Abstract
Restricted and repetitive behaviors are a defining feature of autism which can be expressed as a cognitive flexibility deficit or stereotyped, motor behaviors. There is limited knowledge about the underlying neuropathophysiology contributing to these behaviors. Previous findings suggest that central 5HT2A receptor activity is altered in autism, while recent work indicates that systemic 5HT2A receptor antagonist treatment reduces repetitive behaviors in an idiopathic model of autism. 5HT2A receptors are expressed in the orbitofrontal cortex and striatum. These two regions have been shown to be altered in autism. The present studies investigated whether 5HT2A receptor blockade in the dorsomedial striatum or orbitofrontal cortex in the BTBR mouse strain, an idiopathic model of autism, affects the phenotype related to restricted and repetitive behaviors. Microinfusion of the 5HT2A receptor antagonist, M100907 into the dorsomedial striatum alleviated a reversal learning impairment and attenuated grooming behavior. M100907 infusion into the orbitofrontal cortex increased perseveration during reversal learning and potentiated grooming. These findings suggest that increased 5HT2A receptor activity in the dorsomedial striatum may contribute to behavioral inflexibility and stereotyped behaviors in the BTBR mouse. 5HT2A receptor signaling in the orbitofrontal cortex may be critical for inhibiting a previously learned response during reversal learning and expression of stereotyped behavior. The present results suggest which brain areas exhibit abnormalities underlying repetitive behaviors in an idiopathic mouse model of autism, as well as which brain areas systemic treatment with M100907 may principally act on in BTBR mice to attenuate repetitive behaviors.
Keywords: Caudate, Orbitofrontal Cortex, M100907, Reversal Learning, Grooming, Autism
Introduction
Autism spectrum disorder (ASD) is defined by persistent deficits in social communication and interaction along with restricted interests and repetitive behaviors (RRBs) [American Psychiatric Association, 2013]. RRBs range from motor stereotypies to circumscribed interests, compulsions, and cognitive inflexibility (D'Cruz et al., 2013; Gabriels et al., 2005; Lewis & Bodfish, 1998; Miller et al., 2015). Repetitive behaviors frequently interfere with daily functioning and are particularly challenging for families of affected individuals (Bishop et al., 2007). At present, there are no FDA-approved pharmacotherapies to treat RRBs in ASD, and thus understanding the neurochemical mechanisms in brain circuitry contributing to RRBs can aid in developing effective pharmacotherapies. Findings from several studies suggest that RRBs in ASD occur due to prefrontal cortical and striatal abnormalities (Delmonte et al., 2013; DiMartino et al., 2011; Hollander et al., 2005; Rojas et al., 2006). Specifically, the orbitofrontal cortex and striatum are reported to be altered in ASD and other disorders that are marked by repetitive behaviors (Delmonte et al., 2013; Langen et al., 2012). Although these studies identify brain region abnormalities associated with repetitive behaviors, they do not clarify the neurochemical alterations in these regions related to the expression of RRBs.
The serotonergic system has long been implicated in the etiology of ASD (Schain & Freedman, 1961). In particular, altered serotonin (5HT)2A receptor signaling is suggested to contribute to ASD symptoms based on clinical (McBride et al., 1989) and preclinical (Veenstra-Vanderweele et al., 2012) findings. Related, systemic treatment with a 5HT2A receptor antagonist facilitates set-shifting in rats and alleviates both a reversal learning deficit and elevated grooming behavior in the BTBR mouse model of autism (Baker et al., 2011; Amodeo et al., 2014; Amodeo et al., 2016). The BTBR mouse is an inbred mouse strain that serves as an idiopathic model of ASD. A benefit of using the BTBR mouse to model ASD is that it displays comparable behavioral features in social deficits, communication impairments, as well as RRBs including cognitive inflexibility and elevated grooming behavior (Amodeo et al., 2012; McFarlane et al., 2008; Moy et al., 2008). The results demonstrating that 5HT2A receptor blockade reduces a reversal learning deficit and grooming behavior in BTBR mice suggest that increased 5HT2A receptor activity in certain brain regions may contribute to repetitive behaviors as observed in the BTBR mouse.
The prefrontal cortex and striatum are two brain regions in which 5HT2A receptors are found in moderate to high density (Ito et al., 1998; Xu & Pandey, 2000). Further, the orbitofrontal cortex and dorsomedial striatum have been shown to be involved in the expression of repetitive behaviors (Boulougouris & Robbins, 2010; Burguière et al., 2013; Chudasama & Robbins, 2003; Kim & Ragozzino, 2005; Palencia & Ragozzino, 2006). Therefore, we hypothesize that altered 5HT2A receptor activity in one or both of these brain regions may affect elevated grooming and reversal learning deficits.
To better understand where in the brain a 5HT2A receptor antagonist may be acting to reduce repetitive behaviors in BTBR mice, the present study determined whether the highly selective 5HT2A receptor antagonist M100907 infused into the dorsomedial striatum or orbitofrontal cortex of BTBR mice could reduce elevated grooming behavior and/or a reversal learning deficit. M100907 is a highly selective and potent antagonist for 5HT2A receptors (Hall et al. 2000; Herth et al., 2009; Kehne et al., 1996; Knauer et al., 2008). Previous studies have demonstrated that M100907 has at least a 100-fold separation from D1-5, alpha1 adrenergic and 5-HT2C receptors (Kehne et al., 1996). An earlier study demonstrated that M100907 is highly selective for the 5HT2A receptor with almost no appreciable affinity for D2 or other 5HT receptor sites (Sorensen et al., 1993). Past experiments have compared BTBR mice with C57BL/6J (B6) mice, a commonly used inbred mouse strain (Pearson et al., 2012; Silverman et al., 2015). To determine whether localized M100907 infusions in BTBR mice alter behavior to a level comparable to B6 controls, we compared different BTBR treatment groups with vehicle-injected B6 mice.
Materials and Methods
Subjects
BTBR T+ Itpr3tf/J and B6 male mice were attained from Jackson Laboratory (Bar Harbor, ME). Mice were singly housed in plastic cages (28 cm wide × 17 cm long × 12 cm high) in an humidity (30%) and temperature (22°C) controlled room. In the housing room, lights turned on at 7:00 am and lights went off at 7:00 pm. All behavioral testing occurred during the light phase. Animal care and use was approved by the Institutional Laboratory Animal Care and Use Committee at the University of Illinois at Chicago.
Surgical Methods
Each mouse (8-12 weeks of age) received stereotaxic surgery to bilaterally implant cannulae aimed at either the dorsomedial striatum or ventral orbitofrontal cortex. Before surgery each mouse received an i.p. injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). A 5 millimeter stainless steel guide cannulae (Plastics One, Roanoke, VA) was implanted at an 8° angle aimed medially. The stereotaxic coordinates for the dorsomedial striatum were the following: 0.4 mm anterior to bregma; ± 1.8 mm lateral; 2.4 mm below the skull. The stereotaxic coordinates for the ventral orbitofrontal cortex were the following: 2.8 mm anterior to bregma; ± 1.2 mm lateral; 1.2 mm below the skull. To minimize pain or discomfort, mice received subcutaneous administration of the anti-inflammatory carprofen immediately after surgery. After 5 days of recovery all mice were food restricted to 85% of their ad libitum body weight. Each mouse had free access to water in their home cage throughout the study. Behavioral training began once mice reached 85% of their ad libitum body weight, which occurred in 4 to 6 days.
