Abstract
Aims/Introduction
A meta‐analysis was carried out to evaluate the efficacy of yoga in adults with type 2 diabetes mellitus.
Materials and Methods
The PubMed, EMBASE and Cochrane databases were searched to obtain eligible randomized controlled trials. The primary outcome was fasting blood glucose, and the secondary outcomes included glycosylated hemoglobin A1c, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglyceride and postprandial blood glucose. Weighted mean differences and 95% confidence intervals (CIs) were calculated. The I 2 statistic represented heterogeneity.
Results
A total of 12 randomized controlled trials with a total of 864 patients met the inclusion criteria. The pooled weighted mean differences were −23.72 mg/dL (95% CI −37.78 to −9.65; P = 0.001; I 2 = 82%) for fasting blood glucose and −0.47% (95% CI −0.87 to −0.07; P = 0.02; I 2 = 82%) for hemoglobin A1c. The weighted mean differences were −17.38 mg/dL (95% CI −27.88 to −6.89; P = 0.001; I 2 = 0%) for postprandial blood glucose, −18.50 mg/dL (95% CI −29.88 to −7.11; P = 0.001; I 2 = 75%) for total cholesterol, 4.30 mg/dL (95% CI 3.25 to 5.36; P < 0.00001; I 2 = 10%) for high‐density lipoprotein cholesterol, −12.95 mg/dL (95% CI −18.84 to −7.06; P < 0.0001; I 2 = 37%) for low‐density lipoprotein cholesterol and −12.57 mg/dL (95% CI −29.91 to 4.76; P = 0.16; I 2 = 48%) for triglycerides.
Conclusions
The available evidence suggests that yoga benefits adult patients with type 2 diabetes mellitus. However, considering the limited methodology and the potential heterogeneity, further studies are necessary to support our findings and investigate the long‐term effects of yoga in type 2 diabetes mellitus patients.
Keywords: Meta‐analysis, Type 2 diabetes, Yoga
Introduction
Type 2 diabetes mellitus is one of the most frequently encountered metabolic syndromes worldwide1. The most recent meta‐analysis showed that the overall prevalence (9.1%) has been increasing among inland residents in China since the 1970s, and it increased rapidly with age2. Effective control of blood glucose to reduce the risk of various complications, including diabetic foot, diabetic neuropathy, cataract and cardiovascular disease, is especially important for type 2 diabetes mellitus management3, 4. Medication, diet and physical activity or exercise are the major components of diabetes management. Training exercises have been recommended by recent evidence‐based clinical studies as a cardinal non‐pharmacotherapy5. Numerous training programs, such as jogging, walking, swimming, housework and other outdoor exercises, have been developed. However, taking into account the increasing prevalence of obesity, and the disabilities and complications associated with a sedentary lifestyle6, 7, few patients participate in conventional physical exercise.
Yoga originated in India over 4,000 years ago as a traditional form of mind–body training that seeks to unite the individual self with the transcendental self8. Yoga asanas (postures) and pranayama (breath control) have recently become very popular, and the role of yoga in several chronic diseases, such as hypertension, asthma, chronic obstructive pulmonary disease and diabetes, has been studied8, 9, 10. Several trials have shown that yoga can reduce fasting blood glucose (FBG) and glycosylated hemoglobin A1c (HbA1c), as well as improve the lipid levels and quality of life of type 2 diabetes mellitus patients11, 12, 13, 14, 15, 16, 17, 18. However, these studies present wide variations in sample size and even inconclusive results. Other studies applied a non‐randomized study design that could affect the final outcomes11, 12, 13. Thus, in the present study, we carried out a meta‐analysis of randomized controlled trials (RCTs) to determine the effectiveness of yoga in patients with type 2 diabetes mellitus.
Materials and Methods
The current meta‐analysis was carried out according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions19, and followed Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines20.
Data sources and searches
The PubMed, Embase and Cochrane databases were searched (until April 2016) for eligible RCTs using the key words ‘yoga’ and ‘diabetes’. Eligible trials were limited to adult human subjects, and only trials published with the full text and written in English were included in this work. To ensure literature saturation, the bibliographies of all potentially eligible studies, including reference lists, citation searches and relevant systematic reviews, were searched by hand.
The available trials followed the PICOS criteria, including: (i) (P) patients: adult type 2 diabetes mellitus patients with or without chronicity and diabetes‐associated complications; (ii) (I) intervention: yoga with or without other treatments; (iii) (C) control: any type of control including usual care or standard treatment; (iv) (O) outcomes: the primary outcome was FBG and the secondary outcomes included HbA1c, postprandial blood glucose (PPBG), total cholesterol (TC), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C) and triglyceride; and (v) (S) study design: RCT.
