Abstract
Estradiol supplementation has been shown to enhance cognitive performance in old ovariectomized rhesus macaques (Macaca mulatta). To determine if similar benefits could be achieved in perimenopausal animals using alternative hormonal supplements we administered dehydroepiandrosterone (DHEA) to old ovary-intact female rhesus macaques for ~2.5 months. Using computerized touchscreen memory tasks, including delayed response (DR) and delayed matching-to-sample (DMS), we observed improved performance with time in all of the animals but failed to detect a significant effect of DHEA. On the other hand, gene expression profiling disclosed a significant correlation between cognitive performance and the expression of several steroidogenic and steroid-responsive genes. DR performance was positively correlated with hippocampal expression of AKR1C3 and STAR, and negatively correlated with the expression of SDRD5A1. A positive correlation was also found between DMS performance and prefrontal cortical expression of AKR1C3, and a negative correlation with STAR, as well as a negative correlation with the hippocampal expression of HSD11B1 and NR3C1. Taken together, the results suggest that steroidogenic gene regulation within the brain may help to maintain cognitive function during the perimenopausal transition period, despite a decline in sex-steroid levels in the circulation.
Keywords: cognition, cortisol, cortisone, dehydroepiandrosterone, estradiol, hippocampus, hormone therapy, menopause, perimenopause, prefrontal cortex
Introduction
Like women, old female rhesus macaques show a marked decline in circulating levels of estradiol (E2) (Downs & Urbanski, 2006; Gilardi, 1997), and this hormonal change is thought to exacerbate many age-associated physiological changes including cognitive decline (LeBlanc et al. 2001; Maki & Hogervorst, 2003; Roberts et al. 1997; Sherwin, 2006). Not surprisingly, therefore, studies performed in ovariectomized female rhesus macaques have mostly reported significant improvements in memory after E2 supplementation (Baxter et al. 2013; Lacreuse, 2006; Lacreuse et al. 2002; Rapp et al., 2003a; Kohama et al. 2016; Tinkler & Voytko, 2005; Voytko, 2000; Voytko et al. 2008). However, few studies have examined the therapeutic potential of other steroid hormones, especially those that serve as precursors for estrogen synthesis in the brain. For example, young adult rhesus macaques and humans both show high concentrations of the adrenal hormone dehydroepiandrosterone (DHEA), and its sulfate DHEAS, in their circulations. In addition, the hippocampus (HPC) expresses genes that encode key enzymes in the intracrine conversion of DHEA to E2 (Sorwell et al. 2012). In theory, therefore, rhesus macaques and humans have the potential to maintain elevated E2 concentrations in cognitive brain areas, even when ovarian-derived E2 levels in the circulation have been significantly attenuated, such as after menopause or as a result of ovariectomy. Unfortunately, the circulating levels of DHEA and DHEAS also decrease markedly during aging (Downs et al. 2008; Labrie et al. 1998, 2007; Rainey et al. 2002; Sorwell & Urbanski, 2010; Sorwell et al. 2012, 2014; Urbanski et al. 2013), suggesting that endogenous levels of DHEA and DHEAS in the elderly may be too low to serve as effective precursors for estrogen synthesis in the brain. On the other hand, this raises the issue of whether daily supplementation with exogenous DHEA can increase brain estrogen levels sufficiently to have therapeutic potential for age-associated cognitive decline, while avoiding negative side effects associated with continuously elevated estrogen levels in the circulation.
DHEA is available in the USA without prescription, as a food supplement, and is already widely used by the elderly for self-medication (Wold et al. 2005). However, the efficacy of DHEA supplementation on improving cognitive function in humans remains unproven, with the majority of clinical studies failing to demonstrate any significant beneficial effect (Davis et al. 2008, 2011; Grimley-Evans et al. 2006; Scheffers et al. 2015). Therefore, the goals of the present study were twofold: 1) to examine the pro-cognitive effects of DHEA supplementation in perimenopausal female rhesus macaques, taking advantage of the highly controlled environmental conditions under which these animals are maintained; and 2) to gain insights into the underlying mechanism, by correlating cognitive performance with steroidogenic gene expression in cognitive centers of the brain. We hypothesized that supplementing perimenopausal females with physiological levels of DHEA would result in significant intracrine conversion of DHEA to E2 in cognitive brain centers and thereby compensate for the perimenopausal decline in estrogen production by the ovaries. Consequently, we expected to observe significantly better cognitive performance in animals while undergoing DHEA supplementation. However, our results failed to support this hypothesis; instead, they support the existence of an alternate neuroendocrine mechanism in the HPC and PFC that may help to maintain cognitive function during the menopausal transition period.
