Abstract
BACKGROUND
No prospective cohort study of high-risk children has used rigorous exposure assessment and optimal diagnostic procedures to examine the perinatal antecedents of autism spectrum disorder (ASD), separately among those with and without cognitive impairment.
OBJECTIVE
To identify perinatal factors associated with increased risk for ASD with and without intellectual disability (ID: IQ < 70) in children born extremely preterm.
STUDY DESIGN
This prospective multi-center (14 institutions in 5 states) birth cohort study included children born at 23-27 weeks gestation in 2002-2004 who were evaluated for ASD and ID at age 10 years. Pregnancy information was obtained from medical records and by structured maternal interview. Cervical-vaginal ‘infection’ refers to maternal report of bacterial infection (n = 4), bacterial vaginosis (n = 30), yeast infection (n = 62), mixed infection (n = 4) or other/unspecified infection (n=43; e.g., chlamydia, trichomonas or herpes, etc.). We do not know the extent to which ‘infection’ per se was confirmed by microbial colonization. We use the terms ‘fetal growth restriction’ and ‘small for gestational age’ interchangeably in light of the ongoing challenge to discern pathologically from constitutionally small newborns. Severe fetal growth-restriction was defined as a birth weight Z-score for gestational age at delivery < - 2 (i.e., 2 standard deviations or more below the median birth weight in a referent sample that excluded pregnancies delivered for preeclampsia or fetal indications). Participants were classified into four groups based on whether or not they met rigorous diagnostic criteria for ASD and ID (ASD+/ID−, ASD+/ID+, ASD−/ID+ and ASD−/ID−). Temporally-ordered multinomial logistic regression models were used to examine the information conveyed by perinatal factors about increased risk for ASD and/or ID (ASD+/ID−, ASD+/ID+ and ASD−/ID+).
RESULTS
889 of 966 (92%) children recruited were assessed at age 10 years, of whom 857 (96%) were assessed for ASD; of these, 840 (98%) children were assessed for ID. ASD+/ID− was diagnosed in 3.2% (27/840), ASD+/ID+ in 3.8% (32/840), and ASD−/ID+ in 8.5% (71/840). Maternal report of presumed cervical-vaginal ‘infection’ during pregnancy was associated with increased risk of ASD+/ID+ (odd ratio [OR], 2.7; 95% CI, 1.2-6.4). The lowest gestational age category (23-24 weeks) was associated with increased risk of ASD+/ID+ (OR, 2.9; 95% CI, 1.3-6.6) and ASD+/ID− (OR, 4.4; 95% CI, 1.7-11). Severe fetal growth restriction was strongly associated with increased risk for ASD+/ID− (OR, 9.9; 95% CI, 3.3-30), whereas peripartum maternal fever was uniquely associated with increased risk of ASD−/ID+ (OR, 2.9; 95% CI, 1.2-6.7).
CONCLUSION
Our study confirms that low gestational age is associated with increased risk for ASD irrespective of intellectual ability, whereas severe fetal growth restriction is strongly associated with ASD without ID. Maternal report of cervical-vaginal infection is associated with increased risk of ASD with ID, and peripartum maternal fever is associated with increased risk for ID without ASD.
INTRODUCTION
Meta-analyses and comprehensive reviews describe inconsistencies in research findings on the perinatal antecedents of ASD as likely reflecting study differences in ascertainment and diagnostic procedures, sample size, exposure assessment, and treatment of potential confounders.1-3 Nevertheless, mounting evidence suggests that a constellation of perinatal factors contribute to increased risk of ASD, including preterm birth,4-9 fetal growth restriction (or lower than expected birth weight for gestational age10),4,6 and their correlates (e.g., placental insufficiency and preeclampsia).11
Risk factors for ASD apparently differ between children who have and who do not have co-occurring intellectual impairment (ID).4,7,12 Yet, no prospective cohort study of high-risk children has used rigorous exposure assessment and optimal diagnostic procedures to examine the perinatal antecedents of ASD, taking into account co-occurring cognitive impairment. The large Extremely Low Gestational Age Newborn (ELGAN) Study cohort of infants born before the 28th week of gestation afforded us the opportunity to examine prospectively the antenatal and neonatal antecedents of ASD diagnosed at age 10 years, separately in children with and without co-occurring ID (IQ < 70).
METHODS
Participants
The ELGAN study is a multi-center observational study designed to identify characteristics and exposures associated with increased risk of structural and functional neurologic disorders in extremely preterm infants.13 During the years 2002-2004, women delivering before 28 weeks gestation at one of 14 participating institutions were asked to enroll in the study; 1249 mothers of 1506 infants consented to participate, and 1198 children survived to 10 years [see Supplement Figure 1 for a flow diagram of study participants]. Of 966 children who were actively recruited for follow-up at age 10 years (because of the availability of blood samples from their first postnatal month), informed consent was obtained for the participation of 889 (92%). The institutional review boards of participating institutions approved the study procedures.
Demographic, pregnancy, delivery and newborn variables
Methods of data collection for demographic, pregnancy, delivery, and newborn variables are described elsewhere,13 and also in detail in eAppendix 1 in the Supplement. In brief, gestational age (GA) estimates were based on a hierarchy of the best information available as described in the Supplement (92% were based on fetal ultrasound; most prior to 14 weeks). Cervical-vaginal ‘infection’ refers to maternal report of bacterial infection (n = 4), bacterial vaginosis (n = 30), yeast infection (n = 62), mixed infection (n = 4) or other/unspecified infection (n=43; e.g., chlamydia, trichomonas or herpes, etc.). Previous research indicates that such information gained through self-report can be more accurate than that obtained from medical records or birth certificates,14-17 but we do not know the extent to which ‘infection’ per se was confirmed by microbial colonization. The terms ‘fetal growth restriction’ and ‘small for gestational age’ meet our needs equally, and we use them interchangeably since accurate differentiation of pathologically small from constitutionally small newborns (see18,19) is an ongoing challenge.20-22 Severe fetal growth restriction was defined by a birth weight Z-score < −2. Birth weight Z-score was calculated as the number of standard deviations each infant's birth weight was above or below the median birth weight in referent samples that excluded pregnancies delivered for preeclampsia or fetal indications.23,24 Physiology, laboratory and therapy data for the first 12 postnatal hours were collected to calculate a Score for Neonatal Acute Physiology–II25 (SNAP-II™).26 Additional data were collected on placenta microbiology and histology,27-31 mode of ventilation and respiratory care,32,33 bacteremia,34 patent ductus arteriosis (PDA),35 illnesses and medications used in the first 28 days post-partum,36 necrotizing enterocolitis,37 and retinopathy.38
Assessment at 10-years of age
The assessment procedures, and all relevant test scores for ASD and ID, are reported in a prior publication.39 Briefly, diagnostic assessment of ASD was conducted with three well-validated measures, administered sequentially. First was the Social Communication Questionnaire (SCQ) with a screen-in score ≥ 11 to increase sensitivity relative to the standard criterion score of ≥ 15.40 Children who met the SCQ criterion were then assessed with the Autism Diagnostic Interview–Revised (ADI-R).41 All children who met ADI-R criteria for autism or ASD, 42 or who had a prior clinical diagnosis of ASD and/or exhibited symptoms of ASD during cognitive testing according to the site psychologist) were then assessed with the Autism Diagnostic Observation Schedule, Second Version (ADOS-2)43 -- the criterion measure of ASD in this study.