Data extraction
Two investigators (JC and JHY) independently extracted all of the data, including the first author, publication year, country, study population and grouping (sample size per group), age, form or style of two groups, yoga protocol, duration, outcomes, study design, and Jadad scale, from the eligible RCTs. Disagreements were resolved by a third investigator (LP).
Quality and risk of bias assessment
The quality of each trial was evaluated according to the Jadad scale21. Randomization (0–2 points), blinding (0–2 points), and dropouts and withdrawals (0–1 point) were identified in the scale. A trial with a score ≤2 indicates low quality, whereas a score of ≥3 indicates high quality22. The risk of bias was assessed by the Cochrane Risk of Bias Assessment tool19.
Statistical analysis
All of the data were combined using Revman 5.3 (The Cochrane Collaboration, Oxford, UK). Weighted mean differences (WMDs) with 95% confidence intervals (CIs) for continuous variables were calculated and pooled using the random effects model23. Heterogeneity was tested using Cochrane's Q‐test and the I 2 statistic, and I 2 values >50% were considered to show significant heterogeneity24. If I 2 >50%, sensitivity analyses was carried out to explore potential sources of heterogeneity and investigate the influence of a single study on the overall pooled estimate. Combined with the demographic data of study participants included in the present study, as well as to minimize the risk of bias as a result of grouping criteria, subgroup analyses were carried out to explore potential heterogeneity and examine the influence of various exclusion criteria on the basis of sample size (>60 vs ≤60), Jadad score (>2 vs ≤2), duration (>3 months vs ≤3 months) and region (India vs non‐India). Publication bias was assessed using Stata version 12.0 (Stata Corporation LP, College Station, TX, USA), and results were analyzed using Begg's and Egger's test25. Finally, two‐sided P‐values <0.05 were considered to show statistical significance.
Results
Search results and study characteristics
Initially, 189 potential studies were retrieved from the electronic databases. After reviewing their titles and abstracts, 164 studies were excluded because they were unrelated to the aims of the present study. Another 13 candidate studies were excluded for various reasons (Figure 1). Finally, 12 RCTs were selected for the present meta‐analysis14, 15, 16, 17, 18, 26, 27, 28, 29, 30, 31, 32.
Figure 1.
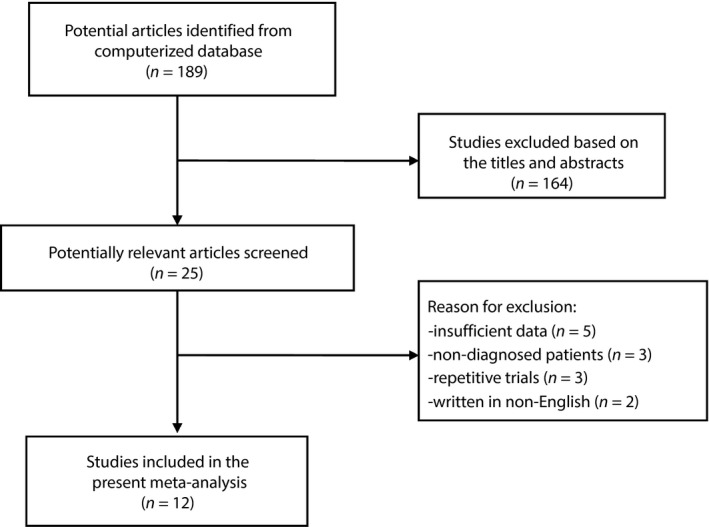
Search strategy and flow chart for this meta‐analysis.
The main characteristics of 12 RCTs involving 864 patients are summarized in Table 1. All RCTs were made available in English between 1992 and 2014. The total sample size ranged from 20 to 277. A total of 11 RCTs were carried out in four countries, including the UK15, 27, India14, 16, 28, 29, 30, 31, 32, Cuba17, 18 and Iran26. Two RCTs were carried out by Gordon et al.17, 18 on the same study population, and another two RCTs were carried out by Shantakumari et al.16, 30, also on the same study population. Follow‐up periods varied from 15 days to 9 months. All RCTs applied different yoga protocols with different exercise times and times per session. Furthermore, Table S1 shows additional information reported in all the randomized controlled trials.
Table 1.
