Abstract
Objective
A 71-year old (MN) with an 11-year history of left onset tremor diagnosed as Parkinson’s disease (PD) completed longitudinal brain magnetic resonance imaging (MRI) and neuropsychological testing. MRI scans showed an asymmetric caudate nucleus (right< left volume). We describe this asymmetry at baseline and the progression over time relative to other subcortical gray, frontal white matter, and cortical gray matter regions of interest. Isolated structural changes are compared to MN’s cognitive profiles.
Method
MN completed yearly MRIs and neuropsychological assessments. For comparison, left onset PD (n=15) and non-PD (n=43) peers completed the same baseline protocol. All MRI scans were processed with FreeSurfer and the FMRIB Software Library (FSL) to analyze gray matter structures and frontal fractional anisotropy (FA) metrics. Processing speed, working memory, language, verbal memory, abstract reasoning, visuospatial, and motor functions were examined using reliable change methods.
Results
At baseline MN had striatal volume and frontal lobe thickness asymmetry relative to peers with mild prefrontal white matter FA asymmetry. Over time only MN’s right caudate nucleus showed accelerated atrophy. Cognitively, MN had slowed psychomotor speed and visuospatial-linked deficits with mild visuospatial working memory declines longitudinally.
Conclusions
This is a unique report using normative neuroimaging and neuropsychology to describe an individual diagnosed with PD who had striking striatal asymmetry followed secondarily by cortical thickness asymmetry and possible frontal white matter asymmetry. His decline and variability in visual working memory could be linked to ongoing atrophy of his right caudate nucleus.
Keywords: neuroimaging, movement disorders, memory, executive function, white matter
Introduction
Parkinson’s disease (PD) motor symptoms typically manifest unilaterally. It has been suggested that laterality corresponds to type of cognitive impairment. A comprehensive review indicated there is a compelling suggestion that cognitive domain specific deficits are related with lateralized motor symptoms (Verreyt, Nys, Santens, & Vingerhoets, 2011; Amick, Grace, & Chou, 2006; Schendan, Amick, & Cronin-Golomb, 2009; Cubo et al., 2010) but results are mixed (see Bentin, Silverberg, & Gordon, 1981; Direnfeld, Albert, & Volicer, 1984; Huber, Freidenberg, Shuttleworth, Paulson, & Clapp, 1989). Right side onset PD correlates with worse verbal fluency, object naming, and verbal memory. There is also evidence of reduced visuospatial orientation and visuospatial memory in left side onset PD. These differences are thought to result from lateralized reductions in dopamine (for a review see Verreyt et al., 2011).
Neuroanatomically, some have shown brain structural asymmetry depending on motor onset side. Motor laterality associates with contralateral neuronal loss in the substantia nigra in individuals with PD (Kempster, Gibb, Stern, & Lees, 1989). While one study showed larger contralateral ventricle volumes in individuals with PD (Lewis et al., 2009) another study did not (Price et al., 2011). There is evidence that individuals with PD have bilateral basal ganglia volume loss regardless of side of onset (Geng, Li, & Zee, 2006) with the putamen the primarily affected structure (Price et al., 2016; Garg et al., 2015; Nemmi et al., 2015). Using shape (morphometric) analyses of subcortical gray matter structures, however, provides evidence of greater contralateral than ipsilateral shape changes in PD for both the putamen and caudate nucleus (Sterling et al., 2013; Caligiuri et al., 2016; Lee et al., 2014). Further, using brain diffusion tensor imaging metrics, it is possible to differentiate between right and left onset PD (Feis, Pelzer, Timmermann, Tittgemeyer, 2013). Overall, existing research provides strong evidence for atrophy of the putamen (and the caudate to a lesser extent) in PD with weaker evidence of lateralized (asymmetric) brain structure differences depending on side of PD symptom onset.
We present a case report of an individual with PD (here called ‘MN’) who was observed to have striking subcortical asymmetry within the caudate nucleus on baseline magnetic resonance imaging (MRI) as part of a longitudinal research investigation. This is a novel case report; there are currently no published longitudinal cognitive and neuroanatomical profiles for individuals with baseline brain structure asymmetry and concurrent parkinsonism symptoms. How subcortical gray matter, white matter, and cortical thickness change over time also has not been investigated. We present MN’s a) relevant history, b) longitudinal subcortical volumes, white, and cortical thickness changes, and c) longitudinal cognitive changes relative to a set of age and disease-matched peers. Given the progressive nature of PD, first involving subcortical and then cortical regions (Braak, Ghebremedhin, Rüb, Bratzke, & Del Tredici, 2004), we hypothesized that MRI would demonstrate continued dominant asymmetry in the subcortical caudate nucleus but also evidence of reduced frontal white matter fractional anisotropy (FA) and frontal cortical thickness over time. We also hypothesized that cognitive changes would associate with side of asymmetry.