All ADOS-2 administrations were independently scored by a second rater with autism diagnostic and ADOS-2 expertise (R.M.J.) who did not have knowledge of the child's SCQ and ADI-R results or prior clinical history. In cases of scoring disagreements, consensus was reached between raters. Item-by-item inter-rater agreement for the 14 ADOS-2 diagnostic algorithm scores was on average .93 (SD = .12). Of 90 ADOS-2 assessments, inter-rater disagreement and consensus scoring resulted in 4 changes of classification, 3 from non-ASD to ASD and 1 from ASD to non-ASD, Cohen's K = .90.
Intellectual ability (IQ) was assessed with the School-Age Differential Ability Scales – II (DAS-II).44 Children with IQ [(Verbal + Nonverbal Reasoning scores)/2] < 70 were classified as having intellectual disability (ID). Because ASD cannot be validly diagnosed in children with significant visual and/or motor impairment accompanied by severe intellectual disability,45 children with these conditions were excluded from diagnostic consideration of ASD. (Supplement Figure S1) Severe gross motor dysfunction was defined as Level 5 (i.e., no self-mobility) on the Gross Motor Function Classification System (GMFCS).46 A child was considered to have severe visual impairment if the parent reported uncorrectable functional blindness in both eyes. No participant had a significant, uncorrected hearing impairment.
Data analyses
We evaluated the null hypothesis that ASD without ID (ASD+/ID−), ASD with ID (ASD+/ID+), and ID without ASD (ASD−/ID+) are not associated with any maternal, pregnancy, delivery, or postnatal characteristic or exposure. We began by classifying children into four groups based on whether or not they met diagnostic criteria for ASD and ID at age 10 years. We then described the percent of children in each group whose mother had selected demographic and pregnancy characteristics or who themselves had perinatal and neonatal characteristics and exposures and who were diagnosed with ASD and/or ID at age 10 years.
Because antepartum phenomena can influence postnatal phenomena, we tested our null hypothesis with temporally-oriented models.47 Primary exposures included inflammation-related phenomena during pregnancy (e.g., maternal report of cervicalvaginal ‘infection’) and at delivery (e.g., intrapartum maternal fever), indicators of fetal growth restriction and its correlates (e.g., birth weight Z-score for gestational age < −2 and preeclampsia) and lowest gestational age category (i.e., 23-24 weeks). We considered variables as confounders if identified in the literature or if in our data they were associated with both the exposure and the outcome with probabilities ≤ .25.48 To construct the time-oriented models, we used a step-down procedure seeking a parsimonious solution without effect modification terms. First, we examined pregnancy information in a multinomial logistic regression model of risk for ASD and ID. Then we added factors measured around the time of delivery, adjusting for those variables with statistically significant associations in the pregnancy-stage model. Finally we added neonatal factors, adjusting for all variables selected in by the earlier models.