Spatial Discrimination Training
Mice were trained for 2–4 days before testing. Training and testing was conducted in a rectangle-shaped maze as described previously (Amodeo et al., 2014). The maze was divided into a start and choice area by a guillotine door. A door opened up at the bottom center of the guillotine door. In the choice area, a piece extended from the back wall, which divided the area into two equally sized and distinct spatial locations. Both choice locations were adorned with distinct visuospatial cues attached to the back and side walls. In each location, a food well was centered and located 3 cm away from the back wall. At the beginning of each training session, mice were placed into the start area. The start door was opened 1 min following placement into the holding chamber, allowing the mouse to freely navigate in the choice area and consume a ½ piece of Fruity Pebbles cereal (Post Foods, St. Louis, MO) from each food well. After cereal pieces were consumed from both choice locations, the guillotine door was raised to allow a mouse to enter the start area. After a mouse had returned to the start area, the guillotine door was closed and the food wells re-baited. The start door was subsequently reopened to begin a new trial. This procedure was repeated until 15 min had elapsed. Mice were considered trained once they successfully completed six trials in a 15 min session across two consecutive days.
Microinfusion procedure
Prior to a test session, mice were restrained and a 33-gauge injection cannula was inserted into each guide cannula. To restrain the subject a folded paper towel and experimenter's palm was placed over the dorsal region of the mouse, while holding the mouse's head with their thumb and index finger. After the injectors were placed into the guide cannula the mouse was allowed to freely move during the duration of the infusion. During infusions the experimenter directed a mouse by the tail to make certain the mouse did not tangle the tubing. Upon completion of the infusion, mice were similarly restrained for removal of injectors and insertion of dummy cannula.
The injection cannula extended 1 mm beyond the guide cannula tip. The injection cannulae were attached to polyethylene tubes (PE-20) connected to separate 10 μl syringes. The syringes were driven by a microinfusion pump with solutions infused in a volume of 0.2 μl per side for 2 min. The total volume infused was 0.2 μl per side. The injection cannulae were left in place for 1 min to allow drug diffusion around the injector tip. Mice were left undisturbed in their home cage for 5 min and behavioral testing began immediately thereafter.
Acquisition and Reversal learning
Acquisition and reversal learning each occurred in a single daily session across two consecutive days. Prior to acquisition, the injection cannulae were inserted into the guide cannulae and left in place for 3 minutes without injecting a solution. Acquisition testing commenced 5 minutes after cannulae removal. This mimicked the microinfusion procedure used in reversal learning. No drug infusion occurred for acquisition as BTBR mice do not exhibit an acquisition deficit (Amodeo et al., 2012, 2014). In testing, only one of the two food wells was baited with a ½ piece of cereal in each trial. One location was designated as the “correct” spatial location and contained a ½ piece of cereal on 80% of trials. On the other 20% of trials, the “incorrect” location was baited with a ½ piece of cereal. The first two trials of each test always contained a food reinforcement in the “correct” arm. Criterion was achieved when a mouse chose the “correct” location on six consecutive trials. If a mouse chose a location with cereal, it was allowed to eat the cereal; the guillotine door was raised and subsequently lowered after a mouse returned to the start area. If a mouse chose a location with no cereal, it was allowed to navigate to the unbaited food well. Subsequently, the guillotine door was raised, allowing a mouse to return to the start area. If a mouse chose an unbaited food well, the baited food well was temporarily removed to prevent a mouse from quickly navigating over to the correct spatial location and obtain a cereal reinforcer after making an incorrect choice. Between trials, the choice area was cleaned with 2% ammonium chloride solution to minimize the use of odor cues.
The retention and reversal learning tests were conducted the day after acquisition. Prior to these tests, a BTBR mouse received an intracranial infusion of vehicle, 0.2μg or 0.6μg of M100907 into the dorsomedial striatum or ventral orbitofrontal cortex. B6 did not receive infusions of M100907 because systemic treatment did not affect probabilistic reversal learning (Amodeo et al., 2014). M100907 was dissolved in 0.01M phosphate buffer saline and 0.1M hydrochloric acid, pH was adjusted to 6.4 using 0.1M sodium hydroxide. Because the main goal of the studies was to determine whether M100907 treatment in dorsomedial striatum or orbitofrontal cortex of BTBR mice rescued the phenotype, each B6 mouse only received a vehicle injection. For the dorsomedial striatum experiment, the following groups were included: B6: vehicle (n = 9); BTBR: vehicle (n = 9), 0.2μg (n = 8), 0.6μg (n = 6). For the orbitofrontal cortex experiment, the following groups were included: B6: vehicle (n = 9); BTBR: vehicle (n = 8), 0.2μg (n = 6), 0.6μg (n = 7). A mouse first received a retention test as in previous experiments (Amodeo et al., 2012, 2014). In the retention test, a mouse was reinforced with 80% probability on trials for choosing the spatial location that was correct in acquisition. Criterion was achieved when a mouse successfully chose the “correct” spatial location (as in acquisition) on five out of six trials. Immediately after achieving retention criterion, reversal learning began. All aspects of the reversal learning test were identical to those in acquisition, except that the opposite spatial location was considered ‘correct’ and reinforced with 80% probability. Criterion was met when a mouse made six consecutive correct choices. All mice tested achieved both acquisition and reversal learning criterion. The time to achieve acquisition criterion ranged from 25-74 minutes. The time to achieve reversal learning criterion ranged from 26 to 124 minutes.
An error analysis of reversal learning was conducted as used previously in rodent models and patient-oriented ASD research (Brown et al., 2012; D'Cruz et al., 2013; Floresco et al., 2006). The first reversal learning trial was not counted as a perseverative error, but served as initial negative feedback. On subsequent trials, if a mouse chose the previously correct spatial location, the choice was recorded as a perseverative error until a mouse first chose the new correct spatial location. After selecting the correct spatial location for the first time, all subsequent entries into the previously reinforced spatial location were scored as regressive errors.