Case Description
At initial screening, MN was a 65-year old right-handed Caucasian male with a five to ten year history of motor symptoms associated with PD. He was found to have a small right caudate nucleus on visual inspection of his MRI. He agreed to participate for longitudinal assessment in order to track caudate nucleus volume and cognition over time. We saw him on four subsequent occasions for repeat neuropsychological assessment and MR imaging as part of two National Institute of Neurological Disorders and Stroke (NINDS) funded studies investigating the contribution of white and gray matter to the cognitive profile of idiopathic PD. We acquired background information during participant interviews and medical record reviews.
Symptom characterization and progression
MN’s initial parkinsonism symptom occurred between the ages of 55 and 60. He reported a slowly progressive left-side shuffling gait and a left hand resting tremor, which led to an initial diagnosis of PD about six months after the symptoms started. He did not start levodopa medication at the time. After 5–10 years of slowly worsening symptoms and in the months before referral for the present study, a board certified neurologist evaluated MN and confirmed his idiopathic PD diagnosis. His Unified Parkinson’s Disease Rating Scale motor score (UPDRS III; Hughes, Ben-Shlomo, Daniel, & Lees, 1992) was 23 at the time (off medication). He did not start any PD-related medication at that time because he did not believe his symptoms interfered significantly with his life. After a 6 month follow-up visit he started taking 1 mg rasagiline at the encouragement of his neurologist. His symptoms have been primarily left-side dominant over the course of the disease with the exception of a mild action tremor and slowing in his right hand. At baseline, cognitive complaints included attention and remembering names, but with these difficulties reportedly present for 20 years.
Neurological examination
MN’s on-medication UPDRS III (motor) scores acquired during our study were: 23 (baseline), 22 (year 1), 33 (year 2), and 22 (year 4). The UPDRS was not given at year 3. Off-medication UPDRS III scores were not acquired during this study. Across all visits his Hoehn and Yahr score (H&Y; Hoehn & Yahr, 1967) remained stage 2. At baseline, he had a mild left hand resting tremor, with slowing and reduction in amplitude in his left hand as well as his left leg. He walked with a shuffling gait and slight left foot drag, but did not require assistance. He had mild difficulty with rapid alternating movements bilaterally. At years 2, 3, and 4, MN reported that his left hand tremor continued to be bothersome and had progressed mildly in severity. Parkinsonism was evident bilaterally (left worse than right) with little self-reported progression in motor symptoms. At year 4, MN stated he no longer found his medications particularly helpful; his neurologist attributed this to under-medication rather than lack of medication efficacy.
Medications
Medications at our baseline evaluation included Requip XL (ropinirole) 8 mg daily, Azilect (rasagiline) 1 mg, Plavix 75 mg, Vytorin, 40 mg, aspirin 81 mg, and five over the counter vitamins and supplements (coenzyme Q10, multivitamin, vitamin C, vitamin D, and omega-3). At year 1 his Requip XL dose had been increased to 16 mg daily. At year 2 he was taking 20 mg of Requip XL and 1 mg of Azliect daily. Requip XL was phased out during year 3 and replaced with carbidopa/levodopa 25/100 (1 tablet, three times per day). By year 4 MN took 2 tablets, four times per day of carbidopa/levodopa 25/100. There were modest changes to MN’s other medications or over the counter supplements; he started finasteride for benign prostatic hyperplasia, replaced omega-3 with krill oil supplement, and started taking curcumin extract.
Medical history
MN had two mild head injuries without loss of consciousness as an adult. He also had a heart attack and surgical cardiac stent placement at age 64. MN’s hyperlipidemia and hypotension were well controlled pharmaceutically during the entire course of the study. MN remained slightly overweight over the study (BMI range: 25.59 – 26.96). He was diagnosed with atrial fibrillation at year 4 but symptoms are well controlled. Contrasted MRIs acquired prior to the study were read as unremarkable other than a cerebral capillary telangiectasia near the right caudate nucleus that was reported by the neuroradiologist as small, benign, and stable in size. A recent (2015) clinical single-photon emission computed tomography (SPECT) with ioflupane iodine-123 (DaTscan) showed significant reduction in right basal ganglia uptake and reduced left putamen uptake. The scan was interpreted as supporting a diagnosis of severe PD.