We present magnitudes of association as odds ratios (OR) with 95% confidence intervals (CI). Associations were statistically significant when the 95% confidence interval did not include the null estimate (i.e., OR 1.0). The primary outcomes ASD+/ID− and ASD+/ID+ affected 3-4% of our sample of ELGANs, giving us 80% power to detect associations with a minimal detectable odds ratio of 3.2, assuming an exposure prevalence of 0.3. We also describe the prevalence and antecedents of ID without ASD as a secondary outcome.
RESULTS
Of the 996 children recruited to participate at age 10 years, 889 (92%) were enrolled, of whom 840 (95%) were assessed both for ASD and for ID. [Supplement Figure 1] Of the 840 children in our final sample, 7.0% (n = 59) met study criteria for ASD and 12.3% (n = 103) had ID (IQ < 70). ASD+/ID− was diagnosed in 3.2% (27/840), ASD+/ID+ in 3.8% (32/840), and ASD−/ID+ in 8.5% (71/840) of the final sample.
Descriptive univariate analyses
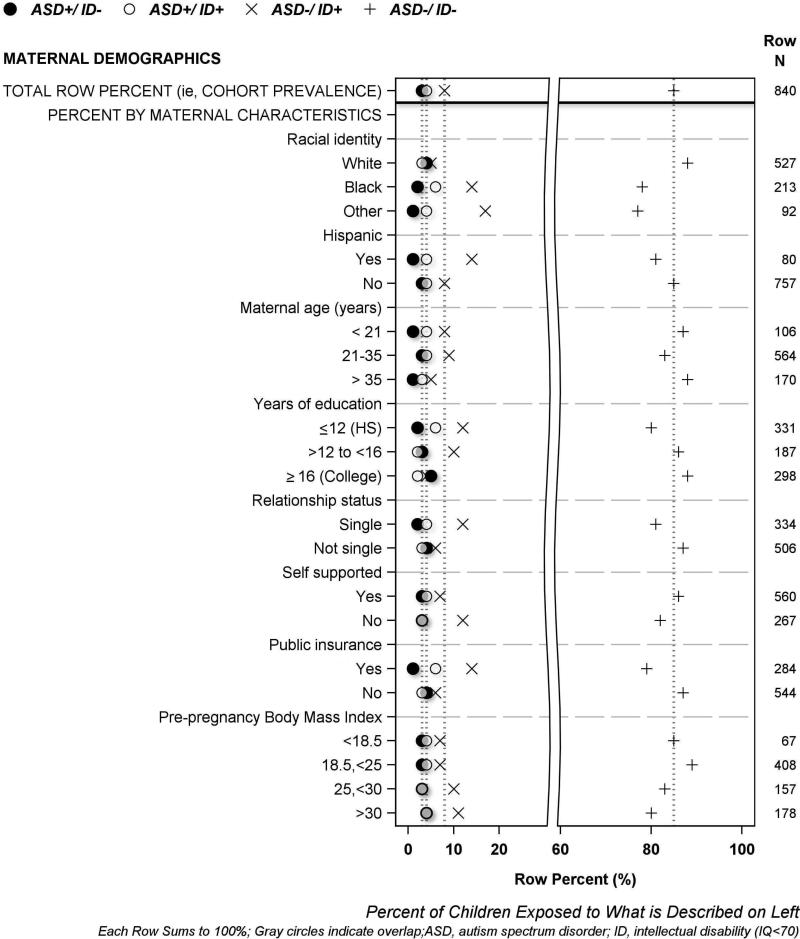
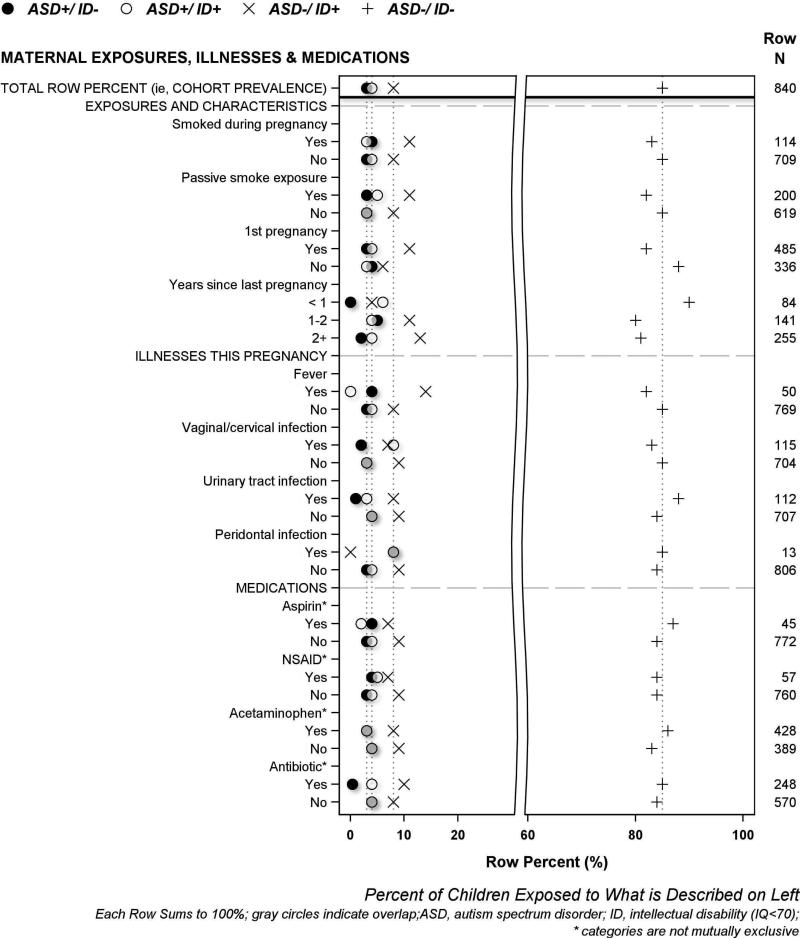
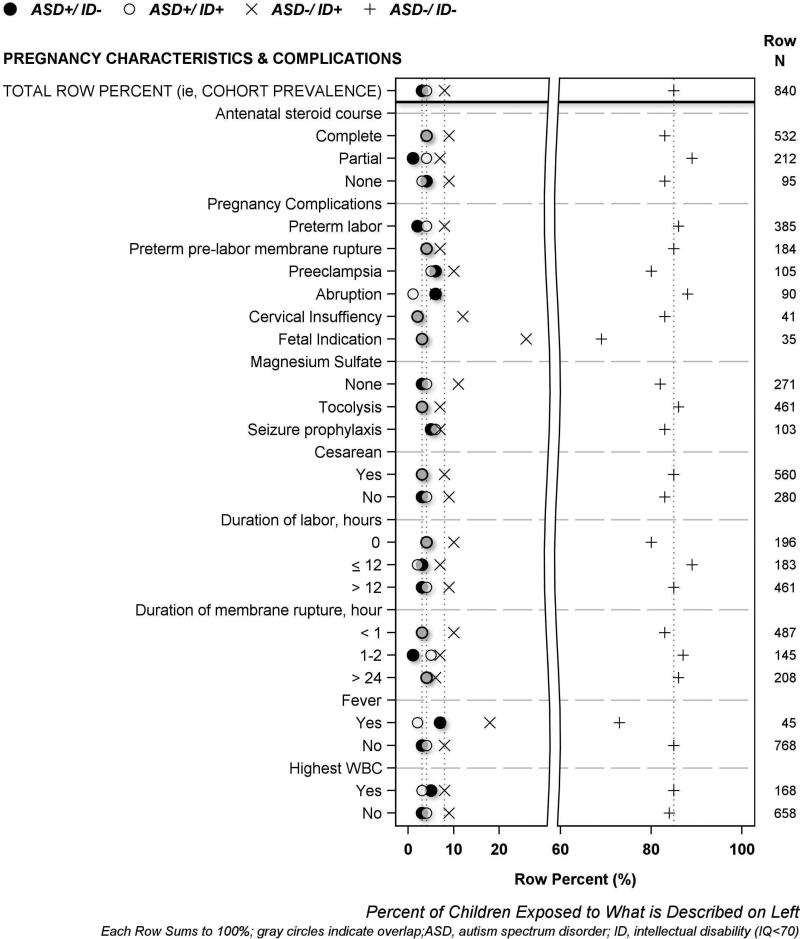
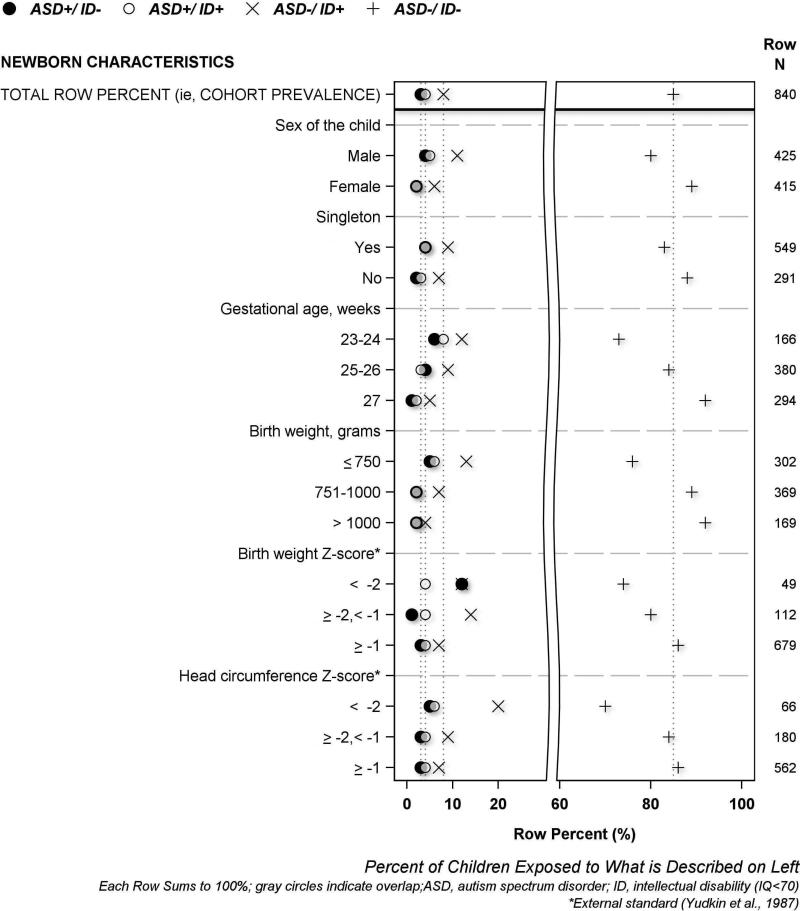
The results of univariate analyses are displayed visually using the same format in four figures, and in four supplement figures. A legend at the top of each figure names the four symbols used to describe each of the four mutually exclusive ASD/ID outcome groups. The horizontal axis labeled at the bottom of each figure indicates row percent (i.e., each row sums to 100%), and the characteristic that each row plotted percent describes is labeled on the left vertical axis. The top row of each figure displays 4 symbols to indicate the cohort prevalence of each of the four ASD/ID groups; dotted vertical lines proceeding downward from each symbol through the plot are provided to enable easy visual comparison between cohort prevalence and each plotted percent (i.e., prevalence among study groups formed according to pregnancy, birth and neonatal characteristics). The total number of children who had or were exposed to each characteristic is shown on the right vertical axis. We describe maternal demographic characteristics in Figure 1, maternal exposures, illnesses and medications in Figure 2, pregnancy characteristics and complications in Figure 3, and newborn characteristics in Figure 4. Supplementary Figures S2-S5 illustrate placenta characteristics, early postnatal characteristics, newborn medications and therapies, and newborn diagnoses and dysfunctions. The accompanying legends provide brief descriptions of the distribution of ASD/ID study groups shown in each figure to represent the entire study population.
