Abstract
Aims/Introduction
To investigate whether donor liver steatosis increases the incidence of new‐onset diabetes after transplantation (NODAT) in liver transplant recipients.
Materials and Methods
We retrospectively analyzed liver transplant recipients at Zhongshan Hospital, Shanghai, China, from April 2001 to December 2014. The final analysis involved 763 patients. The cumulative incidence of NODAT at 1, 3, 5 and 10 years after liver transplantation was investigated. Furthermore, according to the findings of donor liver biopsy before transplantation, patients were divided into steatotic and non‐steatotic donor liver groups, and NODAT incidence was compared between these groups. Multivariate Cox regression was used to explore the risk factors for NODAT in the patients.
Results
Of the 763 donors, 309 (40.5%) had liver steatosis. At the end of follow up, 130 (42.1%) patients in the steatotic donor liver group developed NODAT, an incidence that exceeded that in the non‐steatotic donor liver group (P = 0.001). The cumulative incidence of NODAT among all patients at 1, 3, 5, and 10 years after transplantation was 33, 43, 50 and 56%, respectively. The cumulative incidences of NODAT at 1, 3, 5 and 10 years in the steatotic donor liver group were significantly higher than those in the non‐steatotic donor liver group (P = 0.003). Multivariate Cox regression analyses showed that donor liver steatosis was an independent risk factor for NODAT among liver transplant recipients, after other potential risk factors were adjusted for (hazard ratio 1.774, 95% confidence interval: 1.025–3.073; P = 0.041).
Conclusions
Donor liver steatosis increases NODAT incidence among liver transplant recipients.
Keywords: Donor liver steatosis, Liver transplantation, New‐onset diabetes after transplantation
Introduction
Liver transplantation is the treatment of choice for patients with severe liver failure, end‐stage liver disease and certain metabolic liver diseases for which no alternative therapies are available. As perioperative complications and problems of rejection have been mostly resolved, long‐term survival after liver transplantation has come to increasingly depend on the presence and types of comorbidities. New‐onset diabetes after transplantation (NODAT) is one of the most common long‐term complications in liver transplant recipients. It affects the cardiovascular and neuropsychiatric systems, and increases susceptibility to infections, thereby reducing quality of life and increasing mortality rates1, 2, 3, 4, 5, 6, 7, 8, 9, 10. Previous studies have shown that race, older age, hepatitis C virus infection, family history of diabetes, obesity, preoperative impaired glucose regulation, triglyceride levels, acute rejection (AR), cytomegalovirus infection, liver cirrhosis and immunosuppressant use are risk factors for NODAT11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27.
In recent decades, non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis have become increasingly common worldwide. These are serious health‐related conditions associated with obesity, type 2 diabetes, hypertension and hyperlipidemia28. These conditions also affect the donor population, and the use of steatotic donor livers for liver transplantation might yield poor outcomes29. In the present study, we examined the effect of donor liver steatosis on NODAT among liver transplant recipients at Zhongshan Hospital, Shanghai, China, between April 2001 and December 2014.
Materials and Methods
Participants
We collected hospitalization and outpatient data for patients undergoing liver transplantation between April 2001 and December 2014 at the Liver Transplant Center of Zhongshan Hospital or through clinic follow up and telephone calls. The study was approved by the institutional review board of Zhongshan Hospital, Fudan University, and all participants provided informed consent. Patients who aged were less than 18 years, diabetic before surgery, used glucocorticoids before surgery, did not have complete data, underwent a second transplantation or died within 3 months after surgery were excluded. The preoperative data recorded for patients included age; sex; body mass index (BMI); hepatitis virus infection; history of hypertension; preoperative liver function; the model for end‐stage liver disease score; preoperative levels of fasting plasma glucose (FPG), total cholesterol and total triglycerides; and severity of liver cirrhosis. Additionally, we also reviewed the donor type (living or cadaver) and biopsy results of the donor liver. Postoperative data regarding AR, initial immunosuppressant regimen and the blood levels of immunosuppressant at different points in the patients were also collected. End‐points were defined as death, graft failure and diabetes.