Repetitive Grooming Behavior
Seven days after reversal learning, repetitive self-grooming was tested. In this test, a clear plastic testing chamber was used similar to past studies (McFarlane et al., 2008; Moy et al., 2008). Mice were left undisturbed for 20 min and allowed to freely explore the cage for the entirety of the test. The first 10 min served as a habituation period. During the second 10 min of testing a trained observer recorded cumulative time spent grooming all body regions. The trained observer sat approximately 1.6 m from the test cage. After each mouse was tested, the cage was thoroughly cleaned with a 2% ammonium chloride solution. BTBR mice received a microinfusion of either vehicle, 0.2 or 0.6μg M100907 five minutes before being placed in the test chamber. In the dorsomedial striatum experiment, the following groups were included: B6: vehicle (n = 9); BTBR: vehicle (n = 7), 0.2μg (n = 8), 0.6μg (n = 8). In the orbitofrontal cortex experiment, the following groups were included: B6: vehicle (n = 7); BTBR: vehicle (n = 6), 0.2μg M100907 (n = 7), 0.6μg M100907 (n = 7); musicmol (n = 9). For the BTBR groups, mice were pseudorandomly assigned to a treatment group such that a mouse did not receive the same treatment on grooming as on reversal learning. This is the main reason why the sample sizes for the BTBR mice treatment groups do not match between the reversal learning and grooming tests. In the grooming test for orbitofrontal cortex cannulated mice, a guide cannula in two B6 mice and one BTBR mouse became occluded and thus 3 fewer mice were tested on self-grooming than in reversal learning. Seven days after testing M100907 infusions on repetitive grooming, mice with orbitofrontal cortex cannula received a second grooming test. In this test, mice received microinfusions of sterile saline or 0.2μg of the GABA-A agonist muscimol 5 minutes before measuring grooming duration. Approximately half of the mice in each treatment group from the first grooming test were randomly assigned to the saline group and the other half were assigned to the muscimol group. This experiment was conducted as a comparison to elucidate whether an effect by M100907 may preferentially result from a net increase or decrease in orbitofrontal cortex activity.
Histology
After completion of behavioral testing, mice were given an overdose of sodium pentobarbital. Mice were intracardially perfused with 0.9% saline followed by 4% formaldehyde solution. The brain was removed and stored in formaldehyde until sectioning. Brains were frozen and cut into 50-μm coronal sections on a cryostat. Sections were immediately mounted on slides, dried, and then stained with cresyl violet. Placements were then verified with reference to the stereotaxic atlas of Paxinos and Franklin (2001).
Statistical Analysis
Separate one-way ANOVAs were conducted to determine whether there was a significant difference in trials to criterion for acquisition, retention and reversal learning. Separate one way ANOVAs were conducted to determine differences for perseverative errors, regressive errors and repetitive grooming. Post-hoc Newman–Keuls tests were used to determine significant differences between groups.
Results
The location of cannula tips for mice included in the behavioral analyses are shown in Figure 1. Some mice were excluded from the behavioral analyses due to placements outside of the dorsomedial striatum or ventral orbitofrontal cortex. Six B6 (5 lateral ventricle and 1 dorsolateral placement) and 9 BTBR (7 lateral ventricle and 2 nucleus accumbens placements) were excluded from analysis due to dorsomedial striatum misplacements. Five B6 (4 medial and 1 ventral placement) and 5 BTBR (3 medial and 2 ventral placements) mice were excluded from the analyses due to orbitofrontal cortex misplacements.
Figure 1.
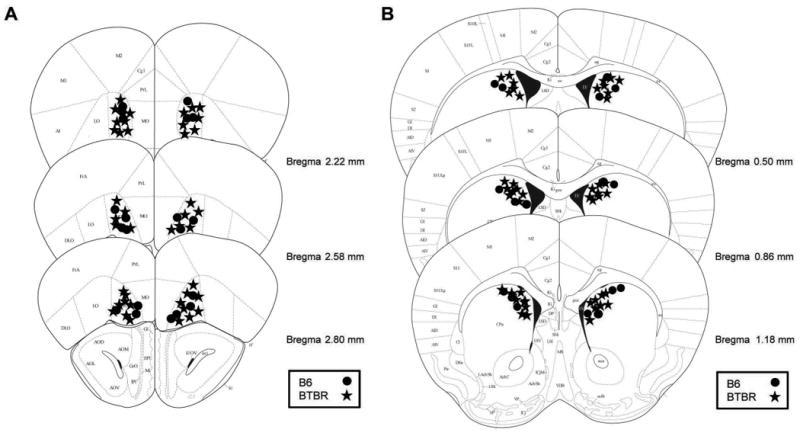
A) Cannula tip placements in the orbitofrontal cortex included in the behavioral analyses. B) Cannula tip placements in the dorsomedial striatum of mice included in behavioral analyses. Mouse brain sections adapted from The Mouse Brain in Stereotaxic Coordinates (Paxinos and Franklin, 2001). ● = B6 and ★ = BTBR
Effect of M100907 Infusions into the Dorsomedial Striatum on Probabilistic Reversal Learning
Figure 2 illustrates the results from the dorsomedial striatum experiment on acquisition and reversal learning. The analysis on trials to criterion for acquisition showed that there was no significant difference among the groups (F3,31 = 0.45, p > 0.05). The analysis on retention trials (data not shown) indicated that there was not a significant difference in trials to criterion among the groups (F3,31 = 1.84, p > 0.05). In the reversal learning test (see Figure 2A), there was a significant group effect (F3,31 = 6.45, p < 0.01). Post-hoc analyses revealed that vehicle-treated BTBR mice required significantly more trials to reach criterion compared to that of vehicle-treated B6 mice (p < 0.01). BTBR mice receiving an infusion of M100907 at 0.2μg or 0.6μg into the dorsomedial striatum significantly reduced reversal learning trials to criterion compared to that of vehicle-treated BTBR mice (p's < 0.05) and to a level that was not significantly different from that of vehicle-treated B6 mice (p's > 0.05).
Figure 2.
