Abstract
The Human Immunodeficiency Virus (HIV), Hepatitis C Virus (HCV), and the treatment of HCV with pegylated interferon and ribavirin (IFN/RBV) have been associated with neurocognitive and psychiatric abnormalities. The goal of this research was to prospectively evaluate neurocognitive functioning among a group of HCV mono-infected and HIV/HCV-co-infected patients during the first 24 weeks of IFN/RBV treatment while accounting for practice effects, normal variations in change over time, and variations in IFN/RBV treatment exposure. Forty-four HCV mono-infected and 30 HIV/HCV co-infected patients were enrolled in a prospective study of patients beginning on IFN/RBV for chronic HCV infection. Patients were administered a depression inventory, a measure of fatigue, a structured psychiatric interview, and a neurocognitive battery at baseline and 24 weeks after initiation of treatment. Analyses were conducted to explore possible associations between neurocognitive functioning and the following: HIV/HCV co-infection versus HCV mono-infection, INF and RBV treatment exposure, psychiatric status, liver disease stage, and other medical characteristics. At baseline there were no significant differences between the two groups’ neuropsychiatric or neurocognitive function other than the mono-infected group had significantly higher reports of fatigue (p = 0.033). Over the course of 24 weeks of treatment after controlling for practice effects, the HIV/HCV co-infected patients experienced significantly greater declines in memory (t(56) = 2.14, p = 0.037) and global neurocognitive functioning (t(53) = 2.28, p = 0.027). In a well-characterized sample of mono- and co-infected patients, it appears that persons with HCV/HIV co-infection are potentially more vulnerable to neurocognitive sequalae during HCV treatment.
Keywords: Hepatitis C, HIV, Pegylated Interferon, Ribavirin, Neurocognitive, Side effects
Infection with Hepatitis C Virus (HCV) is a leading cause of liver disease and may cause a wide range of medical complications including hepatic fibrosis, cirrhosis, and hepatocellular carcinoma. Epidemiological studies suggest that approximately 2.7 million people are infected with HCV within the United States and 130-170 million worldwide (Williams et al. 2011). Globally, of the 33 million people infected with HIV approximately 33% are co-infected with HCV (Averhoff and Glass 2012). Due to similar vectors of transmission, rates of HIV co-infection are highest among patients who have a history of injection drug use or hemophilia (Koziel and Peters 2007). HCV infection is often accompanied by neurocognitive dysfunction, depression, fatigue, and reduced quality of life (Chiu et al. 2014), and HIV co-infection is associated with progressive liver disease, increased morbidity and mortality, and increased risk of mother to child HCV transmission. The etiology of neurocognitive dysfunction among co-infected patients is relatively uncertain given the multiple comorbidities of the patient population, side effects of medical treatments, and inconsistencies in research methods used to assess this. Historically, treatment of Hepatitis C with interferon (IFN) was burdensome, complex, and often was associated neurocognitive abnormalities (Dieperink and Willenbring 2000). With the introduction of direct acting antiviral therapy, IFN-free regimens have become the standard of care for HCV treatment. Fatigue is the most frequently occurring side effect of direct acting antiviral treatment for HCV (Banerjee and Reddy 2016) and fatigue is known to be associated with neurocognitive impairment. Neurocognitive abnormalities associated with HIV and HCV infection persist and the findings of this study remain relevant to this area of clinical concern.
Neurocognitive Complications of HCV/HIV Co-infection
It is well established that patients with HIV-infection experience numerous central and peripheral nervous system deficits (Heaton et al. 2010a; Heaton et al. 2010b). It stands to reason that patients with HCV/HIV co-infection may experience exacerbated neurocognitive impairments due to the additive effects of HIV and HCV infection. However, the literature on the neurocognitive effects of HCV/HIV co-infection is somewhat equivocal. Several studies suggest that HIV/HCV co-infection has an additive effect on neurocognitive impairment compared with HIV mono-infection (Giesen et al. 2004; Martin et al. 2004; Ryan et al. 2004; Clifford et al. 2005; Hinkin et al. 2008). For example, one cross-sectional study by Clifford et al. (2005) demonstrated that HIV/HCV co-infected patients performed significantly worse on global neurocognitive functioning and had higher rates of depression compared to HIV mono-infected patients. In contrast, other studies do not support these findings (Giesen et al. 2004; Ryan et al. 2004; Hilsabeck and Castellon 2005; Perry et al. 2005). One cross-sectional study by Ryan and colleagues (2004) found no significant differences between mono- and co-infected patients on domain specific functioning, however the co-infected group had poorer performance on a measure of executive functioning (the Wisconsin Card Sorting Test, WCST). Another cross-sectional study examining neurocognitive functioning among HIV mono-infected, HCV mono-infected, and HIV/HCV co-infected patients found co-infection was only associated with prolonged reaction time (Giesen et al. 2004). Conversely, a cross-sectional study examining HIV/HCV co-infected and mono-infected patients found that HCV mono-infected patients had deficits in attention, concentration, and psychomotor speed (compared to normative samples), but did not find significant differences between mono- and co-infected patients, after controlling for liver disease severity (Perry et al. 2005). To contextualize these findings, Hilsabeck et al. (2005) conducted a secondary analysis of data from Hinkin et al. (2004) and found that 80% of co-infected patients and 68% of the HIV mono-infected patients were classified as impaired. Further examination revealed that this difference between the groups was largely attributable to psychomotor slowing with 84% of the co-infected group and 56% of the mono-infected group being classified as significantly impaired. Common methodological weaknesses of the discussed studies include limited neurocognitive batteries, small sample sizes, and cross-sectional design.
Treatment of HCV with Interferon and Ribavirin
Interferon (IFN) is an endogenous cytokine prescribed for the treatment of malignant diseases as well as viral hepatitis C. Historically combining IFN alpha (IFN-α) with ribavirin (RBV) had sustained viral response (SVR) 10-40% of the time (McHutchison et al. 1998; Poynard et al. 1998; Schaefer et al. 2007). The advent of pegylated IFN in 2001 (pegylated IFN alpha-2a and pegylated IFN alpha 2-b) caused a much longer half-life, allowing for reducing injections to only one time a week. Additionally when used in combination with RBV, SVR increased to 51-82% (Schaefer et al. 2007). Side effects experienced by many patients treated with IFN-α include developing flu-like symptoms 6-8 hours post injection (Dieperink and Willenbring 2000). Conversely, neurocognitive decline, fatigue, apathy, and other behavioral symptoms commonly develop several weeks after treatment (Dieperink and Willenbring 2000), and are generally more chronic in nature. The etiology of the neurocognitive sequelae associated with IFN/RBV treatment include depression, neurocognitive irregularities, psychosis, irritability, fatigue, somatic complaints, suicide ideation, and relapse in alcohol and drugs (Schaefer et al. 2002; Asnis et al. 2004). Many of these adverse symptoms are already postulated to be caused by the disease itself (Forton et al. 2002; Hilsabeck et al. 2002; McAndrews et al. 2005; Forton and Taylor-Robinson 2006) and further exacerbated due to treatment with IFN and RBV (Fried 2002; Hilsabeck and Hassanein 2005; Reichenberg et al. 2005; Lieb et al. 2006; Tanaka et al. 2006; Thein et al. 2007; Majer et al. 2008; Pawelczyk et al. 2008; Wobrock et al. 2008).
