Abstract
Aims/Introduction
Gestational diabetes mellitus (GDM) is defined as ‘carbohydrate intolerance of varying degrees of severity with onset or first recognition during pregnancy,’ and is associated with increased fetal and maternal risks. The aims of the present study were to investigate the prevalence of GDM in Scotland over 32 years (1981–2012), and using the data from 2012, to assess how GDM related to maternal body mass index, maternal age, parity, smoking, Scottish Index of Multiple Deprivation, infant gender and macrosomia status.
Materials and Methods
GDM prevalence along with anthropometric, obstetric and demographic data were collected on a total of 1,891,097 women with a delivery episode between 1 January 1981 and 31 December 2012 using data extracted from the Scottish Morbidity Record 02. Univariate and multivariate logistic regression analysis was undertaken to investigate their association with GDM.
Results
A ninefold increase in GDM prevalence was observed from 1981 to 2012 (P < 0.001). GDM prevalence in 2012 was 1.9%. Maternal body mass index, age, parity status, Scottish index of multiple deprivation and fetal macrosomia were positively associated with GDM. Reported smoking status at booking was inversely associated with GDM. Multivariable analysis showed that fetal macrosomia was not associated with GDM status.
Conclusions
The present study confirmed that the reporting of GDM is low in Scotland, and that GDM is associated with maternal body mass index, maternal age, multiparity and social deprivation. GDM was negatively associated with smoking and requires further investigation. The lack of association between GDM and macrosomia (following multivariate analysis) might reflect the screening processes undertaken in Scotland.
Keywords: Body mass index, Gestational diabetes mellitus, Macrosomia
Introduction
Gestational diabetes mellitus (GDM) is defined as ‘carbohydrate intolerance of varying degrees of severity with onset or first recognition during pregnancy’1, 2. Although traditionally deemed not as dangerous for the developing fetus as developing diabetes prepregnancy, we now know that GDM has serious long‐term consequences for both the baby and the mother3, 4. The altered intrauterine milieu of hyperglycemia and associated fetal hyperinsulinemia promotes fetal growth and adiposity, which can be referred to as ‘fetal overnutrition’. Neonatal hyperbilirubinemia, hypoglycemia, hypocalcemia, erythremia, poor feeding, respiratory distress syndrome and pre‐eclampsia are also recognized complications of GDM5, 6. Infants born to mothers who have glucose intolerance have 20% higher body fat than infants born to mothers with normal glucose tolerance6. The cesarean section delivery rate is increased in patients with GDM6. This is in part to avoid birth trauma, particularly to avoid the risks of shoulder dystocia and newborn asphyxia, both associated with large‐for‐gestational‐age newborns. Epidemiological research confirms that women who have gestational diabetes have a significant increased risk of type 2 diabetes later in life7, 8, and also suggests that excessive fetal growth is associated with glucose intolerance and obesity in the offspring9, 10.
There are few areas in diabetes that are associated with as much debate and discussion as the diagnosis of GDM11. Evidence suggests early detection and management of gestational diabetes improves outcomes for both mother and child12. In 2001, the Scottish Intercollegiate Network Guidelines (SIGN 55) provided guidelines for the screening of GDM in Scotland. In 2010, based on the International Association of Diabetes and Pregnancy Study Groups Consensus Panel13, SIGN 116 provided further guidelines for GDM with lower plasma glucose levels14. The aim of the present study was to investigate the reported prevalence of GDM in Scotland over 31 years (1981–2012), and to assess how the risk factors of maternal body mass index (BMI), maternal age, parity, patients’ socioeconomic status, smoking status at booking, and the change in the diagnostic criteria related to the reported prevalence of GDM and macrosomia.
Methods
Data source
The linked Scottish Morbidity Record (SMR02) was established in 1975 and collects data from maternity hospitals that are submitted to the Information Services Division (ISD) of National Health Service (NHS) National Services Scotland. Women delivering at home or in non‐NHS hospitals were not included in this data collection. Data were extracted in July 2012 and July 2014 from SMR02, for a total of 1,891,097 women with a delivery episode, discharged from hospital between 1 January 1981 and 31 December 2012. The data extracted were aggregated into the following 5‐year periods, 1981–1985; 1986–1990; 1991–1995; 1996–2000; 2001–2005; 2006–2010, plus the years 2011 and 2012.
Variables
The BMI of pregnant women was defined as <25 kg/m2 (underweight and normal), 25–30 kg/m2 (overweight), 30–40 kg/m2 (obese) or BMI data not known. Data relating to weight and height were optionally recorded before 2003, and have only been made mandatory since April 2011, at antenatal ‘booking’ clinics, which take place first at week 12 of pregnancy.