Immunosuppressant regimen
Most patients received standard triple immunosuppressive treatment with corticosteroids, tacrolimus (FK506) and mycophenolate mofetil; FK506 was switched to cyclosporine‐A (CsA) in the event of mental symptoms, and rapamycin (RAP) was used when the plasma FK506 concentration was not adequate or if renal dysfunction occurred. Some patients received dual immunosuppressive treatment with FK506/CsA and corticosteroids. The initial corticosteroid dose was 1 g intravenous methylprednisolone on the day of surgery and 360 mg on the day after surgery, or 500 mg intravenous methylprednisolone on the day of surgery and 240 mg on the day after surgery. Thereafter, the dose was tapered every day by 40 mg to reach 20 mg oral prednisone daily, and it generally was stopped within 3 months. The doses of FK506, CsA and RAP were adjusted to maintain target blood concentrations, which varied at different points after liver transplantation, as follows: 8–12 ng/mL, 900–1,100 ng/mL and 5–10 ng/mL within 3 months after liver transplantation; 6–10 ng/mL, 600–1,000 ng/mL, and 6–8 ng/mL between 3 and 12 months after liver transplantation; and 4–6 ng/mL, 500–600 ng/mL and 4–6 ng/mL beyond 12 months after liver transplantation, respectively. Because target blood concentrations of immunosuppressant more than 36 months were lower, the duration of evaluation for immunosuppressant concentrations more than 12 months ranged from 12 months to 36 months after transplantation. As an induction agent and to prevent acute rejection, interleukin‐2 receptor antagonists were added from the year 2006. Most patients received basiliximab induction, which was given in doses of 20 mg each on the day of surgery, and on the fourth day after liver transplantation. All patients with hepatitis B virus infection received lamivudine and intravenous low‐dose hepatitis B immunoglobulin after liver transplantation. Treatment was clinically modified in the presence of significant rejection.
Measurements and definitions
We examined BMI, as it is the recommended means to categorize weight relative to height for adults. Hypertension was defined as a usual blood pressure of 140/90 mmHg or higher. Patients who had normal blood pressure, but were using blood pressure medication, were diagnosed with hypertension. We assessed liver function on the basis of Child–Pugh classification, and defined whether liver transplant recipients suffered from cirrhosis according to postoperative pathological diagnosis.
Steatosis of the donor liver was diagnosed when a degree of steatosis ≥5% was determined in donor liver biopsy examination before transplantation30. Donors with a degree of steatosis ≥30% were excluded. A degree of steatosis >5% and <10% was defined as mild steatosis, and a degree of steatosis ≥10% was defined as moderate‐to‐severe steatosis. NODAT was diagnosed on the basis of the 2014 American Diabetes Association criteria. Post‐transplantation fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) was considered to indicate NODAT. Fasting was defined as no caloric intake for at least 8 h. Each criterion was confirmed by repeated testing31. Patients who had normal glucose levels, but were using antidiabetic medication or insulin, were diagnosed with diabetes. Given the stress period after surgery, fasting plasma glucose levels from 1 month after liver transplantation were considered for diagnosis.
Blood glucose management
All liver transplant recipients used micro‐insulin pumps within 3–5 days after transplantation. Thereafter, subcutaneous insulin injections were used to control blood glucose. The insulin dose was adjusted according to the blood glucose levels. The target levels of FPG and 2‐h postprandial plasma glucose were 8–10 mmol/L and 8–14 mmol/L, respectively.
Statistical analysis
Patients were categorized into two groups: those who received steatotic donor livers and those who received non‐steatotic donor livers. The demographic profiles, clinical parameters and immunosuppressive regimen were compared between these groups. Normally distributed variables are shown as mean ± standard deviation. Non‐parametric, χ2 or Fisher's exact tests were used to analyze categorical variables. The cumulative incidence of NODAT in the groups was assessed using the life table, and whether donor liver steatosis predicted NODAT was assessed using a Kaplan–Meier survival analysis. Multivariate Cox regression analysis was used to determine the effect of donor liver steatosis on NODAT. Results are expressed as hazard ratios (HRs) with associated 95% confidence intervals (CIs). P‐values <0.05 were considered statistically significant. All statistical analyses were carried out using Spss 20.0 (SPSS Inc, Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA).
Results
A total of 1,289 patients underwent liver transplantation between April 2001 and December 2014 at our hospital, and the final study cohort consisted of 763 liver transplant recipients (Figure 1). The mean follow‐up duration was 2.56 ± 0.12 years. A total of 309 patients received steatotic donor livers, including 160 mild steatotic donor livers and 149 moderate‐to‐severe steatotic donor livers; 454 received non‐steatotic donor livers. At the end of follow up, 266 liver transplantation patients developed NODAT and 155 patients died.