Multiple longitudinal studies have found associations between IFN/RBV treatment and neurocognitive dysfunction, (Hilsabeck and Hassanein 2005; Majer et al. 2008) whereas some studies have observed neurocognitive decline return to normal upon treatment completion (Kraus et al. 2005; Fontana et al. 2007), and others have not found a clear association between IFN treatment and cognition (Reichenberg et al. 2005). A study by Huckans et al. (2015) examined neurocognitive functioning over six months of 64 HCV mono-infected patients, 33 were treated with IFN/RBV and 31 were not treated. The treated group experienced increased symptoms of depression, fatigue, anxiety, and pain but experienced no significant changes in neurocognitive function. The longitudinal studies mentioned suggest that there may be a relationship between IFN treatment and poorer neurocognitive functioning, however these studies do not address HIV co-infection as a risk factor for neurocognitive decline nor do they adequately control for practice effects related to neurocognitive functioning.
Until the development of direct acting antiviral agents, interferon (IFN) and ribavirin (RBV) were used for the treatment of HCV. Although IFN is no longer used to treat HCV, IFN remains today a treatment option for multiple sclerosis and various cancers including leukemia and melanoma (Calabresi et al. 2014; Mohr et al. 2015; Bohn et al. 2016). Additionally, IFN and RBV may be used in combination for the treatment of special cases of human papillomavirus (HPV) infection (Pavan et al. 2007; Shrestha and Hamrock 2010; Mosa et al. 2014). Thus, the findings of this study may have clinical implications for the treatment of other diseases.
To date, only one study conducted by Cattie et al. (2014) has accounted for practice effects for HCV mono-infected patients undergoing treatment with IFN-RBV. Cattie and colleagues (2014) assessed psychiatric and neurocognitive functioning among 40 mono-infected patients undergoing treatment with IFN-RBV at 10 weeks, 6 months, 12 months, and 18 months after treatment initiation. Utilizing methods by Heaton et al. (1994) domains were averaged to create a global deficit score. Patients scoring above 0.5 were categorized as globally impaired. After controlling for practice effects at 10 weeks post-treatment initiation, the frequency of global neurocognitive impairment significantly increased from 22.5% to 47.5% and patients infected with genotype 1 and pre-existing depression had the most significant increase in impairment. However, this study only included HCV mono-infected patients. Additionally only one study by Thein et al. (2008) longitudinally examined SVR and global neurocognitive functioning among 15 HIV/HCV co-infected and 19 HCV mono-infected patients at 0, 24, and 48 weeks of IFN-RBV treatment. Thein and colleagues found SVR rates to be similar among the groups and the co-infected group performed significantly better on global functioning at 48 weeks. However, this study did not address practice effects and had a relatively small sample size which may have limited the power to detect differences in neurocognitive functioning.
Present Study
The goal of this study was to examine neurocognitive functioning of HCV mono-infected versus HCV/HIV co-infected patients over the first 24 weeks of IFN/RBV treatment while addressing practice effects and variations in IFN-RBV treatment exposure over time. We hypothesized that there would be significantly detectable neurocognitive deficits in both groups at baseline, but the HCV/HIV co-infected group would demonstrate significantly worse neurocognitive decline than HCV mono-infected group at 24 weeks post baseline, independent of virologic response, depression, fatigue, and liver disease severity.
Methods
Participants
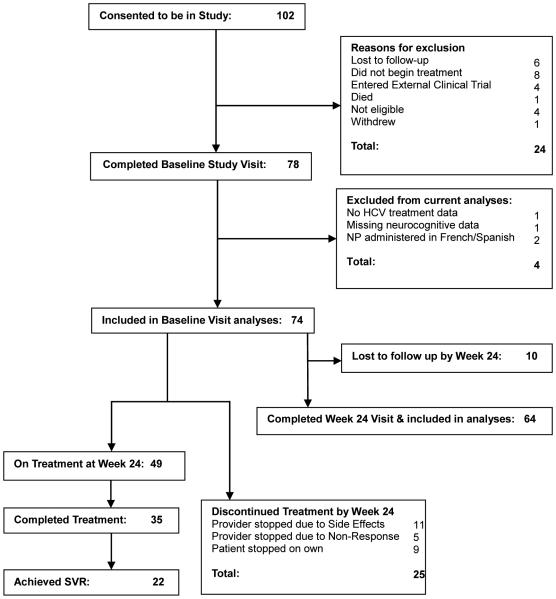
Participants were enrolled in a study at a large academic medical center and Veterans Administration Hospital in New York City examining the longitudinal changes in depression and neurocognitive functioning across 3 time points (0,12,24 weeks) in HCV mono-infected patients and HCV/HIVco-infected patients during the first 24 weeks of treatment with pegylated IFN/RBV. In total, 102 patients were consented and then screened for inclusion criteria. Of those 102, 24 were screened and deemed ineligible to participate in the study (See Figure 1). Participants were selected for this study if (1) they were infected with chronic HCV; (2) they had not received prior treatment with pegylated IFN/RBV; (3) Clinician and patient had decided to begin pegylated IFN/RBV therapy within the next two months; (4) were above the age of 18 at the time of study; and (5) their primary language was English. Eligible participants included in this analysis were 74 mono-infected HCV (n=44) or HCV/HIV (n=30) co-infected patients. The four patients were excluded from the total sample for these analyses due to the following reasons: missing neurocognitive data at baseline, lack of HCV treatment outcome data, or neurocognitive tests being administered in French or Spanish. This study was approved by the Icahn School of Medicine at Mount Sinai and Bronx Veterans Administration Medical Center institutional review boards, and all patients consented prior to completing baseline evaluation and subsequent treatment. Patients were compensated $30 for their baseline visit and $20 for the 24 week visit.
Fig. 1.
Study enrollment, exclusion, retainment, and treatment outcomes
Treatment Design
All patients completed the baseline assessment approximately within two weeks prior to beginning pegylated IFN/RBV treatment, including all of the assessments described below. The mean initial dose of pegylated IFN was 168 mg per week (SD = 29) and RBV was 1067 mg per day (SD = 176). Based on HIV status and HCV genotype, 13.5% of patients were planned to be on 24 weeks of treatment and 86.5% of patients were planned to be on 48 weeks of treatment. Patients were then assessed again at 12 and 24 weeks after beginning treatment. Of the original 74 patients, 68 patients completed assessments at the 12 week visit, and 64 patients completed assessments at the 24 week visit.
Demographic, Medical, and Clinical Characteristics
Demographic and medical characteristics
All patients completed a brief demographic survey at the baseline assessment (e.g., age, gender, years of education). All patients’ medical history was abstracted from hospital medical records in association with HIV (i.e., CD4 count (a measure of immune function) and HCV-related treatment variables (i.e., liver biopsy, cirrhosis status, platelets, hemoglobin, albumin, ALT, AST, bilirubin direct, bilirubin total HCV RNA, and liver disease stage). All patients were chronically infected with HCV as indicated by medical record documentation of HCV RNA at least one year prior to study enrollment. Liver staging was ascertained either through biopsy or clinical diagnosis of cirrhosis. Lifetime medical diagnoses were also abstracted (diabetes, obesity, hypertension, and pulmonary disease).
Clinical characteristics
Depressive symptoms were assessed using the Beck Depression Inventory II at baseline, 12 weeks after starting treatment, and 24 weeks after starting treatment (Beck et al. 1996). For this study the BDI-II Fast Screen (BDI-II FS) was used to minimize the impact of somatic complaints associated with chronic illnesses such as HIV and HCV (Aaron 2000; Krefetz et al. 2004). The BDI-II FS has shown to have good internal consistency and cutoff scores of 4 and higher sensitively detect mood disorders. Additionally, the BDI-FS has also been found to be minimally correlated with age, ethnicity, sex, and specific medical diagnoses, thus making it a sensitive and accurate tool for reliable assessment of depressive symptoms among persons with chronic illness (Beck et al. 1996; Mindt et al. 2014). In addition, both past and current psychiatric and substance use disorders were evaluated by a trained research coordinator using the Structured Clinical Interview for DSM Disorders (SCID) at the baseline visit.