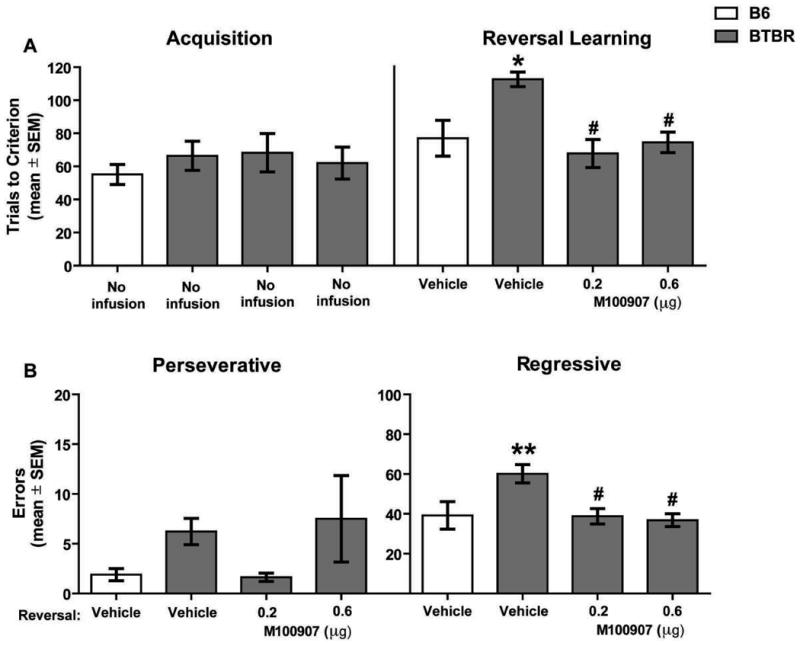
Microinfusions of M100907 into the dorsomedial striatum attenuates a probabilistic reversal learning deficit in BTBR mice by decreasing regressive errors. The treatments on the x-axis represent the treatment received prior to reversal learning. Mice did not receive infusions before acquisition learning. A) Mean (±SEM) trials to criterion on acquisition and reversal learning. This test included the following groups: B6: vehicle (n = 9); BTBR: vehicle (n = 9), 0.2μg (n = 8), 0.6μg (n = 6). Vehicle-treated BTBR mice required significantly more reversal learning trials compared with that of B6 vehicle-treated mice. M100907 at 0.2 and 0.6 μg infused into the dorsomedial striatum significantly attenuated trials needed to reach criterion. *p < 0.01 vs. B6-vehicle; #p < 0.01 vs. BTBR-vehicle. B) Mean (±SEM) perseverative and regressive errors committed during reversal learning. There was no overall group effect on perseverative errors despite vehicle-treated BTBR mice showing an increase in perseverative errors compared to vehicle-treated B6 mice. Vehicle-treated BTBR mice made significantly more regressive errors compared to vehicle-treated B6 mice. Microinfusions of M100907 at 0.2 and 0.6 μg into the dorsomedial striatum significantly attenuated regressive errors in BTBR mice compared to vehicle-treated BTBR mice. *p < 0.01 vs. B6-vehicle; #p < 0.01 vs. BTBR-vehicle.
The different errors committed during reversal learning are illustrated in Figure 2B. There was not a significant difference in perseverative errors among the groups (F3,31 = 2.60, p > 0.05). An ANOVA on regressive errors showed that there was a significant group effect (F3,31 = 4.69, p < 0.05). Post-hoc analyses revealed vehicle-treated BTBR mice committed significantly more regressive errors than vehicle-treated B6 mice (p < 0.01). M100907 infusions at 0.2μg and 0.6μg into the dorsomedial striatum of BTBR mice significantly reduced regressive errors compared to that of vehicle- treated BTBR mice (p's < 0.05) and to a level that was not significantly different from that of vehicle-treated B6 mice (p's > 0.05).
As described above, there were multiple mice that had cannula misplacements and were not included in the final behavioral analyses. To better understand whether a bilateral injection of M100907 into the dorsomedial striatum was critical for the reversal learning effect, we were particularly interested in BTBR mice with cannula misplacements that received either dose of M100907. There were 3 BTBR mice that received either the 0.2 or 0.6 μg dose of M100907 and had a cannula misplacement. These mice had a unilateral cannula placement in the lateral ventricle. The mean trials to criterion for these mice was 122.3 ± 21.4 SEM in reversal learning. Further, the mean number of perseverative errors was 1.7 ± 0.9 and regressive errors was 74.3 ± 20.5.
Effect of M100907 Infusions into the Dorsomedial Striatum on Grooming Behavior
M100907 infusions into the dorsomedial striatum were also found to reduce grooming in BTBR mice (see Figure 3). There was a significant difference in grooming duration among the groups (F3,31 = 11.16, p < 0.01). Vehicle-treated BTBR mice spent significantly more time grooming compared to that of vehicle-treated B6 mice (p < 0.01). M100907 into the dorsomedial striatum significantly reduced grooming behavior at the 0.6μg dose (p < 0.01), but not at the 0.2 μg dose (p > 0.05) compared to that of vehicle-treated BTBR mice. The difference in grooming behavior duration in vehicle-treated B6 mice and BTBR mice receiving M100907 at 0.6μg was not significant (p > 0.05).
Figure 3.
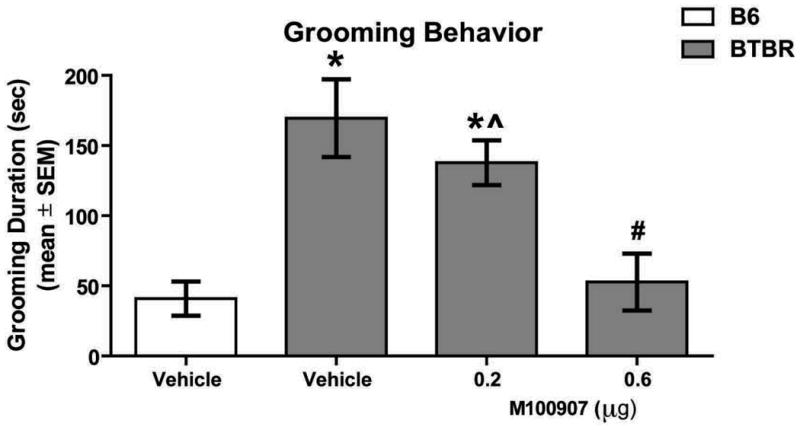
Intra-dorsomedial striatum M100907 attenuates repetitive grooming behavior in BTBR mice. Mean (±SEM) seconds spent grooming. This test included the following groups: B6: vehicle (n = 9); BTBR: vehicle (n = 7), 0.2μg (n = 8), 0.6μg (n = 8).Vehicle-treated BTBR mice spent significantly more time grooming compared to vehicle-treated B6 mice. Infusion of 0.6μg M100907 into dorsomedial striatum significantly attenuated grooming duration in BTBR mice compared to vehicle-treated BTBR mice. *p < 0.01 vs. B6-vehicle; #p < 0.01 vs. BTBR-vehicle; ˆp < 0.01 vs. BTBR-0.6μg M100907.
There was one BTBR mouse that received the 0.6μg dose of M100907 that also had a unilateral cannula placement in the lateral ventricle. This mouse had a grooming duration of 184 seconds.
Effect of M100907 Infusions into the Orbitofrontal Cortex on Probabilistic Reversal Learning
The findings on spatial acquisition and reversal learning are shown in Figure 4. There was no significant group effect for acquisition performance, (F3,25 = 0.38, p> 0.05). The analysis on retention trials (data not shown) indicated that there was not a significant difference among the groups (F3,29 = 0.89, p > 0.05). There was a significant group effect for reversal learning (F3,29 = 30.13, p < 0.01). Post-hoc analyses revealed that all BTBR treatment groups required significantly more trials to reach criterion compared to that of vehicle-treated B6 mice (p's < 0.01). M100907 infusions at 0.2μg or 0.6μg into the orbitofrontal cortex led to reversal learning performance that was not significantly different from that of BTBR controls (p's > 0.05).
Figure 4.