Methods
All imaging studies and neuropsychological tests were administered at the University of Florida Clinical Research Center as part of a federally funded investigation that required Institutional Review Board approval, individual consent, and followed Declaration of Helsinki guidelines. In order to assess for potential abnormalities relative to peers, MN’s neuropsychological profile and structural imaging was compared to left-side onset, non-demented peers (L-PD; n=15) as well as non-PD peers (non-PD; n=43) who were enrolled in a federally funded investigation study Parkinson’s disease and cognition. Two L-PD and three non-PD participants were unable to complete baseline imaging.
Inclusion and exclusion Criteria
Initial inclusion criteria for all participants required right handedness (Briggs & Nebes, 1975), no evidence of severe cognitive impairment (Modified Telephone Interview for Cognitive Status score had to be >34; Cook, Marsiske, & McCoy, 2009), a Dementia Rating Scale-Revised raw score >130 (DRS-2; Matteau et al., 2011), and fluent English. Participants who passed the screening criteria completed full neuropsychological assessment and neuroimaging. For the participants with Parkinson’s disease, a diagnosis with PD was made by board certified movement disorder neurologists using United Kingdom Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria (Hughes et al., 1992). PD participants were also required to have H&Y scores ranging from 1–3. The following were medical exclusions for participants with and without PD: diseases likely to confound cognition (e.g., cerebrovascular accident in the last six months, congestive heart failure, etc.), deep brain stimulation, a diagnosis of secondary/atypical parkinsonism, or major psychiatric disorder. We did not exclude for depression or anxiety because many PD patients report such symptoms.
Magnetic Resonance Imaging
MN, left side onset PD peers (L-PD; n=13), and non-PD peers (n=40) received structural neuroimaging (3T Siemens Verio; 8 channel head coil). L-PD and non-PD peers received imaging only at baseline but MN underwent longitudinal MRI for a total of four scans (baseline, year 1, year 3, and year 4) to examine for cognitive and brain-related changes that might be explained by his baseline caudate asymmetry.
MRI sequences
Both T1-weighted and diffusion-weighted images were acquired. T1-weighted (TR: 2500ms; TE: 3.77ms; 176 sagittal 1mm3 slices) images were post-processed using an automated longitudinal pipeline (FreeSurfer 5.3; Fischl, 2012; Fischl et al., 2002). No significant errors in FreeSurfer processing were found on quality check. Diffusion-weighted imaging (DWI) included two separate single-shot echo planar imaging, with gradients applied along 6 directions (b = 100s/ mm2) and 64 directions (b = 1000s/ mm2), 73 contiguous axial slices, 2mm3 voxels, and TR/TE = 17300/81ms. Both diffusion sequences per participant were merged together to create an acquisition with 70 non-overlapping directions (6 b = 100 and 64 b = 1000) and two b = 0 images. Diffusion images were processed using eddy_correct from the FMRIB Software Library version 5.0 (FSL; Smith et al., 2004) to correct for eddy currents and movement and the FreeSurfer script dt_recon to calculate fractional anisotropy (FA).
Regions of interest (ROIs)
Structures affected by dopaminergic denervation and/or PD pathology and neuron atrophy (caudate nucleus, putamen, globus pallidus, thalamus, hippocampus, and lateral ventricles) were selected a priori for volumetric analyses. All volumes were extracted from FreeSurfer and divided by total intracranial volume (TICV) to adjust for head size. TICV was calculated by manually creating masks filling the entire cranium within the inner boundary of the skull above a straight line between the occipital bone and the clivus (intra- and inter-rater reliabilities for this method were excellent: Dice similarity coefficients = 0.99; Intra-class correlation = 0.96).
Cortical ROIs for thickness measurements were selected based on connectivity to the caudate nucleus and other subcortical structures (Alexander, DeLong, & Strick, 1986). Specifically, we hypothesized that MN’s frontal cortex would demonstrate thickness asymmetry; parietal cortex, as a dissociation, would have symmetric thickness. Lobe ROIs were created by merging cortical parcellations from the Desikan-Killiany Atlas by lobe and then calculating average thickness values for the merged regions.