Figure 1.
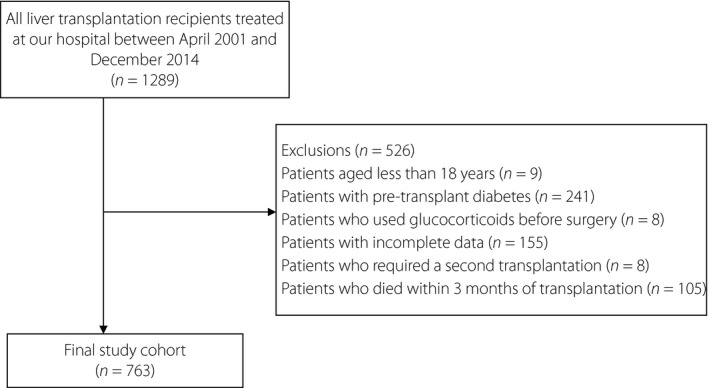
Flow diagram showing patient selection and the final patient cohort after exclusion criteria were applied.
Patient characteristics
Table 1 shows the preoperative baseline characteristics of the patients by group. Age; sex; hepatitis C virus and HBV infection; preoperative liver function; model for end‐stage liver disease score; history of hypertension; preoperative levels of FPG, total cholesterol and total triglycerides; and severity of liver cirrhosis were similar (P > 0.05 for all). However, BMI and donor type differed significantly (P < 0.05 for both). Table 2 shows the postoperative patient characteristics by group. The results showed that the frequency of AR and RAP being the initial immunosuppressant did not differ significantly between the groups (P > 0.05 in both cases). However, the frequency of FK506 and CsA being the initial immunosuppressant did (P < 0.05 in both cases). The levels of FK506, CsA and RAP at different points after liver transplantation were compared between the groups (Figure 2), but showed no significant differences (P > 0.05 in all cases).
Table 1.
Preoperative baseline characteristics of patients in the steatotic and non‐steatotic donor liver groups
| Overall (n = 763) | Steatotic donor liver (n = 309) | Non‐steatotic donor liver (n = 454) | P‐value | |
|---|---|---|---|---|
| Age (years) | 48.78 ± 10.23 | 49.61 ± 9.63 | 48.22 ± 10.59 | 0.064 |
| Male | 651 (85.3%) | 264 (85.4%) | 387 (85.2%) | 0.941 |
| Living donor | 24 (3.1%) | 2 (0.6%) | 22 (4.8%) | 0.001* |
| HBV infection | 666 (87.3%) | 263 (85.1%) | 403 (88.8%) | 0.137 |
| HCV infection | 23 (3.0%) | 11 (3.6%) | 12 (2.6%) | 0.467 |
| BMI (kg/m2) | 23.06 ± 2.40 | 23.28 ± 2.28 | 22.91 ± 2.48 | 0.037* |
| History of hypertension | 72 (9.4%) | 29 (9.4%) | 43 (9.5%) | 0.968 |
| Preoperative liver function (Child–Pugh) | ||||
| A | 465 (60.9%) | 177 (57.3%) | 288 (63.4%) | 0.059 |
| B | 202 (26.5%) | 90 (29.1%) | 112 (24.7%) | |
| C | 96 (12.6%) | 42 (13.6%) | 54 (11.9%) | |
| MELD score | 12.13 ± 6.10 | 12.58 ± 6.14 | 11.82 ± 6.06 | 0.113 |
| Preoperative FPG (mmol/L) | 4.94 ± 0.79 | 5.00 ± 0.81 | 4.90 ± 0.77 | 0.104 |
| Preoperative TC (mmol/L) | 3.88 ± 0.90 | 3.82 ± 0.81 | 3.92 ± 0.96 | 0.170 |
| Preoperative TG (mmol/L) | 1.03 ± 0.37 | 1.03 ± 0.37 | 1.03 ± 0.37 | 0.748 |
| Preoperative liver cirrhosis | ||||
| No | 71 (9.3%) | 25 (8.1%) | 46 (10.1%) | 0.341 |
| Yes | 692 (90.7%) | 284 (91.9%) | 408 (89.9%) | |
Data are mean ± standard deviation or n (%). P‐values refer to comparisons between steatotic and non‐steatotic donor liver groups. *P < 0.05. BMI, body mass index; FPG, fasting plasma glucose; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, model for end‐stage liver disease; TC, total cholesterol; TG, total triglycerides.