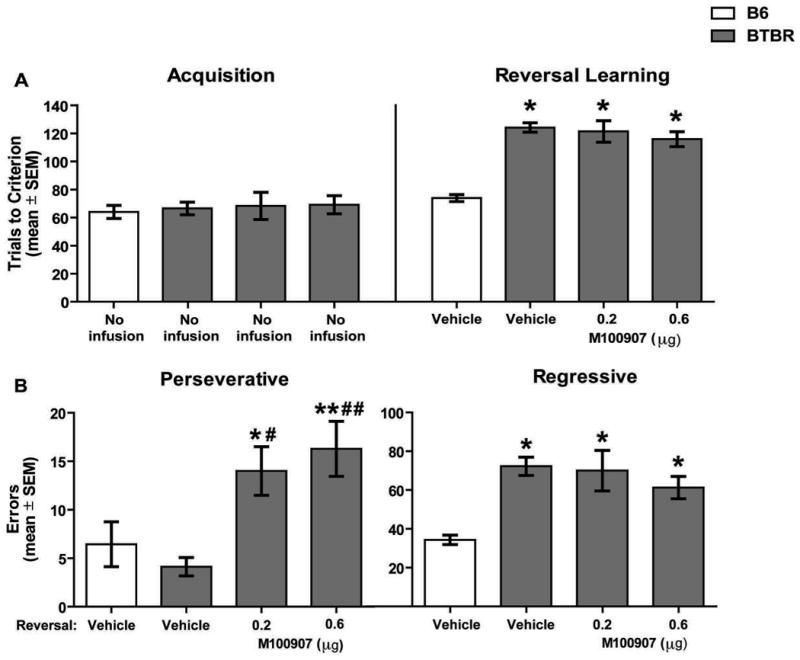
Intra-orbitofrontal cortex M100907 does not attenuate a probabilistic reversal learning deficit, but increases perseveration in BTBR mice. The treatments on the x-axis represent the treatment received prior to reversal learning. Mice did not receive infusions before acquisition. A) Mean (±SEM) trials to criterion on acquisition and reversal learning. This test included the following groups: B6: vehicle (n = 9); BTBR: vehicle (n = 8), 0.2μg (n = 6), 0.6μg (n = 7). Vehicle-treated BTBR mice required significantly more reversal learning trials compared with that of B6 vehicle-treated mice. M100907 at 0.2μg or 0.6μg infused into the orbitofrontal cortex did not affect trials to criterion. *p < 0.01 vs. B6-vehicle. B) Mean (±SEM) perseverative and regressive errors committed during reversal learning. Infusions of M100907 at 0.2μg and 0.6μg into the orbitofrontal cortex significantly increased perseverative errors committed by BTBR mice compared to vehicle treated BTBR mice. B6: vehicle (n = 9); BTBR: vehicle (n = 9); 0.2μg (n = 8); 0.6μg (n = 7). *p < 0.05 vs. B6-vehicle; #p < 0.05, **p < 0.01 vs. B6-vehicle; ##p < 0.01 vs. BTBR-vehicle. Vehicle-treated BTBR mice made significantly more regressive errors compared to vehicle-treated B6 mice. Microinfusions of M100907 at 0.2μg or 0.6μg into the orbitofrontal cortex did not attenuate regressive errors in BTBR mice compared to vehicle-treated BTBR mice. *p < 0.05 vs. B6-vehicle.
Analysis of perseverative errors during reversal learning indicated that there was a significant difference among the groups (F3,29 = 6.80, p < 0.01). M100907 infusions into the orbitofrontal cortex at 0.2 μg and 0.6 μg significantly increased perseverative errors compared to that of vehicle injections in both BTBR and B6 mice (p's < 0.05). In contrast, the difference in perseverative errors between B6 and BTBR controls was not significant (p > 0.05). There was also a significant difference among the groups for regressive errors committed (F3,29 = 10.37, p < 0.01). A post-hoc analysis revealed that vehicle-treated BTBR mice committed significantly more regressive errors compared to that of vehicle-treated B6 mice (p < 0.01). The number of regressive errors following M100907 treatment at 0.2μg and 0.6μg was not significantly different from that of vehicle-treated BTBR mice (p's > 0.05), but was significantly greater than that of vehicle treatment in B6 mice (p's < 0.05).
None of the mice receiving M100907 during reversal learning were found to have cannula misplacements.
Effect of M100907 Infusions into the Orbitofrontal Cortex on Grooming Behavior
The duration of grooming behavior is illustrated in Figure 5. A one-way ANOVA revealed that there was a significant difference among the groups (F4,34 = 16.75, p < 0.01). All BTBR treatment groups demonstrated increased grooming compared to that of B6 mice (p's < 0.01). BTBR mice receiving M100907 0.2μg or vehicle treatment displayed comparable durations of grooming (p > 0.05). However, BTBR mice receiving the M100907 0.6μg infusion in the orbitofrontal cortex exhibited significantly increased grooming compared to that of BTBR mice receiving vehicle or the M100907 0.2μg dose (p's < 0.01).
Figure 5.
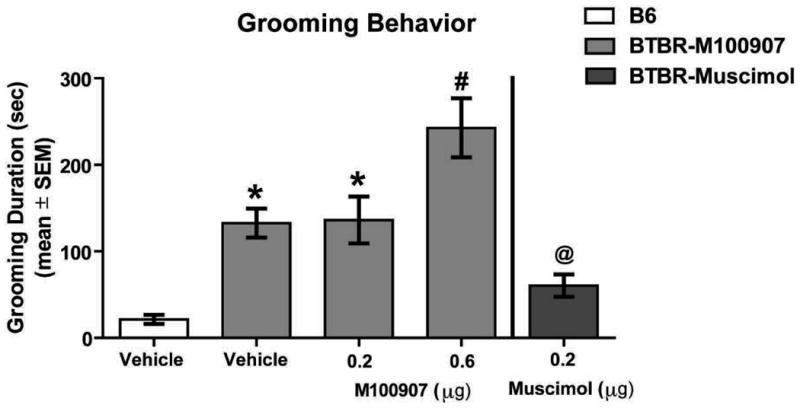
Intra-orbitofrontal cortex M100907 increases repetitive self-grooming in BTBR mice. Mean (±SEM) seconds spent grooming. This included the following groups: B6: vehicle (n = 7); BTBR: vehicle (n = 6), 0.2μg M100907 (n = 7), 0.6μg M100907 (n = 7); musicmol (n = 9). Infusion of 0.6μg M100907 into the orbitofrontal cortex significantly elevated grooming duration in BTBR mice compared to vehicle-treated BTBR mice and 0.2 μg M100907 treated mice. Muscimol treatment reduced grooming behavior in BTBR mice compared to vehicle, 0.2μg M100907 and 0.6μg M100907 treated BTBR mice. *p < 0 .01 vs. B6-vehicle; #p < 0.01 vs. BTBR-vehicle, BTBR-0.2μg M100907 and B6-vehicle; @p < 0.05 vs. BTBR-vehicle, BTBR-0.2μg and BTBR-0.6μg M100907.
None of the mice receiving M100907 during reversal learning were found to have cannula misplacements.
