Abstract
Streptomyces strain KX852460 having antifungal activity against Rhizoctonia solani AG-3 KX852461 that is the causal agent of target spot disease in tobacco leaf. The aim of the study was to determine the antifungal activity of Streptomyces strain KX852460 extract against R. solani AG-3 and to identify bioactive antifungal compounds produced by strain KX852460. Crude substance was produced by submerged fermentation process from Streptomyces strain KX852460. Various solvent was used to extract the culture filtrate. Among all, ethyl acetate extracted supernatant showed great potency against R. solani AG-3 KX852461. The active fractions were purified by silica gel column chromatography having 52 mm zone of inhibition against R. solani AG-3 KX852461. The purified fractions were identified by gas chromatography–mass spectrometry technique. Twenty-seven compounds were identified and most of the compounds were the derivatives of aromatic compounds. Eicosane (C20H42) and dibutyl phthalate (C16H22O4) were found antifungal compounds in this study. While morphinan, 7,8-didehydro-4,5-epoxy-17-methyl-3,6-bis[(trimethylsilyl)oxy]-, (5.Alpha. 6.Alpha)—(C23H35NO3Si2), cyclononasiloxane, octadecamethyl—(C18H54O9Si9) and benzoic acid, 2,5-bis(trimethylsiloxy) (C16H30O4Si3) were the major compounds with highest peak number. These results suggested that Streptomyces strain KX852460 had good general antifungal activity and might have potential biocontrol antagonist against R. solani AG-3 KX852461 to cure the target spot in tobacco leaf.
Keywords: Streptomyces strain KX852460, Rhizoctonia solani AG-3, Gas chromatography–mass spectrometry, Aromatic compounds, Target spot
Introduction
In the Liaoning province of China in 2006 target spot disease of tobacco was investigated, Rhizoctonia solani was the causal agent and caused heavy economic loss regarding to the production and quality of the tobacco (Wu et al. 2012). Anastomosis AG-3 of the R. solani is the causal agent of target spot in tobacco (Johnk et al. 1993). To conflict with phytopathogens biocontrol is most potent and environment friendly practice (Castano et al. 2013). Against the R. solani biocontrol is better strategy even though environmental conditions affected its efficacy (dos Reis Almeida et al. 2007). Actinomycetes are extensively present microorganisms in the environment that have potential to produce bioactive compounds against the phytopathogens (Xue et al. 2013; Zeng et al. 2013). Among the Actinomycetes, Streptomyces is the largest genus and belongs to the family Streptomycetaceae (Kämpfer 2006).
Streptomyces have potential to produced curial compounds for antibiotics and agro-antibiotics (Demain 2009). Streptomycetes species are the source of 75% of antibiotics (Bhavana et al. 2014). Different solvents and elucidation utilized to extract the secondary metabolites and their structure can be finding by different techniques for example gas chromatography–mass spectrometry (GC–MS), liquid chromatography–mass spectrometry (LC–MS), and nuclear magnetic resonance (NMR) (Tiwari et al. 2015). Volatile and semi volatile compounds with lower molecular mass can be separated by using GC–MS (Snyder et al. 2012). The GC–MS is novel technology for isolation of the compounds that present in the secondary metabolites. Recently antibacterial (Khattab et al. 2016), antifungal compound against Lycopersici and Fusarium oxysporum (Jalaluldeen et al. 2015), antifungal compounds against Pyricularia oryzae (Awla et al. 2016), and broad spectrum pharmaceutical compounds (Narasaiah et al. 2014) were extracted by GC–MS. The main objective of this study was extraction, purification and identification of bioactive antifungal compounds produced from Streptomyces strain KX852460 grown under submerged fermentation.
Materials and methods
Microorganism
Streptomyces strain was isolated from soil and identified by 16S rRNA gene sequence technology and sequence was submitted to Gene bank under accession number of KX852460 and also submitted to Chinese general microbial collection center (CGMCC4.7384). The strain was used for the production of antifungal compounds in submerged fermentation. Rhizoctonia solani AG-3 was obtained from naturally infected tobacco leaves in Dandong of China which was also identified by 16S rRNA gene sequence technique and sequence obtained was submitted to the Gene bank and under Accession Number of KX852461 and also submitted to Chinese general microbial collection center (CGMCC3.18223). Other test pathogens obtained from the plant pathology lab of college of plant protection, Shenyang agricultural University China. Fungus pathogens were stored on potato dextrose agar (PDA) at 4 °C.