Figure 1. Percent of women who had selected demographic characteristics whose children were classified at age 10 years as ASD+/ID−, ASD+/ID+, ASD−/ID+ or ASD−/ID−.
[Women who identified as Black, did not graduate from high school, and/or were eligible for government-provided healthcare (public) insurance gave birth to children who later had ASD−/ID+ or ASD+/ID+ more frequently than other women. *Infants may be in more than one category]
Figure 2. Percent of women who had selected pregnancy characteristics or exposures whose children were classified at age 10 years as ASD+/ID−, ASD+/ID+, ASD−/ID+ or ASD−/ID−.
[Children whose mother reported a vaginal/cervical infection, and/or a periodontal infection during this pregnancy, had ASD+/ID+ more frequently than children of other women. By contrast, children of women who consumed antibiotics less frequently received a diagnosis of ASD+/ID− compared to the children of other women, whereas ASD−/ID+ occurred more frequently among children born to women who reported fever during this pregnancy than in children of other women.]
Figure 3. Percent of women who had selected pregnancy complications whose children were classified at age 10 years as ASD+/ID−, ASD+/ID+, ASD−/ID+ or ASD−/ID−.
[Irrespective of their IQ, children whose mother had preeclampsia and/or received magnesium sulfate for seizure prophylaxis developed ASD more frequently than children of other mothers. Children born to women who had placental abruption, and those whose mother had fever within 48 hours before or after delivery, more frequently developed ASD unaccompanied by ID (ASD+/ID−) than children of other mothers.]
Figure 4. Percent of newborns with selected characteristics who were classified at age 10 years as ASD+/ID−, ASD+/ID+, ASD−/ID+ or ASD−/ID−.
[Boys had ASD+/ID+ and ASD+/ID− twice as frequently as girls. The prevalence of ASD increased with decreasing gestational age and, to a lesser extent, with decreasing birth weight, regardless of IQ. Children who had birth head circumference Z-score < −2 also received ASD diagnoses more frequently than other children, irrespective of IQ. Children with the most severe fetal growth restriction (i.e., birth weight Z-score < −2) had the highest percent of ASD+/ID− diagnoses. Antecedents of ID unaccompanied by ASD (ASD−/ID+) included male sex, low gestational age, and fetal growth restriction (including microcephaly).]
Analytic multivariable regression analyses
Time-ordered multinomial logistic regression models (Table 1) were used to examine the extent to which pregnancy, delivery, and neonatal factors are associated with increased risk for ASD+/ID−, ASD+/ID+ or ASD−/ID+, adjusting for potential confounders.
Table 1.
Odds ratios and 95% confidence intervals for the association of ASD+/ID−, ASD+/ID+, ASD−/ID+ and ASD−/ID− with the antecedents listed on the left calculated using a time-oriented multinomial logistic regression model that added variables sequentially as they were identified. Earlier occurring variables that were significantly associated could not be displaced in later models.
| ASD+/ID− |
ASD+/ID+ |
ASD−/ID+ |
ASD−/ID− |
|
|---|---|---|---|---|
| (n = 27) | (n = 32) | (n = 71) | (n = 710) | |
| Pregnancy epoch* | ||||
| Cervical-vaginal infection | 0.9 (0.2, 4.1) | 2.7 (1.2, 6.4) | 0.7 (0.3, 1.6) | 1.0 |
| Receipt of antibiotic | 0.1 (0.01, 0.7) | 0.8 (0.4, 1.9) | 1.3 (0.9, 2.3) | 1.0 |
| Delivery epoch** | ||||
| Fever at delivery | 3.6 (0.98, 13) | 0.6 (0.1, 4.4) | 2.9 (1.2, 6.7) | 1.0 |
| Newborn epoch*** | ||||
| Male | 2.1 (0.9, 5.0) | 2.9 (1.3, 6.8) | 2.1 (1.2, 3.6) | 1.0 |
| GA 23-24 weeks | 4.4 (1.7, 11) | 2.9 (1.3, 6.6) | 1.8 (1.03, 3.3) | 1.0 |
| BW Z-score < −2 | 9.9 (3.3, 30) | 2.1 (0.5, 9.9) | 2.0 (0.7, 5.3) | 1.0 |
Both fixed effects (independent variables) were included in the same multinomial logistic regression model
Adjusted for fixed effects that were significantly associated with ASD−/ID+ risk in the pregnancy epoch model
Adjusted for fixed effects that were significantly associated with ASD−/ID+ risk in the pregnancy and delivery epoch models
odds ratios above 1.0 are interpreted as indicating increased risk of the outcome listed at the top of the column for women or children who were exposed to what is described on the left, whereas odds ratios below 1.0 indicate decreased risk, and confidence intervals that do not include 1.0 indicate statistically significant associations (indicated by bold font).