Obstetric variables characterized GDM status, parity status, smoking status during pregnancy, number of births during the delivery episode, fetal macrosomia status of the offspring and offspring gender. Mothers were defined as having GDM if coded as ‘gestational diabetes’ or if any of the diagnosis were coded as O244 (ICD10) or 6488 (ICD9) in the SMR02 dataset, otherwise they were classified as not having GDM. Parity status of the mothers was defined as multiparous, primiparous or not known. Maternal smoking status defined as yes, no or not known, was derived from self‐reported information obtained from mothers at their antenatal booking visit only from 1993 onwards. The number of births during this delivery episode was defined as singleton or multiple. Fetal macrosomia status of the offspring was defined as babies birthweight <4,000 g (no fetal macrosomia) or ≥4,000 g (fetal macrosomia). Offspring gender was defined as female, male and other/not known.
Demographic variables were characterized by maternal age and Scottish Index of Multiple Deprivation (SIMD) 2012. Maternal age was grouped as <24 years, 25–29 years, 30–34 years, 35–39 years, ≥40 years or not known. The 2012 SIMD quintiles, defined as 1 (most deprived), 2, 3, 4 or 5 (least deprived) and not known, provided an area‐based measure of deprivation of the mother–child dyad. SIMD replaced the Carstairs index in 2004, and ranks the 6,505 geographic data zones in Scotland on the basis of the level of deprivation, with 31 indicators across seven domains, including current income, housing and health15.
The number of delivery episodes and percentages observed within the anthropometric, obstetric, and demographic variables for the intervals 1981–2010, 2006–2010, 2011 and 2012 are summarized in Table 1.
Table 1.
Summary of anthropometric, obstetric and demographic variables for all National Health Service delivery episodes in Scotland from January 1981 to December 2010
| Characteristics | 1981–2010 | 2006–2010 | 2011 | 2012 | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| BMI (kg/m2) | 1,776,845 | 100.0 | 281,983 | 100.0 | 57,018 | 100.0 | 57,234 | 100.0 |
| <25 | 124,041 | 7.0 | 94,608 | 33.6 | 25,434 | 44.6 | 24,995 | 43.7 |
| 25–30 | 67,447 | 3.8 | 51,586 | 18.3 | 13,669 | 24.0 | 23,690 | 41.4 |
| 30–40 | 48,511 | 2.7 | 37,282 | 13.2 | 10,371 | 18.2 | 13,508 | 23.6 |
| Not known | 1,536,846 | 86.5 | 98,507 | 34.9 | 7,544 | 13.2 | 8,444 | 14.8 |
| GDM | 1,776,845 | 100.0 | 281.983 | 100.0 | 57.018 | 100.0 | 57,234 | 100.0 |
| Yes | 8,323 | 0.5 | 2,156 | 0.8 | 723 | 1.3 | 1,098 | 1.9 |
| No | 1,768,522 | 99.5 | 279,827 | 99.2 | 56,295 | 98.7 | 56,136 | 98.1 |
| Parity status | 1,776,845 | 100.0 | 281,983 | 100.0 | 57,018 | 100.0 | 57,234 | 100.0 |
| Multiparous | 971,996 | 54.7 | 145,553 | 51.6 | 29,235 | 51.3 | 31,711 | 55.4 |
| Primiparous | 799,769 | 45.0 | 135,268 | 48.0 | 26,923 | 47.2 | 25,415 | 44.4 |
| Not known | 5,080 | 0.3 | 1,162 | 0.4 | 860 | 1.5 | 108 | 0.2 |
| Smoking status | 1,776,845 | 100.0 | 281,983 | 100.0 | 57,018 | 100.0 | 57,234 | 100.0 |
| Yes | 232,360 | 37.0 | 52,133 | 18.5 | 10,292 | 18.1 | 10,226 | 17.9 |
| No | 657,668 | 13.1 | 197,528 | 70.0 | 43,025 | 75.5 | 43,824 | 76.6 |
| Not known | 886,817 | 49.9 | 32,322 | 11.5 | 3,701 | 6.5 | 3,184 | 5.6 |
| Maternal age | 1,776,845 | 100.0 | 281,983 | 100.0 | 57,018 | 100.0 | 57,234 | 100.0 |
| ≤24 years | 567,929 | 32.0 | 73,786 | 26.2 | 13,776 | 24.2 | 13,341 | 23.3 |
| 25–29 years | 561377 | 31.6 | 75,101 | 26.6 | 15,518 | 27.2 | 15,819 | 27.6 |
| 30–34 years | 435491 | 24.5 | 76,728 | 27.2 | 16,384 | 28.7 | 16,958 | 29.6 |
| ≥35 years | 212037 | 11.9 | 56,368 | 20.0 | 11,340 | 19.9 | 11,114 | 19.4 |
| Not known | 11 | 0.001 | 0 | 0 | 0 | 0.0 | 2 | 0.003 |
| SIMD quintiles | 1,776,845 | 100.0 | 281,983 | 100.0 | 57,018 | 100.0 | 57,234 | 100.0 |
| 1 | 484,447 | 27.3 | 72,605 | 25.7 | 15,161 | 26.6 | 15,100 | 26.4 |
| 2 | 352,668 | 19.8 | 58,677 | 20.8 | 12,105 | 21.2 | 12,197 | 21.3 |
| 3 | 313,108 | 17.6 | 52,733 | 18.7 | 10,638 | 18.7 | 10,930 | 19.1 |