A prefrontal white matter ROI was chosen based on differences observed in our previous research in PD (Price et al., 2016) and because many white matter connections to and from the caudate nucleus pass through prefrontal regions (e.g., Alexander, DeLong, & Strick, 1986). To acquire white matter within the frontal lobe, white matter was extracted from the aseg.mgz files created by FreeSurfer. For each individual, T1 images were aligned with the MNI152 T1 template using an affine registration (12 degrees of freedom; FLIRT; Jenkinson et al., 2002). The matrix from this transformation was then applied to the white matter masks extracted from aseg. Using ITK-SNAP (www.itksnap.org; Yushkevich et al., 2006), all white matter posterior to the rostrum of the corpus callosum was removed. This ‘prefrontal’ white matter mask was inversely transformed into native (FreeSurfer) space and then rigid body alignments from the dt_recon script were applied to transform the prefrontal mask to native DWI space for each time point. Mean FA values within the left and right prefrontal ROIs were then calculated using fslstats.
Normative comparisons
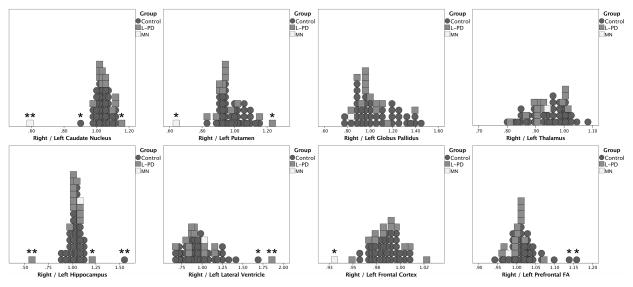
To determine if MN’s subcortical gray and white matter and cortical thickness metrics (volume adjusted for were atypical compared to non-PD and left onset PD peers, standardized z scores were created from the two matched peer groups. Only baseline z scores were calculated because L-PD and non-PD peers did not have longitudinal MRI data. We also created ratio values (right / left) for all raw MRI metrics as a measure of asymmetry. A right / left ROI z score difference that was both greater than 2.00 and more than 1.5 times the interquartile range for right/left structure ratio (see Figure 1) was considered significantly asymmetric.
Figure 1.
Neuropsychology
MN, L-PD, and non-PD peers completed comprehensive neuropsychological assessment as part of a research protocol acquiring baseline and one-year cognitive data. Due to his baseline brain abnormalities and questions about changes over time, MN was followed for an additional three years. For this study, representative measures from all primary cognitive domains were selected to track cognition over time.
The domains and measures included: Processing Speed: Stroop Word Reading (Stroop Color Word Test, total correct in 45 seconds; Golden & Freshwater, 2002), Digit Symbol subtest from the Wechsler Adult Intelligence Scale, 3rd edition (raw score in 120 seconds; WAIS-III; Wechsler, 1997a); Working Memory: Digit Span Backward Span from Wechsler Adult Intelligence Scale, 3rd edition (longest backward span), Spatial Span Backward from the Wechsler Memory Scale, 3rd edition (longest span; WMS-III; Wechsler, 1997b); Language and Language Related Skills: Boston Naming Test (BNT; total raw correct out of 60; Kaplan, Goodglass, & Weintrab, 1983), Controlled Oral Word Association Test (COWAT: FAS; total correct exemplars; Strauss, Sherman, & Spreen, 2006); Memory: Logical Memory II and Visual Reproductions II subtests from the WMS-III (total score); Abstract Reasoning: WAIS-III Matrix Reasoning subtest (total score; Wechsler, 1997a), D-KEFS Tower Test (total achievement score; Delis, Kaplan, & Kramer, 2001); Wisconsin Card Sorting Test (WCST; total errors; Heaton, 1981); Visuospatial: Benton Judgment of Line Orientation (total correct; Benton, Sivan, Hamsher, Varney, & Spreen, 1994), Benton Facial Recognition Test (total correct); Motor: Finger Tapping Test (total; Reitan, 1979). Z scores for cognitive performances were created based on published normative data including adjustment for age and/or education. The z scores were averaged by domain to create composite scores.