Characteristics of randomized controlled trials included in the meta‐analysis
| First author, year and country | Study population | Study group (sample size) | Mean age, years (I/C) | Form or style (I/C) | Yoga protocol | Duration | Outcomes | Study design/Jadad score | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Control group | ||||||||
| Gordon, 2008, 2008, Cuba | 154 patients without complications or malnutrition, trained for T2DM self‐care; 81% F | Yoga (77); Control (77) | 64.0/63.6 | Hatha yoga (pranayamas, dynamic warm‐up exercises, asanas, and savasana) | Usual care (a treatment plan as per their doctors, no active exercise) | 1 class/week, 2‐h class | 6 months | FBG, Lipid, HbA1c | RCT/4 |
| Habibi, 2013, Iran | 26 female patients without taking insulin | Yoga (16); Control (10) | Age range: 45‐60 | Asana and pranayama exercise | Standard care | 3 sessions/week, 75 min/session | 3 months | FBG | RCT/2 |
| Jyotsna, 2014, India | 120 patients with lifestyle modification and oral antidiabetic medication | Yoga (64); Control (56) | 49.92/47.25 | Sudarshan Kriya Yoga + standard treatment | Standard treatment | 3‐day group training followed by classes 1x/week and daily home practice with a total of 25–35 min | 6 months | FBS, PPBG, HbA1c | RCT/3 |
| Monro, 1992, UK | 21 patients with taking medication (13) or on diet control alone (8) | Yoga (11); Control (10) | Age range: 45‐67 | Yoga classes (pranayama, shavasana and asanas) + standard care | Standard care (continuing on medication, diet) | 1‐2 classes/wk + 90 min, 1–5 times/wk at home | 3 months | FBG, HbA1c | RCT/2 |
| Nagarathna, 2012, India | 277 patients with stable dose of oral hypoglycemic agents or insulin for at least 3 wks; 31% F | Yoga (141); Control (136) | 53.46/51.38 | Integrated yoga (yogasanas, pranayama, meditation and lectures on yogic life style) | Physical exercises and life style education. | 1 h/day, 5 days/week for 12 weeks, and then one 2 h class/week and 1 h daily home practice | 9 months | Lipid, FBG, PPBG, HbA1c | RCT/4 |
| Pardasany, 2010, India | 30 patients taking hypoglycemic medications; 38% F | Yoga (15); Control (15) | Age range: 40‐60 | Hatha yoga (asanas and pranayamas) | Oral hypoglycemic medications | 3 times/week | 3 months | FBG, PPBG, Lipid, HbA1c | RCT/2 |
| Shantakumari, 2013,2012, India | 100 patients with hypertension and dyslipidemia; 48% F | Yoga (50); Control (50) | 45.51/44.46 | Asana, pranayama and meditation + standard treatment | Standard treatment (oral hypoglycemic drugs) | Daily for 1 h duration | 3 months | FBG, PPBG, Lipid, | RCT/2 |
| Subramaniyan, 2012, India | 20 adult males patients | Yoga (10); Control (10) | Age range of 55% patients: 31‐40 | Yogic exercises | Brisk walking + routine medicines | 60 min daily between 6AM and 7AM for 15 consecutive days | 15 days | FBG | RCT/3 |
| Skoro‐Kondza, 2009, UK | 59 patients without taking insulin but receiving advice and leaflets on healthy lifestyle and exercise; 61% F | Yoga (29); Control (30) | Total mean age: 60 | Yoga classes (pranayama, gentle stretching and asanas) | Wait list | Twice‐weekly, 90‐min class | 3 months | HbA1c | RCT/3 |
| Vaishali, 2012, India | 57 elderly patients; 36.8% F | Yoga (27); Control (30) | 65.8/64.4 | Individualized yoga asanas and pranayama + educational intervention | Educational Intervention (general healthy lifestyle and exercise) | 6 days a week, 45–60 min/session | 3 months | FBG, Lipid, HbA1c | RCT/3 |
F, female; FBG, fasting blood glucose; FBS, fasting blood sugar; HbA1c, glycosylated hemoglobin A1c; I/C, intervention/control; RCT, randomized controlled trial; PPBG, postprandial blood glucose; T2DM, type 2 diabetes mellitus.
Quality and risk of bias assessment
Two investigators (JC and JHY) agreed on each item of the Jadad score and Cochrane Risk of Bias Assessment tool. The mean Jadad score of the 12 RCTs was 2.8 (SD = 0.8). Risk‐of‐bias assessment showed that all RCTs generated low risk in terms of random sequence generation. None of the trials was double‐blinded, and just three RCTs were single‐blinded17, 18, 28. Details of the quality and risk‐of‐bias assessment of all of the RCTs are shown in Table 1 and Figure S1, respectively.