Children were at increased risk of ASD+/ID− if they were born in the lowest gestational age category (OR, 4.4; 95% CI, 1.7-11) and if they had severe fetal growth restriction (birth weight Z-score < −2) (OR, 9.9; 95% CI, 3.3-30). Maternal fever at delivery was associated with 3.6 times greater risk of ASD+/ID−, though the association was not quite statistically significant (OR, 3.6; 95% CI, 0.98-13). Mother's receipt of an antibiotic during the pregnancy was associated with reduced risk of ASD+/ID− (OR, 0.1; 95% CI, 0.01-0.7).
Children were at increased risk of ASD+/ID+ if the mother reported a cervical-vaginal ‘infection’ during pregnancy (OR, 2.7; 95% CI, 1.2-6.4), if they were boys (OR, 2.9; 95% CI, 1.3-6.8), or if their gestational age was in the lowest category (23-24 weeks) (OR, 2.9; 95% CI, 1.3-6.6).
Risk factors for ID unaccompanied by ASD included maternal fever at delivery (OR, 2.9; 95% CI, 1.2-6.7), male sex (OR, 2.1; 95% CI, 1.2-3.6), and very low gestational age (OR,1.8; 95% CI, 1.03-3.3).
DISCUSSION
In a large, prospectively followed cohort of children born before the 28th week of gestation, we found that low gestational age is a risk factor for rigorously diagnosed ASD irrespective of IQ < or ≥ 70, severe fetal growth restriction (i.e., birth weight Z-score < −2) is strongly associated with increased risk of ASD+/ID−, and maternal report of cervical-vaginal ‘infection’ is strongly associated with increased risk of ASD+/ID+. In addition, prescription of an antibiotic is associated with lowered risk of ASD+/ID−, and peripartum maternal fever is associated with increased risk of ID not accompanied by ASD (ASD−/ID+).
Our study confirms previous observations that preterm birth is associated with increased risk of ASD,4,6,7 and that the risk increases as gestational age decreases,5 even among children born in the narrow window of 23 to 27 weeks of gestation. The only prior study of ASD risk specifically among extremely preterm children did not find an association between gestational age and ASD,8 perhaps because it was based on only 16 diagnosed children, and because of the relatively restricted range of gestational age (< 26 weeks) of the study sample.
Very low gestational age is associated with increased risk not only for ASD with ID, but also for ASD with relatively intact or normal cognitive function. Some of the increased risk might reflect vulnerability of cerebral maturation processes,49 paucity of neuroprotective factors,50 postnatal physiologic instability,51 and/or inflammatory phenomena that appear to increase the risk of brain damage in very preterm newborns.52
Lower than expected birth weight for gestational age,4 and its correlates (i.e., preeclampsia, placental insufficiency, and cesarean delivery11) have also been associated with increased risk of ASD.10 We use the terms ‘fetal growth restriction’ and ‘small for gestational age’ interchangeably, since the accurate differentiation of pathologically small from constitutionally small newborns (see18,19) is an ongoing challenge.20-22 Our findings are novel in indicating that impaired fetal growth among extremely preterm newborns is the factor most strongly associated with ASD among children without cognitive impairment. The co-occurrence of severe growth restriction with very preterm birth might result in “double jeopardy”,53 placing children with both characteristics at especially high risk of developmental disability, perhaps due to the tendency of such children to have a more intense systemic inflammatory response compared to their peers who are not growth restricted.54 The strong association of fetal growth restriction with ASD might also reflect epigenetic phenomena. Not only is fetal growth restriction strongly associated with developmental programming that has been attributed to DNA methylation55,56 and histone acetylation,57 ASD has also been associated with epigenetic changes.58-60 Here, too, “double jeopardy” might come into play because epigenetic phenomena have also been associated with inflammation.61-64
Our finding that maternal report of cervical-vaginal ‘infection’ in pregnancy and peripartum maternal fever are associated with ID with and without ASD adds further support to the role of immune responses in the genesis of perinatal brain disorders.24,65,66 We do not know the extent to which maternal reported cervical-vaginal infections involved a documented immune response to microbial colonization (i.e., true infection). Previous research indicates that information gained through self-report during maternal interview can be more accurate than that obtained from medical records or birth certificates,14-17 though modest reliability has also been reported for some obstetric morbidity surveys.67,68 In the ELGAN Study, newborns whose mother reported a genitourinary infection during pregnancy were more frequently exposed to inflammation of the chorionic plate, chorion, decidua, fetal stem vessels, and umbilical cord than were those whose mother did not.69 These children also had higher neonatal blood concentrations of inflammation-associated proteins than other newborns.70 Likewise, prior studies suggest that children with ASD diagnoses or autistic mannerisms are more frequently exposed to certain viruses and higher titers of antibodies to these viruses,71,72 have elevated peripheral blood or dried newborn blood spot concentrations of some inflammation-related proteins,73 and have higher rates of immune-mediated conditions than other children.74 Preclinical evidence additionally supports the view that perinatal inflammation-initiating conditions make the brain more vulnerable to subsequent insults, and that the timing of an inflammatory exposure might alter the course of subsequent brain injury.66
Very preterm delivery for maternal or fetal indications is associated with a higher risk of ASD+/ID+ than is delivery for spontaneous indications. Because women who deliver for maternal or fetal indications are less likely to receive antibiotics than women who deliver for spontaneous indications,75 it is possible that the variable for receipt of antibiotics conveys information about spontaneous preterm deliveries, which were at reduced risk of ASD without ID. Thus, the receipt of antibiotics may merely be an indicator or correlate of reduced risk that is not involved in lowering risk.
Strengths and limitations
Strengths of our study include the large, prospectively followed cohort of infants enrolled on the basis of gestational age rather than birth weight,76 and confirmation of the diagnosis of ASD at age 10 years with rigorous diagnostic procedures. As with all observational studies, we were limited in our ability to infer causation from associations. While ASD was more prevalent in our sample of ELGANs than in the general population, only 3.8% of our sample had ASD with ID, and 3.2% had ASD without ID. As a consequence, our analyses had adequate statistical power to detect only relatively strong underlying associations. Nonetheless, we did identify a number of antecedents associated with both of these outcomes.
Conclusions and relevance
Among children born before the 28th week of gestation, those who were in the lowest gestational age category were at increased risk of ASD irrespective of cooccurring ID, whereas severe fetal growth restriction was strongly associated with increased risk of ASD without ID, and cervical-vaginal infection was specifically associated with increased risk of ASD with ID. Peripartum maternal fever was uniquely associated with increased risk of cognitive impairment not accompanied by ASD. These findings support other evidence that immaturity, epigenetic phenomena, and inflammation contribute to the occurrence of ASD.
Supplementary Material
ACKNOWLEDGMENTS
The authors express their gratitude to the children and their families who participated in this study. They also gratefully acknowledge the contributions of the ELGAN Study Investigators, listed below.