| 4 | 297,438 | 16.7 | 50,788 | 18.0 | 9,916 | 17.4 | 9,728 | 17.0 |
| 5 | 290,896 | 16.4 | 45,993 | 16.3 | 8,898 | 15.6 | 8,952 | 15.6 |
| Not known | 38,288 | 2.2 | 1,187 | 0.4 | 300 | 0.5 | 327 | 0.6 |
| Fetal macrosomia | – | – | – | – | – | – | 57,234 | 100.0 |
| No | – | – | – | – | – | – | 49,380 | 86.3 |
| Yes | – | – | – | – | – | – | 7,854 | 13.7 |
| Births this pregnancy | – | – | – | – | – | – | 57234 | 100.0 |
| Multiple | – | – | – | – | – | – | 1,691 | 3.0 |
| Singleton | – | – | – | – | – | – | 55,543 | 97.0 |
| Offspring gender | 57,234 | 100.0 | ||||||
| Female | – | – | – | – | – | – | 27,868 | 48.7 |
| Male | – | – | – | – | – | – | 29,360 | 51.3 |
| Other/not Known | – | – | – | – | – | – | 6 | 0.01 |
Summary of anthropometric (body mass index [BMI]), obstetric (gestational diabetes mellitus [GDM] status, parity status, smoking status during pregnancy, number of births during the delivery episode, fetal macrosomia status, infant gender) and demographic (maternal age, Scottish Index of Multiple Deprivation [SIMD]) variables for all national Health Service delivery episodes in Scotland from 1 January 1981 to 31 December 2010 (dataset reference: IR2012‐01211; n = 1,776,845), 1 January 2006 to 31 December 2010 (dataset reference: subgroup from IR2012‐01211, n = 281,983), 1 January 2011 to 31st December 2011 (Dataset reference: IR2013‐02036; n = 57,018) and 1st January 2012 to 31 December 2012 (dataset reference: IR2015‐00505_macrosomia; n = 57,234). The datasets also include cases labeled as ‘not known’.
Data analysis
GDM prevalence estimates were determined as a proportion of the total number of NHS delivery episodes for each of the following 5‐year periods, 1981–1985, 1986–1990, 1991–1995, 1996–2000, 2001–2005, 2006–2010 plus 2011 and 2012. The results were reported as estimated GDM prevalence ± standard error (%). Standard error (%) was calculated to illustrate the uncertainty of the GDM prevalence estimates using the following formula: , where ‘x’ is the number of GDM cases and ‘n’ is the total number of NHS delivery episodes diagnosed with GDM plus total number of NHS delivery episodes not diagnosed with GDM.
The fold‐change in estimated GDM prevalence was calculated before and after the introduction of the SIGN 116 guidance to gain insight into how changes in screening and diagnostic criteria affected the estimated GDM prevalence levels. Likewise an online survey carried out by Stirrat et al. investigating the screening and management of GDM in 15 Scottish maternity units have been published elsewhere16.
Statistical analysis
All statistical analysis was carried out using IBM spss 19 (IBM, Armonk, NY, USA). The χ2‐test was carried out to (i) assess trends of estimated GDM prevalence over the time‐period of 1981–2012; all the data extracted from the linked SMR02 was included in this analysis; and (ii) assess trends/associations between GDM status and risk factor variables using the 2006–2010 subgroup from the NHS delivery episode dataset (reference dataset: IR2012‐01211) and 2012 NHS delivery episodes dataset (reference dataset: IR2015‐00505_macrosomia; Table 2. Using the 2012 NHS delivery episodes dataset, univariate logistic regression models were created to examine the magnitude of association between the dependent variable, GDM status, and the following independent variables (i) maternal BMI status; (ii) maternal age groups; (iii) parity status; (iv) smoking status; (v) SIMD quintile status; or (vi) fetal macrosomia status. The results were reported as odds ratio (OR) with their respective 95% confidence interval (CI). Finally, a multivariate logistic regression model was created to identify factors that have a significant independent influence on GDM status and their magnitude of effect. The results were reported as adjusted odds ratio (AOR) with their respective 95% CI. When creating the logistic regression statistical models, cases with one or more data points classified as ‘not known’ were excluded (n = 9,944). The working file contained 47,290 cases for the statistical analysis results presented in Table 2.