Fatigue symptoms were assessed using the Fatigue Severity Scale (Krupp et al. 1989) (FSS) at baseline, 12 weeks after starting treatment, and 24 weeks after starting treatment. The FSS is a 9-item scale validated for the use of patients with HCV and other chronic illnesses (Kleinman et al. 2000). Change scores were computed for both depression and fatigue to assess differences between baseline and 24 weeks and baseline.
IFN/RBV Treatment Exposure
Length of treatment completed and any dose reductions of pegylated IFN or RBV during treatment were recorded for all patients from the medical chart. Combined with these two measurements, self-reported adherence to pegylated IFN and RBV was assessed and used to calculate cumulative drug exposure for each participant at weeks 12 and 24. The calculation of the IFN and RBV Treatment Exposure variables were calculated utilizing the following three components: (1) drug persistence – the number of weeks on treatment; (2) drug adherence - percentage of prescribed doses self-reported as taken; and (3) dose reductions (Weiss et al. 2009).
Neurocognitive Assessment
Neurocognitive function was assessed using a battery of tests, which measure neurocognitive domains suspected to show deficits in HCV- and HIV-infected populations. All patients completed a detailed neuropsychological test battery administered and scored by trained research staff using standardized procedures (Woods et al. 2004). Alternate forms of the verbal learning/memory test (Hopkins Verbal Learning Test-Revised, HVLT-R) (Brandt 1991) were used for the follow-up neurocognitive assessments. Table 1 summarizes the 45 minute battery, which consists of measures assessing the following six ability domains: processing speed, learning, memory, executive function, verbal function, and motor function. Raw scores were converted into demographically corrected T-scores. To evaluate the rates of neurocognitive impairment across domains, Domain Average T-scores were derived from the mean T-scores of the individual tests in that particular domain, and the Global neurocognitive average T-score is the mean of all individual neurocognitive test T-scores. Neurocognitive Global and Domain Average T-scores below 40 were considered impaired (Woods et al. 2004).
Table 1.
Neuropsychological test battery and normative data arranged by the seven major ability areas for computation of average T-scores.
| Neuropsychological Domain and Tests | Normative Data Sources |
|---|---|
| Processing Speed | |
| Trail Making Test (Part A) Symbol Digit Modalities Test (SDMT) |
Heaton, Grant & Matthews (1991) 1, 2, 3,
Smith (1982) 1,2 |
| Learning | |
| Hopkins Verbal Learning Test-Revised (Total Recall) |
Benedict, Schretlen, Groninger & Brandt (1998) 1 |
| Memory | |
| Hopkins Verbal Learning Test (Delayed Recall Trial) |
Benedict, Schretlen, Groninger & Brandt (1998) 1 |
| Executive Function | |
| Wisconsin Card Sorting Task-64 Item Version (Perseverative Respsonses) |
Kongs, Thompson, Iverson & Heaton (2000) 1, 2 |
| Trail Making Test (Part B) | Heaton, Grant & Matthews (1991) 1, 2, 3 |
| Verbal Function | |
| Controlled Oral Word Association Test (F-A-S) |
Heaton, Grant & Matthews (1991) 1, 2, 4 |
| Motor | |
| Grooved Pegboard Time (dominant hand) | Heaton, Grant & Matthews (1991) 1, 2, 3 |
| Grooved Pegboard Time (non-dominant hand) | Heaton, Grant & Matthews (1991) 1, 2, 3 |
Notes. Normative data corrects for the following demographic characteristics indicated by superscript number:
Age;
Education;
Gender
Race
In order to assess change in neurocognitive functioning across the study visits (baseline and 24 weeks), we utilized Cysique and colleagues’(2011) regression-based change score approach in order to assess change while accounting for practice effects on neurocognitive functioning. For the purpose of the current study, we report on the neurocognitive data from baseline and 24 weeks in order to describe the cumulative impact of the course of the first 24 weeks of treatment.
Statistical Analysis
This study used cross-sectional data and prospective longitudinal data (both within-group and between groups). The study sample was described using descriptive statistics and frequencies, then analyzed for differences using independent t-tests and Pearson chi-square tests. Initial group comparisons were performed for baseline neurocognitive data between HCV mono-infected and HCV/HIV co-infected patients. Independent-samples t-test and Chi-square analyses were used to determine if differences observed in baseline neurocognitive performance between mono- and co-infected patients were significant and if other variables contributed to observed differences. The non-parametric Wilcoxon's Signed-Rank test was used to compare baseline and follow up lab values.
For longitudinal analyses, normative data published by Cysique et al. (2011) were used to generate z-scores for each of the ten neurocognitive tests. The resulting z-scores indicate how well or poorly the groups did at 24 weeks relative to a neurocognitively stable control based on age, education, gender ethnicity (Caucasian versus others), and practice effects (Cysique et al. 2011). These z-scores were then averaged to create summary regression-based change scores (sRCS) for six neurocognitive domains and global neurocognitive functioning at 24-weeks (Heaton et al. 2015) .
A series of bivariate analyses were conducted in order to identify whether any demographic factors, clinical characteristics, or treatment exposure to IFN or RBV were potential covariates. Of note, there were three treatment variables, including total weeks on treatment (continuous variable), and two dichotomous treatment exposure variables were created with a cut point of ≥ 80% exposure to either IFN or RBV. To be included as a covariate, the variable must have been significantly different between the HCV mono-infected and HCV/HIV co-infected groups (p <.05) and significantly correlated with the sRCS variable (p <.05). In order to examine the study hypotheses, a series of independent t-test or ANCOVA analyses (if covariates were included) were computed to compare sRCS scores between the HCV mono-infected and HCV/HIV co-infected groups to determine if significant change occurred between-groups from baseline to the 24-week visit. All analyses were conducted with SPSS 22.
Results
Demographic, medical, and clinical characteristics
As Table 2 illustrates, there were no significant differences at baseline between the HCV mono-infected and HCV/HIV co-infected groups in age, gender, estimated premorbid intelligence (via the Wide Range Achievement Test), or ethnicity. However, the HCV mono-infected group had significantly more years of education than the HIV/HCV co-infected group t(72) = 2.05, p < 0.04.
Table 2.
Demographic characteristics for sample (n = 74)
|
HCV
Mono-infected n = 44 |
HCV/HIV
Co-infected n = 30 |
|||
|---|---|---|---|---|
|
| ||||
| M (SD)/ % | M (SD)/ % | t(dj) or χ2 | p | |
| Age (years) | 53.52 (12.2) | 49.07 (10.5) | 1.63 (72) | .11 |
| Gender | 0.03 | .87 | ||
| Male (%) | 75.0 | 76.7 | ||
| Female (%) | 25.0 | 23.3 | ||
| Education (years) | 13.48 (2.4) | 12.1 (3.2) | 2.1 (72) | .04 |
| WRAT-III Reading Test T-Score | 46.02 (8.2) | 44.1 (10.3) | 0.9 (71) | .37 |
| Ethnicity | 4.91 | .09 | ||
| Black (%) | 40.9 | 23.3 | ||
| Hispanic (%) | 20.5 | 43.3 | ||
| White (%) | 38.6 | 33.3 | ||
Notes. WRAT-III=Wide Range of Achievement Test – 3rd edition.