Meta‐analyses of primary outcome
Nine RCTs reported FBG as a primary outcome14, 17, 26, 27, 28, 29, 30, 31, 32. The pooled WMDs were −23.72 mg/dL (95% CI −37.78 to −9.65; P = 0.001; P for heterogeneity <0.00001; I 2 = 82%) for FBG (Figure 2). Heterogeneity was clearly significant for the primary end‐point. We carried out sensitivity analyses to investigate the potential sources of heterogeneity. However, regardless of which study was excluded from our analysis, the source of heterogeneity was not observed and the overall combined WMDs, which ranged from −27.90 mg/dL (95% CI −41.84 to −13.96; P < 0.0001) to −20.61 mg/dL (95% CI −33.99 to −7.23; P = 0.003), were not significantly altered. Next, we carried out subgroup analyses to examine the influence of various exclusion criteria with respect to FBG according to sample size (>60 vs ≤ 60), Jadad score (>2 vs ≤2), duration (>3 months vs ≤3 months) and region (India vs non‐India). The detailed results are shown in Table 2. We found that the overall combined effects of the trials, regardless of their quality, sample size or follow‐up period, were poor. Furthermore, non‐Indian patients might benefit from yoga more than Indian patients.
Figure 2.
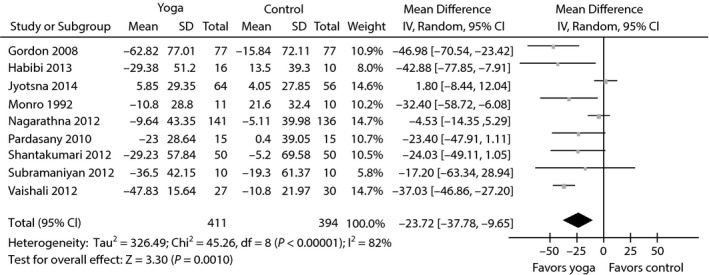
Forest plots of evaluating the effect of yoga on fasting blood glucose.
Table 2.
Subgroup analyses based on various exclusion criteria for fasting blood glucose
| Various exclusion criteria | n (N) | WMDs, mg/dL (95% CI) | P‐value | I 2 (%) | P heterogeneity |
|---|---|---|---|---|---|
| All included trials38, 39, 40, 41, 42, 43, 44, 45, 46 | 805 (9) | −23.72 (−37.78 to −9.65) | 0.001 | 82 | <0.00001 |
| Jadad scores ≥3 | 628 (5) | −19.96 (−40.02 to 0.09) | 0.05 | 90 | <0.00001 |
| Jadad scores ≤2 | 177 (4) | −28.82 (−42.29 to −15.36) | <0.0001 | 0 | 0.80 |
| Sample sizes >60 | 651 (4) | −15.16 (−32.37 to 2.04) | 0.08 | 81 | 0.001 |
| Sample sizes ≤60 | 154 (5) | −34.73 (−42.97 to −26.50) | <0.00001 | 0 | 0.77 |
| Duration >3 months | 551 (3) | −13.19 (−32.99 to 6.60) | 0.19 | 86 | 0.001 |
| Duration ≤3 months | 254 (6) | −33.69 (−41.51 to −25.87) | <0.00001 | 0 | 0.78 |
| Region (India) | 604 (6) | −16.70 (−33.15 to −0.25) | 0.05 | 86 | <0.00001 |
| Region (non‐India) | 201 (3) | −40.97 (−56.66 to −25.28) | <0.00001 | 0 | 0.72 |
CIs, confidence intervals; n, number of patients; N, number of trials; WMDs, weighted mean differences.
Meta‐analyses of secondary outcomes
The aggregated results suggested that the WMDs were –0.47% (95% CI −0.87 to −0.07; P = 0.02; P for heterogeneity <0.00001; I 2 = 82%) for HbA1c (Figure 3a), −17.38 mg/dL (95% CI −27.88 to −6.89; P = 0.001; P for heterogeneity = 0.73; I 2 = 0%) for PPBG (Figure 3b), −18.50 mg/dL (95% CI −29.88 to −7.11; P = 0.001; P for heterogeneity = 0.003; I 2 = 75%) for TC (Figure 4), −12.95 mg/dL (95% CI −18.84 to −7.06; P < 0.0001; P for heterogeneity = 0.18; I 2 = 37%) for LDL‐C (Figure 4), −12.57 mg/dL (95% CI −29.91 to 4.76; P = 0.16; P for heterogeneity = 0.12; I 2 = 48%) for triglycerides (Figure 4) and 4.30 mg/dL (95% CI 3.25 to 5.36; P < 0.00001; P for heterogeneity = 0.34; I 2 = 10%) for HDL‐C (Figure 5).
Figure 3.