Table 2.
Postoperative characteristics of patients in steatotic and non‐steatotic donor liver groups
| Overall (n = 763) | Steatotic donor liver (n = 309) | Non‐steatotic donor liver (n = 454) | P‐value | |
|---|---|---|---|---|
| AR | 67 (8.8%) | 24 (7.8%) | 43 (9.5%) | 0.414 |
| FK506 | 642 (84.1%) | 271 (87.7%) | 371 (81.7%) | 0.026* |
| CsA | 47 (6.2%) | 10 (3.2%) | 37 (8.1%) | 0.006* |
| RAP | 74 (9.7%) | 28 (9.1%) | 46 (10.1%) | 0.624 |
Data are n (%). P‐values refer to comparisons between the steatotic and non‐steatotic donor liver groups. *P < 0.05. AR, acute rejection; CsA, cyclosporine; FK506, tacrolimus; RAP, rapamycin.
Figure 2.
Comparison of blood concentrations of immunosuppressant at different points after liver transplantation between recipients of steatotic and non‐steatotic donor livers. (a) Tacrolimus (FK506). (b) Cyclosporine‐A (CsA). (c) Rapamycin (RAP).
Incidence of NODAT
As aforementioned, 266 among the 763 liver transplantation patients (34.6%) developed NODAT. The incidence of NODAT was significantly higher in the steatotic donor liver group (130 patients, 42.1%; 95% CI: 36.5–47.6%) than the non‐steatotic donor liver group (136 patients, 30.0%; 95% CI: 25.7–34.2%; P = 0.001; Figure 3). The cumulative incidence of NODAT among all patients at 1, 3, 5, and 10 years after liver transplantation was 33, 43, 50 and 56%, respectively. Furthermore, the cumulative incidence of NODAT at all these time‐points was significantly higher in the steatotic donor liver group than the non‐steatotic donor liver group (P = 0.003; Figure 4). Most patients developed NODAT within 1 year after liver transplantation. The difference in NODAT incidence was not significant between patients who received mildly steatotic donor livers and those who received moderately to severely steatotic donor livers (P = 0.868).
Figure 3.
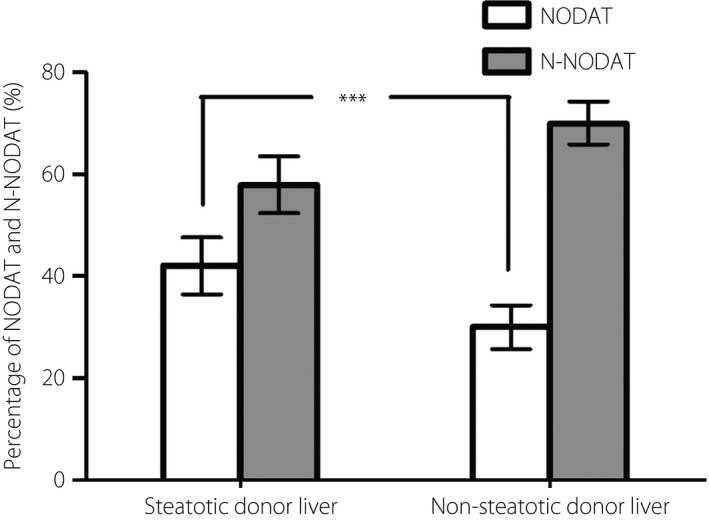
Percentage of patients with new‐onset diabetes after transplantation (NODAT) and without NODAT (N‐NODAT) at the end of follow up in the steatotic and non‐steatotic donor liver groups.
Figure 4.
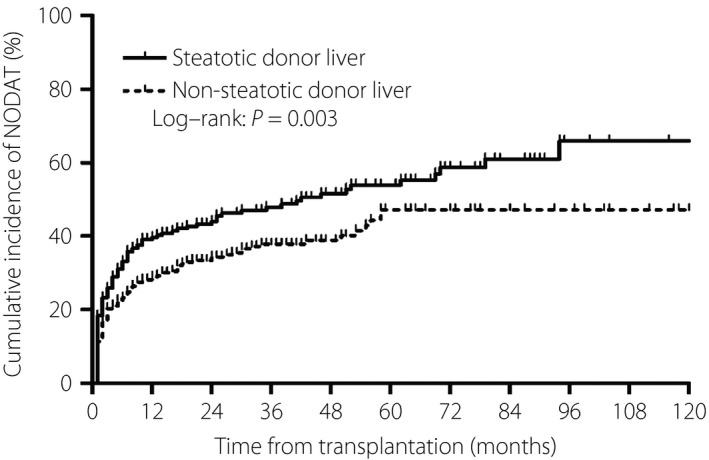
Cumulative incidence of new‐onset diabetes after transplantation (NODAT) in the steatotic and non‐steatotic donor liver groups. Steatotic donor liver group: n = 309; non‐steatotic donor liver group: n = 454.