Boston Children's Hospital, Boston MA
Janice Ware, Taryn Coster, Brandi Henson, Rachel Wilson, Kirsten McGhee, Patricia Lee, Aimee Asgarian, Anjali Sadhwani
Tufts Medical Center, Boston MA
Ellen Perrin, Emily Neger, Kathryn Mattern, Jenifer Walkowiak, Susan Barron
University of Massachusetts Medical School, Worcester MA
Jean Frazier, Lauren Venuti, Beth Powers, Ann Foley, Brian Dessureau, Molly Wood, Jill Damon-Minow
Yale University School of Medicine , New Haven, CT
Richard Ehrenkranz, Jennifer Benjamin, Elaine Romano, Kathy Tsatsanis, Katarzyna Chawarska, Sophy Kim, Susan Dieterich, Karen Bearrs
Wake Forest University Baptist Medical Center, Winston-Salem NC
T. Michael O'shea, Nancy Peters, Patricia Brown, Emily Ansusinha, Ellen Waldrep, Jackie Friedman, Gail Hounshell, Debbie Allred
University Health Systems of Eastern Carolina, Greenville, NC
Stephen C. Engelke, Nancy Darden-Saad, Gary Stainback
North Carolina Children's Hospital, Chapel Hill, NC
Diane Warner, Janice Wereszczak, Janice Bernhardt, Joni McKeeman, Echo Meyer
Helen DeVos Children's Hospital, Grand Rapids, MI
Steve Pastyrnak, Wendy Burdo-Hartman, Julie Rathbun, Sarah Nota, Teri Crumb,
Sparrow Hospital, Lansing, MI
Madeleine Lenski, Deborah Weiland, Megan Lloyd
University of Chicago Medical Center, Chicago, IL
Scott Hunter, Michael Msall, Rugile Ramoskaite, Suzanne Wiggins, Krissy Washington, Ryan Martin, Barbara Prendergast, Megan Scott
William Beaumont Hospital, Royal Oak, MI
Judith Klarr, Beth Kring, Jennifer DeRidder, Kelly Vogt
Financial Support: This study was supported by the National Institute of Neurological Disorders and Stroke (5U01NS040069-05; 2R01NS040069 - 06A2), the National Institute of Child Health and Human Development (5P30HD018655-28), and the Wayne State University Perinatal Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions: Drs Joseph, Korzeniewski, O'shea, Leviton, and Kuban and Ms Allred conceptualized and designed the study, and contributed to the drafting of the initial manuscript. Ms Allred and Dr Herren conducted statistical analyses. All authors contributed to the interpretation of the data, reviewed and revised the manuscript, approved the final manuscript as submitted, and agree to be held accountable for all aspects of the work.
Disclosures: The authors have no financial disclosures or competing interests.
REFERENCES
- 1.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011 Aug;128(2):344–355. doi: 10.1542/peds.2010-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guinchat V, Thorsen P, Laurent C, Cans C, Bodeau N, Cohen D. Pre-, peri- and neonatal risk factors for autism. Acta Obstet Gynecol Scand. 2012 Mar;91(3):287–300. doi: 10.1111/j.1600-0412.2011.01325.x. [DOI] [PubMed] [Google Scholar]
- 3.Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch. Pediatr. Adolesc. Med. 2007 Apr;161(4):326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- 4.Abel KM, Dalman C, Svensson AC, et al. Deviance in fetal growth and risk of autism spectrum disorder. A. J. Psychiatry. 2013 Apr;170(4):391–398. doi: 10.1176/appi.ajp.2012.12040543. [DOI] [PubMed] [Google Scholar]
- 5.Leavey A, Zwaigenbaum L, Heavner K, Burstyn I. Gestational age at birth and risk of autism spectrum disorders in Alberta, Canada. J. Pediatr. 2013 Feb;162(2):361–368. doi: 10.1016/j.jpeds.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 6.Losh M, Esserman D, Anckarsater H, Sullivan PF, Lichtenstein P. Lower birth weight indicates higher risk of autistic traits in discordant twin pairs. Psychol. Med. 2012 May;42(5):1091–1102. doi: 10.1017/S0033291711002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schendel D, Bhasin TK. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics. 2008 Jun;121(6):1155–1164. doi: 10.1542/peds.2007-1049. [DOI] [PubMed] [Google Scholar]
- 8.Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism spectrum disorders in extremely preterm children. J. Pediatr. 2010 Apr;156(4):525–531.e522. doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 9.Kuzniewicz MW, Wi S, Qian Y, Walsh EM, Armstrong MA, Croen LA. Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J. Pediatr. 2014 Jan;164(1):20–25. doi: 10.1016/j.jpeds.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing G. Autism risk in small- and large-forgestational-age infants. Am. J. Obstet. Gynecol. 2012 Apr;206(4):314.e311–319. doi: 10.1016/j.ajog.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr. 2015 Feb;169(2):154–162. doi: 10.1001/jamapediatrics.2014.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langridge AT, Glasson EJ, Nassar N, et al. Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS One. 2013;8(1):e50963. doi: 10.1371/journal.pone.0050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Shea TM, Allred EN, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early human development. 2009 Nov;85(11):719–725. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baheiraei A, Banihosseini SZ, Heshmat R, Mota A, Mohsenifar A. Association of self-reported passive smoking in pregnant women with cotinine level of maternal urine and umbilical cord blood at delivery. Paediatr Perinat Epidemiol. 2012 Jan;26(1):70–76. doi: 10.1111/j.1365-3016.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- 15.Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit. 2009 Feb;31(1):14–30. doi: 10.1097/FTD.0b013e3181957a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gartland D, Lansakara N, Flood M, Brown SJ. Assessing obstetric risk factors for maternal morbidity: congruity between medical records and mothers’ reports of obstetric exposures. American journal of obstetrics and gynecology. 2012 Feb;206(2):152 e151–110. doi: 10.1016/j.ajog.2011.10.863. [DOI] [PubMed] [Google Scholar]
- 17.Srisukhumbowornchai S, Krikov S, Feldkamp ML. Self-reported maternal smoking during pregnancy by source in Utah, 2003-2007. Birth Defects Res A Clin Mol Teratol. 2012 Dec;94(12):996–1003. doi: 10.1002/bdra.23058. [DOI] [PubMed] [Google Scholar]
- 18.Das UG, Sysyn GD. Abnormal fetal growth: intrauterine growth retardation, small for gestational age, large for gestational age. Pediatr. Clin. North Am. 2004 Jun;51(3):639–654, viii. doi: 10.1016/j.pcl.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Chauhan SP, Gupta LM, Hendrix NW, Berghella V. Intrauterine growth restriction: comparison of American College of Obstetricians and Gynecologists practice bulletin with other national guidelines. Am. J. Obstet. Gynecol. 2009 Apr;200(4):409.e401–406. doi: 10.1016/j.ajog.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Chauhan SP, Beydoun H, Chang E, et al. Prenatal detection of fetal growth restriction in newborns classified as small for gestational age: correlates and risk of neonatal morbidity. Am. J. Perinatol. 2014 Mar;31(3):187–194. doi: 10.1055/s-0033-1343771. [DOI] [PubMed] [Google Scholar]