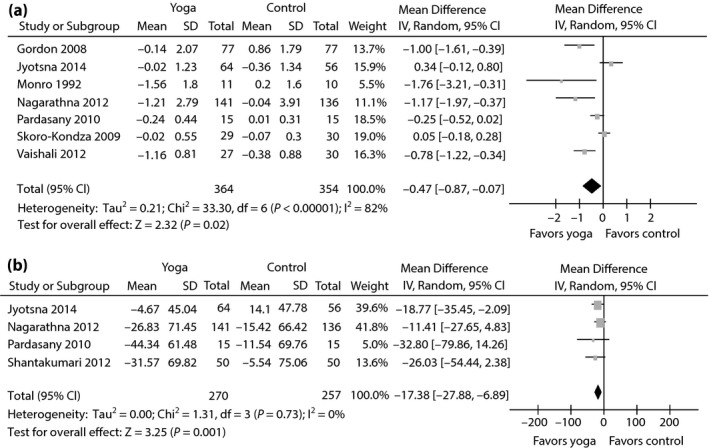
Forest plots of evaluating the effect of yoga on (a) glycosylated hemoglobin A1c and (b) postprandial blood glucose.
Figure 4.
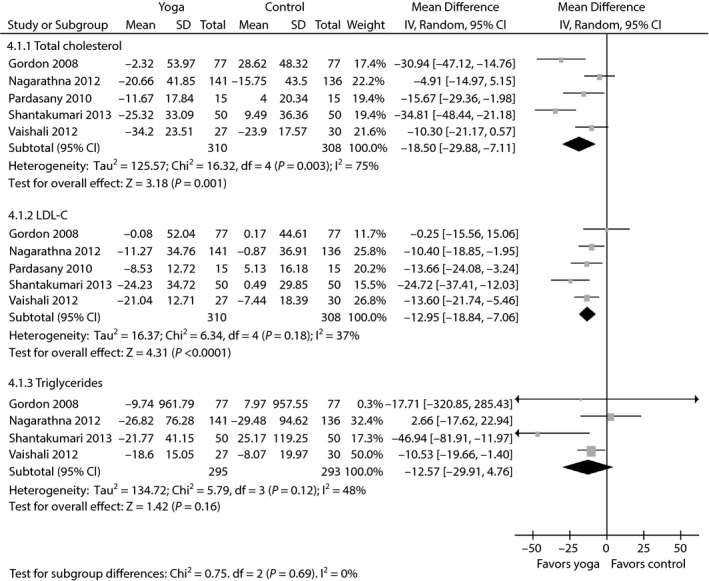
Forest plots of evaluating the effect of yoga on total cholesterol, low‐density lipoprotein cholesterol (LDL‐C), and triglyceride.
Figure 5.
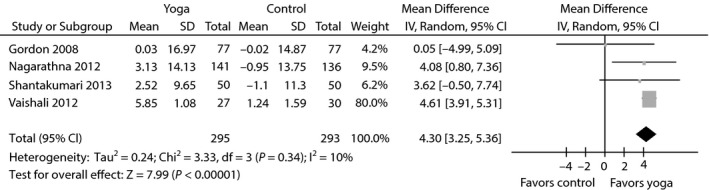
Meta‐analysis of evaluating the effect of yoga on high‐density lipoprotein cholesterol.
Publication bias
Publication bias is shown in Figure 6. The results of the Begg's and Egger's tests suggested that no evidence of publication bias was found from funnel plots and associated statistics for FBG (P Begg = 0.917; P Egger = 0.328).
Figure 6.
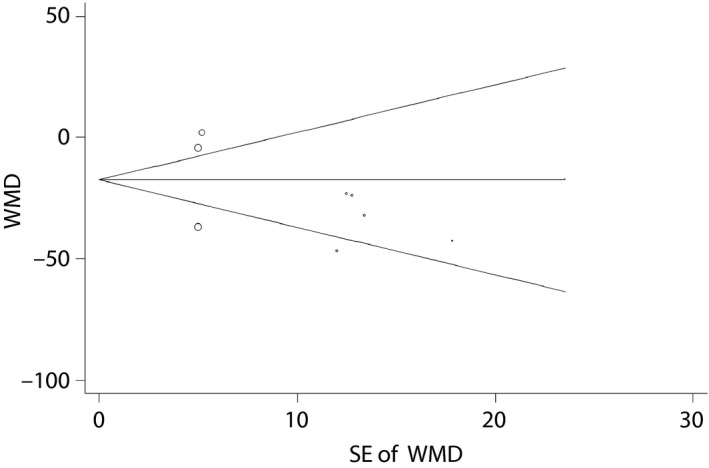
Publication bias. Begg's funnel plot of pseudo 95% confidence intervals. SE, standard error; WMD, weighted mean differences.
