. However, the mechanism underlying the lipid‐lowering effect of dipeptidyl peptidase‐4 inhibitors remains unclear. Here, we investigated the lipid‐lowering efficacy of anagliptin in a hyperlipidemic animal model, and examined the mechanism of action.
Materials and Methods
Male low‐density lipoprotein receptor‐deficient mice were administered 0.3% anagliptin in their diet. Plasma lipid levels were assayed and lipoprotein profile was analyzed using high‐performance liquid chromatography. Hepatic gene expression was examined by deoxyribonucleic acid microarray and quantitative polymerase chain reaction analyses. Sterol regulatory element‐binding protein transactivation assay was carried out in vitro.
Results
Anagliptin treatment significantly decreased the plasma total cholesterol (14% reduction, P < 0.01) and triglyceride levels (27% reduction, P < 0.01). Both low‐density lipoprotein cholesterol and very low‐density lipoprotein cholesterol were also decreased significantly by anagliptin treatment. Sterol regulatory element‐binding protein‐2 messenger ribonucleic acid expression level was significantly decreased at night in anagliptin‐treated mice (15% reduction, P < 0.05). Anagliptin significantly suppressed sterol regulatory element‐binding protein activity in HepG2 cells (21% decrease, P < 0.001).
Conclusions
The results presented here showed that the dipeptidyl peptidase‐4 inhibitor, anagliptin, exhibited a lipid‐lowering effect in a hyperlipidemic animal model, and suggested that the downregulation of hepatic lipid synthesis was involved in the effect. Anagliptin might have beneficial effects on lipid metabolism in addition to a glucose‐lowering effect.
Keywords: Anagliptin, Dipeptidyl peptidase‐4 inhibitor, Lipid metabolism
Introduction
Incretins, such as glucagon‐like peptide‐1 (GLP‐1), are important factors involved in the regulation of glucose metabolism. GLP‐1 is produced by L‐cells in the intestine after meals, leading to secretion of insulin by pancreatic β‐cells. However, plasma GLP‐1 is cleaved by dipeptidyl peptidase‐4 (DPP‐4), and inactivated within a few minutes. Therefore, inhibition of DPP‐4 activity is an effective approach to lowering blood glucose levels by preventing the degradation of plasma GLP‐1. Since the approval by the US Food and Drug Administration in 2006 of the first DPP‐4 inhibitor, sitagliptin1, many DPP‐4 inhibitors have been used to treat patients with type 2 diabetes.
In addition to glycemic control, DPP‐4 inhibitors, such as sitagliptin, vildagliptin and anagliptin, were recently shown to have beneficial effects on lipid metabolism. Meta‐analyses showed lipid‐lowering effects of DPP‐4 inhibitors, including sitagliptin and vildagliptin2, 3. Some, but not all studies, showed that sitagliptin reduced serum cholesterol and triglyceride levels4, 5, 6, 7, 8, 9. Significant changes in cholesterol levels were observed in all vildagliptin trials included in the meta‐analysis by Monami et al.2, but the triglyceride‐lowering effect was not consistent among the trials10, 11, 12, 13. With regard to the lipid‐lowering effect of anagliptin, plasma total cholesterol, low‐density lipoprotein cholesterol (LDL‐C) and triglyceride levels were decreased in pooled analysis of phase III clinical trials, and all of the trials showed decreased lipid levels14, 15, 16. These clinical findings suggest that DPP‐4 inhibitors contribute to a reduction in plasma lipid level, although the underlying mechanism of this action of DPP‐4 inhibitors remains unclear.
In the present study, we investigated the lipid‐lowering efficacy with DPP‐4 inhibitor, anagliptin, in LDL receptor (LDLR)‐deficient mice as a hyperlipidemic animal model. As significant reductions in cholesterol and triglyceride levels were both observed in mice treated with anagliptin, we used this agent to investigate the mechanism underlying the lipid‐lowering effects of DPP‐4 inhibitors. The present results showed that anagliptin reduced the lipid levels in LDLR‐deficient mice and decreased hepatic lipid synthesis. DPP‐4 inhibitor, anagliptin, might have beneficial effects on lipid metabolism in addition to glycemic control.