Table 2.
Univariate and multivariate associations with gestational diabetes mellitus status and risk factors
| Total | No. GDM (referent) | GDM yes | OR | 95% CI | AOR † | 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | χ2‐test (P)2 | AOR ‡ | 95% CI | |||||
| BMI (kg/m2) | 47,290 | 100 | 46,317 | 100 | 973 | 100 | χ2(1) = 758.8 § | ||||||
| <25 | 24,221 | 51.2 | 24,049 | 51.9 | 172 | 17.7 | P < 0.001 | 1.0 | Reference | 1.0 | Reference | ||
| 25–30 | 13,071 | 27.6 | 12,826 | 27.7 | 245 | 25.2 | 2.7 | 2.2–3.3 | 2.5 | 2.1–3.1 | |||
| 30–40 | 9,998 | 21.1 | 9,442 | 20.4 | 556 | 57.1 | 8.2 | 6.9–9.8 | 7.7 | 6.5–9.2 | |||
| Maternal age | 47,290 | 100 | 46,317 | 100 | 973 | 100 | χ2(1) = 170.8 § | ||||||
| ≤24 years | 10,920 | 23.1 | 10,806 | 23.3 | 114 | 11.7 | P < 0.001 | 1.0 | Reference | ||||
| 25–29 years | 13,090 | 27.7 | 12,880 | 27.8 | 210 | 21.6 | 1.5 | 1.2–1.9 | |||||
| 30–34 years | 14,030 | 29.7 | 13,714 | 29.6 | 316 | 32.5 | 2.2 | 1.8–2.7 | |||||
| ≥35 years | 9,250 | 19.6 | 8,917 | 19.3 | 333 | 34.2 | 3.5 | 2.9–4.4 | |||||
| Parity status | 47,290 | 100 | 46,317 | 100 | 973 | 100 | χ2(1) = 170.8 ¶ | ||||||
| Primiparous | 20,698 | 43.8 | 20,335 | 43.9 | 363 | 37.3 | P < 0.001 | 1.0 | Reference | ||||
| Multiparous | 26,592 | 56.2 | 25,982 | 56.1 | 610 | 62.7 | 1.3 | 1.2–1.5 | |||||
| Smoking status | 47,290 | 100 | 46,317 | 100 | 973 | 100 | χ2(1) = 12.4 ¶ | ||||||
| No | 38,369 | 81.1 | 37,537 | 81.0 | 832 | 85.5 | P < 0.001 | 1.0 | Reference | ||||
| Yes | 8,921 | 18.9 | 8,780 | 19.0 | 141 | 14.5 | 0.7 | 0.6–0.9 | |||||
| SIMD quintile | 47,290 | 100 | 46,317 | 100 | 973 | 100 | χ2(1) = 6.5 § | ||||||
| 1 ‐ Most deprived | 11,305 | 23.9 | 11,052 | 23.9 | 253 | 26.0 | P = 0.011 | 1.3 | 1.1–1.7 | ||||
| 2 | 10,264 | 21.7 | 10,043 | 21.7 | 221 | 22.7 | 1.3 | 1.0–1.6 | |||||
| 3 | 9,501 | 20.1 | 9,305 | 20.1 | 196 | 20.1 | 1.2 | 1.0–1.5 | |||||
| 4 | 8,484 | 17.9 | 8,311 | 17.9 | 173 | 17.8 | 1.2 | 1.0–1.5 | |||||
| 5 ‐ Least deprived | 7,736 | 16.4 | 7,606 | 16.4 | 130 | 13.4 | 1.0 | Reference | |||||
| Fetal macrosomia | 47,290 | 100 | 46,317 | 100 | 973 | 100 | χ2(1) = 8.1 ¶ | ||||||
| No | 40,742 | 86.2 | 39,934 | 86.2 | 808 | 83.0 | P = 0.005 | 1.0 | Reference | 1.0 | Reference | ||
| Yes | 6,548 | 13.8 | 6,383 | 13.8 | 165 | 17.0 | 1.3 | 1.1–1.5 | 1.0 | 0.8–1.2 | |||
| Births | 47,290 | 100 | 46,317 | 100 | 973 | 100 | χ2(1) = 1.4 ¶ | ||||||
| Single | 45,936 | 97.1 | 44,997 | 97.2 | 939 | 96.5 | P = 0.233 | ||||||
| Multiple | 1,354 | 2.9 | 1,320 | 2.8 | 34 | 3.5 | |||||||