Materials and methods
Experimental animals
This study was performed with approval from the OHSU Institutional Animal Care and Use Committee at the Oregon National Primate Research Center (ONPRC), using female rhesus macaques (Macaca mulatta). The animals were housed indoors under controlled environmental conditions: 24° C temperature; 12-h light and 12-h dark photoperiods (lights on at 07:00 h). They were pair-caged and fed High Protein Monkey Chow (LabDiet Inc., St. Louis, MO) twice daily, supplemented with fresh fruits and vegetables; drinking water was available ad libitum. Animal care was provided by the ONPRC Division of Comparative Medicine in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Characterization of hormonal status and DHEA supplementation
Eight old females (mean ± SEM age: 23.2 ± 0.44 years) were trained for un-sedated blood collection. At the start of the study they showed the following mean (± SEM) serum hormone levels: anti-Müllerian hormone (AMH) = 1.55 ± 0.56 ng/ml; follicle–stimulating hormone (FSH) = 1.35 ng/ml; DHEAS = 28.8 ± 4.38 ng/ml. All of these hormonal levels are consistent with animals starting their transition into perimenopause (Downs and Urbanski, 2006; Sorwell et al. 2012), which was corroborated by half of the animals already showing irregular menstrual cycles or complete acyclicity before the start of the cognitive testing. Preliminary tests found this administration paradigm to mimic the youthful circulatory patterns of DHEAS, characterized by daily morning peaks generally in the range of 160 – 400 ng/ml 1-2 hours after DHEA administration. Consequently, the animals each received 5 mg of oral DHEA inside a marshmallow treat, daily at 07:45 h for ~2.5 months.
Behavioral testing apparatus
Training and testing on behavioral tasks were performed in sound-insulated computerized behavioral testing chambers. Each chamber consisted of a modified cage (24” L × 24” W × 32” H) with 3”-spaced bars in the front to allow access to a 17” Elo-Touch Systems CRT Touchmonitor (Menlo Park, CA, USA). Chambers were also equipped with a light, two fans, and a 1” closed-circuit video camera to allow for unobtrusive monitoring of behavior. During training and testing, animals remained unrestrained with full access to the touch screen. Food rewards were delivered into a rubber food receptacle in front of the touch screen by either a Med Associates mini M&M candy dispenser or a custom-built universal food dispenser mounted on the top of the testing chambers. These food rewards were chosen based on individual animals’ preferences and generally consisted of a mixture of candy, nuts, and dried fruit. Animals were not strictly food-restricted; however, training and testing was performed in the morning after an overnight fast. The morning meal was provided immediately after the training/testing session. Task stimulus presentation and reward contingencies were controlled individually for each chamber by a suite of custom programs written in True Basic and run on Macintosh iMac computers.
Cognitive training and testing
A detailed outline of the cognitive testing schedule is shown in Figure 1. The animals were first trained to touch a physical target outside their home cage. Later, animals were acclimated to the testing chambers and trained to transfer the targeting response to the touch screen for manually-controlled rewards. Once able to target the screen without assistance, they were placed on an automated training program and rewarded for hitting a large red square presented in random locations on the screen. The size of the square was gradually reduced until animals were able to hit a 3” × 3” square with 90% accuracy over 80 trials. They were then trained on the two tasks described below at successive delays of 1 to 5 seconds, and were required to reach a criterion of 85% accuracy at the 5-second delay in each task before advancing to the testing battery.
Figure 1.
Cognitive tests. In the DR task, animals were shown a red square on either the left or right side of a touch screen in the stimulus presentation phase (a) and, after a delay, were required to select the square on the same side during the query phase (b). In the DMS task, monkeys were shown a picture at the center of a touch screen in the stimulus presentation phase (c) and, after a delay, were required to select the previously shown image in the query phase (d). Correct responses resulted in a small palatable food reward.
In the delayed response (DR) task, the subject was presented with a red square on either the left or right side of the touch screen, and acknowledged its presence by touching it (Fig. 1a). After a variable delay (1, 5, 15, or 30 seconds, presented in random order), the subject was presented with two red squares, one on each side of the screen (Fig. 1b). In the delayed matching-to-sample (DMS) task, the subject first acknowledged the presence of a clipart picture displayed in the center of the touch screen by touching it (Fig. 1c). After a delay (5, 30, 60, 120, or 240 seconds, again presented in random order), the previously-shown picture and a novel picture were displayed randomly on the left and right sides of the touch screen (Fig. 1d). The subject indicated its selection by touching one of the two pictures. A correct response (selection of the square on the previously shown side in DR; selection of the previously shown image in DMS) was associated with a small palatable food reward delivered into a hopper in front of the screen, a positively-conditioned auditory tone, and a 1.5-second inter-trial interval (ITI). An incorrect response resulted in no food reward, a negatively-conditioned auditory tone, and a 3-second ITI. Each testing battery consisted of 5 days of DR testing, with 20 trials/day at each of the 4 delays (total of 100 trials per delay), and 10 days of DMS testing, with 10 trials/day at each delay (total of 100 trials per delay). In both tasks, the primary outcome measure was the percent correct responses at each delay across all testing days. While no images were repeated in the DMS task over the course of a single testing session, the same bank of images was accessed for each session, allowing for the potential repetition of images across testing days.