Donor liver steatosis and NODAT risk
Multivariate Cox regression analyses after adjusting for various potential risk factors (age; sex; donor type; hepatitis virus infection; BMI; history of hypertension; preoperative liver function; preoperative levels of FPG, total cholesterol and total triglycerides; severity of liver cirrhosis; AR; postoperative transaminase levels; initial immunosuppressant regimen type; and postoperative immunosuppressant levels) showed that donor liver steatosis was an independent risk factor for NODAT (HR 1.774, 95% CI: 1.025–3.073; P = 0.041). Additionally, preoperative FPG levels (HR 1.765, 95% CI: 1.209–2.577; P = 0.003) and blood FK506 concentration at 3–12 months after liver transplantation (HR 1.110, 95% CI: 1.013–1.216; P = 0.026) differed significantly between the steatotic and non‐steatotic donor liver groups (Table 3). However, no other risk factors differed significantly between these groups. We also separately analyzed the predictive factors of early‐ (within 1 year) and late‐onset diabetes after transplantation using multivariate Cox regression analyses. The results showed that donor liver steatosis (HR 1.987, 95% CI: 1.411–3.283; P < 0.001) and preoperative FPG levels (HR 2.152, 95% CI: 1.007–1.112; P = 0.024) were risk factors for early‐onset diabetes after transplantation (Table 3). Blood FK506 concentration at 3–12 months after liver transplantation (HR 1.422, 95% CI: 1.060–1.907; P = 0.019) was a risk factor for late‐onset diabetes after transplantation (Table 3).
Table 3.
Multivariate Cox regression analysis showing the predictors of new‐onset diabetes after transplantation in liver transplant recipients
| Variables | HR | 95% CI | P‐value |
|---|---|---|---|
| NODAT | |||
| Preoperative FPG | 1.765 | 1.209–2.577 | 0.003* |
| FK506 (3–12 months after LT) | 1.110 | 1.013–1.216 | 0.026* |
| Donor liver steatosis | 1.774 | 1.025–3.073 | 0.041* |
| Early NODAT | |||
| Preoperative FPG levels | 2.152 | 1.411–3.283 | <0.001* |
| Donor liver steatosis | 1.987 | 1.045–3.780 | 0.036* |
| Late NODAT | |||
| FK506 (3–12 months after LT) | 1.422 | 1.060–1.907 | 0.019* |
Variables were analyzed after adjusting for age; sex; donor type; hepatitis virus infection; body mass index; history of hypertension; preoperative liver function; preoperative levels of fasting plasma glucose, total cholesterol and total triglycerides; severity of liver cirrhosis; acute rejection; postoperative transaminase levels; initial immunosuppressant type; and postoperative immunosuppressant levels. *P < 0.05. CI, confidence interval; FK506, tacrolimus; FPG, fasting plasma glucose; HR, hazard ratio; LT, liver transplantation; NODAT, new‐onset diabetes after transplantation.
Discussion
The reported incidence of NODAT varies from 2 to 53% across studies26, probably because of variations in the NODAT definition, immunosuppressive regimen and predisposing factors for NODAT development. The cumulative incidence of NODAT was high in the present study, ranging from 33 to 56% depending on the duration of follow up. Most patients developed NODAT within 1 year after liver transplantation, possibly because patients require high‐dose immunosuppressant therapy for 1 year after transplantation. Because we did not have information on the 2‐h postprandial glucose and glycated hemoglobin levels, which can be used for diagnosing diabetes on the basis of the 2014 American Diabetes Association criteria, we used FPG levels to diagnose NODAT. Thus, we might have underestimated the incidence of NODAT. In contrast, as diabetes was defined as one of the study end‐points, patients with transient diabetes (those meeting the diagnostic criteria, but with normal glucose levels beyond 1 year after liver transplantation) were misdiagnosed with NODAT. Additionally, previous studies reported that FK506 could more easily lead to NODAT than CsA and RAP32, 33. Among our liver transplantation patients, most received FK506 as part of the triple immunosuppressive regimen. All these factors could underlie the higher incidence of NODAT detected in the present study.