| Gender | 47,290 | 100 | 46,317 | 100 | 973 | 100 | χ2(1) = 0.8 ¶ | ||||||
| Male | 24,297 | 51.4 | 23,811 | 51.4 | 486 | 49.9 | P = 0.367 | ||||||
| Female | 22,993 | 48.6 | 22,506 | 48.6 | 487 | 50.1 | |||||||
Univariate and multivariate associations with gestational diabetes mellitus (GDM) status and risk factors (maternal body mass index [BMI]/maternal age/maternal parity status/maternal smoking status during pregnancy/maternal Social Index of Multiple Deprivation (SIMD)/fetal macrosomia/number of births during the delivery episode/infant gender). †Adjusted for maternal BMI, maternal age, parity status, smoking status and maternal SIMD status. ‡Adjusted for maternal BMI, maternal age, parity status, smoking status, maternal SIMD status and fetal macrosomia. National Health Service delivery episodes in Scotland from 1 January 2012 to 31 December 2012 (dataset reference: IR2015‐00505_macrosomia; n = 57,234) with n = 9,944, data‐points classified as ‘not known’ removed from the sample. §The χ2‐test for trend. ¶Pearson's χ2‐test. AOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
Results
During the survey period 1981–2010, estimated GDM prevalence, calculated as a proportion of total number of NHS delivery episodes in each 6‐year period, increased fourfold from 0.21 ± 0.01% (n = 690/325,953) in 1981–1985, to 0.76 ± 0.02% (n = 2,156/281,983) in 2006–2010 (χ2[1] = 1243.0; P < 0.001). After the introduction of the 2010 SIGN 116, a further 2.5‐fold increase in estimated GDM prevalence was observed between time‐points 2006–2010 and 2012. Overall, a ninefold increase in GDM prevalence was observed from 1981 to 2012 (χ2[1] = 2,987.8; P < 0.001). GDM prevalence in 2012 was 1.92 ± 0.06% (n = 1,097/57,231; Figure 1).
Figure 1.
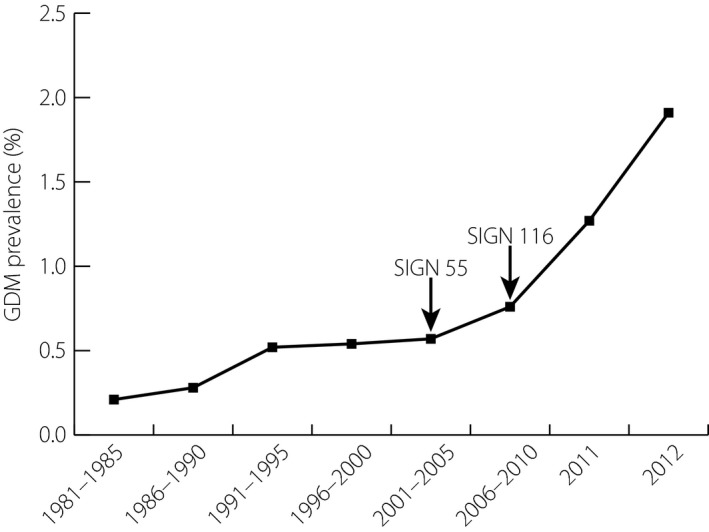
Reported prevalence of gestational diabetes mellitus (GDM) across the 5‐year periods from 1 January 1981 to 31 December 2010 (dataset reference: IR2012‐01211; n = 1,776,845), from 1 January 2011 to 31 December 2011 (dataset reference: IR2013‐02036; n = 57,018) and from 1 January 2012 to 31 December 2012 (dataset reference: IR2015‐00505_macrosomia; n = 57,234) based on data submitted to the Information Services Division of NHS National Services Scotland from Scottish Maternity Hospitals. The vertical bars represent standard errors for each point estimate of GDM prevalence. The arrows illustrate the introduction of the Scottish Intercollegiate Network Guidelines SIGN 55 and 116 ‘Management of Diabetes’ guidelines. The χ2‐test for trend (1) = 2,987.8; P < 0.001.