Preparation of inoculum
Fermentation was performed in two stages, seed growth and production of active antifungal substance. Streptomyces Strain KX852460 was grown on plates of Gause’ s synthetic agar medium at 28 °C for 5 days after spore production used in liquid fermentation medium. Two spore cakes (5 mm) were used to inoculate a 250 ml flask having medium volume of 40 ml and then incubated at 28 °C with agitation speed of 160 rpm for 48 h.
Fermentation technique
For the production of antifungal compounds, 40 ml of fermentation medium [47 g soluble starch, 3 g yeast extract, 22 g peanut meal, 2.7 g (NH4)2 SO4, 2.7 g NaCl, 2.7 g CaCO3 dissolved in 1 L distilled water and pH was adjusted to 6.8–7.2] was taken in 250 ml flask and sterilized. After sterilization, the medium was inoculated with 5% (v/v) seed culture and incubated at 28 °C in rotatory shaker with agitation speed of 160 rpm for 96 h. After the termination of fermentation process, the culture was centrifuged and the supernatant was stored at −4 °C for further work (Gao et al. 2015).
Antifungal activity
Antifungal activities were determined by oxford cup method (Wang et al. 2010a, b) and measured the inhibition zone.
Stability test of the cultural filtrate of Streptomyces KX852460
Thermal stability, pH stability, illuminated light stability, and UV light stability were performed according to Zhao and Wu (2006). All the experiment were performed in triplicates and antifungal activity determined by oxford cup method mentioned above.
Extraction of the culture filtrate
The culture filtrate (500 ml) was extracted two times with ethyl acetate as solvent. The solvent was added to the filtrate in the ratio of 1:1(v/v) and shaken vigorously for 20 min. The ethyl acetate phase that contains antibiotic was separated from the aqueous phase using separating funnel. Ethyl acetate layer was concentrated by evaporating to dryness at 50 °C and residue obtained was purified using methanol to (1.8 g) brown crude extract (Ahmed 2007).
Purification and identification of the compound
The purification of the antimicrobial compound was carried out using silica gel column chromatography as described by Atta et al. (2009). Ethyl acetate was used as eluting solvent. The column was packed with silica gel (60–120 mesh). The sample to be separated was loaded on the packed column and eluted with the solvent at the flow rate of one drop per minute. A conical flask was placed at the bottom of the column to collect the eluted fractions. Antifungal activity was checked and most active fractions were used for further analysis. The antifungal compounds were identified by using gas chromatography–mass spectrometer technique (GC–MS). Agilent technologies 6890–5973 N with capillary column TG-5 ms Phenyl Methyl Siloxane (30 m × 250 μm × 0.25 μm) system were used. Mass detector used in split mode, and helium gas with flow rate of 1.0 ml/min was used as a carrier. Injector was operated at 230 °C and oven temperature for initial setup was 60 °C for 2 min, ramp 10/min to 280 °C for 8 min.