Table 3 summarizes the sample’s clinical and medical characteristics at baseline. The HCV mono-infected group had a significantly higher rate of current psychiatric disorders (52.3% vs. 20.0%, respectively; χ2 (1, n = 74) = 7.80, p = 0.005) than the HIV/HCV co-infected group, but the groups did not differ on lifetime psychiatric disorders. Table 3 also illustrates the medical characteristics of the two groups. The mono-infected group had significantly higher rates of hypertension (59.5% vs. 25.0%, respectively; χ2 (2, n = 65) = 7.66, p = 0.010). Conversely the HIV/HCV co-infected group had higher rates of pulmonary disease (26.7% vs. 9.1%, respectively; χ2 (2, n = 56) = 9.61, p = 0.008). Liver staging was established for 63 of 74 patients either by liver biopsy (n=59) or clinical diagnosis of cirrhosis (n=4). There were no clinically significant differences between the groups for platelets, hemoglobin, albumin, ALT, AST, bilirubin direct, bilirubin total, HCV viral load, or liver disease stage established by biopsy or clinical diagnosis of cirrhosis. (all p’s > .05).
Table 3.
Clinical and Medical Characteristics at Baseline (n = 74)
|
HCV
Mono-infected n = 44 |
HCV/HIV
Co-infected n = 30 |
|||
|---|---|---|---|---|
|
| ||||
| M (SD) | M (SD) | t(df) or χ2 | p | |
| Psychiatric/Substance Disorders | ||||
| Current Psychiatric Disorder | 52.3% | 20.0% | 7.8 | <.01 |
| Lifetime Psychiatric Disorder | 65.9% | 66.7% | 0.01 | .95 |
| Current Substance Disorder | 6.8% | 0.0% | 2.13 | .14 |
| Lifetime Substance Disorder | 36.4% | 46.7% | 379 | .38 |
| Lifetime medical diagnoses | ||||
| Diabetes | 16.7% | 20.0% | .10 | .75 |
| Obesity | 16.1% | 7.7% | 0.93 | .33 |
| Hypertension | 50.0% | 23.3% | 9.22 | .01 |
| Pulmonary Disease | 9.1% | 23.3% | 9.61 | <.01 |
| Clinical Characteristic | ||||
| Platelet | 195.3 (76.9) | 170.1 (68.2) | 1.5 (72) | .15 |
| Hemoglobin | 14.1 (1.3) | 13.8 (1.6) | .71 (72) | .48 |
| Albumin | 4.2 (0.4) | 4.1 (0.6) | 0.7 (72) | .49 |
| Alanine Aminotransferase (ALT) | 76.3 (66.4) | 70.7 (50.8) | 0.4 (72) | .69 |
| Aspartate Aminotransferase (AST) | 70.1 (59.6) | 67.4 (37.7) | 0.2 (72) | .82 |
| Bilirubin, total | 0.65 (0.4) | 0.9 (0.5) | −2.1 (72) | .05 |
| Bilirubin, direct (n = 41) | 0.3 (0.3) | 0.4 (0.3) | −1.1 (39) | .30 |
| HCV RNA (log10 IU/ml) | 6.17 (0.6) | 6.2 (1.1) | −0.61 (72) | .54 |
| HCV genotype (type 1 %) | 7.29 | .06 | ||
| Type 1 | 84.1% | 63.3% | ||
| Type 2 | 13.6% | 16.7% | ||
| Type 3 | 2.3% | 13.3% | ||
| Type 4 | 0% | 6.7% | ||
| Liver Biopsy Stage (n = 63, %) | 6.58 | .09 | ||
| Stage 1 | 18.9% | 7.7% | ||
| Stage 2 | 16.2% | 42.3% | ||
| Stage 3 | 35.1% | 19.2% | ||
| Stage 4 | 29.7% | 30.8% | ||
| HIV Characteristics | – | – | ||
| HIV+ Duration (years) | – | 13.4 (6.7) | – | – |
| CD4 cell count | – | 506.4 (251.2) | – | – |
| % AIDS (CD4<200) | – | 10% | – | – |
| % HIV Detectable Viral Load (>1000) | – | 3.3% | – | – |
Notes. HCV = Hepatitis C Virus, RNA = Ribonucleic acid, HIV = Human Immunodeficiency Virus; CD4 = T–lymphocyte cell bearing CD4 receptor; AIDS = Acquired Immunodeficiency Syndrome.
After 12 weeks of treatment there were significant difference in mean levels of Platelets (M= 184.79, SD= 58.95; Z = −5.62, p < .001), Hemoglobin (M= 11.80, SD= 1.84; Z = −6.85, p < .001), Albumin (M= 4.02, SD= 0.43; Z = −4.33, p < .001), ALT (M= 40.5, SD= 30.72; Z = −5.95, p < .001), and AST (M= 47.97, SD= 29.75; Z = −4.63, p < .001). After 24 weeks of treatment there were significant differences in mean levels of Platelets (M= 146.10, SD= 80.59; Z = −5.06, p < .001), Hemoglobin (M= 11.78, SD=1.78; Z = −6.87, p < .001), Albumin (M= 4.03, SD= 0.57; Z = −3.52, p < .001), ALT (M= 41.51, SD= 41.38; Z = −4.84, p < .001), and AST (M= 51.10, SD= 45.49; Z = −4.45, p < .001). However, there were no significant differences between the co-infected and the mono-infected groups. As would be expected on interferon treatment (Cha et al. 2014), CD4 did drop significantly from baseline (M= 506.43, SD= 251.26) to 12 weeks (M= 364.41, SD= 216.04; Z = −4.39, p < .001) and from baseline to 24 weeks (M= 324.65, SD= 193.6;5 Z = −3.57, p < .001). There were no significant differences in virologic suppression at either time points.
Neurocognitive function and change
Table 4 presents average T-scores for global and domain neurocognitive functioning, fatigue, and depressive symptomology at baseline and 24 weeks for descriptive purposes. There was no significant relationship found between baseline psychiatric variables (SCID, FSS change scores, BDI-FS change scores) and neurocognitive change. The only neurocognitive domain on which the two groups differed was memory at 24 weeks, with the HIV/HCV co-infected group being more impaired than the HCV mono-infected group (p = .02). More specifically, at baseline the HIV/HCV co-infected group’s memory functioning fell within normal limits, but at 24 weeks declined to the mildly impaired range. There were no differences between the groups on baseline and 24 weeks on depression scores. Although the groups did significantly differ at baseline on fatigue (p = 0.03) with the mono-infected group experiencing a significantly higher rate of fatigue than the HIV/HCV-co-infected group, the groups did not significantly differ at 24 weeks (p = .07). Correlational analyses demonstrated that fatigue was not related to neurocognitive functioning at baseline (all p’s>.05).
Table 4.
Mean FSS Scores, Mean BDI-FS Scores, and Mean Neurocognitive Functioning (Average T-scores) at Baseline and 24 Weeks of Treatment.