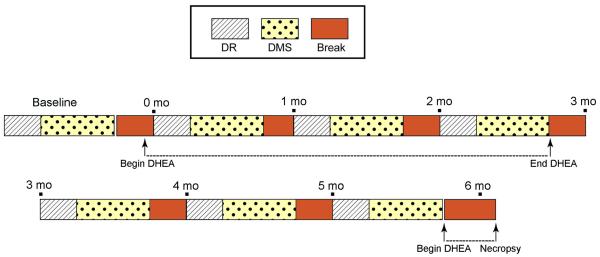
The behavioral testing schedule is shown in Figure 2. An initial testing battery was completed before the start of DHEA administration, followed by testing batteries starting one week, one month, and two months after the start of DHEA administration. Additional testing batteries began one week, one month, and two months after the cessation of DHEA supplementation.
Figure 2.
Schematic of the cognitive testing protocol. Animals completed one Baseline testing battery, and then underwent additional testing batteries one week, one month, and two months after the start of DHEA supplementation. They subsequently completed three more testing batteries without DHEA supplementation, and then an additional final testing battery while on DHEA supplementation.
Statistical analysis of cognitive data
Scores for each cognitive battery were calculated as percent correct at each delay for the five days of DR (50 trials per delay) and ten days of DMS (100 trials per delay). To examine the effect of DHEA administration on cognitive performance, percent improvement in score from the first (Baseline) battery test of each phase (on DHEA and off DHEA) to the third battery test was calculated. As roughly equivalent levels of improvement were seen within each phase of the study, this manipulation normalized performance to the beginning of each study phase and controlled for individual variation between animals. These improvement scores were analyzed using a repeated-measures ANOVA, with treatment and delay as within-subjects factors. An additional repeated-measures ANOVA was performed with treatment and delay as within-subjects factors and hormonal status as a between-subjects factor. Where the assumption of sphericity was not met as determined by Mauchly’s test of sphericity, the Greenhouse-Geisser ε was used as a correction. In all figures, data are presented as means ± standard error of the mean (SEM).
Serum hormone measurements
As previously reported (Downs and Urbanski, 2006; Sorwell et al. 2010, 2012), AMH was assayed using a commercially available ELISA kit (Diagnostic Systems Laboratories, Inc., Webster, TX, USA), while FSH and DHEAS were measured by RIA; the intra-assay coefficients of variation of these assays were <10%. E2 was assayed by electro-chemiluminescence (ECL) using the Elecsys 2010 Platform (Roche Diagnostics, Indianapolis, IN, USA); the intra-assay coefficient of variation was <6% (Sorwell et al. 2012).
Tissue processing and quantification of RNA
Postmortem brain tissue was obtained from the eight old DHEA-supplemented animals, as described above. Total RNA was extracted from the hippocampi (HPC) and dorsolateral prefrontal cortices (PFC) using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), and converted to cDNA using the RT2 First Strand Kit (SABiosciences, Qiagen, Valencia, CA, USA). The samples were then run on custom RT2 Profiler PCR Array plates (SABiosciences) in accordance with the kit instructions. Genes selected for inclusion on this plate were chosen for their involvement in either steroidogenesis (i.e., steroidogenic enzymes or transport proteins such as steroidogenic acute regulatory protein [StAR]) or steroid responses (i.e., steroid receptors and intracellular signaling proteins involved in the estrogen response). All genes were normalized against the arithmetic mean expression value of the three housekeeping genes, ALG9, GAPDH, and RPL13A.
Correlation of cognitive performance with gene expression
To allow for correlations between gene expression and cognitive performance, animals were classified based on their scores on the 15-second delay in the DR task and the 60-second delay in the DMS task during the final testing battery. These specific delays were chosen as they demonstrated the widest variability in performance among the animals. The final testing battery was chosen for analysis as this allowed animals to learn and practice the task as much as possible after meeting the baseline criteria. Animals were ranked based on their mean performance at this delay, and ranks were correlated with gene expression using Kendall’s τ b. The threshold level of significance was defined as α = 0.05.