Results
Streptomyces strain KX852460 was isolated from the soil and screened against the R. solani AG-3 that is the causal agent of target spot in tobacco leaf. This strain had great potency against the pathogen. Twenty litter fermentation broth was produced by the Streptomyces strain KX852460 and broth was active against different plant pathogens including R. solani AG-3 having inhibition zone diameter of 45.78 mm. The strain also showed strong activity against Sclerotinia sclerotiorum with inhibition zone diameter of 50.4 mm (Table 1). Antifungal activity of the fermentation broth was found stable at various temperature levels from 60 to 90 °C, while at 100 °C activity was decreased (Fig. 1a). At different pH values antifungal activity of fermentation broth was observed, having peak activity at pH 6, while extreme pH conditions (pH 2, 14) resulted decreased antifungal activity (Fig. 1b). Fermentation broth treated with illuminated light showed stability in the activity against the pathogen (Fig. 1c). Under UV light treated broth was affected within duration of treatment and activity decreased abruptly (Fig. 1d). Solvent extraction method used to extract the bioactive compounds with several organic polar and non-polar solvents. All the extracts showed some inhibition effect against the pathogen ranging from 1.43 to 44.36 mm inhibition zone. But extract with ethyl acetate showed strong antifungal activity against the R. solani AG-3 KX852461 (Fig. 2). For further work based on this result ethyl acetate was selected and resulted antifungal activity against R. solani AG-3 KX852461 (Fig. 3). Crude extract dried at 50 °C and brown color powdery substance obtained that further purified by silica gel column chromatography by using ethyl acetate as eluent. Several fractions were obtained and most active fractions against the R. solani. In (Fig. 4) fraction number 8 had strongly inhibited the R. solani AG-3 with diameter of inhibition zone 52 mm. Active fraction was further analyzed by gas chromatography–mass spectrometer (GC–MS). GC–MS analysis detected 27 bioactive compounds (Table 2). By comparison of mass spectra of the constituent with NIST library twenty-seven peaks obtained (Fig. 5a). Among 27 different compounds, 16 compounds were the constituent of aromatic compounds while others were derivatives of different hydrocarbons. Eicosane (C20H42) and dibutyl phthalate (C16H22O4), having retention time of 13.641 (Fig. 5b) 18.852 (Fig. 5c) respectively were two antifungal compounds identified.
Table 1.
Antimicrobial effects of the cultural filtrate
| Test pathogens | Inhibition spectrum (mm) of cultural filtrate |
|---|---|
| Alternaria alternata | 33.96 |
| Botrytis cinerea | 40.16 |
| Alternaria solani | 38.62 |
| Rhizoctonia solani AG-3 | 45.78 |
| Fusarium oxysporum | 24.78 |
| Sclerotinia sclerotiorum | 50.4 |
| Bipolaris maydis | 33.72 |
| Colletotrichum capsici | 25.48 |
Fig. 1.
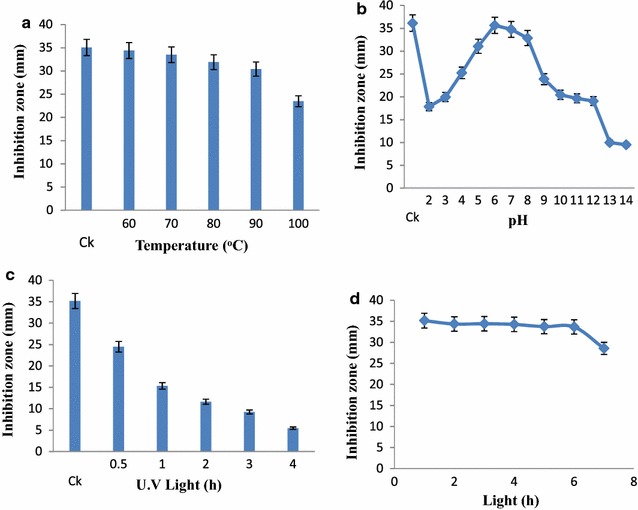
Effect of temperature (a), pH (b), illuminated light (c) and ultra violet light (d) on the stability of fermentation broth. Ck represents control of each treatment
Fig. 2.
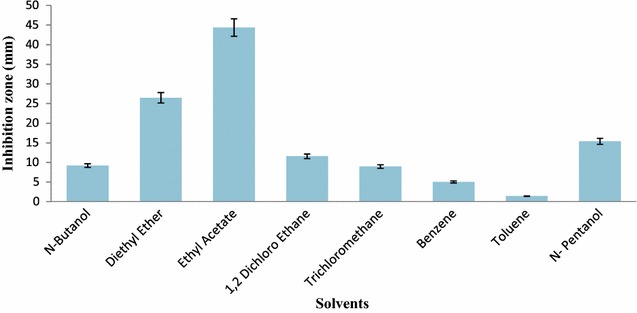
Effect of different solvents on activity of bioactive compound produced from Streptomyces strain KX852460
Fig. 3.

Activity of solvent extracted supernatant with ethyl acetate against R. solani AG-3
Fig. 4.

Antifungal activity of purified fractions by silica gel column chromatography and 1, 2, 3, 4, 5, 6, 7, 8, and 9 represented the numbers of purified fractions, obtained by silica gel column chromatography
Table 2.