Discussion
The aim of the present meta‐analysis of the existing data is to quantitatively assess the role of yoga in patients with type 2 diabetes mellitus. The available evidence from 12 RCTs with a total of 864 patients suggested that yoga can significantly decrease patient FBG, PPBG, HbA1c, TC and LDL‐C levels, and increase their HDL‐C.
Several systematic reviews focusing on yoga for adult patients with type 2 diabetes mellitus have been published33, 34, 35, 36. Although differences between our meta‐analysis and these previous studies can be noted, our principal findings are consistent with the published results. Three studies carried out by Innes et al.33, 34, 35 were mainly narrative reviews. Although a recent systematic review34 also meta‐analyzed several clinical endpoints, including glucose control, lipid levels and body composition, only studies reporting significant changes were included in that work. We believe that pooled results are not suitable for inclusion in the present study because, they can lead to selection bias, and affect the objectivity and authenticity of our findings. Additionally, another previous systematic review enrolling just five RCTs with a total of 362 participants was published in 200836. In comparison with that review, the present meta‐analysis included 12 RCTs with a total of 864 patients. Considering the limited data on the topic, we combined existing RCTs to increase the sample size, strengthen our analyses and produce more robust results.
The present results showed that yoga significantly decreased FBG, PPBG, HbA1c, TC and LDL‐C levels, and increased HDL‐C in patients with type 2 diabetes mellitus. Significant heterogeneity was noted during our analyses of FBG and HbA1c. Given that we specified the primary end‐point was FBG, sensitivity and subgroup analyses were carried out to explore the potential sources of heterogeneity for FBG. We found that exclusion of each of the RCTs considered in this work did not resolve the heterogeneity issue or materially alter the overall combined FBG. We thus believe that the heterogeneity observed across trials could be viewed as a result of clinical and methodological differences. Subgroup analyses were carried out to investigate the impact of various exclusion criteria according to sample size, Jadad score, duration and region. The overall combined effects of the trials, regardless of their quality, sample size or follow‐up period, were poor. Furthermore, non‐Indian patients might benefit from yoga more than Indian patients. The exact reason is still unknown, and it might be related to racial differences, and different diet and lifestyle habit, and also might be derived from the limited data or other related bias, such as the existing heterogeneity. Thus, we believe that robust, well‐designed and larger‐scale trials should be carried out to substantiate the long‐term effects of yoga in type 2 diabetes mellitus patients, especially those who are not from India.
Combining the present results with those reported in the related literature, the following considerations might help direct future clinical research on the effects of yoga on type 2 diabetes mellitus management. First, exercise is a key factor for diabetes management. As the optimal exercise form and appropriate exercise parameters for type 2 diabetes mellitus patients are unknown, the development of exercise regimens for these patients seems to be warranted. Second, the aspects of yoga that benefit patients with type 2 diabetes mellitus remain unknown, and objective outcome measurements, such as peripheral nerve modulation, quality of life, blood pressure, overall survival, inflammatory mediators and immune cell function, especially at the cellular and molecular levels, are not carried out in most studies. Therefore, further research should focus on improving measurement modalities to better address potential mechanisms and obtain more reliable evidence of the role of yoga‐based training in type 2 diabetes mellitus patients. Third, the follow‐up periods of the RCTs included in the present study ranged from 15 days to 9 months, and the long‐term effects of yoga remain unknown. Most of the RCTs included in the present study were not blinded. Considering that blinding prevents bias and protects the sequence after allocation37, appropriate blinding, such as blind‐outcome assessments, should be carried out.
Compared with previous reviews, our meta‐analysis was carried out in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines and the Cochrane Collaboration, which is one of its main strengths. Another major strength is that we enrolled well‐designed RCTs with relatively large sample sizes and performed subgroup analyses according to various exclusion criteria, including sample size, Jadad score, duration and region, thereby improving the critical significance of the present findings for clinical practice.
When interpreting the results, several limitations should be taken into account: (i) different characteristics of study participants, yoga forms and protocols, and exercise durations are the most crucial confounders of the RCTs, and could result in risk of bias and heterogeneity; (ii) except for three RCTs17, 18, 28, all other RCTs surveyed were not blinded, which could result in performance and detection bias; (iii) considering that 12 RCTs with a wide variation in sample size were incorporated into our analysis, the effects of overestimation of treatment efficiency may be significant; and (iv) missing and unpublished data, as well as the exclusion of non‐English language studies, could result in effect size bias.