Materials and Methods
Animals
Male LDLR‐deficient mice (B6.129S7‐Ldlrtm1Her/J) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) through Charles River Laboratories Japan (Yokohama, Japan) at 5 weeks‐of‐age. Mice were housed under 12‐h light/dark cycle (07.00/19.00 h), and fed a normal chow diet (CE‐2; CLEA Japan, Tokyo, Japan) and sterilized water ad libitum. Anagliptin was provided by Sanwa Kagaku Kenkyusho (Nagoya, Japan). From the age of 6 weeks, LDLR‐deficient mice were given a diet containing 0.3% anagliptin17, 18, for 4 weeks. In the experiments for sampling at night, anagliptin at 0.3% was administered for 2 weeks for sampling from 22.00 to 02.00 h. In this condition, plasma concentration of anagliptin was approximately 600 ng/mL, and approximately 80% inhibition of DPP‐4 activity was observed, similar to a clinical report16. The animal care and experimental procedures were approved by the Animal Care Committee of Tokyo New Drug Research Laboratories, Kowa Company (Tokyo, Japan).
Plasma cholesterol and triglyceride levels
Blood samples were collected to measure plasma glucose, cholesterol and triglyceride levels using assay kits from Wako Pure Chemical Industries (Osaka, Japan). Lipid profile was analyzed by high‐performance liquid chromatography (LC‐20A; Shimadzu, Kyoto, Japan). Plasma samples were diluted with phosphate‐buffered saline containing 1 mmol/L ethylenediaminetetraacetic acid and separated using a Superose 6 gel filtration column (GE Healthcare, Buckinghamshire, UK), followed by on‐line reaction with a lipid assay kit and detected at 600 nm19.
Gene expression analysis using deoxyribonucleic acid microarray and real‐time quantitative polymerase chain reaction
Ribonucleic acid extracted from tissue samples was subjected to deoxyribonucleic acid (DNA) microarray analysis (Mouse GE 4x44K v2; Agilent Technologies, Santa Clara, California, USA), and analyzed using GeneSpring 13.0 (Agilent Technologies). Genes with >1.2‐fold change were regarded as differentially expressed and used for pathway analysis. For real‐time quantitative polymerase chain reaction, an ABI 7900HT Fast Real‐Time PCR System (Thermo Fisher Scientific, Waltham, Massachusetts, USA) was used with the following probes: Sterol regulatory element‐binding protein (SREBP)‐1c, (forward) ATCGGCGCGGAAGCTGTCGGGGTAGCGTC, (reverse) TGAGCTGGAGCATGTCTTCAA, (probe) FAM‐ACCACGGAGCCATGGATTGCACATT‐TAMRA20; SREBP‐2, Mm01306292_m1; Actb, Mm00607939_s1.
Plasmid vector construction
Tandem sterol responsive element (3 × SRE) was obtained using synthetic oligo DNA as follows21: (sense) CCGCTCGAGAAAATCACCCCACTGCAAAATCACCCCACTGCAAAATCACCCCACTGCAAACTCCTCCCCCTGCGAT and (antisense) ATCGCAGGGGGAGGAGTTTGCAGTGGGGTGATTTTGCAGTGGGGTGATTTTGCAGTGGGGTGATTTTCTCGAGCGG. They were annealed and inserted into the pGL4.28 vector containing firefly luciferase (Promega, Madison, Wisconsin, USA) with EcoRV and XhoI sites (SRE‐luc).
SREBP transactivation assay
HepG2 cells were purchased from American Type Culture Collection (ATCC; Manassas, Virginia, USA). HepG2 cells were cultured at 1 × 104 cells/well in 96‐well plates. The next day, the cells were transfected with luciferase vector using X‐treamGENE HP reagent (Roche, Mannheim, Germany). pRL‐SV40 (Promega) was co‐transfected for correction of transfection efficiency. After 24 h, medium was changed to fresh medium containing 5% lipoprotein‐deficient serum, and stimulated with the test compound for 24 h. Cell lysates were subject to Dual‐Glo Luciferase Assay System (Promega) according to the manufacturer's instructions.
Statistical analysis
The results are presented as mean ± standard error of the mean. Differences between groups were examined for statistical significance using Student's t‐test or Dunnett's test. In all analyses, P < 0.05 was taken to show statistical significance.