Compounds identified in ethyl acetate extract of Streptomyces KX852460 by GC–MS
| Peak # | Retention time | Area % | Name of the compound | Chemical formula | Molecular weight |
|---|---|---|---|---|---|
| 1 | 4.786 | 3.92 | Benzoic acid, 2-methoxy-, methyl ester | C9H10O3 | 166 |
| 2 | 8.024 | 5.36 | Undecane | C11H24 | 156 |
| 3 | 8.106 | 1.8 | Undecane | C11H24 | 268 |
| 4 | 11.373 | 0.98 | Cyclohexasiloxane, dodecamethyl- | C12H36O6Si6 | 444 |
| 5 | 13.641 | 0.98 | Eicosane | C20H42 | 282 |
| 6 | 13.87 | 0.72 | Phenol, 2,4-bis(1,1-dimethylethyl) | C14H22O | 206 |
| 7 | 13.935 | 1.02 | Butylated hydroxytoluene | C15H22O | 220 |
| 8 | 16.009 | 0.74 | N-(Glycyl)alanine | C5H10N2O3 | 146 |
| 9 | 16.955 | 1.98 | 1,3-Diphenyl-4H-1,2,4-triazoline-5-thione | C14H11N3S | 253 |
| 10 | 17.378 | 1.09 | Cyclononasiloxane, octadecamethyl- | C18H54O9Si9 | 666 |
| 11 | 17.901 | 5.9 | 1,2-Benzenedicarboxylic acid, bis (2-methylpropyl) ester | C16H22O4 | 278 |
| 12 | 18.406 | 4.67 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 |
| 13 | 18.852 | 6.44 | Dibutyl phthalate | C16H22O4 | 278 |
| 14 | 18.929 | 1.63 | Cyclodecasiloxane, eicosamethyl- | C20H66O10Si10 | 740 |
| 15 | 19.264 | 2.04 | p-Dicyclohexylbenzene | C18H26 | 242 |
| 16 | 19.74 | 1.1 | p-Dicyclohexylbenzene | C18H26 | 242 |
| 17 | 20.092 | 3.44 | 9,10-Anthracenedione, 2-ethyl- | C16H20O2 | 236 |
| 18 | 20.357 | 5.95 | Cyclodecasiloxane, eicosamethyl- | C20H60O10Si10 | 740 |
| 19 | 20.515 | 1.4 | Octahydrotripheylene | C18H20 | 236 |
| 20 | 21.649 | 5.35 | 7-Chloro-10-ethyl-1-[[2-[[2-hydroxyethyl] amino] ethyl] amino]-3-[4- | C26H25ClF3N3O2 | 503 |
| 21 | 22.689 | 1.48 | Hexanedioic acid, bis(2-ethylhexyl) ester | C22H42O4 | 370 |
| 22 | 22.836 | 6.56 | Morphinan, 7,8-didehydro-4,5-epoxy-17-methyl-3, 6-bis [(trimethylsilyl) oxy]-, (5.alpha. 6. Alpha.)- | C23H35NO3Si2 | 429 |
| 23 | 23.947 | 14.46 | Cyclononasiloxane, octadecamethyl- | C18H54O9Si9 | 666 |
| 24 | 25.11 | 7.55 | Benzoic acid, 2,5-bis(trimethylsiloxy)-, trimethylsilyl ester | C16H30O4Si3 | 370 |
| 25 | 26.497 | 8.32 | 1,2,4-Benzenetricarboxylic acid, 4-butyl 1,2-dimethyl ester | C15H18O6 | 294 |
| 26 | 28.23 | 4.15 | Cyclotrisiloxane, hexamethyl- | C6H18O3Si3 | 222 |
| 27 | 30.468 | 0.97 | Cyclotrisiloxane, hexamethyl- | C6H18O3Si3 | 222 |
Fig. 5.