| Baseline Visit | 24 Weeks of Treatmen | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
|
HCV
Mono-infected n = 44 |
HCV/HIV
Co-infected n = 30 |
HCV
Mono-infected n = 36 |
HCV/HIV
Co-infected n = 28 |
|||||||
|
|
||||||||||
| M (SD) | M (SD) | t | p | d | M (SD) | M (SD) | t | p | d | |
| FSS | 4.94 (2.39) | 3.76 (2.19) | 2.17 | .03 | 0.52 | 5.85 (2.34) | 4.79 (2.21) | 1.83 | .07 | 0.46 |
| BDI-II FS | 2.61 (3.39) | 1.97 (2.64) | .88 | .38 | 0.22 | 3.19 (3.88) | 2.57 (2.61) | .73 | .47 | 0.18 |
| Neurocognitive Domains | ||||||||||
| Processing Speed | 40.4 (7.4) | 42.1 (10.1) | −.81 | .42 | −0.19 | 43.6 (6.74) | 43.4 (9.15) | .12 | .91 | 0.03 |
| SDMT | 39.0 (7.8) | 38.8 (11.5) | .09 | .93 | 0.02 | 42.2 (8.82) | 38.5 (11.7) | 1.02 | .31 | 0.36 |
| TMT-A | 41.8 (10.0) | 45.3 (10.4) | −1.45 | .15 | −0.34 | 45.0 (8.21) | 47.2 (8.71) | −1.01 | .32 | −0.26 |
| Learning | 39.1 (11.0) | 38.8 (13.4) | .12 | .91 | 0.02 | 40.9 (10.7) | 39.6 (12.1) | .45 | .67 | 0.11 |
| HVLT-R Recall | 39.1 (11.0) | 38.8 (13.4) | .12 | .91 | 0.02 | 40.9 (10.7) | 39.6 (12.1) | .45 | .67 | 0.11 |
| Memory | 41.8 (9.9) | 40.7 (10.7) | .44 | .66 | 0.11 | 41.2 (10.1) | 35.4 (9.77) | 2.25 | .03 | 0.58 |
| HVLT-R Delay | 38.3 (11.5) | 38.2 (12.3) | .02 | .99 | 0.01 | 40.2 (12.4) | 34.1 (9.99) | 2.07 | .04 | 0.53 |
| HVLT-R Recog. | 45.3 (11.2) | 43.1 (12.89) | .74 | .46 | 0.18 | 42.1 (11.7) | 36.6 (12.9) | 1.74 | .09 | 0.45 |
| Executive | 42.5 (8.1) | 42.9 (8.1) | −.22 | .83 | −0.05 | 46.6 (10.7) | 44.9 (7.23) | .70 | .49 | 0.18 |
| TMT-B | 43.9 (10.1) | 42.0 (13.6) | .71 | .48 | 0.15 | 45.2 (11.9) | 44.3 (11.6) | .29 | .77 | 0.08 |
| WCST-64 PR | 41.0 (12.2) | 43.8 (9.9) | −1.05 | .30 | −0.26 | 47.9 (14.5) | 45.5 (9.85) | .74 | .46 | 0.19 |
| Verbal | 44.4 (11.6) | 44.4 (11.6) | −.02 | .99 | 0.00 | 44.4 (9.7) | 46.5 (12.4) | −.74 | .46 | −0.19 |
| COWAT-FAS | 44.4 (11.6) | 44.4 (11.6) | −.02 | .99 | 0.00 | 44.4 (9.7) | 46.5 (12.4) | −.74 | .46 | −0.19 |
| Motor | 40.5 (7.8) | 39.9 (7.5) | .35 | .73 | 0.08 | 41.1 (7.54) | 40.1 (8.81) | .47 | .64 | 0.12 |
| GPDH | 40.4 (8.8) | 40.7 (7.9) | −.13 | .90 | −0.04 | 41.0 (7.71) | 40.7 (9.82) | .15 | .88 | 0.03 |
| GPNDH | 40.2 (8.9) | 39.1 (8.2) | .57 | .57 | 0.13 | 41.4 (8.34) | 39.6 (9.26) | .81 | .42 | 0.21 |
| Global functioning | 41.6 (5.2) | 41.8 (6.3) | −.09 | .93 | −0.03 | 43.0 (6.15) | 41.9 (6.47) | .64 | .52 | 0.17 |
Notes. BDI-II FS =Beck Depression Inventory-2nd edition Fast Screen; FSS = Fatigue Severity Scale; SDMT = Symbol Digit Modalities Test; TMT = Trail Making Test; HVLT-R = Hopkins Verbal Learning Test-Revised; WCST-64 PR = Wisconsin Card Sorting Test-64 Perseverative Responses; COWAT = Controlled Oral Word Associations Test; GPDH = Grooved Pegboard Dominant Hand; GPNDH = Grooved Pegboard Non-Dominant Hand.
Before assessing group differences on sRCS scores we conducted either chi-square or t-tests to assess whether demographic, clinical, and medical characteristics that were significantly different among the groups were related to sRCS scores. There were no significant relationships found between education, hypertension, pulmonary disease, current psychiatric disorders, lab values and the sRCS scores and thus these variables were not included as covariates.
In order to test our hypotheses regarding the impact of HCV/HIV co-infection status (mono- vs. co-infected) on neurocognitive change while controlling for practice effects we computed a series of independent samples t-test analyses to comapare the groups’ sRCS scores. Shown in Table 5, the results revealed that the HCV/HIV co-infected group demonstrated significantly greater decline in memory functioning (t(56) = 2.14, p = 0.04) and overall global functioning (t(53) = 2.28, p = 0.03) during the study period, with large effect sizes noted (Cohen’s d’s = .55 & .61, respectively). No other significant differences were observed (all p’s>.05).
Table 5.
Mean FSS Change Scores, BDI-FS Change Scores, and sRCS z-scores after 24-Weeks of Treatment.
| Baseline - 24 Weeks | |||||
|---|---|---|---|---|---|
|
| |||||
|
HCV
Mono-infected n = 36 |
HCV/HIV
Co-infected n = 28 |
||||
|
| |||||
| M (SD) | M (SD) | t | p | d | |
| FSS Change Score | .72 (2.30) | 1.31 (1.73) | −1.111 | .27 | −0.28 |
| BDI-FS Change Score | .58 (3.65) | .86 (1.94) | −0.36 | .72 | −0.09 |
| Neurocognitive Domain sRCSs | |||||
| Processing Speed | 0.29 (.70) | .31 (1.01) | −0.09 | .93 | −0.02 |
| Learning | 1.01 (2.51) | .14 (2.10) | 1.41 | .17 | 0.37 |
| Memory | −3.18 (7.43) | −7.79 (8.97) | 2.14 | .04 | 0.57 |
| Executive | −1.19 (.93) | −.79 (.65) | −1.83 | .07 | −0.49 |
| Verbal | −.24 (1.29) | −.14 (1.18) | −0.30 | .76 | −0.08 |
| Motor | −.61 (.81) | −.63 (1.09) | .09 | .93 | 0.02 |
| Global Neurocognitive Functioning | −.86 (1.71) | −1.93 (1.78) | 2.28 | .03 | 0.61 |
Notes. FSS = Fatigue Severity Scale; BDI-II FS =Beck Depression Inventory-2nd edition Fast Screen; sRCS = summary Regression-based Change Scores.
In order to assess if the decline in CD4 among the co-infected group impacted neurocognitive functioning, an independent t-test was conducted to evaluate if there was a relationship between the individuals who did decline and the sRCS scores. Within the co-infected group decline in CD4 count was associated with poorer speed of information processing at 24 weeks (non-decliners, M= 0.48, SD= 0.82; and decliners, M= −0.38, SD= 0.91) t(21) = 2.32, p = 0.03. Conversely, CD4 decline was inversely related to higher performance on motor functioning at 24 weeks (non-decliners, M= −0.89 SD= 0.61; and decliners, M= −0.28, SD= 1.08) t(20) = −3.26, p = 0.04.
Lastly, Of the 22 who achieved SVR by week 24, the coinfected group scored significantly worse on learning (M= −1.02, SD= 2.22 vs. M= .98, SD= 1.02, respectively t(14)= 2.42, p = .03), memory (M= −11.04, SD= 9.35 vs. M= −1.46, SD= 8.01, respectively t(14)= 2.21, p = 04), and global functioning (M= −2.67 , SD= 2.01 vs. M= −0.52, SD= 1.73, respectively; t(14)= 2.31, p = 03).
Treatment exposure
In order to better characterize our sample and contextualize our findings regarding co-infection status, we examined treatment exposure. Seven mono-infected and 3 co-infected patients who were otherwise eligible were lost to follow-up and were not included in these analyses, however, this did not differ significantly between the groups with (15.9% vs. 10%, respectively; χ2 (2, n = 74) = .533, p = 0.47.