Results
Effect of DHEA supplementation on cognitive performance
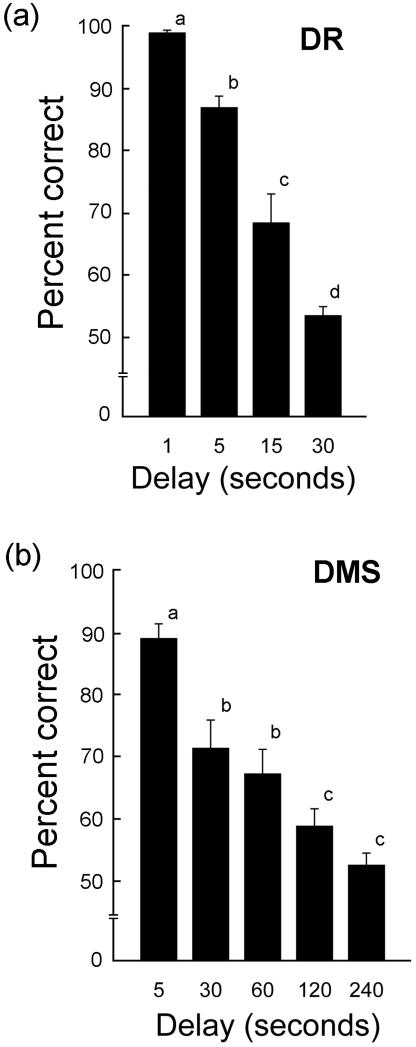
As expected, in the DR task there was a significant within-subjects effect of delay (F3,18 = 63.211, P < 0.001), showing that animals performed worse at longer delays (Fig. 3a). Similarly in the DMS task, there was a significant effect of delay (assumption of sphericity was not met, Greenhouse-Geisser ε 1.377,8.265 = 10.621, P = 0.008) (Fig. 3b). At 30-sec delays for the DR task, and 120-sec delays for the DMA task, the animals appeared to be at their cognitive limits, achieving scores of only ~50% correct.
Figure 3.
Baseline cognitive performance in aged female rhesus macaques in the DR (a) and DMS (b) tasks, with variable delays. As expected cognitive performance decreased as delay intervals increased, with performance at a delay of 15 seconds being considered a true measure of memory in the DR task and performance a delay of 120 seconds a true measure of memory in the DMS task. Each bar represents the mean of 8 animals and the vertical lines represent SEMs. Values with different letters were significantly different (P < 0.01), except for the difference in DMS performance at 60-sec and 120-sec delays (P < 0.05; ANOVA followed by the Newman-Keuls test).
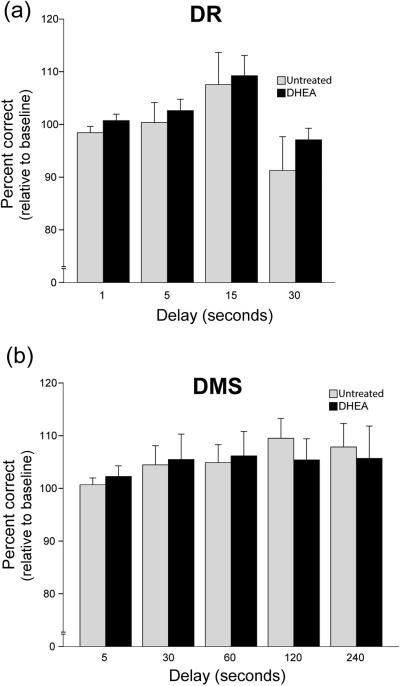
Figure 4a shows cognitive performance (at each delay in the DR task) observed at the end of the DHEA administration period, expressed as a percentage of Baseline performance. For reference it also shows cognitive performance at the end of the untreated period (see Fig. 2 for schematic of the cognitive testing timeline). Values greater than 100% indicate an improvement in performance across each of the two three-month testing phases; this demonstrates a practice effect on performance and validates the normalization of the results relative to Baseline performance at the beginning of each testing phase. A repeated-measures ANOVA revealed no significant effect of delay (P > 0.05), no significant effect of treatment (P > 0.05), and no significant delay-by-treatment interaction (P > 0.05). Thus, DHEA supplementation did not affect change in performance over time in this task.
Figure 4.
DR and DMS performance in aged female rhesus macaques treated with DHEA relative to untreated baseline. Performance is presented as scores starting at the 2-month time point after the start of each testing phase (ON and OFF), relative to the baseline of each testing phase. This manipulation of the data corrected for the practice effect observed that resulted in higher scores as the study progressed. No significant effect of treatment was observed in DR (a) performance nor in DMS (b) performance.
Figure 4b shows cognitive performance (at each delay in the DMS task) observed at the end of the DHEA administration period, expressed as a percentage of Baseline performance. For reference it also shows cognitive performance at the end of the untreated period. Similarly to the DR task, there was no significant effect on change in performance of treatment (P > 0.05) or delay (P > 0.05), nor was there any significant delay-by-treatment interaction (P > 0.05).