Results
Lipid‐lowering effect of anagliptin in LDLR‐deficient mice
The effect of DPP‐4 inhibitor on lipid profile was investigated. LDLR‐deficient mice treated with anagliptin and control groups showed similar bodyweight (Figure 1a). Plasma glucose and insulin levels were at normal levels in both groups. Anagliptin‐treated mice showed significantly decreased plasma total cholesterol and triglyceride levels (Figure 1b,c). High‐performance liquid chromatography analysis showed that very low‐density lipoprotein cholesterol and LDL‐C were significantly decreased (Figure 2a,b). High‐density lipoprotein cholesterol was slightly lowered in the anagliptin treatment group, but not significantly (Figure 2c), indicating that the reduction of plasma total cholesterol level was mainly due to a decrease in LDL‐C in mice treated with anagliptin. As significantly reduced cholesterol and triglyceride levels were observed with anagliptin treatment in the in vivo model, this agent was used in subsequent experiments as a lipid‐lowering DPP‐4 inhibitor.
Figure 1.
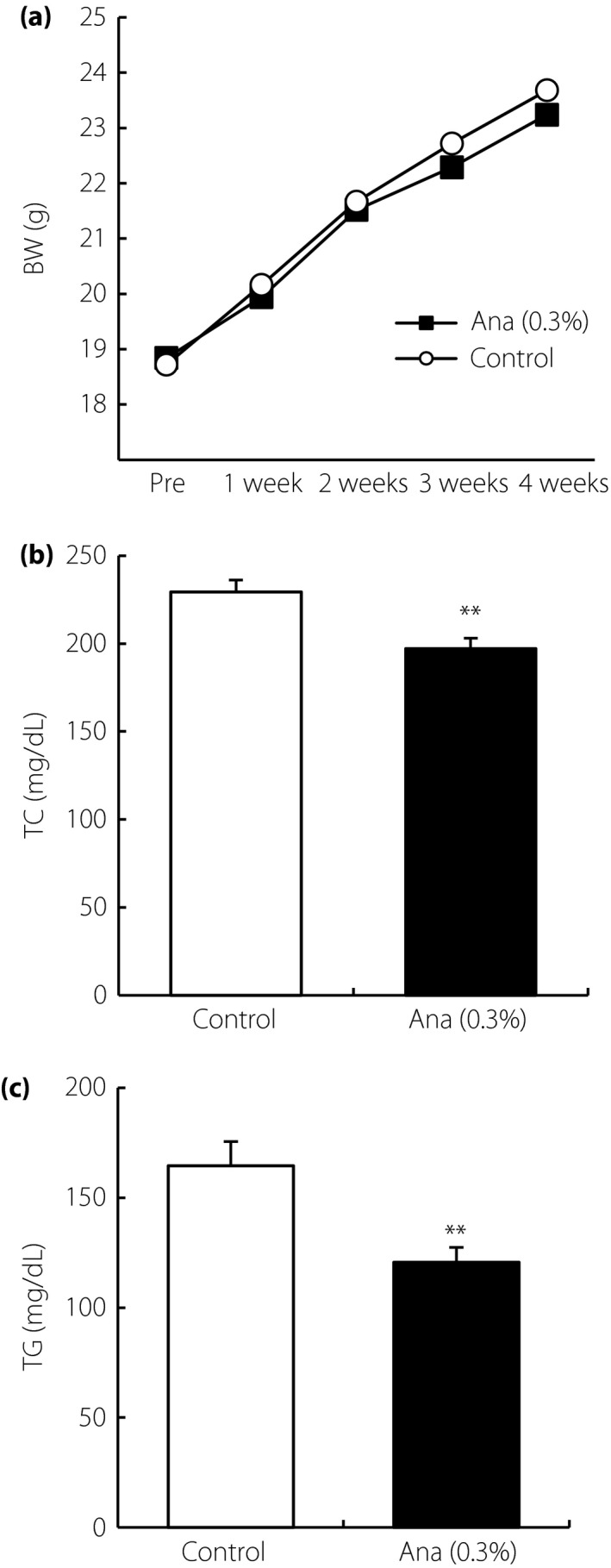
Lipid‐lowering effects of dipeptidyl peptidase‐4 inhibitors in low‐density lipoprotein receptor‐deficient mice with anagliptin (Ana). (a) Bodyweight (BW). Plasma concentrations of (b) total cholesterol (TC) and (c) triglyceride (TG) after 4 weeks of treatment. Data are shown as mean ± standard error of the mean (n = 10). **P < 0.01.
Figure 2.