Cognitive comparison
MN’s cognitive composites were compared to 15 L-PD and 43 age and education matched participants without PD (non-PD; see Supplemental Table 1). One L-PD participant and three non-PD participants did not complete the one year neuropsychological assessment. MN’s cognitive changes from baseline to year 1 were calculated using a robust linear bivariate regression Reliable Change Index (RCI) method that accounts for L-PD or non-PD group mean change score, practice effects, and regression to the mean (McSweeny, Naugle, Chelune, & Lüders, 1993; Martin et al., 2002). Baseline to one year individual reliable change scores (SRC) for each cognitive and composite score were calculated using the following equation SRC = (YO − YP) / SEest, where YO is the observed one year score, YP is the predicted one-year score, and SEest is the bivariate linear regression standard error of the estimate. The predicted one-year score (YP) equals βX + C, where β is the regression coefficient, X is the baseline score, and C is the regression constant (intercept). Significant cognitive change was determined as a SRC score ≤-1.645 or≥ +1.645 (p < 0.05 in each distribution tail; Jacobson & Truax, 1991).
Results
Within-Subject and Group Comparisons at Baseline MN’s right versus left subcortical structures.
Relative to the left side, the right caudate nucleus volume was 41% smaller, the right putamen was 36% smaller, the right globus pallidus (both internal and external) was 17% smaller, and the right thalamus was 11% smaller. MN’s right hippocampus volume was 9.6% larger than the left with his right lateral ventricle 4.6% larger in volume than left.
Right versus left subcortical gray matter structure asymmetry for all participants
MN had more right / left caudate nucleus and putamen asymmetry than any L-PD or non-PD peer (Figure 1). There were no between-group (L-PD versus non-PD, excluding MN) differences in ROI metrics (all p values > 0.084). L-PD had a trend toward smaller bilateral putamen volumes than non-PD (Left p = 0.084, Right p = 0.098). Asymmetry also did not differ between groups for any ROI (all p values > 0.188).
Relative to L-PD
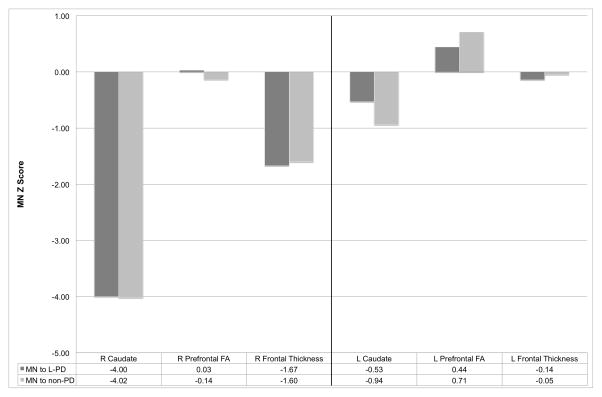
MN’s right caudate nucleus was more than four standard deviations smaller than L-PD peers (Figure 2; Right: z= −4.00; Left: z= −0.53). Also asymmetric was the right putamen (Right: z= −1.75; Left: z= 1.57) with the right globus pallidus marginally asymmetric (Right z= −2.14; Left: z= −0.12). MN’s thalamus (Right: z= −0.04; Left: z= 0.26), hippocampus (Right z= −0.81, Left z= − 1.27), and lateral ventricle (Right z= −0.22, Left z= −0.33) volumes did not demonstrate asymmetry relative to peers.
Figure 2.
Relative to non-PD
MN’s right caudate nucleus volume was markedly smaller and asymmetric (Figure 2; Right: z= −4.02; Left: z= −0.94). Also smaller relative to peers was MN’s right putamen (Right: z= −3.31; Left: z= 0.56). His right globus pallidus was marginally asymmetric (Right z= −2.29; Left: z= 0.00). MN’s thalamic hemispheres were not asymmetric or smaller than peers’ (Right: z= −0.20; Left: z= 0.33). Hippocampus (right z= −0.97, left z= −1.20) and lateral ventricle (right z= 0.07, left z= −0.07) volumes were also similar across hemispheres, relative to peers.
MN’s right versus left cortical thickness
The right frontal cortex (2.24 mm) was 6.67% thinner than left frontal cortex (2.40 mm). No L-PD or non-PD peer had frontal lobe thickness as asymmetric (Figure 1). In comparison, parietal thickness was similar across hemispheres (right: 2.19 mm; left: 2.21 mm).
Relative to L-PD
MN’s right frontal lobe cortex was thinner than the left (Figure 2; MN Right z= −1.67; Left z= −0.14) but parietal lobes were relatively symmetric (Right: z= −0.48; Left: z= −0.09).
Relative to non-PD
MN’s right frontal lobe cortex was thinner than the left (Figure 2; MN Right z= −1.60; Left z= −0.05) with parietal lobes less asymmetric (Right: z= −0.51; Left: z= −0.18).