- 21.Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for predicting intrauterine growth restriction: a systematic review and meta-analysis. Bjog. 2013 May;120(6):681–694. doi: 10.1111/1471-0528.12172. [DOI] [PubMed] [Google Scholar]
- 22.Savchev S, Sanz-Cortes M, Cruz-Martinez R, et al. Neurodevelopmental outcome of full-term small-for-gestational-age infants with normal placental function. Ultrasound Obstet. Gynecol. 2013 Aug;42(2):201–206. doi: 10.1002/uog.12391. [DOI] [PubMed] [Google Scholar]
- 23.Yudkin PL, Aboualfa M, Eyre JA, Redman CWG, Wilkinson AR. NEW BIRTH-WEIGHT AND HEAD CIRCUMFERENCE CENTILES FOR GESTATIONAL AGES 24 TO 42 WEEKS. Early Hum. Dev. 1987 Jan;15(1):45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 24.Leviton A, Paneth N, Reuss ML, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Pediatr. Res. 1999 Nov;46(5):566–575. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J. Pediatr. 2001 Jan;138(1):92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 26.Dammann O, Naples M, Bednarek F, et al. SNAP-II and SNAPPE-II and the risk of structural and functional brain disorders in extremely low gestational age newborns: the ELGAN study. Neonatology. 2010;97(2):71–82. doi: 10.1159/000232588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leviton A, Allred EN, Kuban KC, et al. Microbiologic and histologic characteristics of the extremely preterm infant's placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr. Res. 2010 Jan;67(1):95–101. doi: 10.1203/PDR.0b013e3181bf5fab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olomu IN, Hecht JL, Onderdonk AO, Allred EN, Leviton A. Perinatal Correlates of Ureaplasma urealyticum in Placenta Parenchyma of Singleton Pregnancies That End Before 28 Weeks of Gestation. Pediatrics. 2009 May;123(5):1329–1336. doi: 10.1542/peds.2008-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht JL, Onderdonk A, Delaney M, et al. Characterization of chorioamnionitis in 2nd-trimester C-section placentas and correlation with microorganism recovery from subamniotic tissues. Pediatr. Dev. Pathol. 2008 Jan-Feb;11(1):15–22. doi: 10.2350/07-06-0285.1. [DOI] [PubMed] [Google Scholar]
- 30.Hecht JL, Allred EN, Kliman HJ, et al. Histological characteristics of singleton placentas delivered before the 28th week of gestation. Pathology (Phila) 2008 Jun;40(4):372–376. doi: 10.1080/00313020802035865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecht JL, Kliman HJ, Allred EN, et al. Reference weights for placentas delivered before the 28th week of gestation. Placenta. 2007 Oct;28(10):987–990. doi: 10.1016/j.placenta.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Laughon M, Allred EN, Bose C, et al. Patterns of Respiratory Disease During the First 2 Postnatal Weeks in Extremely Premature Infants. Pediatrics. 2009 Apr;123(4):1124–1131. doi: 10.1542/peds.2008-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laughon M, Bose C, Allred EN, et al. Antecedents of chronic lung disease following three patterns of early respiratory disease in preterm infants. Archives of Disease in Childhood-Fetal and Neonatal Edition. 2011 Mar;96(2):F114–F120. doi: 10.1136/adc.2010.182865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel S, Dammann O, Martin CR, Allred EN, Leviton A, Investigators ES Presumed and definite bacteremia in extremely low gestational age newborns. Acta Paediatr. 2011 Jan;100(1):36–41. doi: 10.1111/j.1651-2227.2010.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartholomew J, Martin CR, Allred E, et al. Risk factors and correlates of neonatal growth velocity in extremely low gestational age newborns: the ELGAN Study. Neonatology. 2013;104(4):298–304. doi: 10.1159/000351020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Shea TM, Shah B, Allred EN, et al. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain. Behav. Immun. 2013 Mar;29:104–112. doi: 10.1016/j.bbi.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh R, Shah B, Allred EN, et al. The antecedents and correlates of necrotizing enterocolitis and spontaneous intestinal perforation among infants born before the 28th week of gestation. J Neonatal Perinatal Med. 2016 May 19; doi: 10.3233/NPM-16915100. [DOI] [PubMed] [Google Scholar]
- 38.Lee JW, VanderVeen D, Allred EN, Leviton A, Dammann O. Prethreshold retinopathy in premature infants with intrauterine growth restriction. Acta Paediatr. 2015 Jan;104(1):27–31. doi: 10.1111/apa.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joseph RM, O'Shea TM, Allred EN, et al. Prevalence and associated features of autism spectrum disorder in extremely low gestational age newborns at age 10 years. Autism Res. 2016 May 25; doi: 10.1002/aur.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- 41.Le Couteur A, Lord C, Rutter M. The autism diagnostic interview-revised (ADI-R) Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- 42.Risi S, Lord C, Gotham K, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2006 Sep;45(9):1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- 43.Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule–2 (ADOS-2) Western Psychological Corporation; Los Angeles, CA: 2012. [Google Scholar]
- 44.Elliott CD. Differential Ability Scales. 2nd ed. Pearson; San Antonio, TX: 2007. [Google Scholar]
- 45.APA . Diagnostic and statistical manual of mental disorders : DSM-5. 5th ed. American Psychiatric Association; Washington, D.C.: 2013. [Google Scholar]
- 46.Palisano RJ, Hanna SE, Rosenbaum PL, et al. Validation of a model of gross motor function for children with cerebral palsy. Phys Ther. 2000 Oct;80(10):974–985. [PubMed] [Google Scholar]
- 47.Laughon M, O'Shea MT, Allred EN, et al. Chronic Lung Disease and Developmental Delay at 2 Years of Age in Children Born Before 28 Weeks’ Gestation. Pediatrics. 2009 Aug;124(2):637–648. doi: 10.1542/peds.2008-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dales LG, Ury HK. An improper use of statistical significance testing in studying covariables. Int J Epidemiol. 1978 Dec;7(4):373–375. doi: 10.1093/ije/7.4.373. [DOI] [PubMed] [Google Scholar]
- 49.Penn AA, Gressens P, Fleiss B, Back SA, Gallo V. Controversies in preterm brain injury. Neurobiol. Dis. 2015 Oct 15; doi: 10.1016/j.nbd.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reuss ML, Paneth N, Susser M. Does the loss of placental hormones contribute to neurodevelopmental disabilities in preterm infants? Dev. Med. Child Neurol. 1994 Aug;36(8):743–747. [PubMed] [Google Scholar]