In the present study, the baseline characteristics of liver transplantation patients were generally similar. Only BMI, donor type, and the frequency of FK506 and CsA as the initial immunosuppressant differed significantly between the groups. To predict risk factors of NODAT among our liver transplant recipients, we used multivariate Cox regression analysis to eliminate the effect of confounding factors. After adjusting for potential risk factors of NODAT, we found that donor liver steatosis remained an independent risk factor. The risk of developing NODAT in the steatotic donor liver group was twice that in the non‐steatotic donor liver group. These results showed that donor liver steatosis could predict NODAT development among liver transplant recipients. In turn, the greater risk of developing NODAT in the steatotic donor liver group shows an association between donor liver biopsy before transplantation and NODAT. We found that a high preoperative FPG level and FK506 concentration at 3–12 months after liver transplantation were also associated with NODAT, a result that agreed with those of previous studies. Additionally, we found that donor liver steatosis and high preoperative FPG level were associated with early NODAT. However, FK506 concentration at 3–12 months after liver transplantation was a risk factor for late NODAT. It has been reported that preoperative FPG and 2‐h postprandial glucose levels predict NODAT development11. Therefore, in future studies, corticosteroids should be withdrawn early, a suitable immunosuppressant regimen should be planned, and FPG and 2‐h postprandial glucose levels should be monitored closely after liver transplantation in patients with impaired glucose regulation preoperatively. FK506 is known to inhibit AR, and is currently gaining increasing popularity for clinical use among liver transplant recipients. However, it is considered an independent risk factor of NODAT and could increase NODAT incidence. The possible underlying mechanism is that FK506 causes vacuolation of pancreatic β‐cells and insulin shortage. Furthermore, it might inhibit insulin secretion by reducing messenger ribonucleic acid transcription of the insulin gene and increase insulin resistance34, 35, 36, 37. Surprisingly, older age, hepatitis C virus infection, obesity, AR and cirrhosis were not identified as risk factors for NODAT in the present study, perhaps because of a sampling error or overcorrection in the multivariate regression analysis. To our knowledge, corticosteroids are an important factor for NODAT. However, corticosteroids were usually withdrawn within 3 months, and the cumulative dose of corticosteroids was similar among all liver transplant recipients in the present study. In addition, it is well known that corticosteroid increases postprandial and pre‐meal glucose levels, but not fasting plasma glucose level. However, we only used FPG levels after transplantation to diagnose NODAT. Therefore, we were not able to accurately evaluate the effects of corticosteroids on NODAT.
Vetelainen et al.38 postulated that steatosis has a negative effect on graft survival. Furthermore, Burke and Lucey29 believed that the use of steatotic donor livers was associated with an increased risk of primary non‐function of the allograft. They also suggested that liver steatosis was associated with insulin resistance. Although data that directly address the impact of insulin resistance on NODAT in liver transplant recipients are limited, insulin resistance is known to affect liver steatosis. Liver steatosis in turn aggravates insulin resistance by stimulating gluconeogenesis and c‐Jun N‐terminal kinase, which causes lipid accumulation in hepatocytes and disturbs the insulin signal. Eventually, this leads to NODAT development in liver transplant recipients. However, the detailed mechanism remains unclear and requires future investigation.
Like type 2 diabetes, NODAT can result from increased insulin resistance and decreased insulin production by pancreatic β‐cells. Because of the retrospective nature of the present study, however, we could not estimate pancreatic function in the liver transplant recipients. In subsequent studies, we hope to acquire fasting glucose and insulin data to assess pancreatic function in liver transplant recipients, which might shed light on the mechanism of NODAT in these patients.
According to the present results, donor liver steatosis increased the incidence of NODAT in liver transplant recipients. NODAT is associated with a higher risk of complications, and affects the long‐term survival of liver transplant patients. Therefore, patients with donor liver steatosis should be followed up very closely after liver transplantation. In addition, the donor population needs to be chosen very carefully, in order to reduce the incidence of NODAT, improve the recipients’ quality of life and increase long‐term survival.
In conclusion, donor liver steatosis is a predictor of NODAT in liver transplant recipients. Given that liver transplantation improves the short‐term survival of patients with end‐stage liver disease, the choice of donor livers is very important in order to lower the chances of NODAT and increase long‐term recipient survival.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors have no funding sources to report.