As shown in Table 6, there were no significant differences between weeks on treatment between the mono- vs. co-infected groups. Between baseline and 24 weeks there were also no significant differences between the percentages of mono-infected vs. co-infected groups who achieved ≥ 80% treatment exposure to either IFN or RBV(all p’s>.10). There was also no significant difference between sustained virologic response (SVR) achieved by each group: 31.8% of the mono-infected group and 26.7% of the co-infected group (p = .63). As Figure 1 illustrates, at 24 weeks, 49 patients were still on treatment, 65.1% of the mono-infected group and 66.7 of the co-infected group χ2 (1, n = 74) = 0.005, p = 0.95). For the entirety of the study, 35 patients completed the entire course of treatment and a total of 22 reached SVR. Lastly, we computed a series of independent samples t-test analyses comparing treatment status groups (≥ 80% vs. <80% exposure), regardless of co-infection status, on the neurocognitive sRCS scores. There were no significant differences observed based on IFN or RBV treatment exposure (all p’s>.05). However, the Cohen’s d effect sizes indicate moderate effects for excecutive functioning (in both IFN & RBV exposure; d’s = −.38 to −.42, respectively), learning (IFN exposure; d = .42) and motor functioning (IFN exposure; d = −.50), such that greater treatment exposure was associated with less neurocognitive decline.
Table 6.
Medication Exposure and Treatment Outcomes
| Baseline - 24 Weeks | |||||
|---|---|---|---|---|---|
|
| |||||
|
HCV
Mono-infected n = 44 |
HCV/HIV
Co-infected n = 30 |
||||
|
| |||||
| Treatment Exposure | M (SD)/ % | M (SD)/ % | t(df) or χ2 | p | d/φ |
| Weeks on Treatment | 31.6 (18.8) | 32.3 (22.2) | −.16 | .87 | −.03 |
| ≥ 80% Interferon Exposure | 65.9 | 63.3% | .05 | .82 | .03 |
| ≥ 80% Ribavirin Exposure | 61.4 | 60.0 | .01 | .91 | .01 |
| Completed 24 Weeks of Treatment | 65.9% | 66.7% | .01 | .95 | .01 |
| Completed Entire Course of Treatment | 50.0% | 43.3% | .32 | .57 | −.07 |
| SVR Result | 31.8% | 26.7% | .23 | .63 | −.05 |
Notes. SVR - Sustained viral response.
Discussion
The current study prospectively examined the neurocognitive function of HCV mono-infected and HCV/HIV co-infected patients treated with pegylated IFN/RBV over a 24-week course to assess whether HIV co-infection would increase risk of neurocognitive decline among HCV infected patients undergoing IFN/RBV treatment. A secondary aim of this study was to provide a fine-grained analysis of pegylated IFN/RBV treatment exposure and whether it was associated with neurocognitive change. Our study identified HIV co-infection as a significant risk factor for neurocognitive decline longitudinally. Specifically, at baseline there were no significant differences in neurocognitive functioning between the mono- and co-infected groups. However, over the course of 24 weeks of HCV treatment, HIV/HCV co-infection significantly predicted neurocognitive decline in memory and global functioning – even after accounting for practice effects. Although the mono-infected group reported significantly more fatigue at baseline, there were no between-group differences in fatigue at 24 weeks. Moreover, fatigue was not associated with neurocognitive decline. In terms of treatment exposure for all patients, regardless of co-infection, we found that greater treatment exposures was associated with moderate effect sizes in the domains of executive functioning, learning, and memory, such that greater treatment exposure was associated with less neurocognitive decline.
It is not surprising that HIV/HCV co-infected group did worse on neurocognitive functioning considering it is well established that both HIV mono-infection and HCV mono-infection are associated with neurocognitive decline. Therefore, it could be inferred that HIV/HCV co-infection synergistically increases neurocognitive decline when challenged by IFN/RBV treatment. Several explanations have been postulated to explain the potential neuropathological mechanisms of HIV/HCV co-infection. As Hinkin et al. (2008) notes, HCV has been recovered in cerebrospinal fluid, and infects the same monocytes and macrophages of the same cells that are a target of HIV, suggesting similar mechanisms of neuropathogenesis. Another shared similarity in viral pathology between HIV and HCV, is that in both viruses there is an association between macrophage expression of pro-inflammatory cytokines which can contribute to neural degeneration (Gill and Kolson 2014), and perhaps increase likelihood of neurocognitive decline. Additionally, one cross-sectional study from the CHARTER group utilizing structural MRI methods found that HIV/HCV co-infected subjects had greater loss of white matter in the brain compared to HIV mono-subjects (Jernigan et al. 2011).
The primary clinical implication of this study is that it presents evidence that HCV/HIV co-infected patients are at significantly greater risk for decline in memory and global neurocognitive functioning compared with HCV monoinfected patients while undergoing HCV treatment (in this case 24 weeks of treatment with pegylated IFN/RBV). HCV mono-infected and HCV/HIV co-infected groups had similar percentages of dosage exposure to IFN and RBV; therefore observed neurocognitive function differences were not due to different dose exposure. This finding highlights the need for care providers to monitor the neurocognitive functioning of their HCV/HIV co-infected patients closely during HCV treatment by including baseline neurocognitive screening and longitudinal assessment. Should patients demonstrate incident neurocognitive decline, they may consider the need for potential medication adherence interventions and/or assistance with developing compensatory strategies to cope with any neurocognitive declines.
Our study is unique and extends the prior literature in two important ways. First, this study addresses major limitations of the previous longitudinal neurocognitive studies noted above. Few studies have taken into account naturally occurring change and practice effects on repeated neurocognitive test performance, which is a possible explanation of the diverging findings for past studies on both HCV/HIV co-infection (Giesen et al. 2004; Ryan et al. 2004; Clifford et al. 2005; Hilsabeck and Castellon 2005; Hinkin et al. 2008) and pegylated IFN/RBV treatment studies (Fried 2002; Hilsabeck and Hassanein 2005; Fontana et al. 2007; Wobrock et al. 2008; Fontana et al. 2010; Group et al. 2014; Huckans et al. 2015). We controlled for practice effects by adopting the standard regression based approach, which utilized norms for change (McSweeny et al. 1993; Hinton-Bayre 2010a; Maassen 2010; Hinton-Bayre 2010b; Cysique et al. 2011). In doing so, the current study provides a more accurate representation of neurocognitive change over the course of treatment. One final methodological strength of this study, which was not addressed in previous studies, is that it took into account the proportional amount of prescribed IFN/RBV each individual receives given the frequent occurrence of dose reductions, early treatment discontinuation, and patient missed doses. Although neurocognitive measurements were done at set time points, some patients could exhibit other non-neurocognitive related side effects resulting in the treating physician decreasing the dosage of IFN or RBV instead of simply stopping therapy. Since proportion of prescribed drug actually received can contribute to within-group variance, we treated it as a variable in the analyses. Second, this study offers an exceptionally well-characterized sample with a granular analysis of treatment exposure, as well as clinical and psychiatric variables, in order to better contextualize the impact of HCV/HIV co-infection status. Given the methodological advances provided in this study, it is clear that co-infected patients are at increased risk for declines in memory and global neurocognitive functioning compared to their mono-infected counterparts.
Although this study addresses limitations of several previous studies, there are still some important limitations that merit discussion. First, it is important to note that our present findings cannot conclusively determine the etiology of neurocognitive dysfunction considering the methodology of the present study. The present study did not employadvanced brain imaging techniques alongside our methodsto better understand the neural underpinnings of the current findings. The size of our sample also could have limited power for between-subjects analyses. Additionally, this study did not assess whether neurocognitive decline sustained or improved upon treatment discontinuation. Past research (e.g., Fontana et al. (2007; 2010)) leads us to assume upon the discontinuation of IFN/RBV treatment neurocognitive functioning would likely be improved. Lastly, the introduction of direct acting antiviral therapy for the treatment of Hepatitis C has dramatically transformed the treatment of HCV among both mono- and co-infected patients such that IFN is no longer used. Fatigue does however remain the most frequently occurring side effect of direct acting antiviral treatment for HCV (Banerjee and Reddy 2016) and fatigue is known to be correlated with neurocognitive impairment. We assert that these study findings are relevant and informative for the larger literature on the impact of co-infection with HIV and HCV on neurocognitive functioning. Our findings suggest that co-infected patients may be at increased risk for neurocognitive decline compared to mono-infected patients, and that care providers should carefully monitor neurocognitive status in order to address patient needs and provide support and resources accordingly.