- 51.Leviton A, Blair E, Dammann O, Allred E. The wealth of information conveyed by gestational age. J. Pediatr. 2005 Jan;146(1):123–127. doi: 10.1016/j.jpeds.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 52.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatric research. 2014 Mar;75(3):376–380. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Regev RH, Reichman B. Prematurity and intrauterine growth retardation--double jeopardy? Clin. Perinatol. 2004 Sep;31(3):453–473. doi: 10.1016/j.clp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 54.Leviton A, Fichorova RN, O'Shea TM, et al. Two-hit model of brain damage in the very preterm newborn: small for gestational age and postnatal systemic inflammation. Pediatr. Res. 2013 Dec 7;73(3):362–370. doi: 10.1038/pr.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hillman SL, Finer S, Smart MC, et al. Novel DNA methylation profiles associated with key gene regulation and transcription pathways in blood and placenta of growth-restricted neonates. Epigenetics. 2015;10(1):50–61. doi: 10.4161/15592294.2014.989741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Januar V, Desoye G, Novakovic B, Cvitic S, Saffery R. Epigenetic regulation of human placental function and pregnancy outcome: considerations for causal inference. Am. J. Obstet. Gynecol. 2015 Oct;213(4 Suppl):S182–196. doi: 10.1016/j.ajog.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 57.Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J. Biol. Chem. 2008 May 16;283(20):13611–13626. doi: 10.1074/jbc.M800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loke YJ, Hannan AJ, Craig JM. The Role of Epigenetic Change in Autism Spectrum Disorders. Front Neurol. 2015;6:107. doi: 10.3389/fneur.2015.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grayson DR, Guidotti A. Merging data from genetic and epigenetic approaches to better understand autistic spectrum disorder. Epigenomics. 2016 Jan;8(1):85–104. doi: 10.2217/epi.15.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciernia AV, LaSalle J. The landscape of DNA methylation amid a perfect storm of autism aetiologies. Nature reviews. Neuroscience. 2016 Jul;17(7):411–423. doi: 10.1038/nrn.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Hoyo C, Murphy S, et al. DNA methylation at imprint regulatory regions in preterm birth and infection. American journal of obstetrics and gynecology. 2013 May;208(5):395.e391–397. doi: 10.1016/j.ajog.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parets SE, Bedient CE, Menon R, Smith AK. Preterm birth and its long-term effects: methylation to mechanisms. Biology. 2014 Aug 21;3(3):498–513. doi: 10.3390/biology3030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Claycombe KJ, Brissette CA, Ghribi O. Epigenetics of inflammation, maternal infection, and nutrition. The Journal of nutrition. 2015 May;145(5):1109s–1115s. doi: 10.3945/jn.114.194639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knight AK, Smith AK. Epigenetic Biomarkers of Preterm Birth and Its Risk Factors. Genes. 2016 Apr 13;7(4) doi: 10.3390/genes7040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci. 2015 Aug;16(8):469–486. doi: 10.1038/nrn3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hagberg H, Mallard C, Ferriero DM, et al. The role of inflammation in perinatal brain injury. Nature reviews. Neurology. 2015 Apr;11(4):192–208. doi: 10.1038/nrneurol.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Souza JP, Cecatti JG, Pacagnella RC, et al. Development and validation of a questionnaire to identify severe maternal morbidity in epidemiological surveys. Reprod Health. 2010 Jul 21;7:16. doi: 10.1186/1742-4755-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dietz P, Bombard J, Mulready-Ward C, et al. Validation of self-reported maternal and infant health indicators in the Pregnancy Risk Assessment Monitoring System. Matern Child Health J. 2014 Dec;18(10):2489–2498. doi: 10.1007/s10995-014-1487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leviton A, Allred EN, Kuban KC, et al. The Development of Extremely Preterm Infants Born to Women Who Had Genitourinary Infections During Pregnancy. Am. J. Epidemiol. 2016 Jan 1;183(1):28–35. doi: 10.1093/aje/kwv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fichorova RN, Beatty N, Sassi RR, et al. Systemic inflammation in the extremely low gestational age newborn following maternal genitourinary infections. Am. J. Reprod. Immunol. 2015 Feb;73(2):162–174. doi: 10.1111/aji.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gentile I, Zappulo E, Bonavolta R, et al. Exposure to Varicella Zoster Virus Is Higher in Children with Autism Spectrum Disorder than in Healthy Controls. Results from a Case-control Study. In Vivo. 2014;28(4):627–631. 07-08. [PubMed] [Google Scholar]
- 72.Sakamoto A, Moriuchi H, Matsuzaki J, Motoyama K, Moriuchi M. Retrospective diagnosis of congenital cytomegalovirus infection in children with autism spectrum disorder but no other major neurologic deficit. Brain Dev. 2015 Feb;37(2):200–205. doi: 10.1016/j.braindev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Mitchell RHB, Goldstein BI. Inflammation in Children and Adolescents With Neuropsychiatric Disorders: A Systematic Review. J. Am. Acad. Child Adolesc. Psychiatry. 2014 Mar;53(3):274–296. doi: 10.1016/j.jaac.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Zerbo O, Leong A, Barcellos L, Bernal P, Fireman B, Croen LA. Immune mediated conditions in autism spectrum disorders. Brain, behavior, and immunity. 2015 May;46:232–236. doi: 10.1016/j.bbi.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martinez de Tejada B. Antibiotic use and misuse during pregnancy and delivery: benefits and risks. Int J Environ Res Public Health. 2014 Aug;11(8):7993–8009. doi: 10.3390/ijerph110807993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am. J. Epidemiol. 1991 Sep 15;134(6):604–613. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
- 77.Glinianaia SV, Ghosh R, Rankin J, Pearce MS, Parker L, Pless-Mulloli T. No improvement in socioeconomic inequalities in birthweight and preterm birth over four decades: a population-based cohort study. BMC Public Health. 2013;13:345. doi: 10.1186/1471-2458-13-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DiGuiseppi CG, Daniels JL, Fallin DM, et al. Demographic profile of families and children in the Study to Explore Early Development (SEED): Case-control study of autism spectrum disorder. Disability and health journal. 2016 doi: 10.1016/j.dhjo.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rai D, Lewis G, Lundberg M, et al. Parental socioeconomic status and risk of offspring autism spectrum disorders in a Swedish population-based study. Journal of the American Academy of Child and Adolescent Psychiatry. 2012 May;51(5):467–476 e466. doi: 10.1016/j.jaac.2012.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.