In sum, based on the evidence, yoga significantly reduces FBG levels and alters other significant clinical outcomes in patients with type 2 diabetes mellitus. These results support the idea that yoga‐based training is a possible alternative exercise for type 2 diabetes mellitus management. However, given the aforementioned limitations and potential bias of our analyses, more large‐scale and robust RCTs must be carried out to verify our current findings and substantiate the long‐term effects of yoga in type 2 diabetes mellitus patients.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1| Additional information reported in all the randomized controlled trials.
Figure S1| Risk‐of‐bias analysis. (a) Risk‐of‐bias graph: the authors’ judgments about each risk‐of‐bias item presented as percentages across all included studies. (b) Risk‐of‐bias summary: the authors’ judgments about each risk‐of‐bias item for the each included studies.
J Diabetes Investig 2017; 8: 201–209
References
- 1. Ali S, Davies MJ, Brady EM, et al Guidelines for managing diabetes in Ramadan. Diabet Med 2016. doi: 10.1111/dme.13080. [DOI] [PubMed] [Google Scholar]
- 2. Yang L, Shao J, Bian Y, et al Prevalence of type 2 diabetes mellitus among inland residents in China (2000–2014): a meta‐analysis. J Diabetes Investig 2016; 7: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joseph JJ, Golden SH. Type 2 diabetes and cardiovascular disease: what next? Curr Opin Endocrinol Diabetes Obes 2014; 21: 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Standards of medical care in diabetes–2014. Diabetes Care 2014; 37(Suppl 1): S14–S80. [DOI] [PubMed] [Google Scholar]
- 5. Colberg SR, Sigal RJ, Fernhall B, et al Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 2010; 33: e147–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Temelkova‐Kurktschiev T, Stefanov T. Lifestyle and genetics in obesity and type 2 diabetes. Exp Clin Endocrinol Diabetes 2012; 120: 1–6. [DOI] [PubMed] [Google Scholar]
- 7. Lin CC, Li CI, Liu CS, et al Impact of lifestyle‐related factors on all‐cause and cause‐specific mortality in patients with type 2 diabetes: the Taichung Diabetes Study. Diabetes Care 2012; 35: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu XC, Pan L, Hu Q, et al Effects of yoga training in patients with chronic obstructive pulmonary disease: a systematic review and meta‐analysis. J Thorac Dis 2014; 6: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raub JA. Psychophysiologic effects of Hatha Yoga on musculoskeletal and cardiopulmonary function: a literature review. J Altern Complement Med 2002; 8: 797–812. [DOI] [PubMed] [Google Scholar]
- 10. Chandler K. The emerging field of yoga therapy. Hawaii Med J 2001; 60: 286–287. [PubMed] [Google Scholar]
- 11. Singh S, Kyizom T, Singh KP, et al Influence of pranayamas and yoga‐asanas on serum insulin, blood glucose and lipid profile in type 2 diabetes. Indian J Clin Biochem 2008; 23: 365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kyizom T, Singh S, Singh KP, et al Effect of pranayama & yoga‐asana on cognitive brain functions in type 2 diabetes‐P3 event related evoked potential (ERP). Indian J Med Res 2010; 131: 636–640. [PubMed] [Google Scholar]
- 13. Popli U, Subbe CP, Sunil K. Research letter‐the role of yoga as a lifestyle modification in treatment of diabetes mellitus: results of a pilot study. Altern Ther Health Med 2014; 20: 24–26. [PubMed] [Google Scholar]
- 14. Jyotsna VP, Dhawan A, Sreenivas V, et al Completion report: Effect of Comprehensive Yogic Breathing program on type 2 diabetes: A randomized control trial. Indian J Endocrinol Metab 2014; 18: 582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skoro‐Kondza L, Tai SS, Gadelrab R, et al Community based yoga classes for type 2 diabetes: an exploratory randomised controlled trial. BMC Health Serv Res 2009; 9: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shantakumari N, Sequeira S, El deeb R. Effects of a yoga intervention on lipid profiles of diabetes patients with dyslipidemia. Indian Heart J 2013; 65: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gordon LA, Morrison EY, McGrowder DA, et al Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. BMC Complement Altern Med 2008; 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gordon L, Morrison EY, McGrowder DA, et al Changes in clinical and metabolic parameters after exercise therapy in patents with type 2 diabetes. Arch Med Sci 2008; 4: 427–437. [Google Scholar]
- 19. Higgins JP, Altman DG, Gotzsche PC, et al The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jadad AR, Moore RA, Carroll D, et al Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 22. Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Ann Intern Med 2001; 135: 982–989. [DOI] [PubMed] [Google Scholar]