Acknowledgements
We thank the men and women who participated in the study and the medical providers who referred patients to the study.
Source of funding
This work was supported by the National Institute of Mental Health of the National Institutes of Health (K23MH071177). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
The study was approved by Institutional Review Boards at all participating institutions; participants provided written informed consent in accordance with institutional requirements prior to enrollment.
Conflict of interest
The authors declare that they have no competing interests.
References
- Aaron BT. BDI-Fast Screen for Medical Patients: Manual. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Asnis, Miller D. Ribavirin may be an important factor in IFN-induced neuropsychiatric effects. 2004 doi: 10.4088/jcp.v65n0420b. [DOI] [PubMed] [Google Scholar]
- Averhoff, Glass Global burden of hepatitis C: considerations for healthcare providers in the United States. 2012 doi: 10.1093/cid/cis361. doi: 10.1093/cid/cis361. [DOI] [PubMed] [Google Scholar]
- Banerjee D, Reddy KR. Review article: safety and tolerability of direct-acting anti-viral agents in the new era of hepatitis C therapy. Alimentary pharmacology & therapeutics. 2016;43(6):674–696. doi: 10.1111/apt.13514. [DOI] [PubMed] [Google Scholar]
- Beck, Steer, Brown Beck depression inventory-II. 1996 [Google Scholar]
- Bohn J-P, Gastl G, Steurer M. Long-term treatment of hairy cell leukemia with interferon-α: still a viable therapeutic option. Memo. 2016;9:63–65. doi: 10.1007/s12254-016-0269-1. doi: 10.1007/s12254-016-0269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5:125–142. doi: 10.1080/13854049108403297. [Google Scholar]
- Calabresi PA, Kieseier BC, Arnold DL, Balcer LJ. Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. 2014 doi: 10.1016/S1474-4422(14)70068-7. [DOI] [PubMed] [Google Scholar]
- Cattie J, Letendre S, Woods S, Barakat F, Perry W, Cherner M, et al. Persistent neurocognitive decline in a clinic sample of hepatitis C virus-infected persons receiving interferon and ribavirin treatment. Journal of NeuroVirology. 2014;20:561570. doi: 10.1007/s13365-014-0265-3. doi: 10.1007/s13365-014-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha L, Berry CM, Nolan D, Castley A, Fernandez S, French MA. Interferon-alpha, immune activation and immune dysfunction in treated HIV infection. Clinical & Translational Immunology. 2014;3(2):e10. doi: 10.1038/cti.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W-C, Tsan Y-T, Tsai S-L, et al. Hepatitis C viral infection and the risk of dementia. European journal of neurology?: the official journal of the European Federation of Neurological Societies. 2014;21:1068–e59. doi: 10.1111/ene.12317. doi: 10.1111/ene.12317. [DOI] [PubMed] [Google Scholar]
- Clifford, Evans, Yang, Gulick The neuropsychological and neurological impact of hepatitis C virus co-infection in HIV-infected subjects. 2005 doi: 10.1097/01.aids.0000192072.80572.43. doi: 10.1097/00002030-200510003-00011. [DOI] [PubMed] [Google Scholar]
- Cysique, Abramson F., Jr Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. 2011 doi: 10.1080/13803395.2010.535504. doi: 10.1080/13803395.2010.535504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieperink, Willenbring Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. 2000 doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- Fontana, Bieliauskas, Back-Madruga Cognitive function does not worsen during long-term low-dose peginterferon therapy in patients with chronic hepatitis C. 2010 doi: 10.1038/ajg.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana, Bieliauskas, Lindsay Cognitive function does not worsen during pegylated interferon and ribavirin retreatment of chronic hepatitis C. 2007 doi: 10.1002/hep.21633. doi: 10.1002/hep.21633. [DOI] [PubMed] [Google Scholar]
- Forton, Taylor-Robinson Central nervous system changes in hepatitis C virus infection. 2006 doi: 10.1097/00042737-200604000-00005. [DOI] [PubMed] [Google Scholar]
- Forton, Thomas, Murphy, Allsop Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. 2002 doi: 10.1053/jhep.2002.30688. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- Fried Side effects of therapy of hepatitis C and their management. 2002 doi: 10.1002/hep.1840360730. [Google Scholar]
- Giesen H-J, Heintges T, Abbasi-Boroudjeni N, et al. Psychomotor slowing in hepatitis C and HIV infection. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2004;35:131–137. doi: 10.1097/00126334-200402010-00005. doi: 10.1097/00126334-200402010-00005. [DOI] [PubMed] [Google Scholar]
- Gill A, Kolson D. Chronic inflammation and the role for cofactors (hepatitis C, drug abuse, antiretroviral drug toxicity, aging) in HAND persistence. Current HIV/AIDS reports. 2014;11:325–35. doi: 10.1007/s11904-014-0210-3. doi: 10.1007/s11904-014-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Kirson D, Velin RA, Grant I. The utility of clinical ratings for detecting cognitive change in HIV infection. 1994 [Google Scholar]
- Heaton, Clifford, Franklin, Woods HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. 2010a doi: 10.1212/WNL.0b013e318200d727. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. 2015 doi: 10.1093/cid/ciu862. doi: 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R, Franklin D, Ellis R, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of NeuroVirology. 2010b;17:3–16. doi: 10.1007/s13365-010-0006-1. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsabeck, Castellon Neuropsychological aspects of coinfection with HIV and hepatitis C virus. 2005 doi: 10.1086/429494. doi: 10.1086/429494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsabeck, Hassanein Effect of Interferon-[alpha] on cognitive functioning in patients with chronic hepatitis C. 2005 doi: 10.1017/S1355617705050022. [DOI] [PubMed] [Google Scholar]
- Hilsabeck R, Perry W, Hassanein T. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35:440–446. doi: 10.1053/jhep.2002.31257. doi: 10.1053/jhep.2002.31257. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Aronow HA, Hilsabeck RC. Psychiatric and cognitive concomitants of HIV and HCV co-infection; National Institute on Drug Abuse (NIDA) symposium on issues in the medical management of HIV/HCV co-infection in IDUs; Washington, DC. 2004. [Google Scholar]
- Hinkin, Castellon, Levine Neurocognition in individuals co-infected with HIV and hepatitis C. 2008 doi: 10.1300/j069v27n02_02. doi: 10.1300/J069v27n02_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton-Bayre Deriving Reliable Change statistics from test–retest normative data: Comparison of models and mathematical expressions. 2010a doi: 10.1093/arclin/acq008. [DOI] [PubMed] [Google Scholar]
- Hinton-Bayre Specificity of reliable change models and review of the within-subjects standard deviation as an error term. 2010b doi: 10.1093/arclin/acq087. doi: 10.1093/arclin/acq087. [DOI] [PubMed] [Google Scholar]
- Huckans M, Fuller B, Wheaton V, et al. A longitudinal study evaluating the effects of interferon-alpha therapy on cognitive and psychiatric function in adults with chronic hepatitis C. Journal of psychosomatic research. 2015;78:184–192. doi: 10.1016/j.jpsychores.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan T, Archibald S, Fennema-Notestine C, et al. Clinical factors related to brain structure in HIV: the CHARTER study. Journal of neurovirology. 2011;17:248–57. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman, Zodet, Hakim, Aledort Psychometric evaluation of the fatigue severity scale for use in chronic hepatitis C. 2000 doi: 10.1023/a:1008960710415. doi: 10.1023/A:1008960710415. [DOI] [PubMed] [Google Scholar]