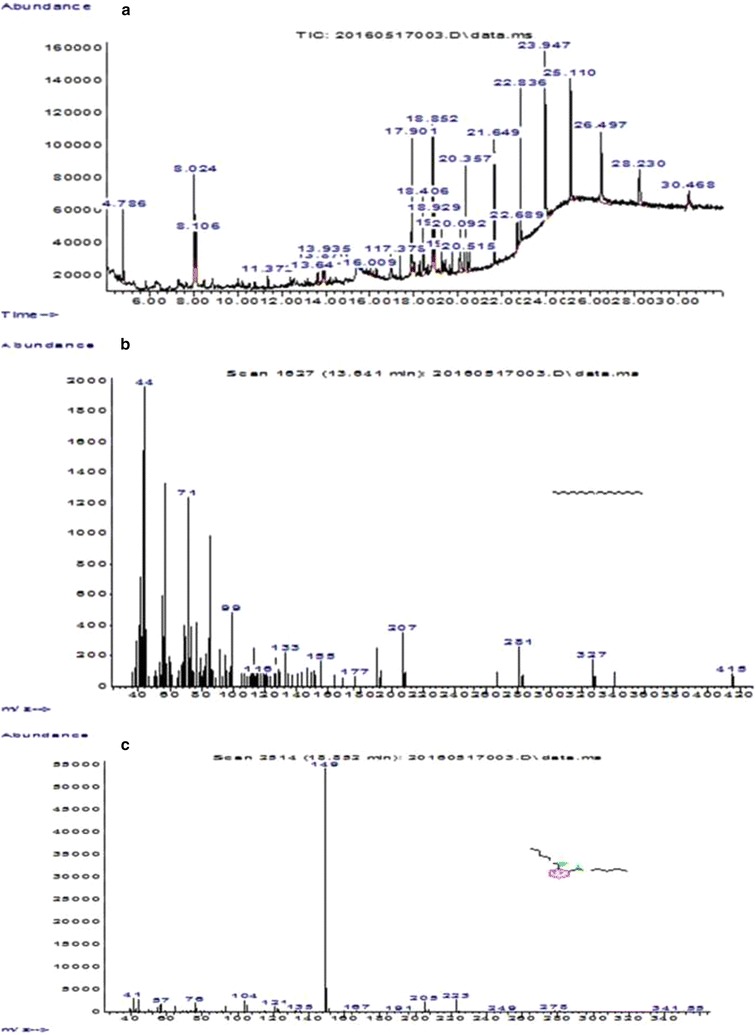
Gas chromatography-mass spectrometer (GC–MS) analysis of the purified active fraction (a), detection of eicosane (b) and dibutyl phthalate (c) from purified active fraction
Discussion
In this study Streptomyces strain KX852460 was screened against the R. solani AG-3 that is the causal agent of target spot disease in tobacco. Strain KX852460 belongs to Streptomyces which strongly inhibits the pathogen and could be curing the target spot in tobacco. The effects of the R. solani diseases are very severe throughout the world and affected the quality and yield of the several crops. For the control of R. solani, bacterial antagonist could be an environment friendly substituent. Various bacterial antagonists against the R. solani, including Bacillus subtilis CA32 in eggplant (Abeysinghe 2009), Pseudomonas fluorescens In5 (Michelsen and Stougaard 2011), Burkholderia cepacia T1A-2B and Pseudomonas sp. T4B-2A in tomato (De Curtis et al. 2010), inoculum of GB7 and 3Re4-18 in lettuce (Grosch et al. 2012), endophytic Streptomyces damping off growth promotion in tomato (Goudjal et al. 2014) have been reported as effective biological control agents. Antagonist activity of Streptomyces sp. CACIS-1.16CA against different phytopathogens including R. solni was also investigated by Evangelista-Martínez (2014).
In this study stability of the active cultural filtrate was determined at various temperatures, pH values, and treated with illuminated light and UV light. At 60–90 °C the antifungal activity of cultural filtrate of the Streptomyces strain KX852460 remained same, however above these conditions, the activity become decreased. pH values remained stable between 5.0 and 8.0 pH, while pH values above and lower beyond this were not stable. Uddin et al. (2013) reported stability of antimicrobial filtrate at different temperature and pH values. Treated with illumination light cultural filtrate was stable and showed good activity. Treated with UV light for long time was not stable, while treated for 30 min and for 1 h was stable. Stability of antimicrobial cultural filtrate from Streptomyces was also reported by Zhao and Wu (2006).