MN’s baseline frontal white matter
MN had right < left prefrontal FA values but both values were within normal limits (e.g., within 2 standard deviations). 15% of his L-PD peers and 10% of his non-PD peers had similar or greater right/left prefrontal FA asymmetry (Figure 1).
Relative to L-PD
L-PD participants had a mean left prefrontal FA value = 0.34±0.02 and a mean right prefrontal FA value = 0.34±0.02. Relatively, MN’s right frontal white matter FA was within 0.5 standard deviations of his left (Figure 2; Right: FA z score = 0.03; Left: FA z score = 0.44).
Relative to non-PD
Non-PD participants had a mean left prefrontal FA value = 0.33±0.02 and a mean right prefrontal FA value = 0.34±0.02. Relatively, MN’s right frontal white matter FA was within one standard deviation of his left (Figure 2; Right: FA z score = −0.14, Left: FA z score = 0.71).
MN’s Longitudinal MRI Changes
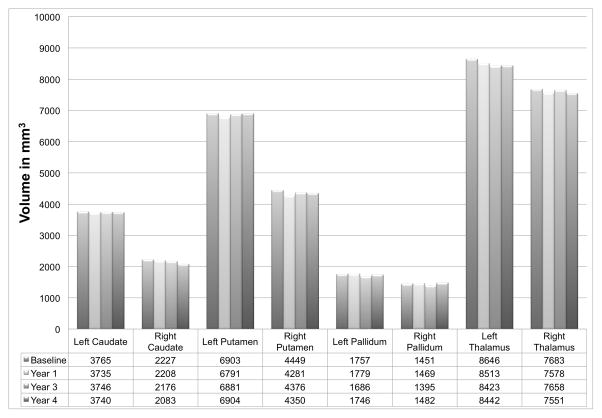
Based on the findings above, we assessed only subcortical structures and frontal thickness for comparison of change over time. MN’s left caudate nucleus volume decreased an average of 0.17% per year. The right caudate nucleus volume decreased an average of 1.61% per year. Other subcortical structures decreased in volume at a rate of less than 0.59% per year (Figure 3). MN’s lateral ventricles increased in volume (left: 0.58% per year; right: 1.22% per year). His right hippocampus volume decreased 0.40% per year and left hippocampus increased an average of 0.41% per year. Right frontal thickness decreased 0.24% per year and left frontal thickness decreased 0.19% per year. Right prefrontal FA decreased 0.76% per year and left prefrontal FA decreased 1.60% per year.
Figure 3.
MN’s Baseline Cognitive Performance in Comparison to L-PD and Non-PD Peers
At baseline, verbal processing speed (Stroop Word Reading) and motor functioning (Finger Tapping) were low average, visual working memory (Spatial Span Backward), verbal memory (Logical Memory II) and abstract reasoning (Matrix Reasoning, Tower achievement, WCST errors) were high average, and visual memory (Visual Reproductions II) was superior. His other neurocognitive functioning scored average and comparable to his PD and non-PD peers.
MN’s Reliable Change from Baseline to Year 1
After controlling for practice effects and regression to the mean with peer groups, MN’s one-year visual memory and abstract reasoning reliably improved relative to his L-PD peers. Only his visuospatial and language composites scored more than one standard deviation lower relative to L-PD peers but this was not significant, however, with a reliable change cut-point of 1.645. His cognition remained stable in all other domains.
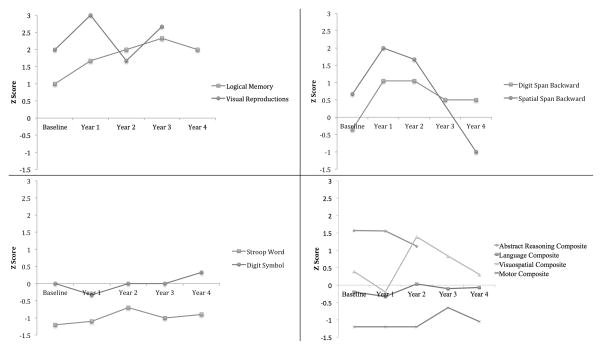
MN’s Neuropsychological Profile Across 5 Years
MN’s visuospatial working memory (Spatial Span backwards) was high average at baseline, improved to superior performance at the second and third year visits, and then declined to low average at year 4. Other domains were stable over time.