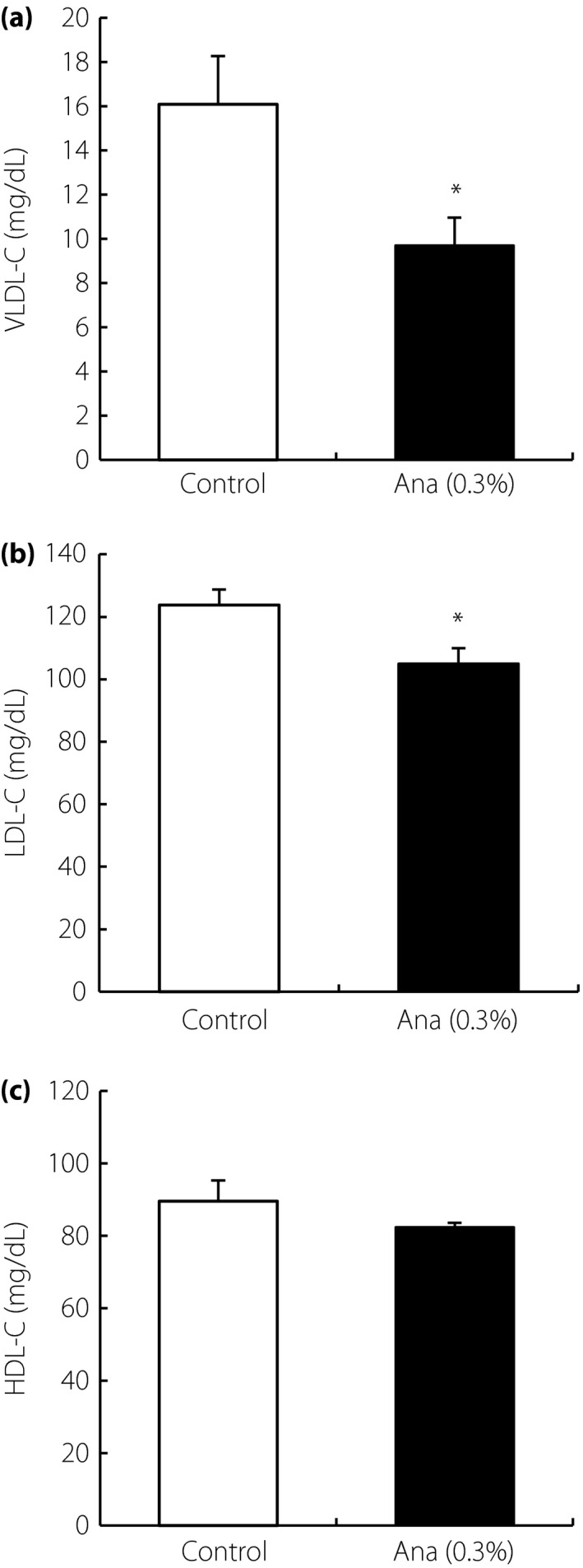
Lipid profiling by high‐performance liquid chromatography in low‐density lipoprotein receptor‐deficient mice with anagliptin (Ana). Plasma (a) very low‐density lipoprotein cholesterol (VLDL‐C), (b) low‐density lipoprotein cholesterol (LDL‐C) and (c) high‐density lipoprotein cholesterol (HDL‐C) concentrations after 4 weeks of anagliptin treatment. Data are shown as mean ± standard error of the mean (n = 10). *P < 0.05.
Hepatic gene expression analysis involved in lipid metabolism
To investigate the mechanism underlying the lipid‐lowering effect of anagliptin, hepatic gene expression was analyzed by DNA microarray and pathway analyses. Comparison of gene expression patterns between anagliptin‐treated and control liver samples showed associations with pathways related to lipid metabolism (Table 1). Hepatic de novo triglyceride synthesis tended to be reduced in anagliptin‐treated mice (Figure S1). Next, the expression levels of SREBP were examined, as it is an important factor for lipid syntheses. The level of SREBP‐1c expression was not altered, but SREBP‐2 expression was significantly decreased in the anagliptin‐treated group at night (Figure 3). These data suggested that anagliptin might regulate hepatic gene expressions involved in lipid synthesis.
Table 1.
Pathways involved in lipid metabolism
| Pathway list | P‐value |
|---|---|
| Mm_Nuclear_receptors_in_lipid_metabolism_and_toxicity_WP431_47744 | 0.0089 |
| Mm_Fatty_Acid_Biosynthesis_WP336_41307 | 0.0202 |
| Mm_Statin_Pathway_WP1_41296 | 0.0384 |
| Mm_Triglyceride_Synthesis_WP386_45646 | 0.0791 |
Liver samples from low‐density lipoprotein receptor‐deficient mice treated with anagliptin or untreated controls were subjected to deoxyribonucleic acid microarray analysis (n = 4). Associated pathways with differentially expressed genes as determined by deoxyribonucleic acid microarray analysis are shown.