The crude extract obtained by solvent extraction with ethyl acetate showed strong activity against the R. solani. Isolation of crude extract by solvent extraction is very important phenomenon, to find a good solvent that have the potential to extract high yield and most potent bioactive compounds. Studies demonstrated that the extract of ethyl acetate have wide antimicrobial spectrum against the bacterial and fungus pathogens (Khamna et al. 2009; Kobayashi et al. 1994). Extract from Streptomyces EF37141 contains 27 different organic compounds. GC–MS analysis showed that the majority of the compounds were derivatives of the aromatic compounds. These compounds were antimicrobial and antifungal. Volatile organic compounds and polycyclic aromatic derivatives have the antifungal potential (Müller et al. 2009; Memić et al. 2011).
GC–MS is a novel technique to identify the secondary metabolites from the Streptomyces fermentation broth and analysis of GC–MS is very reliable to identify the compound in complex biochemical product. From current study some compounds were reported antifungal. Eicosane reported as antifungal compound (Karanja et al. 2012; Nandhini 2015) and dibutyl phthalate also reported antifungal compound (Nandhini 2015; Roy et al. 2006). Morphinan, 7,8-didehydro-4,5-epoxy-17-methyl-3,6-bis[(trimethylsilyl)oxy]-, (5.alpha, 6.alpha)-, cyclononasiloxane, octadecamethyl- and 1,2,4-benzenetricarboxylic acid, 4-butyl 1,2-dimethyl ester showed highest peaks and the area percent of these compounds also more than other compounds. On the base of GC–MS analysis highest peak number and area percent indicated that these three compounds were major in the extract of Streptomyces EF37141. These compounds consider active substances against the R. solani. Extract from the Streptomyces shows the same effectiveness as the oxine benzoate and fungicide (Sabaratnam and Traquair 2002).
Authors’ contributions
Planning and designing of study: YW; Experimentation: TA; Result Analysis: JC, XZ; Manuscript Drafting: MI. All authors contributed in the final approval of manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors are thankful to the technical staff of the Department of Plant Pathology, College Plant Protection, Shenyang Agricultural University, P. R. China.
Competing interests
The authors declare that they have no competing interests.
Ethical approval
No data was used in this article which needs approval.
Funding
This study is supported by Project Number 183/2010 from Liaoning research center of tobacco China
Abbreviations
- PDA
potato dextrose agar
- GC–MS
gas chromatography–mass spectrophotometry
- NMR
nuclear magnetic resonance
Contributor Information
Taswar Ahsan, Email: babbar.jatt@hotmail.com.
Jianguang Chen, Email: chenjianguang2010@126.com.
Xiuxiang Zhao, Email: zhaoxx0772@163.com.
Muhammad Irfan, Email: m.irfan@uos.edu.pk.
Yuanhua Wu, Email: wuyh7799@163.com.
References
- Abeysinghe S. Effect of combined use aí Bacillus subtilis CA32 and Trichoderma harzianum RUOI on biological control of Rhizoctonia satani on Solanum melongena and Capsicum annuum. Plant Pathol J. 2009;8:9–16. doi: 10.3923/ppj.2009.9.16. [DOI] [Google Scholar]
- Ahmed AA. Production of antimicrobial agent by Streptomyces violachromogenes. Saudi J Biol Sci. 2007;14:7–16. [Google Scholar]
- Atta H, Dabour S, Desoukey S. Sparsomycin antibiotic production by Streptomyces sp. AZ-NIOFD1: taxonomy, fermentation, purification and biological activities. Agric Environ Sci. 2009;5:368–377. [Google Scholar]
- Awla HK, Kadir J, Othman R, Rashid TS, Wong MY. Bioactive compounds produced by Streptomyces sp. isolate UPMRS4 and antifungal activity against Pyricularia oryzae. Am J Plant Sci. 2016;7:1077. doi: 10.4236/ajps.2016.77103. [DOI] [Google Scholar]