Unclear is whether enhanced grooming behavior following an orbitofrontal cortex infusion of M100907 at 0.6μg may result from a net increase or decrease in orbitofrontal cortex activity. As a comparison, a subset of BTBR mice received a second grooming test seven days later with an orbitofrontal cortex infusion of the GABA-A agonist, muscimol (0.2μg). A muscimol infusion into the orbitofrontal cortex significantly attenuated repetitive grooming in BTBR mice compared to that of all other BTBR groups (p's < 0.05), but showed comparable grooming duration to that of vehicle-treated B6 mice (p > 0.05). Thus, orbitofrontal cortex inactivation with muscimol decreased grooming while M100907 injection into the orbitofrontal cortex enhanced grooming behavior.
One BTBR mouse that received muscimol had a bilateral cannula misplacement with the cannulas located in the forceps minor of the corpus callosum. This mouse had a grooming duration of 140 seconds following an infusion of muscimol.
Discussion
The present experiments investigated whether 5HT2A receptor mechanisms in the dorsomedial striatum and orbitofrontal cortex of the BTBR mouse play a role in repetitive behaviors. 5HT2A receptor blockade in these two areas led to distinct effects on probabilistic reversal learning and grooming behavior. A M100907 infusion into the dorsomedial striatum attenuated a probabilistic reversal learning deficit and self-grooming behavior in BTBR mice. M100907 injected into the orbitofrontal cortex increased perseveration during reversal learning and potentiated self-grooming behavior in BTBR mice. The findings suggest that increased 5HT2A receptor activity in the dorsomedial striatum may contribute to behavioral inflexibility and stereotyped behaviors in the BTBR mouse. In contrast, 5HT2A receptor activity in the orbitofrontal cortex of BTBR mice may be critical for stopping perseveration of a previous response in reversal learning and attenuating grooming behavior. Although infusion of the 5HT2A receptor antagonist had opposite behavioral effects when infused into the dorsomedial striatum versus the orbitofrontal cortex, the drug infusion did affect ASD-like features when infused into either the dorsomedial striatum or orbitofrontal cortex. The reversal learning test has a significant cognitive component in which a subject must inhibit a previously learned choice pattern and acquire a new, choice pattern. Unlike the reversal learning test, grooming behavior does not have a significant cognitive component, but represents a highly stereotyped pattern of movements which may model obsessive, compulsive-like behaviors when elevated in rodents (Kalueff et al., 2016). Despite the distinctness of the two different behavioral measures used in the present investigation, manipulation of 5HT2A receptor activity in either the dorsomedial striatum or orbitofrontal cortex affected both probabilistic reversal learning and grooming behavior suggesting that similar mechanisms may contribute to these behaviors.
As described above, M100907 infusions into the dorsomedial striatum attenuated elevated grooming, as well as a probabilistic reversal learning deficit. Because the dorsomedial striatum is juxtaposed to the lateral ventricles one possibility is that the M100907 infusion into the dorsomedial striatum spread into the lateral ventricle producing behavioral effects due to actions outside of the dorsomedial striatum. This is unlikely as BTBR mice with a cannula located in the lateral ventricle and who received M100907 exhibited a behavioral pattern similar to BTBR mice that received a vehicle injection. In particular, BTBR mice with a unilateral placement in the lateral ventricle and receiving either 0.2 or 0.6μg of M100907 required a high number of trials to achieve criterion and also exhibited a large number of regressive errors. In addition, a BTBR mouse that also had one cannula located in the lateral ventricle and received the 0.6μg of M100907 had a grooming duration similar to that of BTBR vehicle-injected mice. Thus, only a bilateral infusion of M100907 into the dorsomedial striatum was sufficient to attenuate grooming behavior and a reversal learning deficit in BTBR mice. This pattern of results is comparable to past studies in rats, in which drugs aimed at the dorsomedial striatum were only effective with accurate bilateral cannula placements while placements in the lateral ventricle did not lead to a behavioral effect (Baker & Ragozzino, 2014; McCool et al., 2008; Palencia & Ragozzino, 2004).
The effects of M100907 when infused into the dorsomedial striatum mimics those with a systemic injection of M100907 (Amodeo et al., 2014; Amodeo et al., 2016). This pattern suggests that systemic treatment with M100907 may be due, in part, to actions in the dorsomedial striatum. Attenuation of reversal learning and grooming behavior following 5HT2A receptor blockade in the dorsomedial striatum unlikely results from altered 5HT2A receptor density because a previous study found no differences in 5HT2A receptor density in the striatum of BTBR mice compared to that of B6 mice (Gould et al., 2011). Although, one possibility is that 5HT2A receptor signaling and/or receptor density within a specific striatal cell population is altered in BTBR mice, which may lead to a reversal learning deficit and elevated grooming. Related to this point, earlier studies have found that systemic or striatal injections of a 5HT2A receptor agonist preferentially activate preprotachykinin mRNA expression in rats (Gresch & Walker, 1999). Preprotachykinin upregulation is associated with elevated activation of the direct basal ganglia pathway (Liste et al., 1999; Reiner & Anderson, 1990). Therefore, activation of 5HT2A receptors may preferentially activate striatal direct pathway neurons. One possibility is that over activation of the direct pathway in BTBR mice contributes to impaired reversal learning and elevated grooming behavior. Thus, infusion of a 5HT2A receptor antagonist directly into the dorsomedial striatum may regulate reversal learning and grooming behavior by reducing direct pathway activity and producing a greater balance between the basal ganglia direct and indirect pathways. An imbalance between these two pathways has been associated with increased stereotyped behaviors (Lewis et al., 2007; Tanimura et al., 2010). Furthermore, a 5HT2A receptor agonist infusion into the striatum can increase stereotyped behavior which is attenuated by a 5HT2A receptor antagonist (Bishop et al., 2004). These findings suggest that certain repetitive behaviors in ASD may result from increased 5HT2A receptor signaling in the striatum which then leads to over activation of the basal ganglia direct pathway.
In contrast to the dorsomedial striatum, 5HT2A receptor blockade in the orbitofrontal cortex of BTBR mice did not improve reversal learning, but increased perseveration. This is somewhat comparable to studies showing M100907 infused into the rat orbitofrontal cortex impairs reversal learning (Boulougouris et al., 2010; Furr et al., 2012) and that 5HT depletion in the marmoset orbitofrontal cortex impairs reversal learning by increasing perseveration (Clarke et al., 2007). The increased perseveration observed in this study is similar to that observed with a systemic injection of 0.1 mg/kg M100907 which increased perseverative errors during reversal learning in BTBR mice (Amodeo et al., 2014). Therefore, the combined effects of M100907 infusions into the orbitofrontal cortex and dorsomedial striatum reproduce the effects observed with a peripheral injection of M100907 in which there is an initial increase in perseverative errors, but a subsequent decrease in regressive errors that improves reversal learning performance overall.