Figure 3.
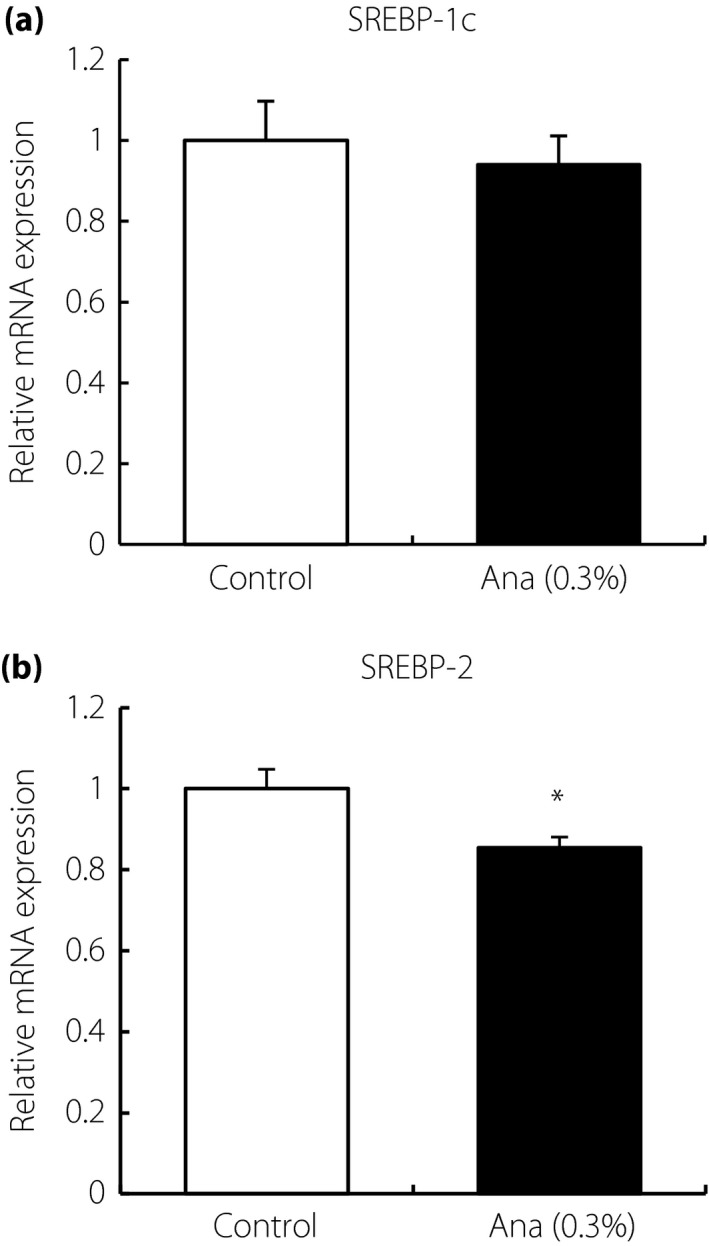
Hepatic gene expression in low‐density lipoprotein receptor‐deficient mice after anagliptin (Ana) treatment. Hepatic (a) sterol regulatory element‐binding protein (SREBP)‐1c and (b) SREBP‐2 expression levels at night. Data are shown as mean ± standard error of the mean (n = 10), *P < 0.05. mRNA, messenger ribonucleic acid.
SREBP activity in HepG2 cells treated with anagliptin
SREBP transactivation assay was carried out in HepG2 cells using luciferase vector with the SRE promoter to investigate the direct effects of anagliptin on lipid metabolism. SREBP activity was significantly suppressed by anagliptin after 24 h of stimulation (Figure 4). These observations suggested that the lipid‐lowering mechanism of anagliptin might involve downregulation of SREBP activity.
Figure 4.