Correlations between cognitive performance and steroid-related gene expression
Correlations between rank-ordered cognitive data and gene expression data in the HPC and PFC are depicted in Figure 5, and the results of the corresponding Kendall’s τ b statistical analyses are shown in Table 1. In the HPC, better cognitive performance in the DMS task was correlated with lower levels of expression of HSD11B1 (the gene encoding 11β-hydroxysteroid dehydrogenase, the enzyme that synthesizes cortisol from cortisone) and NR3C1 (glucocorticoid receptor). Better cognitive performance in the DR task was associated with higher levels of expression of AKR1C3 (also known as HSD17B5, the enzyme that converts E1 into E2), and STAR (steroidogenic acute regulatory protein, the first protein necessary for synthesis of steroids from cholesterol), and with lower levels of SRD5A1 (5α-reductase type 1, the enzyme synthesizing 5αDHT from testosterone). In the PFC, better performance in the DMS task was correlated with higher levels of expression of AKR1C3, and with lower levels of expression of STAR.
Figure 5.
Correlation of cognitive performance with steroidogenic and steroid-related gene expression in the hippocampus (HPC) and prefrontal cortex (PFC) of aged female rhesus macaques. Animals were ranked by their score in the final Off-DHEA battery on the 15-second delay in the delayed response (DR) task and the 60-second delay in the delayed matching-to-sample (DMS) task. These ranks were then correlated with relative gene expression using Kendall’s τ b. Only significant correlations are presented. See Table 1, and text for details of the statistical analysis definition of gene names.
Table 1.
Correlation of cognitive performance with steroidogenic and steroid-related gene expression in the hippocampus and prefrontal cortex
| Hippocampus | Prefrontal Cortex | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| DR | DMS | DMS | ||||||
|
|
|
|
||||||
| Gene | Kendall's τb | P value | Gene | Kendall's τb | P value | Gene | Kendall's τb | P value |
| AKR1C3 | 0.500 | 0.039 | HSD11B1 | −0.473 | 0.010 | AKR1C3 | 0.400 | <0.001 |
| SRD5A1 | −0.643 | <0.001 | NR3C1 | −0.327 | 0.031 | STAR | −0.473 | 0.017 |
| STAR | 0.429 | 0.014 | ||||||
Animals were ranked by their score in the final Off-DHEA battery on the 15-second delay in the delayed response (DR) task and the 60-second delay in delayed matching-to-sample (DMS) task. These ranks were then correlated with relative gene expression using Kendall’s τ b. Only significant correlations are presented. See text for definition of gene names.
Discussion
Evidence for interaction between the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) axes in perimenopausal women (Pluchino et al. 2005; Crawford et al. 2009) suggests compensation between circulating E2 and DHEAS; however, the effect of supplemental DHEA or DHEAS on circulating E2 is unclear, with studies demonstrating gradual increases or no effect (Caufriez et al. 2013; Labrie et al. 2007; Labrie et al. 2008). In the current study we hypothesized that DHEA supplementation in perimenopausal rhesus macaques would increase central levels of E2 and thereby result in significant improvement of cognitive performance. This hypothesis was tested using aged female rhesus macaques as they were approaching their menopausal years, but before they had experienced extreme attenuation of their circulating E2 levels. The rationale for this experimental design stemmed from reports that cognitive function is enhanced in women undergoing estrogen replacement therapy when the treatment is initiated close to the time of menopause (Shao et al. 2012), but not when the treatment is significantly delayed (Rapp et al., 2003b, Espeland et al. 2004).
Based on our previous observation that circulating DHEAS levels are already significantly attenuated before the onset of menopause (Sorwell et al. 2012), we expected to observe a significant improvement of cognitive performance after ~2.5 months of supplementary DHEA; however, supplementation had no significant effect on either DR or DMS performance. Consequently, our results are consistent with the earlier negative findings by Herndon et al. (1999), which failed to show a correlation between DHEAS and cognitive performance in rhesus macaques. There are many possible reasons for the lack of obvious pro-cognitive effects of DHEA in humans and nonhuman primates. First, the duration of the DHEA treatment in the current study may have been too short to elicit a significant effect, although previously we observed cognitive improvements in ovariectomized macaques after only 2 months of treatment with E2 (Kohama et al. 2016). It’s also possible that the 5-mg oral dose of DHEA that we administered daily was suboptimal at maintaining elevated circulating levels of DHEAS throughout the entire day (Urbanski et al. 2014). Another possible cause for the negative results is that our old females were still able to maintain elevated E2 levels in their cognitive brain centers, despite showing perimenopausal hormonal changes in the circulation. It may be, therefore, that pro-cognitive effects of supplementary hormones only become evident when the endogenous levels of estrogens in the brain are significantly reduced, which might not occur in rhesus macaques until after the animals have completely transitioned into menopause and/or after ovariectomy. While this does not rule out effects of the more complete loss of ovarian steroids seen in post-menopause, it does suggest that the cognitive deficits associated with menopause in aged female macaques occur later in the reproductive lifespan than examined in the current study.