- Bhavana M, Talluri VP, Kumar KS, Rajagopal S. Optimization of culture conditions of Streptomyces carpaticus (MTCC-11062) for the production of antimicrobial compound. Int J Pharm Pharm Sci. 2014;6:281–285. [Google Scholar]
- Castano R, Borrero C, Trillas M, Avilés M. Selection of biological control agents against tomato Fusarium wilt and evaluation in greenhouse conditions of two selected agents in three growing media. BioControl. 2013;58:105–116. doi: 10.1007/s10526-012-9465-z. [DOI] [Google Scholar]
- De Curtis F, Lima G, Vitullo D, De Cicco V. Biocontrol of Rhizoctonia solani and Sclerotium rolfsii on tomato by delivering antagonistic bacteria through a drip irrigation system. Crop Prot. 2010;29:663–670. doi: 10.1016/j.cropro.2010.01.012. [DOI] [Google Scholar]
- Demain AL. Antibiotics: natural products essential to human health. Med Res Rev. 2009;29:821–842. doi: 10.1002/med.20154. [DOI] [PubMed] [Google Scholar]
- dos Reis Almeida FB, Cerqueira FM, Silva RN, Ulhoa CJ, Lima AL. Mycoparasitism studies of Trichoderma harzianum strains against Rhizoctonia solani: evaluation of coiling and hydrolytic enzyme production. Biotechnol Lett. 2007;29:1189–1193. doi: 10.1007/s10529-007-9372-z. [DOI] [PubMed] [Google Scholar]
- Evangelista-Martínez Z. Isolation and characterization of soil Streptomyces species as potential biological control agents against fungal plant pathogens. World J Microbiol Biotechnol. 2014;30:1639–1647. doi: 10.1007/s11274-013-1568-x. [DOI] [PubMed] [Google Scholar]
- Gao X, He Q, Jiang Y, Huang L. Optimization of nutrient and fermentation parameters for antifungal activity by Streptomyces lavendulae Xjy and its biocontrol efficacies against Fulvia fulva and Botryosphaeria dothidea. J Phytopathol. 2015;164(3):155–165. doi: 10.1111/jph.12440. [DOI] [Google Scholar]
- Goudjal Y, Toumatia O, Yekkour A, Sabaou N, Mathieu F, Zitouni A. Biocontrol of Rhizoctonia solani damping-off and promotion of tomato plant growth by endophytic actinomycetes isolated from native plants of Algerian Sahara. Microbiol Res. 2014;169:59–65. doi: 10.1016/j.micres.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Grosch R, Dealtry S, Schreiter S, Berg G, Mendonça-Hagler L, Smalla K. Biocontrol of Rhizoctonia solani: complex interaction of biocontrol strains, pathogen and indigenous microbial community in the rhizosphere of lettuce shown by molecular methods. Plant Soil. 2012;361:343–357. doi: 10.1007/s11104-012-1239-y. [DOI] [Google Scholar]
- Jalaluldeen AM, Sijam K, Othman R, Ahmad ZAM. Growth characteristics and production of secondary metabolites from selected Streptomyces species isolated from the Rhizosphere of Chili Plant. Int J Anh Res Sci Techol Eng. 2015;4(1):1–8. doi: 10.15623/ijret.2015.0401001. [DOI] [Google Scholar]
- Johnk JS, Jones R, Shew H, Carling D. Characterization of populations of Rhizoctonia solani AG-3 from potato and tobacco. Phytopathol. 1993;83:854–858. doi: 10.1094/Phyto-83-854. [DOI] [Google Scholar]
- Kämpfer P. The family Streptomycetaceae, part I: taxonomy. In: Dworkin M, editor. The prokaryotes. Berlin: Springer; 2006. pp. 538–604. [Google Scholar]
- Karanja E, Boga H, Muigai A, Wamunyokoli F, Kinyua J, Nonoh J (2012) Growth characteristics and production of secondary metabolites from selected novel Streptomyces species isolated from selected Kenyan national parks. In: Scientific conference proceeding
- Khamna S, Yokota A, Peberdy JF, Lumyong S. Antifungal activity of Streptomyces spp. isolated from rhizosphere of Thai medicinal plants. Int J Integr Biol. 2009;6:143–147. [Google Scholar]
- Khattab AI, Babiker EH, Saeed HA. Streptomyces: isolation, optimization of culture conditions and extraction of secondary metabolites. Int Curr Pharm J. 2016;5:27–32. doi: 10.3329/icpj.v5i3.26695. [DOI] [Google Scholar]