The results following 5HT2A receptor blockade in the orbitofrontal cortex on reversal learning contrasts those observed with a 5HT2C receptor antagonist when infused into the orbitofrontal cortex or injected systemically (Alsio et al., 2015). In particular, treatment with the selective 5HT2C receptor antagonist, SB 242084 reduced perseverative errors during reversal learning in rats. Unknown is whether treatment with a 5HT2C receptor antagonist would be effective in alleviating behavioral flexibility deficits in mouse models of autism. We previously reported that ASD individuals are impaired on probabilistic reversal learning due to a selective increase in regressive errors (D'Cruz et al., 2013). However, other studies have found that ASD individuals can preferentially exhibit perseverative errors or a deficit in initially shifting away from an originally learned strategy (Liss et al., 2001; Westwood et al., 2016). ASD is known to express a heterogeneous phenotype. One possibility is that treatment with a 5HT2C receptor antagonist, but not a 5HT2A receptor antagonist, may be effective in reducing cognitive rigidity in a subgroup of ASD individuals who exhibit perseveration.
Besides increasing perseveration in reversal learning, M100907 infusion into the orbitofrontal cortex also exacerbated elevated grooming behavior in BTBR mice. In the cortex, 5HT2A receptors are expressed on pyramidal neurons and interneurons (Jakab & Goldman-Rakic, 2000; Puig & Gulledge, 2011), where 5HT or selective agonists produce a general depolarizing effect via Gq-type proteins (Marek & Aghajanian, 1999; Puig & Gulledge, 2011). In an attempt to clarify whether M100907 may be preferentially acting on output or interneurons in the orbitofrontal cortex, the effect of the GABA-A agonist, muscimol infused into the orbitofrontal cortex was examined. Muscimol infusions into the orbitofrontal cortex dramatically reduced grooming behavior in BTBR mice. Thus, muscimol had the opposite effect of M100907 on grooming when infused into the orbitofrontal cortex. This raises the possibility that 5HT2A receptor blockade in the orbitofrontal cortex may lead to a net increase in orbitofrontal cortex output by decreasing interneuron activity.
Comparison of the two experiments indicate that M100907 had opposite effects by attenuating repetitive behaviors when infused into the dorsomedial striatum, but potentiating repetitive behaviors when injected into the orbitofrontal cortex. This neuropharmacological approach to rescue a phenotype in a mouse model of autism has the advantage of better understanding what brain areas exhibit abnormalities underlying specific behaviors related to autism. By targeting specific neurotransmitter receptors this approach can also identify what receptor mechanisms in specific neural systems are altered that contribute to a particular phenotype.
Taken together, the effects of M100907 into the dorsomedial striatum and orbitofrontal cortex on reversal learning mimic results with systemic M100907 treatment. Because of this the systemic effect of M100907 at 0.1 mg/kg may have increased initial perseverative behavior by principally acting at the orbitofrontal cortex while facilitating the reliable execution of a new choice pattern due to actions in the dorsomedial striatum. The differential effects on probabilistic reversal learning observed with M100907 when infused into the orbitofrontal cortex versus dorsomedial striatum also has implications about what brain circuitry may be altered that contributes to a reversal learning deficit in ASD. Similar to the BTBR mouse, ASD individuals are impaired on probabilistic reversal learning due to a selective increase in regressive errors (D'Cruz et al., 2013). This raises the possibility that similar 5HT mechanisms in the striatum of ASD individuals are altered that lead to a reversal learning deficit. At minimum, the findings suggest that the dorsomedial striatum exhibits enough plasticity such that infusion of a 5HT2A receptor antagonist can rescue the reversal learning deficit and elevated grooming. While previous studies have found 5HT2A receptor changes in ASD (Hranilovic et al., 2015; McBride et al., 1989), the present findings highlight how blocking 5HT2A receptor activity in specific brain systems can attenuate repetitive behaviors that are a core symptom domain in ASD.
Acknowledgments
This research was supported by NIH grant P50 HD055751 (EHC). Dr. John Sweeney has served as a consultant with Eli Lilly, Janssen, BMS and Takeda International pharmaceutical companies. All other authors do not have any conflict of interest disclosures.
References
- Alsiö J, Nilsson SRO, Gastambide F, Wang RAH, Dam SA, Mar AC, Tricklebank M, Robbins TW. The role of 5-HT2C receptors in touchscreen visual reversal learning in the rat: a cross-site study. Psychopharmacology. 2015;232:4017–4031. doi: 10.1007/s00213-015-3963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Autism spectrum disorder. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Risperidone and the 5-HT2A Receptor Antagonist M100907 Improve Probabilistic Reversal Learning in BTBR T+ tf/J Mice. Autism Res. 2014;7:555–567. doi: 10.1002/aur.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo DA, Rivera E, Dunn JT, Ragozzino ME. M100907 attenuates elevated grooming behavior in the BTBR mouse. Behav Brain Res. 2016;313:67–70. doi: 10.1016/j.bbr.2016.06.064. [DOI] [PubMed] [Google Scholar]
- Baker PM, Thompson JL, Sweeney JA, Ragozzino ME. Differential effects of 5-HT2A and 5-HT2C receptor blockade on strategy-switching. Behav Brain Res. 2011;219:123–131. doi: 10.1016/j.bbr.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PM, Ragozzino ME. Contralateral disconnection of the rat prelimbic cortex and dorsomedial striatum impairs cue-guided behavioral switching. Learn Mem. 2014;21:368–379. doi: 10.1101/lm.034819.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C, Tessmer JL, Ullrich T, Rice KC, Walker PD. Serotonin 5-HT2A receptors underlie increased motor behaviors induced in dopamine-depleted rats by intrastriatal 5-HT2A/2C agonism. JPET. 2004;310:687–694. doi: 10.1124/jpet.104.066365. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Richler J, Cain AC, Lord C. Predictors of perceived negative impact in mothers of children with autism spectrum disorder. Am J Mental Retardation. 2007;112:450–461. doi: 10.1352/0895-8017(2007)112[450:POPNII]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;3:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, Amodeo DA, Sweeney JA, Ragozzino ME. The selective serotonin reuptake inhibitor, escitalopram, enhances inhibition of prepotent responding and spatial reversal learning. J Psychopharm. 2012;26:1443–1455. doi: 10.1177/0269881111430749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguière E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- D'Cruz AM, Ragozzino ME, Mosconi MW, Shrestha S, Cook EH, Sweeney JA. Reduced behavioral flexibility in autism spectrum disorders. Neuropsych. 2013;27:152–160. doi: 10.1037/a0031721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmonte S, Gallagher L, O'Hanlon E, McGrath J, Balsters JH. Functional and structural connectivity of frontostriatal circuitry in autism spectrum disorder. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, Milham MP. Aberrant striatal functional connectivity in children with autism. Biol Psych. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharm. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Furr A, Lapiz-Bluhm MD, Morilak DA. 5-HT2A receptors in the orbitofrontal cortex facilitate reversal learning and contribute to the beneficial cognitive effects of chronic citalopram treatment in rats. Int J Neuropsychopharmacol. 2012;15:1295–1305. doi: 10.1017/S1461145711001441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriels RL, Cuccaro ML, Hill DE, Ivers BJ, Goldson E. Repetitive behaviors in autism: Relationships with associated clinical features. Res Dev Disabilities. 2005;26:169–181. doi: 10.1016/j.ridd.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors,and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresch PJ, Walker PD. Serotonin-2 receptor stimulation normalizes striatal preprotachykinin messenger RNA in an animal model of Parkinson's disease. Neurosci. 1999;93:831–841. doi: 10.1016/s0306-4522(99)00238-9. [DOI] [PubMed] [Google Scholar]