Association between maternal characteristics and GDM prevalence
Within the 2006–2010 NHS delivery episode sample, GDM prevalence showed a positive monotonic association with maternal BMI (χ2[1] = 1,215.8, P < 0.001), maternal age (χ2[1] = 328.4, P < 0.001) and maternal parity status (χ2[1] = 66.5, P < 0.001). GDM subgroup analysis of BMI distribution within the 2012 GDM sample (n = 973) showed that 17.7% (172/973) had BMI <25 kg/m2, 25.1% (245/973) had BMI 25–30 kg/m2 and 57.1% (556/973) had BMI 30–40 kg/m2. Maternal smoking status was inversely associated with GDM (χ2[1] = 12.4, P < 0.001; Table 2). In the 2011 and 2012 NHS delivery episode samples, similar GDM prevalence trends were observed across the maternal BMI, maternal age, maternal parity status and maternal smoking status stratified groups. In the 2012 sample, there was a clear monotonic decrease in GDM prevalence as maternal deprivation decreased from the most deprived SIMD quintile 1, to the least deprived SIMD quintile 5, (χ2[1] = 6.5, P = 0.011; Table 2). There was no association between single or multiple births, or the sex of the baby and GDM.
Association between GDM and fetal macrosomia
The univariate model showed that the presence of maternal GDM was a significant positive predictor of fetal macrosomia (OR 1.3, 95% CI 1.1–1.5). However, after adjusting for maternal BMI, SIMD, maternal age, parity and maternal smoking status, fetal macrosomia was not associated with maternal GDM status (AOR 1.0, 95% CI 0.8–1.2).
Association between fetal macrosomia and SIMD after adjustment for maternal BMI
Both maternal obesity and the presence of GDM were observed to be positively associated with the presence of fetal macrosomia. Fetal macrosomia was not influenced by either maternal BMI or maternal age when SIMD status was taken into account. Fetal macrosomia was inversely associated with SIMD quintile 1 (AOR 0.7, 95% CI 0.7–0.8), SIMD quintile 2 (AOR 0.8, 95% CI 0.8–0.9), SIMD quintile 3 (AOR 0.9, 95% CI 0.8–1.0) and positively associated with SIMD quintile 4 (AOR 1.1, 95% CI 1.0–1.2), when compared with the least deprived SIMD quintile 5 after adjustment for maternal BMI. The addition of maternal age to the model did not change the observed AORs reported above.
Association between fetal macrosomia and maternal age
The prevalence of fetal macrosomia in the sample of women who were obese (BMI 30–40 kg/m2) was 15.2% (318/2,094), 19.4% (567/2,928), 21.1% (600/2,847), and 19.0% (405/2,129) in those aged ≤24 years, 25–29 years, 30–34 years and ≥35 years, respectively. In those with a BMI <25 kg/m2 and aged ≤24 years, 25–29 years, 30–34 years or ≥35 years, the prevalence of fetal macrosomia was 8.5% (522/6118), 10.9% (725/6629), 11.6% (836/7179) and 12.5% (536/4295), respectively. After adjustment for maternal BMI, it was observed that mothers aged ≤24 years were less likely to give birth to an offspring with fetal macrosomia (AOR 0.7, 95% CI 0.7–0.8) compared with those aged ≥35 years. Obese mothers (BMI 30–40 kg/m2) were 1.9‐fold (95% CI 1.8–2.0) more likely to have a baby with fetal macrosomia, after adjusting the model for maternal age. Obese mothers were 8.0‐fold (95% CI 6.7–9.5) more likely to be diagnosed with GDM, so it was unexpected that fetal macrosomia was not associated with GDM.
Association between fetal macrosomia and smoking during pregnancy
Further analysis showed that smoking at booking was found to be inversely associated with fetal macrosomia (AOR 0.4, 95% CI 0.4–0.4) before and after adjusting the model for the confounding factors describing maternal characteristics. There was a minimal association observed between fetal macrosomia and maternal SIMD quintile 1 (AOR 0.9, 95% CI 0.8–1.0), SIMD quintile 2 (AOR 1.0, 95% CI 0.9–1.1), SIMD quintile 3 (AOR 1.0, 95% CI 0.9–1.1) and SIMD quintile 4 (AOR 1.1, 95% CI 1.0–1.2) compared with SIMD quintile 5, after adjustment for just smoking status. The prevalence of smoking at booking was 32.1% (3,626/11,305), 23.4% (2,402/10,264), 15.9% (1,511/9,501), 10.9% (924/8,484), and 5.9% (458/7,736) in SIMD quintile 1, 2, 3, 4 and 5, respectively.