Additionally, from a cognitive perspective, ~2.5 months of testing may not have been sufficient time to show a deviation in the rate of improvement in these animals. All animals were required to meet testing criteria prior to the beginning of the study, resulting in significant selection bias, as those aged females showing significant cognitive deficits were excluded from participation in this experiment. Therefore, all animals included in the study were successful “cognitive agers,” and may have been less likely to show cognitive decline over such a short (less than one year) period of time.
Though our experimental design was aimed at an earlier intervention than is typically used in humans, in order to avoid an extended period of E2 deprivation, this design may have inadvertently resulted in the opposite effect: any cognitive impact of low DHEA levels may have been masked by the maintained E2 levels in these animals. Thus, a ceiling effect may have been reached by the endogenous circulating E2, making any additional locally-synthesized E2 resulting from the DHEA supplementation superfluous, as animals may have already been performing at maximal levels.
We also observed no significant increase in circulating E2 with DHEA supplementation, contrary to what we expected according to the hypothesis that DHEA is a significant source of active estrogens. Our prediction was that levels of E2 would increase, as DHEA is a precursor of E2; however, this lack of change in circulating E2 does not necessarily indicate that E2 levels within various peripheral tissues were not increased by the treatment. Labrie and colleagues (1998) have estimated that in premenopausal women, roughly 75% of active estrogens in tissues are produced locally; thus, a detailed analysis of E2 concentrations in multiple tissues on and off DHEA treatment would be necessary to assess the true effect of DHEA supplementation on E2 exposure. As this experiment was a within-subjects design, it was not feasible to perform these analyses in the current study. Additionally, circulating DHEA and E2 have been shown to be inversely correlated during the menopausal transition and estrogen supplementation (Crawford et al. 2009; Pluchino et al. 2005). Thus, ovarian E2 may be down regulated by an increase in E2 derived from exogenous DHEA.
The results of correlations between cognitive performance and central gene expression provide insights into the potential roles of steroidogenic and steroid-related genes in cognition. In the HPC, performance in the DMS task was negatively correlated with HSD11B1, suggesting that the local action of this enzyme in the conversion of cortisone to cortisol may negatively impact cognition. Although no direct evidence of central cortisol production in the nonhuman primate has yet been published, studies of aging and memory in mice have observed a significant negative impact of central corticosterone production on memory, and have noted that the expression of HSD11B1 is associated with greater cognitive impairment (Holmes et al. 2010; Holmes & Seckl, 2006). It has also been demonstrated that inhibition or knockout of HSD11B1 helps to maintain spatial memory during aging to a greater extent than in wild-type controls (Yau et al. 2007; Sooy et al. 2010). Furthermore, this mechanism is thought to involve increased activation of mineralocorticoid receptors rather than glucocorticoids receptors (Yau et al. 2011). Consistent with this previous report, we found the expression of the glucocorticoid receptor (NR3C1) to be negatively correlated with cognitive performance in our old monkeys. Taken together, therefore, our findings give support to the idea these two genes work in concert to protect the HPC from stress-induced memory impairments, and that individuals retaining the ability to down regulate their glucocorticoid receptors during aging are more resistant to cognitive deficits induced by stress and hypercortisolemia (McEwen 2002).
Also of note was the significant positive correlation between expression of AKR1C3 (the gene encoding HSD17B5) in the HPC and better cognitive performance in the DR task, as well as the significant positive correlation between the expression of AKR1C3 in the PFC and better cognitive performance in the DMS task. The AKR1C3 enzyme plays a key role in the de novo synthesis of sex steroids in the brain by participating in the metabolism of DHEA into testosterone and/or E1 into E2. Given that E2 has known cognitive benefits in monkeys, the observed positive correlations are not surprising. On the other hand, it is unclear whether testosterone itself can directly affect cognitive function or whether its influence is mediated by its two primary bioactive metabolites E2 and 5αDHT. However, higher expression of enzymes that promote the local production of E2 appear to enhance memory performance, whereas higher levels of SRD5A1 (encoding 5α-reductase type 1, an enzyme that converts testosterone to 5αDHT) appear to be associated with worse performance. Thus a decrease in the expression of SRD5A1 would allow more testosterone to be aromatized to E2 rather than being converted to 5αDHT. If true, this would suggest that during aging pro-cognitive effects of sex-steroids may ultimately be mediated by estrogen receptors rather than androgen receptors. Interestingly, STAR, which encodes the steroid acute regulatory protein and represents a rate limiting gene in the de novo synthesis of steroids, showed a significant positive correlation between its expression in the HPC and better cognitive performance in the DR task, but showed a significant negative correlation between its expression in the PFC and better cognitive performance in the DMS task. The reason for these two radically different STAR expression patterns is unclear. However, we have previously observed that key genes involved in the conversion of DHEA to E2 (e.g., 3BHSD1/2 and AKR1C3) are more highly expressed in the rhesus macaque PFC than in the HPC. Consequently, it is plausible that attenuated expression of STAR helps to protect the PFC from excessive steroid levels by moderating de novo synthesis.