- Kobayashi A, Koguchi Y, Kanzaki H, Kajiyama S, Kawazu K. A new type of antimicrobial phenolics produced by plant peroxidase and its possible role in the chemical defense systems against plant pathogens. Z Naturforsch C. 1994;49:411–414. doi: 10.1515/znc-1994-7-804. [DOI] [PubMed] [Google Scholar]
- Memić M, Selović A, Sulejmanović J. Antifungal activity of polycyclic aromatic hydrocarbons against ligninolytic fungi. Hem Ind. 2011;65:575–581. doi: 10.2298/HEMIND110408039M. [DOI] [Google Scholar]
- Michelsen CF, Stougaard P. A novel antifungal Pseudomonas fluorescens isolated from potato soils in Greenland. Curr Microbiol. 2011;62:1185–1192. doi: 10.1007/s00284-010-9846-4. [DOI] [PubMed] [Google Scholar]
- Müller H, Westendorf C, Leitner E, Chernin L, Riedel K, Schmidt S, Eberl L, Berg G. Quorum-sensing effects in the antagonistic rhizosphere bacterium Serratia plymuthica HRO-C48. FEMS Microbiol Ecol. 2009;67:468–478. doi: 10.1111/j.1574-6941.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- Nandhini SU. Gas chromatography–mass spectrometry analysis of bioactive constituents from the marine Streptomyces. Asi J Pharm Clin Res. 2015;8:244–246. [Google Scholar]
- Narasaiah BC, Leelavathi V, Sudhakar G, Mariyadasu P, Swapna G, Manne AK. Isolation and structural confirmation of bioactive compounds produced by the strain Streptomyces albus CN-4. IOSR J Pharm Biol Sci. 2014;9:49–54. [Google Scholar]
- Roy R, Laskar S, Sen S. Dibutyl phthalate, the bioactive compound produced by Streptomyces albidoflavus 321.2. Microbiol Res. 2006;161:121–126. doi: 10.1016/j.micres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Sabaratnam S, Traquair JA. Formulation of a Streptomyces biocontrol agent for the suppression of Rhizoctonia damping-off in tomato transplants. Biol Control. 2002;23:245–253. doi: 10.1006/bcon.2001.1014. [DOI] [Google Scholar]
- Snyder LR, Kirkland JJ, Glajch JL. Practical HPLC method development. New York: Wiley; 2012. [Google Scholar]
- Tiwari V, Roy R, Tiwari M. Antimicrobial active herbal compounds against Acinetobacter baumannii and other pathogens. Front Microbiol. 2015;6:618. doi: 10.3389/fmicb.2015.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Mahmud N, Anwar N, Manchur MA. Bioactive metabolite production by Streptomyces albolongus in favourable environment. J Microbiol Infect Dis. 2013;3(2):75–82. doi: 10.5799/ahinjs.02.2013.02.0085. [DOI] [Google Scholar]
- Wang X, Huang L, Kang Z, Buchenauer H, Gao X. Optimization of the fermentation process of actinomycete strain Hhs. 015 T. Biomed Res. 2010 doi: 10.1155/2010/141876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YH, Fang XL, Li YP, Zhang X. Effects of constant and shifting dissolved oxygen concentration on the growth and antibiotic activity of Xenorhabdus nematophila. Biores Technol. 2010;101:7529–7536. doi: 10.1016/j.biortech.2010.04.070. [DOI] [PubMed] [Google Scholar]
- Wu YH, Zhao YQ, Fu Y, Zhao XX, Chen JG. First report of target spot of flue-cured tobacco caused by Rhizoctonia solani AG-3 in China. Plant Dis. 2012;96:1824. doi: 10.1094/PDIS-06-12-0551-PDN. [DOI] [PubMed] [Google Scholar]
- Xue L, Xue Q, Chen Q, Lin C, Shen G, Zhao J. Isolation and evaluation of rhizosphere actinomycetes with potential application for biocontrol of Verticillium wilt of cotton. Crop Prot. 2013;43:231–240. doi: 10.1016/j.cropro.2012.10.002. [DOI] [Google Scholar]
- Zeng Q, Huang H, Zhu J, Fang Z, Sun Q, Bao S. A new nematicidal compound produced by Streptomyces albogriseolus HA10002. Antonie Van Leeuwenhoek. 2013;103:1107–1111. doi: 10.1007/s10482-013-9890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Wu YH. Antimicrobial spectrum and stability of the fermentation broth of Streptomyces. J Agrochem. 2006;8:006. [Google Scholar]