Discussion
The major strength of our observations is that they were taken from a national database, containing a large number of patient data, with a proven track record of accuracy and quality of data17, 18. The method of record linkage used in Scotland is estimated to result in mismatched records in less than 2% of cases16. In the present study we showed a fourfold increase in reported GDM over 31 years (1981–2010), with a further significant rise after the introduction of new guidelines in 2010. The prevalence of GDM in 2012 in the present study was 1.9%, which is very similar to the recently published Scottish data from Stirrat et al.19 The present study confirmed the association of GDM with maternal BMI, maternal age, multiparity and social deprivation20, 21. However, we found that GDM was negatively associated with smoking. In addition, after adjusting for maternal BMI, SIMD, maternal age, parity and maternal smoking status, we showed that fetal macrosomia was not associated with maternal GDM status. There was no association between single, multiple births or the sex of the baby and GDM.
A few studies have investigated the association between smoking and GDM, and have shown both positive and negative correlations22, 23. For example, the Nurse Cohort study involving USA women showed a significant association, with a relative risk for GDM of 1.43 in pregravid current smokers24. Contrary to the popular belief that smokers have a lower BMI compared with non‐smokers25, the present study showed that within the GDM sample 58.0% of smokers were obese, whereas just 21.2% of smokers had BMI <25 kg/m2. There are, however, some limitations to the way data relating to smoking was collected in the present study. We do not know if patients continued to smoke during their pregnancy or if smoking had an effect on maternal weight during the pregnancy. Although smoking seems to be associated with reduced infant weight, it masks potential short‐term and long‐term adverse health conditions in both the mother and infant. Further investigation is clearly required to understand the association between smoking and GDM.
We also found that after multivariate analysis, fetal macrosomia was not associated with GDM. This might be explained by the fact that over 80% of the sample had a BMI <30 kg/m2 at booking. It is possible that maternal obesity and the associated insulin resistance is a bigger influence on macrosomia than GDM. Another explanation might lie in the screening process. Estimates of up to 4–6% of pregnancies having GDM are reported in the majority of the studies across Europe and North America.11, 26 We are aware that in Scotland there are significant inconsistencies between health boards related to risk factor screening, and that we are missing a significant number of patients with GDM19. The lack of association between macrosomia and GDM might reflect that we are screening more overweight/obese, socially deprived pregnant patients and missing the less heavy, less deprived patients. Systematic review and meta‐analysis have found that screening using risk factors alone produces low sensitivities (50–69%) and low specificities (58–69%)27. Maternity units that are using screening risk factors alone, or only screening at a higher BMI might be significantly under diagnosing GDM.
This then begs the questions as to whom, how and when should screening take place? We know from large and well‐powered randomized controlled trials plus observational studies that appropriate management reduces the risks of GDM28, 29, 30. These studies have purportedly clarified some of the main issues relating to GDM and have informed national clinical guidelines. However, there are still no internationally agreed diagnostic criteria11, 31. Agreed criteria are required to train clinicians about GDM screening, to improve clinical practice and to aid further research. With increased provision of information and appropriate screening, we anticipate that the reported prevalence of GDM will increase significantly. In an already stretched NHS, the diagnosis of more GDM patients will put more pressure on available resources. Obesity, with its associated problems, appears to be the main driver in the development GDM. Concerted efforts by national government, together with community services, schools, sports clubs and local councils along with the NHS are required to raise awareness and campaign for optimal lifestyle management during childhood, adolescence, particularly pregnancy and adult life to curb the continuing obesity epidemic.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2017; 8: 161–167
References
- 1. Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop‐Conference on Gestational Diabetes Mellitus. Diabetes Care 1998; 21(Suppl 2): 161–167. [PubMed] [Google Scholar]
- 2. World Health Organisation (WHO) . Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Geneva: WHO; 2013. Available from: http://apps.who.int/iris/bitstream/10665/85975/1/WHO_NMH_MND_13.2_eng.pdf Accessed December 5, 2015. [PubMed] [Google Scholar]
- 3. Ovesen PG, Jensen DM, Damm P, et al Maternal and neonatal outcomes in pregnancies complicated by gestational diabetes. A nation‐wide study. J Matern Fetal Neonatal Med 2015; 28: 1720–1724. [DOI] [PubMed] [Google Scholar]
- 4. Xiong X, Saunders L, Wang F, et al Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J Gynecol Obstet 2001; 75: 221–228. [DOI] [PubMed] [Google Scholar]
- 5. Lawlor DA, Smith GD, O'Callaghan M, et al Epidemiologic Evidence for the Fetal Overnutrition Hypothesis: findings from the Mater‐University Study of Pregnancy and Its Outcomes. Am J Epidemiol 2007; 165: 418–424. [DOI] [PubMed] [Google Scholar]
- 6. Stotland NE, Caughey AB, Breed EM, et al Risk factors and obstetric complications associated with macrosomia. Int J Gynaecol Obstet 2004; 87: 220–226. [DOI] [PubMed] [Google Scholar]
- 7. Bellamy L, Casas J, Hingorani A, et al Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta‐analysis. Lancet 2009; 373: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 8. Engeland A, Bjørge T, Daltveit AK, et al Risk of diabetes after gestational diabetes and preeclampsia. A registry‐based study of 230,000 women in Norway. Eur J Epidemiol 2011; 26: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lawlor DA, Fraser A, Lindsay R, et al Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 2010; 53: 89–97. [DOI] [PubMed] [Google Scholar]
- 10. Wright C, Rifas‐Shiman S, Rich‐Edwards J, et al Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens 2009; 22: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bilous R. Diagnosis of gestational diabetes, defining the net, refining the catch. Diabetologia 2015; 58: 1965–1968. [DOI] [PubMed] [Google Scholar]
- 12. Scottish Intercollegiate Guidelines Network (SIGN) . Management of Diabetes. A national clinical guideline SIGN 2001; no. 55. Available from: www.elib.scot.nhs.uk/portal/upload/sign55.pdf Accessed December 5, 2015.