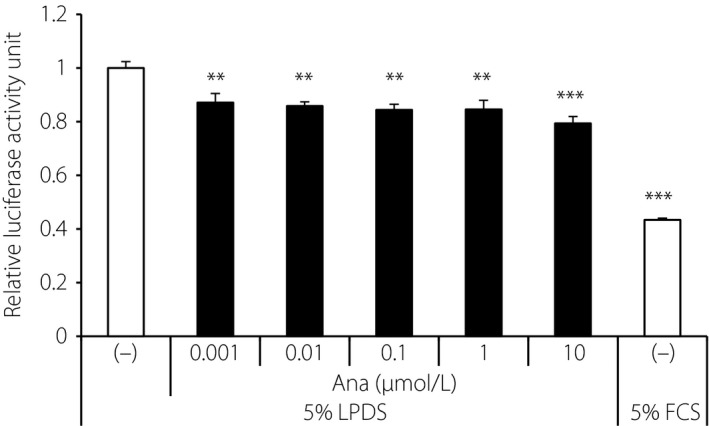
Sterol regulatory element‐binding protein (SREBP) transactivation assay in vitro. HepG2 cells transfected with luciferase vector with SRE were incubated with anagliptin at various concentrations (0.001–10 μmol/L) in medium containing 5% lipoprotein‐deficient serum (LPDS). Data are shown as mean ± standard error of the mean. **P < 0.01, ***P < 0.001 vs control group by Dunnett's test (n = 4). FCS, fetal calf serum.
Discussion
In addition to glycemic control, clinical trials with DPP‐4 inhibitors have shown that these agents also show lipid‐lowering effects. Meta‐analyses showed lipid‐lowering effects of several DPP‐4 inhibitors, including sitagliptin and vildagliptin2, 3. Anagliptin was shown to reduce total cholesterol, LDL‐C and triglyceride levels in clinical trials14, 15, 16. Thus, DPP‐4 inhibitors might have beneficial effects on lipid metabolism, but the underlying mechanism is not fully understood.
Mice and rats lacking DPP‐4 activity showed decreased levels of hepatic SREBP‐1c and FAS expression22, 23. The lipid‐lowering effects of DPP‐4 inhibitors in vivo were described previously in animal models of type 2 diabetes. Sitagliptin analog and vildagliptin were reported to decrease lipogenic gene expression in a mouse model of diet‐induced obesity24, 25. Recently, MK‐0626 was shown to decrease lipogenic gene expressions in ob/ob mice26. These reports showed that DPP‐4 inhibitors ameliorated not only blood glucose level, but also lipid metabolism. In the present study, we used LDLR‐deficient mice fed normal chow as a normoglycemic hyperlipidemic animal model to eliminate the effects of glycemic control27.
First, we confirmed the efficacy of DPP‐4 inhibitor, anagliptin, on plasma lipid levels in vivo. Anagliptin significantly reduced both total cholesterol and triglyceride levels in LDLR‐deficient mice. LDL‐C was also decreased significantly in mice with anagliptin treatment. These observations suggested that anagliptin might have a beneficial effect on lipid metabolism in clinical and experimental settings. In a previous clinical study, plasma cholesterol levels were decreased by anagliptin treatment in patients treated with or without statins14. Statins are known to suppress cholesterol synthesis, and enhance the uptake of LDL‐cholesterol mediated by LDLR. In the present study, however, LDLR‐deficient mice treated with anagliptin showed decreased cholesterol levels, suggesting that this drug might regulate plasma lipid levels through a mechanism different from that of statins.
Next, we investigated the mechanisms underlying the lipid‐lowering effects of DPP‐4 inhibitor. These experiments were carried out using anagliptin as a lipid‐lowering DPP‐4 inhibitor. DNA microarray analysis showed significant associations with pathways involved in lipid metabolism in the livers of mice with anagliptin. The level of SREBP‐1c expression was unchanged, but SREBP transactivation assay in hepatocytes suggested the downregulation of triglyceride synthesis by anagliptin treatment. Although the effects of DPP‐4 inhibitors on triglyceride synthesis and secretion have not yet been reported, hepatic de novo triglyceride synthesis tended to be reduced in anagliptin‐treated mice, and our preliminary experiments showed that anagliptin treatment did not alter the rate of triglyceride secretion in vivo (Figure S1). With regard to cholesterol metabolism, SREBP‐2 expression level was significantly decreased in the anagliptin‐treated group at night, and SREBP transactivation assay in hepatocytes also suggested the downregulation of SREBP‐2 by anagliptin treatment. Thus, anagliptin treatment might reduce plasma cholesterol levels mediated by the downregulation of SREBP‐2. Although further studies are required to determine the mechanism by which anagliptin regulates lipid metabolism, these results suggested that the plasma triglyceride and cholesterol lowering effects of anagliptin treatment might involve its interactions with the SREBP pathway. The in vitro result suggested that anagliptin could regulate lipid metabolism directly. The present results suggested that the lipid‐lowering effect of DPP‐4 inhibitor treatment might involve a decrease in hepatic lipid synthesis, which support previous clinical findings indicating that anagliptin decreased a marker of cholesterol synthesis28. In addition, there remains the possibility that other tissues are involved in the lipid‐lowering effects of anagliptin. The intestine is an important tissue for lipid metabolism, because the lipids present in foods are absorbed, gastrointestinal hormones are produced and lipids are excreted with bile acids in the intestine. In a review article, Ussher et al.29 reported that activation of GLP‐1R signaling inhibits lipoprotein production in the intestine. Anti‐inflammatory effects might also be involved, as a recent study showed that anagliptin improved inflammation in the liver and adipose tissue, suggesting a beneficial effect on chronic inflammation in metabolic diseases30.