- 23. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG, Deeks JJ, et al Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egger M, Davey Smith G, Schneider M, et al Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Habibi N, Farsani Z, Yazdani B, et al The influence of yoga on risk profiles programs in women with diabetes type II. Adv Environ Biol 2013; 7: 550–555. [Google Scholar]
- 27. Monro R, Power J, Coumar A, et al Yoga therapy for NIDDM: a controlled trial. Complement Med Res 1992; 6: 66–68. [Google Scholar]
- 28. Nagarathna R, Usharani MR, Rao AR, et al Efficacy of yoga based life style modification program on medication score and lipid profile in type 2 diabetes‐a randomized control study. Int J Diabetes Dev Ctries 2012; 32: 122–130. [Google Scholar]
- 29. Vaishali K, Kumar KV, Adhikari P, et al Effects of yoga‐based program on glycosylated hemoglobin level serum lipid profile in community dwelling elderly subjects with chronic type 2 diabetes mellitus–a randomized controlled trial. Phys Occup Ther in Geriatr 2012; 30: 22–30. [Google Scholar]
- 30. Shantakumari N, Sequeira S, Eldeeb R. Effect of a yoga intervention on hypertensive diabetic patients. J Adv Intern Med 2012; 1: 60–63. [Google Scholar]
- 31. Subramaniyan TG, Subramaniyan N, Chidambaram M. Brisk walking and yoga as adjuvant therapy in management of type 2 diabetes mellitus. Int J Stud Res 2012; 2: 43–46. [Google Scholar]
- 32. Pardasany A, Shenoy S, Sandhu JS. Comparing the efficacy of tai chi chuan and hatha yoga in type 2 diabetes mellitus patients on parameters of blood glucose control and lipid metabolism. Indian J Physiother Occup Ther 2010; 4: 11–16. [Google Scholar]
- 33. Innes KE, Vincent HK. The influence of yoga‐based programs on risk profiles in adults with type 2 diabetes mellitus: a systematic review. Evid Based Complement Alternat Med 2007; 4: 469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Innes KE, Selfe TK. Yoga for Adults with Type 2 Diabetes: A Systematic Review of Controlled Trials. J Diabetes Res 2016; 2016: 6979370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de GR Hansen E, Innes KE. The benefits of yoga for adults with type 2 diabetes: a review of the evidence and call for a collaborative, integrated research initiative. Int J Yoga Therap 2013; 23: 71–83. [PubMed] [Google Scholar]
- 36. Aljasir B, Bryson M, Al‐Shehri B. Yoga Practice for the Management of Type II Diabetes Mellitus in Adults: A systematic review. Evid Based Complement Alternat Med 2010; 7: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet 2002; 359: 696–700. [DOI] [PubMed] [Google Scholar]
- 38. Rennard SI, Calverley PM, Goehring UM, et al Reduction of exacerbations by the PDE4 inhibitor roflumilast–the importance of defining different subsets of patients with COPD. Respir Res 2011; 12: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rabe KF, Bateman ED, O'Donnell D, et al Roflumilast–an oral anti‐inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005; 366: 563–571. [DOI] [PubMed] [Google Scholar]
- 40. Calverley PM, Sanchez‐Toril F, McIvor A, et al Effect of 1‐year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 176: 154–161. [DOI] [PubMed] [Google Scholar]
- 41. Lee SD, Hui DS, Mahayiddin AA, et al Roflumilast in Asian patients with COPD: a randomized placebo‐controlled trial. Respirology 2011; 16: 1249–1257. [DOI] [PubMed] [Google Scholar]
- 42. Calverley PM, Rabe KF, Goehring UM, et al Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009; 374: 685–694. [DOI] [PubMed] [Google Scholar]
- 43. Fabbri LM, Calverley PM, Izquierdo‐Alonso JL, et al Roflumilast in moderate‐to‐severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009; 374: 695–703. [DOI] [PubMed] [Google Scholar]
- 44. O'Donnell DE, Bredenbroker D, Brose M, et al Physiological effects of roflumilast at rest and during exercise in COPD. Eur Respir J 2012; 39: 1104–1112. [DOI] [PubMed] [Google Scholar]
- 45. Turk DC. Clinical effectiveness and cost‐effectiveness of treatments for patients with chronic pain. Clin J Pain 2002; 18: 355–365. [DOI] [PubMed] [Google Scholar]
- 46. Manninen P, Riihimaki H, Heliovaara M, et al Physical exercise and risk of severe knee osteoarthritis requiring arthroplasty. Rheumatology (Oxford) 2001; 40: 432–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1| Additional information reported in all the randomized controlled trials.
Figure S1| Risk‐of‐bias analysis. (a) Risk‐of‐bias graph: the authors’ judgments about each risk‐of‐bias item presented as percentages across all included studies. (b) Risk‐of‐bias summary: the authors’ judgments about each risk‐of‐bias item for the each included studies.