Taken together, these data suggest that modulation of local steroid synthesis may play an important role in maintaining cognitive function in aged female macaques, and may explain why no further enhancement of cognitive function was observed after DHEA supplementation.
Acknowledgements
This work was supported by National Institutes of Health grants AG023477, AG029612, AG036670 and OD011092. The authors confirm that no actual, or potential, conflicts of interest exist.
Footnotes
eTOC text
Fig 5. Correlation of cognitive performance with gene expression in the hippocampus and prefrontal cortex of aged female rhesus macaques.
References
- Baxter MG, Roberts MT, Gee NA, Lasley BL, Morrison JH, Rapp PR. Multiple clinically relevant hormone therapy regimens fail to improve cognitive function in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2013;34:1882–1890. doi: 10.1016/j.neurobiolaging.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caufriez A, Leproult R, L’Hermite-Balériaux M, Kerkhofs M, Copinschi G. Effects of a 3-week dehydroepiandrosterone administration on sleep, sex steroids and multiple 24-h hormonal profiles in postmenopausal women: a pilot study. Clin Endocrinol (Oxf) 2013;79:716–724. doi: 10.1111/cen.12201. [DOI] [PubMed] [Google Scholar]
- Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94:2945–2951. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab. 2008;93:801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab. 2011;96:1642–1653. doi: 10.1210/jc.2010-2888. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Gilardi KVK, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Grimley Evans J, Malouf R, Huppert F, van Niekerk JK. Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database Syst Rev. 2006:CD006221. doi: 10.1002/14651858.CD006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Lacreuse A, Ladinsky E, Killiany RJ, Rosene DL, Moss MB. Age-related decline in DHEAS is not related to cognitive impairment in aged monkeys. NeuroReport. 1999;10:3507–3511. doi: 10.1097/00001756-199911260-00008. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Seckl JR. The role of 11beta-hydroxysteroid dehydrogenases in the brain. Mol Cell Endocrinol. 2006;248:9–14. doi: 10.1016/j.mce.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Carter RN, Noble J, Chitnis S, Dutia A, Paterson JM, Mullins JJ, Seckl JR, Yau JL. 11beta-hydroxysteroid dehydrogenase type 1 expression is increased in the aged mouse hippocampus and parietal cortex and causes memory impairments. J Neurosci. 2010;30:6916–6920. doi: 10.1523/JNEUROSCI.0731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Bélanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez JL, Candas B. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–328. doi: 10.1016/s0039-128x(98)00007-5. [DOI] [PubMed] [Google Scholar]
- Labrie F, Bélanger A, Labrie C, Candas B, Cusan L, Gomez JL. Bioavailability of oral and percutaneous dehydroepiandrosterone in postmenopausal women. J Steroid Biochem Mol Biol. 2007;107:57–69. doi: 10.1016/j.jsbmb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Labrie F, Cusan L, Gomez JL, Martel C, Bérubé R, Bélanger P, Chaussade V, Deloche C, Leclaire J. Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA. J Steroid Biochem Mol Biol. 2008;110:1–9. doi: 10.1016/j.jsbmb.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Wilson M,E, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2002;23:589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Lacreuse A. Effects of ovarian hormones on cognitive function in nonhuman primates. Neuroscience. 2006;138:859–867. doi: 10.1016/j.neuroscience.2005.09.006. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Janowsky J, Chan BKS, Nelson HD. Hormone replacement therapy and cognition: A systematic review and meta-analysis. JAMA. 2001;285:1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- Maki PM, Hogervorst E. HRT and cognitive decline. Best Practices Res Clin Endocrinol Metab. 2003;17:105–122. doi: 10.1016/s1521-690x(02)00082-9. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Genazzani AD, Bernardi F, Casarosa E, Pieri M, Palumbo M, Picciarelli G, Gabbanini M, Luisi M, Genazzani AR. Tibolone, transdermal estradiol or oral estrogen-progestin therapies: effects on circulating allopregnanolone, cortisol and dehydroepiandrosterone levels. Gynecol Endocrinol. 2005;20:144–149. doi: 10.1080/09513590400021169. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003a;2:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Henderson VW, Brunner RL, Manson JE, Gass MLS, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women. JAMA. 2003b;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- Kohama SG, Renner L, Landauer N, Weiss AR, Urbanski HF, Park B, Voytko ML, Neuringer M. Effect of ovarian hormone therapy on cognition in the aged female rhesus macaque. J Neurosci. 2016;36:10416–10424. doi: 10.1523/JNEUROSCI.0909-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Gilardi KVK, Lasley B, Rapp PR. Reproductive senescence predicts cognitive decline in aged female monkeys. NeuroReport. 1997;8:2047–2051. doi: 10.1097/00001756-199705260-00048. [DOI] [PubMed] [Google Scholar]