Taken together, the results presented here suggest that DPP‐4 inhibitor, anagliptin, might have beneficial effects on lipid metabolism in addition to glycemic control, and therefore ameliorate metabolic diseases and type 2 diabetes mellitus.
Disclosure
WY, NI, SI, TI, MY, YY, SH, TN, KI and ST are employees of Kowa Company, Ltd. MG is an employee of Sanwa Kagaku Kenkyusho Co., Ltd. KK is an advisor to, received honoraria for lectures from, and received scholarship grants from Novo Nordisk Pharma, Sanwa Kagaku Kenkyusho, Takeda Pharmaceutical, Taisho Pharmaceutical, MSD, Kowa Company, Sumitomo Dainippon Pharma, Novartis Pharma, Mitsubishi Tanabe Pharma, AstraZeneca, Nippon Boehringer Ingelheim, Chugai Pharmaceutical, Daiichi Sankyo and Sanofi. None of the authors have any financial interests related to this work.
Supporting information
Figure S1 ¦ Hepatic triglyceride synthesis and secretion assay.
Acknowledgment
We thank all the members of the laboratory animal administration for their excellent animal care.
J Diabetes Investig 2017; 8: 155–160
References
- 1. Drucker D, Easley C, Kirkpatrick P. Sitagliptin. Nat Rev Drug Discov 2007; 6: 109–110. [DOI] [PubMed] [Google Scholar]
- 2. Monami M, Lamanna C, Desideri CM, et al DPP‐4 inhibitors and lipids: systematic review and meta‐analysis. Adv Ther 2012; 29: 14–25. [DOI] [PubMed] [Google Scholar]
- 3. Monami M, Vitale V, Ambrosio ML, et al Effects on lipid profile of dipeptidyl peptidase 4 inhibitors, pioglitazone, acarbose, and sulfonylureas: meta‐analysis of placebo‐controlled trials. Adv Ther 2012; 29: 736–746. [DOI] [PubMed] [Google Scholar]
- 4. Rosenstock J, Brazg R, Andryuk PJ, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Clin Ther 2006; 28: 1556–1568. [DOI] [PubMed] [Google Scholar]
- 5. Yoon KH, Shockey GR, Teng R, et al Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase‐4 inhibitor, and pioglitazone on glycemic control and measures of β‐cell function in patients with type 2 diabetes. Int J Clin Pract 2011; 65: 154–164. [DOI] [PubMed] [Google Scholar]
- 6. Charbonnel B, Karasik A, Liu J, et al Efficacy and safety of the dipeptidyl peptidase‐4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29: 2638–2643. [DOI] [PubMed] [Google Scholar]
- 7. Bergenstal RM, Wysham C, Macconell L, et al Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION‐2): a randomised trial. Lancet 2010; 376: 431–439. [DOI] [PubMed] [Google Scholar]
- 8. Pratley R, Nauck M, Bailey T, et al One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel‐group, open‐label trial. Int J Clin Pract 2011; 65: 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams‐Herman D, Johnson J, Teng R, et al Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 442–451. [DOI] [PubMed] [Google Scholar]
- 10. Rosenstock J, Kim SW, Baron MA, et al Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab 2007; 9: 175–185. [DOI] [PubMed] [Google Scholar]
- 11. Dejager S, Razac S, Foley JE, et al Vildagliptin in drug‐naïve patients with type 2 diabetes: a 24‐week, double‐blind, randomized, placebo‐controlled, multiple‐dose study. Horm Metab Res 2007; 39: 218–223. [DOI] [PubMed] [Google Scholar]
- 12. Bolli G, Dotta F, Colin L, et al Comparison of vildagliptin and pioglitazone in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Obes Metab 2009; 11: 589–595. [DOI] [PubMed] [Google Scholar]
- 13. Schweizer A, Couturier A, Foley JE, et al Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug‐naïve patients with Type 2 diabetes. Diabet Med 2007; 24: 955–961. [DOI] [PubMed] [Google Scholar]
- 14. Kaku K. Effects of anagliptin on serum lipids in Japanese patients with type 2 diabetes ‐ A pooled analysis of long‐term therapy with anagliptin. Jpn Pharmacol Ther 2012; 40: 771–784 (Japanese). [Google Scholar]