- Koziel, Peters Viral hepatitis in HIV infection. 2007 doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M, Schäfer A, Wißmann S, et al. Neurocognitive Changes in Patients with Hepatitis C Receiving Interferon alfa-2b and Ribavirin. Clinical Pharmacology & Therapeutics. 2005;77:90–100. doi: 10.1016/j.clpt.2004.09.007. doi: 10.1016/j.clpt.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Krefetz, Steer, Jermyn Screening HIV-infected patients with chronic pain for anxiety and mood disorders with the Beck Anxiety and Depression Inventory-Fast Screens for medical settings. 2004 doi: 10.1023/B:JOCS.0000045348.28440.82. [Google Scholar]
- Krupp, LaRocca, Muir-Nash The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. 1989 doi: 10.1001/archneur.1989.00520460115022. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Lieb, Engelbrecht, Gut, et al. Cognitive impairment in patients with chronic hepatitis treated with interferon alpha (IFNα): results from a prospective study. 2006 doi: 10.1016/j.eurpsy.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Maassen The two errors of using the within-subject standard deviation (WSD) as the standard error of a Reliable Change Index. 2010 doi: 10.1093/arclin/acq036. doi: 10.1093/arclin/acq036. [DOI] [PubMed] [Google Scholar]
- Majer, Welberg L, Capuron, Pagnoni IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. 2008 doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, Novak, Fendrich Stroop performance in drug users classified by HIV and hepatitis C virus serostatus. 2004 doi: 10.1017/S135561770410218X. [DOI] [PubMed] [Google Scholar]
- McAndrews M, Farcnik K, Carlen P, et al. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology (Baltimore, Md) 2005;41:801–8. doi: 10.1002/hep.20635. doi: 10.1002/hep.20635. [DOI] [PubMed] [Google Scholar]
- McHutchison, Gordon, Schiff Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. 1998 doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- McSweeny, Naugle, Chelune “T scores for change”: An illustration of a regression approach to depicting change in clinical neuropsychology. 1993 doi: 10.1080/13854049308401901. [Google Scholar]
- Mindt M, Miranda C, Arentoft A, et al. Aging and HIV/AIDS: Neurocognitive Implications for Older HIV-Positive Latina/o Adults. Behavioral Medicine. 2014;40:116–123. doi: 10.1080/08964289.2014.914464. doi: 10.1080/08964289.2014.914464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr P, Hauschild A, Trefzer U, et al. Intermittent High-Dose Intravenous Interferon Alfa-2b for Adjuvant Treatment of Stage III Melanoma: Final Analysis of a Randomized Phase III Dermatologic Cooperative Oncology Group Trial. J Clin Oncol. 2015;33:4077–84. doi: 10.1200/JCO.2014.59.6932. doi: 10.1200/JCO.2014.59.6932. [DOI] [PubMed] [Google Scholar]
- Mosa C, Trizzino A, Trizzino A, et al. Treatment of human papillomavirus infection with interferon alpha and ribavirin in a patient with acquired aplastic anemia. Int J Infect Dis. 2014;23:25–7. doi: 10.1016/j.ijid.2013.11.021. doi: 10.1016/j.ijid.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Pavan MH, Velho PE, Vigani AG, et al. Treatment of human papillomavirus with peg-interferon alfa-2b and ribavirin. Braz J Infect Dis. 2007;11:383–4. doi: 10.1590/s1413-86702007000300017. [DOI] [PubMed] [Google Scholar]
- Pawelczyk T, Pawelczyk A, Strzelecki D, Rabe-Jablonska J. Pegylated interferon and ribavirin therapy may induce working memory disturbances in chronic hepatitis C patients. General hospital psychiatry. 2008;30:501–508. doi: 10.1016/j.genhosppsych.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Perry, Carlson, Barakat, Hilsabeck Neuropsychological test performance in patients co-infected with hepatitis C virus and HIV. 2005 doi: 10.1097/01.aids.0000192074.18691.31. doi: 10.1097/00002030-200510003-00013. [DOI] [PubMed] [Google Scholar]
- Poynard T, Marcellin P, Lee S, et al. Randomised trial of interferon 2b plus ribavirin for 48 weeks or for 24 weeks versus interferon 2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. The Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Gorman J, Dieterich D. Interferon-induced depression and cognitive impairment in hepatitis C virus patients: a 72 week prospective study. AIDS (London, England) 2005;19(Suppl 3):S174–8. doi: 10.1097/01.aids.0000192087.64432.ae. [DOI] [PubMed] [Google Scholar]
- Ryan, Morgello, Isaacs, et al. Neuropsychiatric impact of hepatitis C on advanced HIV. 2004 doi: 10.1212/01.wnl.0000115177.74976.6c. doi: 10.1212/01.WNL.0000115177.74976.6C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, Hinzpeter, Mohmand, Janssen Hepatitis C treatment in “difficult-to-treat” psychiatric patients with pegylated interferon-alpha and ribavirin: Response and psychiatric side effects. 2007 doi: 10.1002/hep.21791. doi: 10.1002/hep.21791. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Engelbrechta M, Gut O, et al. Interferon alpha (IFN ) and psychiatric syndromes: a review. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26:731–746. doi: 10.1016/s0278-5846(01)00324-4. [DOI] [PubMed] [Google Scholar]
- Shrestha NK, Hamrock DJ. Successful treatment of disseminated human papillomavirus infection with pegylated interferon and ribavirin. Clin Infect Dis. 2010;51:e4–6. doi: 10.1086/653428. doi: 10.1086/653428. [DOI] [PubMed] [Google Scholar]
- Statistical Package for Social Sciences for Windows, Version 22.0. Chicago, Illinois: 2015. [Google Scholar]
- Tanaka, Maeshima, Shigekawa, Ueda Neuropsychological impairment and decreased regional cerebral blood flow by interferon treatment in patients with chronic hepatitis: a preliminary study. 2006 doi: 10.1007/s10238-006-0107-6. doi: 10.1007/s10238-006-0107-6. [DOI] [PubMed] [Google Scholar]
- Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. Aids. 2008 doi: 10.1097/QAD.0b013e32830e6d51. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- Thein, Maruff, Krahn, Kaldor Cognitive function, mood and health-related quality of life in hepatitis C virus (HCV)-monoinfected and HIV/HCV-coinfected individuals commencing HCV. 2007 doi: 10.1111/j.1468-1293.2007.00452.x. doi: 10.1111/j.1468-1293.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- Weiss, Bräu, Stivala, Swan Review article: adherence to medication for chronic hepatitis C–building on the model of human immunodeficiency virus antiretroviral adherence research. 2009 doi: 10.1111/j.1365-2036.2009.04004.x. doi: 10.1111/j.1365-2036.2009.04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I, Bell B, Kuhnert W, Alter M. Incidence and transmission patterns of acute hepatitis C in the United States, 1982-2006. Archives of internal medicine. 2011;171:242–248. doi: 10.1001/archinternmed.2010.511. doi: 10.1001/archinternmed.2010.511. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Mihm U, Löhr C, et al. Cognition in hepatitis C patients treated with pegylated interferon. The world journal of biological psychiatry?: the official journal of the World Federation of Societies of Biological Psychiatry. 2008;10:819–26. doi: 10.1080/15622970701714362. doi: 10.1080/15622970701714362. [DOI] [PubMed] [Google Scholar]
- Woods, Rippeth, Frol, Levy Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. 2004 doi: 10.1080/13803390490509565. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]