J Diabetes Investig 2017; 8: 181–187
References
- 1. Parekh J, Corley DA, Feng S. Diabetes, hypertension and hyperlipidemia: prevalence over time and impact on long‐term survival after liver transplantation. Am J Transplant 2012; 12: 2181–2187. [DOI] [PubMed] [Google Scholar]
- 2. Wilkinson A, Davidson J, Dotta F, et al Guidelines for the treatment and management of new‐onset diabetes after transplantation. Clin Transplant 2005; 19: 291–298. [DOI] [PubMed] [Google Scholar]
- 3. Pagadala M, Dasarathy S, Eghtesad B, et al Posttransplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl 2009; 15: 1662–1670. [DOI] [PubMed] [Google Scholar]
- 4. John PR, Thuluvath PJ. Outcome of patients with new‐onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl 2002; 8: 708–713. [DOI] [PubMed] [Google Scholar]
- 5. Moon JI, Barbeito R, Faradji RN, et al Negative impact of new‐onset diabetes mellitus on patient and graft survival after liver transplantation: Long‐term follow up. Transplantation 2006; 82: 1625–1628. [DOI] [PubMed] [Google Scholar]
- 6. Watt KD, Pedersen RA, Kremers WK, et al Evolution of causes and risk factors for mortality post‐liver transplant: results of the NIDDK long‐term follow‐up study. Am J Transplant 2010; 10: 1420–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharif A, Hecking M, de Vries AP, et al Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant 2014; 14: 1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lane JT, Dagogo‐Jack S. Approach to the patient with new‐onset diabetes after transplant (NODAT). J Clin Endocrinol Metab 2011; 96: 3289–3297. [DOI] [PubMed] [Google Scholar]
- 9. Samuelson AL, Lee M, Kamal A, et al Diabetes mellitus increases the risk of mortality following liver transplantation independent of MELD score. Dig Dis Sci 2010; 55: 2089–2094. [DOI] [PubMed] [Google Scholar]
- 10. Oufroukhi L, Kamar N, Muscari F, et al Predictive factors for posttransplant diabetes mellitus within one‐year of liver transplantation. Transplantation 2008; 85: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 11. Xu X, Ling Q, He ZL, et al Post‐transplant diabetes mellitus in liver transplantation: Hangzhou experience. Hepatobiliary Pancreat Dis Int 2008; 7: 465–470. [PubMed] [Google Scholar]
- 12. Tsochatzis E, Koskinas J, Manesis EK, et al Liver transplantation in Greek patients: epidemiological data, morbidity, and mortality of 71 patients from a single center with 6 years of mean follow‐up. Transplant Proc 2007; 39: 1505–1507. [DOI] [PubMed] [Google Scholar]
- 13. Fallahi P, Ferrari SM, Colaci M, et al Hepatitis C virus infection and type 2 diabetes. Clin Ter 2013; 164: e393–e404. [DOI] [PubMed] [Google Scholar]
- 14. Navasa M, Bustamante J, Marroni C, et al Diabetes mellitus after liver transplantation: prevalence and predictive factors. J Hepatol 1996; 25: 64–71. [DOI] [PubMed] [Google Scholar]
- 15. Konrad T, Steinmuller T, Vicini P, et al Regulation of glucose tolerance in patients after liver transplantation: impact of cyclosporin versus tacrolimus therapy. Transplantation 2000; 69: 2072–2078. [DOI] [PubMed] [Google Scholar]
- 16. Pinheiro BM, de Francesco DE, de Matos ER, et al Historical cohort with diabetes mellitus after kidney transplantation and associated factors of its development in adult patients of a transplantation reference center in the State of Ceara. Brazil. Transplant Proc 2014; 46: 1698–1704. [DOI] [PubMed] [Google Scholar]
- 17. Mora PF. Post‐transplantation diabetes mellitus. Am J Med Sci 2005; 329: 86–94. [DOI] [PubMed] [Google Scholar]
- 18. Markell M. New‐onset diabetes mellitus in transplant patients: pathogenesis, complications, and management. Am J Kidney Dis 2004; 43: 953–965. [DOI] [PubMed] [Google Scholar]
- 19. Baid S, Cosimi AB, Farrell ML, et al Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation 2001; 72: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 20. Rakel A, Karelis AD. New‐onset diabetes after transplantation: risk factors and clinical impact. Diabetes Metab 2011; 37: 1–14. [DOI] [PubMed] [Google Scholar]