Discussion
At baseline, MN was a cognitively intact individual diagnosed with left onset idiopathic PD who was observed to have asymmetric subcortical volumes. Specifically, MN’s right striatum (caudate more than putamen) were reduced relative to his left. That this is highly abnormal was particularly evident when using normative neuroimaging based on L-PD and non-PD peers. Similarly extensive striatal asymmetry was not found in matched peer groups. MN’s prefrontal white matter FA was within normal limits but his right prefrontal FA was almost one standard deviation lower than that of his left. A left and right difference that large existed in 15% of L-PD and 10% of non-PD peers, which suggests that MN’s frontal white matter FA asymmetry is not unique. Cortically, MN had moderate and unique frontal lobe asymmetry despite having cortical thickness similar to peers. Ventricle and hippocampal regions were symmetric.
Overall, baseline neuroimaging shows a noteworthy combination of dominant striatal asymmetry, mild frontal white matter asymmetry, and moderate frontal cortex asymmetry. Over four years of measurement, the gray matter atrophy of MN’s right caudate nucleus (1.62% in volume per year) was prominent relative to his other gray matter atrophy. His right lateral ventricular volume paralleled this change. By contrast, his left caudate nucleus and ventricle were remarkably stable in volume (left caudate nucleus showed 0.17% decrease in volume per year). Although there was atrophy in other subcortical and cortical regions only the caudate nucleus had asymmetric atrophy. Additionally, prefrontal FA in both hemispheres decreased over time but with the left hemisphere decreasing faster than right (i.e., decreasing asymmetry of values). The cause of decreased left prefrontal FA longitudinally is unknown and might be independent from PD-related degeneration. Further, a change in FA only indicates aspects of orientation of diffusion within tissue are different over time. Decreased FA does not necessarily imply reduced white matter integrity or loss of axons (Jones, Knösche, & Turner, 2013) and increases in FA have been shown in the brains of individuals with neurodegenerative disease (e.g., Douaud et al., 2011), which might explain the accelerated decrease in left prefrontal FA relative to right prefrontal FA. Left side white matter changes, however, might also result from right hemisphere gray matter atrophy, given the extensive bilateral connectivity of caudate nuclei (Robinson et al., 2012). For all of these reasons, the cause and implications of decreased frontal FA in MN are not clear. Thus, the right caudate nucleus is likely the focal point of MN’s atrophy in the context of broader bilateral gray and white matter changes that could be independent from MN’s accelerated right caudate atrophy.
Cognitively, there was evidence of lateralized strengths/weaknesses. He displayed superior verbal and visual memory across all visits with at least average verbal working memory and stable language functions. His abstract reasoning abilities were also high average to superior and relatively stable over time. Relative to L-PD peers, MN appeared to have improved abstract reasoning at year one but this is largely because his abilities remained relatively stable instead of declining slightly. By contrast, MN showed yearly variability in visuospatial and visual working memory coupled with consistent reduction in psychomotor and finger tapping motor speed. Visuospatial abilities and visuospatial working memory fluctuated between high average, superior, and finally low average performance in the fourth year. Processing speed and motor speed routinely scored low average to average. Left side motor speed was consistently reduced relative to right side. MN’s overall weakness in psychomotor and finger tapping speed is consistent with PD. It is possible that his relative stability of motor speed over time is due a combination of increased dopaminergic medications over the course of the study and relative stability in volumes of subcortical structures more involved in motor abilities than the caudate nucleus is (e.g., putamen, thalamus). His visuospatial variability and possible fourth year relative deficit can also be consistent with PD. It is likely that MN’s shrinking right caudate nucleus played a role in those cognitive changes.
Briefly, functions involving processing speed and working memory are most commonly associated with the frontal cortex (Stuss & Alexander, 2000), subcortical nuclei (e.g., caudate, thalamus), and associated frontal-subcortical and cortical-cortical white matter connections (Alexander, DeLong, & Strick, 1986). General disruption within the frontostriatal circuits is a primary contributor to the cognitive profile of PD and specifically processing speed and working memory deficits of PD (Zgaljardic et al., 2006). Elegant animal and human lesion research implicates the prefrontal cortex, specifically the dorsolateral prefrontal cortex (DLPFC), as the primary site of working memory (Foster, Eskes, & Stuss, 1994; Goldman-Rakic, 1987; Petrides, 1994), which is known to be impaired in PD. Research demonstrating normal prefrontal function as evaluated by probing for working memory deficits are also highly dependent on the aminergic neurotransmitters known to be deregulated in PD, specifically dopamine (Williams & Goldman-Rakic, 1995; Zahrt, Taylor, Mathew, & Arnsten, 1997).