- 15. Kakuda H, Kobayashi J, Kakuda M, et al The effect of anagliptin treatment on glucose metabolism and lipid metabolism, and oxidative stress in fasting and postprandial states using a test meal in Japanese men with type 2 diabetes. Endocrine 2015; 48: 1005–1009. [DOI] [PubMed] [Google Scholar]
- 16. Nishio S, Abe M, Ito H. Anagliptin in the treatment of type 2 diabetes: safety, efficacy, and patient acceptability. Diabetes Metab Syndr Obes 2015; 8: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ervinna N, Mita T, Yasunari E, et al Anagliptin, a DPP‐4 Inhibitor, Suppresses Proliferation of Vascular Smooth Muscles and Monocyte Inflammatory Reaction and Attenuates Atherosclerosis in Male apo E‐Deficient Mice. Endocrinology 2013; 154: 1260–1270. [DOI] [PubMed] [Google Scholar]
- 18. Nakaya K, Kubota N, Takamoto I, et al Dipeptidyl peptidase‐4 inhibitor anagliptin ameliorates diabetes in mice with haploinsufficiency of glucokinase on a high‐fat diet. Metabolism 2013; 62: 939–951. [DOI] [PubMed] [Google Scholar]
- 19. Yoshinaka Y, Shibata H, Kobayashi H, et al A selective ACAT‐1 inhibitor, K‐604, stimulates collagen production in cultured smooth muscle cells and alters plaque phenotype in apolipoprotein E‐knockout mice. Atherosclerosis 2010; 213: 85–91. [DOI] [PubMed] [Google Scholar]
- 20. Awazawa M, Ueki K, Inabe K, et al Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun 2009; 382: 51–56. [DOI] [PubMed] [Google Scholar]
- 21. Sanchez HB, Yieh L, Osborne TF. Cooperation by sterol regulatory element‐binding protein and Sp1 in sterol regulation of low density lipoprotein receptor gene. J Biol Chem 1995; 270: 1161–1169. [DOI] [PubMed] [Google Scholar]
- 22. Conarello SL, Li Z, Ronan J, et al Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA 2003; 100: 6825–6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ben‐Shlomo S, Zvibel I, Shnell M, et al Glucagon‐like peptide‐1 reduces hepatic lipogenesis via activation of AMP‐activated protein kinase. J Hepatol 2011; 54: 1214–1223. [DOI] [PubMed] [Google Scholar]
- 24. Shirakawa J, Fujii H, Ohnuma K, et al Diet‐induced adipose tissue inflammation and liver steatosis are prevented by DPP‐4 inhibition in diabetic mice. Diabetes 2011; 60: 1246–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flock G, Baggio LL, Longuet C, et al Incretin receptors for glucagon‐like peptide 1 and glucose‐dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes 2007; 56: 3006–3013. [DOI] [PubMed] [Google Scholar]
- 26. Ohyama T, Sato K, Yamazaki Y, et al MK‐0626, a selective DPP‐4 inhibitor, attenuates hepatic steatosis in ob/ob mice. World J Gastroenterol 2014; 20: 16227–16235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah Z, Kampfrath T, Deiuliis JA, et al Long‐term dipeptidyl‐peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 2011; 124: 2338–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aoki K, Ijima T, Kamiyama H, et al Anagliptin decreases serum lathosterol level in patients with type 2 diabetes: a pilot study. Expert Opin Pharmacother 2015; 16: 1749–1754. [DOI] [PubMed] [Google Scholar]
- 29. Ussher JR, Drucker DJ. Cardiovascular Actions of Incretin‐Based Therapies. Circ Res 2014; 114: 1788–1803. [DOI] [PubMed] [Google Scholar]
- 30. Shinjo T, Nakatsu Y, Iwashita M, et al DPP‐4 inhibitor anagliptin exerts anti‐inflammatory effects on macrophages, adipocytes, and mouse livers by suppressing NF‐κB activation. Am J Physiol Endocrinol Metab 2015; 309: E214–E223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 ¦ Hepatic triglyceride synthesis and secretion assay.