- 21. Hjelmesaeth J, Sagedal S, Hartmann A, et al Asymptomatic cytomegalovirus infection is associated with increased risk of new‐onset diabetes mellitus and impaired insulin release after renal transplantation. Diabetologia 2004; 47: 1550–1556. [DOI] [PubMed] [Google Scholar]
- 22. Hjelmesaeth J, Hagen M, Hartmann A, et al The impact of impaired insulin release and insulin resistance on glucose intolerance after renal transplantation. Clin Transplant 2002; 16: 389–396. [DOI] [PubMed] [Google Scholar]
- 23. Sun K, Lu J, Jiang Y, et al Low serum potassium level is associated with nonalcoholic fatty liver disease and its related metabolic disorders. Clin Endocrinol (Oxf) 2014; 80: 348–355. [DOI] [PubMed] [Google Scholar]
- 24. Montori VM, Basu A, Erwin PJ, et al Posttransplantation diabetes: a systematic review of the literature. Diabetes Care 2002; 25: 583–592. [DOI] [PubMed] [Google Scholar]
- 25. Pham PT, Pham PC, Lipshutz GS, et al New onset diabetes mellitus after solid organ transplantation. Endocrinol Metab Clin North Am 2007; 36: 873–890. [DOI] [PubMed] [Google Scholar]
- 26. Caillard S, Eprinchard L, Perrin P, et al Incidence and risk factors of glucose metabolism disorders in kidney transplant recipients: role of systematic screening by oral glucose tolerance test. Transplantation 2011; 91: 757–764. [DOI] [PubMed] [Google Scholar]
- 27. Chakkera HA, Knowler WC, Devarapalli Y, et al Relationship between inpatient hyperglycemia and insulin treatment after kidney transplantation and future new onset diabetes mellitus. Clin J Am Soc Nephrol 2010; 5: 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poordad F, Gish R, Wakil A, et al De novo non‐alcoholic fatty liver disease following orthotopic liver transplantation. Am J Transplant 2003; 3: 1413–1417. [DOI] [PubMed] [Google Scholar]
- 29. Burke A, Lucey MR. Non‐alcoholic fatty liver disease, non‐alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant 2004; 4: 686–693. [DOI] [PubMed] [Google Scholar]
- 30. Tiniakos DG. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: histological diagnostic criteria and scoring systems. Eur J Gastroenterol Hepatol 2010; 22: 643–650. [DOI] [PubMed] [Google Scholar]
- 31. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37: S81–S90. [DOI] [PubMed] [Google Scholar]
- 32. Krok KL, Thuluvath PJ. Perioperative and postoperative use of immunosuppressive agents in liver transplantation. Int Anesthesiol Clin 2006; 44: 51–68. [DOI] [PubMed] [Google Scholar]
- 33. Mehrabi A, Fonouni H, Kashfi A, et al The role and value of sirolimus administration in kidney and liver transplantation. Clin Transplant 2006; 20: 30–43. [DOI] [PubMed] [Google Scholar]
- 34. Lawrence MC, Bhatt HS, Watterson JM, et al Regulation of insulin gene transcription by a Ca(2 + )‐responsive pathway involving calcineurin and nuclear factor of activated T cells. Mol Endocrinol 2001; 15: 1758–1767. [DOI] [PubMed] [Google Scholar]
- 35. Oetjen E, Baun D, Beimesche S, et al Inhibition of human insulin gene transcription by the immunosuppressive drugs cyclosporin A and tacrolimus in primary, mature islets of transgenic mice. Mol Pharmacol 2003; 63: 1289–1295. [DOI] [PubMed] [Google Scholar]
- 36. Paty BW, Harmon JS, Marsh CL, et al Inhibitory effects of immunosuppressive drugs on insulin secretion from HIT‐T15 cells and Wistar rat islets. Transplantation 2002; 73: 353–357. [DOI] [PubMed] [Google Scholar]
- 37. Panz VR, Bonegio R, Raal FJ, et al Diabetogenic effect of tacrolimus in South African patients undergoing kidney transplantation1. Transplantation 2002; 73: 587–590. [DOI] [PubMed] [Google Scholar]
- 38. Vetelainen R, van Vliet A, Gouma DJ, et al Steatosis as a risk factor in liver surgery. Ann Surg 2007; 245: 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]