- Scheffers CS, Armstrong S, Cantineau AE, Farquhar C, Jordan V. Dehydroepiandrosterone for women in the peri- or postmenopausal phase. Cochrane Database Syst Rev. 2015:CD011066. doi: 10.1002/14651858.CD011066.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantineau AE, Farquhar C, Jordan V. Dehydroepiandrosterone for women in the peri- or postmenopausal phase. Cochrane Database Syst Rev. 2015:CD011066. doi: 10.1002/14651858.CD011066.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Shao H, Breitner JCS, Whitmer RA, Wang J, Hayden K, Wengreen H, Corcoran C.. Tschanz, J., Norton M, Munger R, Welsh-Bohmer K, Zandi PP. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County study. Neurology. 2012;79:1846–1852. doi: 10.1212/WNL.0b013e318271f823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooy K, Webster SP, Noble J, Binnie M, Walker BR, Seckl JR, Yau JL. Partial deficiency or short-term inhibition of 11beta-hydroxysteroid dehydrogenase type 1 improves cognitive function in aging mice. J Neurosci. 2010;30:13867–13872. doi: 10.1523/JNEUROSCI.2783-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorwell KG, Urbanski HF. Dehydroepiandrosterone and age-related cognitive decline. Age (Dordr) 2010;32:61–67. doi: 10.1007/s11357-009-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorwell KG, Kohama SG, Urbanski HF. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol Aging. 2012;33:1487e1–e13. doi: 10.1016/j.neurobiolaging.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorwell KG, Kohama SG, Urbanski HF. Testosterone increases circulating dehydroepiandrosterone sulfate levels in the male rhesus macaque. Front Endocrinol (Lausanne) 2014;5:101. doi: 10.3389/fendo.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkler GP, Voytko ML. Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:423–431. doi: 10.1016/j.pnpbp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Mattison JA, Roth GS, Ingram DK. Dehydroepiandrosterone sulfate (DHEAS) as an endocrine marker of aging in calorie restriction studies. Exp Gerontol. 2013;48:1136–1139. doi: 10.1016/j.exger.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Sorwell KG, Garyfallou VT, Garten J, Weiss A, Renner L, Neuringer M, Kohama SG. Androgen supplementation during aging: development of a physiologically appropriate protocol. Rejuvenation Research. 2014;17:150–153. doi: 10.1089/rej.2013.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML. The effects of long-term ovariectomy and estrogen replacement therapy on learning and memory in monkeys. Behav Neurosci. 2000;114:1078–1087. [PubMed] [Google Scholar]
- Voytko ML, Higgs CJ, Murray R. Differential effects on visual and spatial recognition memory of a novel hormone therapy regimen of estrogen alone or combined with progesterone in older surgically menopausal monkeys. Neurosci. 2008;154:1205–1217. doi: 10.1016/j.neuroscience.2008.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold RS, Lopez ST, Yau CL, Butler LM, Pareo-Tubbeh SL, Waters DL, Garry PJ, Baumgartner RN. Increasing trends in elderly persons’ use of nonvitamin, nonmineral dietary supplements and concurrent use of medications. J Am Diet Assoc. 2005;105:54–63. doi: 10.1016/j.jada.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Yau J.L,, McNair, K.M., Noble J, Brownstein D, Hibberd C, Morton N, Mullins JJ, Morris RG, Cobb S, Seckl JR. Enhanced hippocampal long-term potentiation and spatial learning in aged 11beta-hydroxysteroid dehydrogenase type 1 knock-out mice. J Neurosci. 2007;27:10487–10496. doi: 10.1523/JNEUROSCI.2190-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Noble J, Seckl JR. 11beta-hydroxysteroid dehydrogenase type 1 deficiency prevents memory deficits with aging by switching from glucocorticoids receptor to mineralocorticoid receptor-mediated cognitive control. J Neurosci. 2011;31:4188–4193. doi: 10.1523/JNEUROSCI.6145-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]