- 13. International Association of Diabetes and Pregnancy Study Groups Consensus Panel , Metzger BE, Gabbe SG, et al International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scottish Intercollegiate Guidelines Network (SIGN) . Management of Diabetes. A national clinical guideline. SIGN; 2010; no. 116. Available from: www.sign.ac.uk/pdf/sign116.pdf Accessed December 5, 2015. [Google Scholar]
- 15. The Scottish Government (Riaghaltas na h‐Alba) .Statistics: Scottish Index of Multiple Deprivation. 2009. Available from: www.scotland.gov.uk/Topics/Statistics/SIMD/ Accessed December 5, 2015.
- 16. Harley K, Jones C. Quality of Scottish Morbidity Record (SMR) data. Health Bull (Edin) 1996; 54: 410–417. [PubMed] [Google Scholar]
- 17. Kendrick S, Clarke J. The Scottish record linkage system. Health Bull (Edinb) 1993; 51: 72–79. [PubMed] [Google Scholar]
- 18. NHS Information Services Division (ISD) published report on SMR02 . Available from: http://www.isdscotland.org/Health-Topics/Maternity-and-Births/Births/DQA-Assessment-of-Maternity-Data-SMR02-2008-to-2009.pdf Accessed December 5, 2015.
- 19. Stirrat LI, Denison FC, Love CDB, et al Screening and management of gestational diabetes mellitus in Scottish obstetric units: a national survey. Scot Med J 2015; 60: 37–43. [DOI] [PubMed] [Google Scholar]
- 20. Janghorbani M, Stenhouse EA, Jones RB, et al Is neighbourhood deprivation a risk factor for gestational diabetes mellitus? Diab Med 2006; 23: 313–317. [DOI] [PubMed] [Google Scholar]
- 21. Heslehurst N, Ells L, Simpson H, et al Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15‐year period. BJOG 2007; 114: 187–194. [DOI] [PubMed] [Google Scholar]
- 22. Wendland E, Pinto M, Duncan B, et al Cigarette smoking and risk of gestational diabetes: a systematic review of observational studies. BMC Pregnancy Childbirth 2008; 8: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terry PD, Weiderpass E, Östenson C, et al Cigarette smoking and the risk of gestational and pregestational diabetes in two consecutive pregnancies. Diabetes Care 2003; 26: 2994–2998. [DOI] [PubMed] [Google Scholar]
- 24. Solomon C, Willett W, Carey V, et al A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 1997; 278: 1078–1083. [PubMed] [Google Scholar]
- 25. Chiolero A, Faeh D, Paccaud F, et al Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr 2008; 87: 801–809. [DOI] [PubMed] [Google Scholar]
- 26. Buckley BS, Harreiter J, Damm P, et al Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabet Med 2012; 29: 844–854. [DOI] [PubMed] [Google Scholar]
- 27. Scott DA, Loveman E, McIntyre L, et al Screening for gestational diabetes: a systematic review and economic evaluation. Health Technol Assess 2002; 6: 1–161. [DOI] [PubMed] [Google Scholar]
- 28. Crowther CA, Hiller JE, Moss JR, et al Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005; 352: 2477–2486. [DOI] [PubMed] [Google Scholar]
- 29. HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 30. Landon MB, Spong CY, Thom E, et al A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009; 361: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meek CL, Lewis HB, Patient C, et al Diagnosis of gestational diabetes mellitus: falling through the net. Diabetologia 2015; 58: 2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