- Hall H, Farde L, Halldin C, Lundkvist C, Sedvall G. Autoradiographic localization of 5-HT2A receptors in the human brain using [3H] M100907 and [11C] M100907. Synapse. 2000;38:421–431. doi: 10.1002/1098-2396(20001215)38:4<421::AID-SYN7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Herth MM, Kramer V, Piel M, Palner M, Riss PJ, Knudsen GM, Rösch F. Synthesis and in vitro affinities of various MDL 100907 derivatives as potential 18 F-radioligands for 5-HT 2A receptor imaging with PET. Bioorganic & medicinal chemistry. 2009;17:2989–3002. doi: 10.1016/j.bmc.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, Wassermana S, Sooryaa L, Buchsbauma M, Buchsbaum M. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol Psych. 2005;58:226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Blazevic S, Stefulj J, Zill P. DNA methylation analysis of HTR2A regulatory region in leukocytes of autistic subjects. Autism Res. 2015;2:204–209. doi: 10.1002/aur.1519. [DOI] [PubMed] [Google Scholar]
- Ito H, Nyberg S, Halldin C, Lundkvist C, Farde L. PET imaging of central 5-HT2A receptors with carbon-11-MDL 100,907. J Nuclear Med. 1998;39:208–214. [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–348. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nature Rev Neurosci. 2016;17:45–59. doi: 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehn e JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. Journal of Pharmacology and Experimental Therapeutics. 1996;277:968–981. [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer CS, Campbell JE, Galvan B, Bowman C, Osgood S, Buist S, Buchholz L, Henry B, Wong EHR, Shahid M, Grimwood S. Validation of a rat in vivo [3 H] M100907 binding assay to determine a translatable measure of 5-HT 2A receptor occupancy. Eur J Pharmacol. 2008;591:136–141. doi: 10.1016/j.ejphar.2008.06.063. [DOI] [PubMed] [Google Scholar]
- Langen M, Leemans A, Johnston P, Ecker C, Daly E, Murphy CM, Dell'acqua F, Durston S, AIMS Consortium. Murphy DG. Fronto-striatal circuitry and inhibitory control in autism: findings from diffusion tensor imaging tractography. Cortex. 2012;48:183–193. doi: 10.1016/j.cortex.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Bodfish JW. Repetitive behavior disorders in autism. Mental retardation and developmental disabilities research reviews. 1998;4:80–89. [Google Scholar]
- Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss M, Fein D, Allen D, Dunn M, Feinstein C, Morris R, Waterhouse L, Rapin I. Executive functioning in high-functioning children with autism. J Child Psychol Psychiatry. 2001 Feb;42(2):261–70. [PubMed] [Google Scholar]
- Liste I, Rodriguez-Pallares J, Caruncho HJ, Labandeira-Garcia JL. Locomotor-activity-induced changes in striatal levels of preprotachykinin and preproenkephalin mRNA. Regulation by the dopaminergic and glutamatergic systems. Brain Res Mol Brain Res. 1999;70:74–83. doi: 10.1016/s0169-328x(99)00140-0. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- McBride PA, Anderson GM, Hertzig ME, Sweeney JA, Kream J, Cohen DJ, Mann JJ. Serotonergic responsivity in male young adults with autistic disorder. Results of a pilot study. Arch Gen Psychiatry. 1989;46:213–221. doi: 10.1001/archpsyc.1989.01810030019003. [DOI] [PubMed] [Google Scholar]
- McCool MF, Patel S, Talati R, Ragozzino ME. Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiol Learn Mem. 2008;89:114–124. doi: 10.1016/j.nlm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+ tf/J mice. Genes, Brain & Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Miller HL, Ragozzino ME, Cook EH, Sweeney JA, Mosconi MW. Cognitive set shifting deficits and their relationship to repetitive behaviors in autism spectrum disorder. J Autism Dev Disord. 2015;45:805–815. doi: 10.1007/s10803-014-2244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiol Learn Mem. 2004;82:81–89. doi: 10.1016/j.nlm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The effect of N-methyl-D-aspartate receptor blockade on acetylcholine efflux in the dorsomedial striatum during response reversal learning. Neurosci. 2006;143:671–678. doi: 10.1016/j.neuroscience.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates 2. San Diego: Academic Press; 2001. [Google Scholar]
- Pearson BL, Bettis JK, Meyza KZ, Yamamoto LY, Blanchard DC, Blanchard RJ. Absence of social conditioned place preference in BTBR T+ tf/J mice: relevance for social motivation testing in rodent models of autism. Behav Brain Res. 2012;233:99–104. doi: 10.1016/j.bbr.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Gulledge AT. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol. 2011;44:449–464. doi: 10.1007/s12035-011-8214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Anderson KD. The patterns of neurotransmitter and neuropeptide co-occurrence among striatal projection neurons: conclusions based on recent findings. Brain Res Brain Res Rev. 1990;15:251–265. doi: 10.1016/0165-0173(90)90003-7. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregellas JR. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiat. 2006;6:56. doi: 10.1186/1471-244X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–320. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Gastrell PT, Karras MN, Solomon M, Crawley JN. Cognitive abilities on transitive inference using a novel touchscreen technology for mice. Cereb Cortex. 2015;25:1133–1142. doi: 10.1093/cercor/bht293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, Taylor VL, Schmidt CJ. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: Behavioral electrophysiological and neurochemical studies. J Pharm Exp Ther. 1993;266:684–691. [PubMed] [Google Scholar]
- Tanimura Y, Vaziri S, Lewis MH. Indirect basal ganglia pathway mediation of repetitive behavior: attenuation by adenosine receptor agonists. Behav Brain Res. 2010;210:116–122. doi: 10.1016/j.bbr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood H, Stahl D, Mandy W, Tchanturia K. The set-shifting profiles of anorexia nervosa and autism spectrum disorder using the Wisconsin Card Sorting Test: a systematic review and meta-analysis. Psychol Med. 2016:1–19. doi: 10.1017/S0033291716000581. [DOI] [PubMed] [Google Scholar]
- Xu T, Pandey SC. Cellular localization of serotonin 2A (5HT 2A) receptors in the rat brain. Brain Res Bull. 2000;51:499–505. doi: 10.1016/s0361-9230(99)00278-6. [DOI] [PubMed] [Google Scholar]