MN’s working memory disruption is largely visuospatial as measured by the spatial span test. He had possible secondary visual deficits in the fourth year for the Benton Facial Recognition Test, although his scores varied up and down over time (e.g., Year 1 z = −1.0, Year 3 z = 0.59); the relatively weaker and more variable performance might be related to caudate atrophy and underlying lateralized Parkinson’s disease. It is also possible that some of the observed variability reflects test-retest reliability, potential medication status, and regression to the mean. Performing a spatial span task typically results in activation of bilateral dorsolateral prefrontal cortex (D'Esposito, Postle, & Rypma, 2000) but with the right hemisphere thought to be more involved (Bor, Duncan, Lee, Parr, & Owen, 2006). Although facial recognition involves mid-fusiform gyrus (Gauthier, Tarr, Anderson, Skudlarski, & Gore, 1999) and inferior occipital regions (Hadjikhani & de Gelder, 2002), striatal dopamine is involved in visuospatial attention tasks (Tomasi et al., 2009), with deficits impacting frontostriatal and frontoparietal networks. Taken together, MN’s relative weaknesses on visuospatial working memory could be explained primarily by dopaminergic deficits, right-predominant striatal atrophy, and striatofrontal network deficits; such deficits are common in idiopathic PD and are not necessarily unique to MN.
We speculate the right dorsal caudate nucleus head may be most implicated in MN’s weaknesses. Dorsal anterior caudate nucleus regions connect prefrontally with ventral caudate nucleus connecting to orbitofrontal regions (Lehericy et al., 2004). The head of the caudate nucleus connects primarily with frontal regions, while the body and tail connect mainly but not exclusively to posterior and temporal brain regions (e.g., the tail of the caudate nucleus connects to ventrolateral prefrontal cortex; Kehagia, Barker, & Robbins, 2013; Robinson et al., 2012). Dopamine loss in particular appears to affect rostral and dorsal portions of the caudate nucleus initially with ventral and caudal loss occurring as PD progresses (Kish, Shannak, & Hornykiewicz, 1988). The structural neuroimaging results from this case report (striatum volume loss greater than cortical thinning) tentatively support the concept of subcortical to cortical progression of changes associated with PD (Bohnen et al., 2015). At baseline MN had considerable basal ganglia volume asymmetry. However, while MN’s right caudate nucleus atrophied at a rapid rate his left caudate nucleus remained stable in volume, which we speculate is partially compensatory for his declining right caudate nucleus volume. We also speculate that if MN has contralateral volumetric compensation it might explain his relatively stable cognition over time. It is also possible that the accelerated atrophy is not clinically meaningful because of his slower atrophy of other brain regions.
Although controversial, side of disease onset in PD has been associated with different motor, cognitive, and behavioral deficits (Foster, Yung, Drago, Crucian, & Heilman, 2013; Katzen, Levin, & Weiner, 2006; Tomer, Levin, & Weiner, 1993). Laterality of motor symptoms during early stages of the disease have related to asymmetric dopamine depletion in the substantia nigra (Kempster et al., 1989), asymmetric dysfunction of cortico-striatal circuitry (Middleton & Strick, 2000a), and might be associated with specific cognitive deficits (Middleton & Strick, 2000b). Patients with right-sided symptoms (left brain) perform poorly on verbal tasks while patients with left-sided symptoms (right brain) show reduced performance on visuospatial tasks (Bentin et al., 1981; Blonder, Gur, Gur, Saykin, & Hurtig, 1989; Cooper et al., 2009; Spicer, Roberts, & LeWitt, 1988).
By contrast, other studies have found either no association with lateralization of motor deficits and cognition (Riklan, Stellar, & Reynolds, 1990) or that individuals with right-sided symptoms show sparing of performances across cognitive tasks (Direnfeld et al., 1984) and those with left sided symptoms show more widespread cognitive deficits (Tomer et al., 1993). Discrepancies across studies might relate to methodological differences, sample size, and disease severity of participants. Additionally, motor lateralization might not consistently affect cognition due to the heterogeneity of the disease in clinical presentation and variability of cognitive decline across individuals (Cubo et al., 2010). The relationship between lateralization of motor symptoms and cognition is complex and can be influenced by the type of dominant motor symptom. For example, Katzen and colleagues found that individuals who have initial bradykinesia or rigidity show cognitive deficits regardless of laterality of symptoms, individuals with left side tremor onset show verbal learning and memory deficits, and individuals with right-sided tremor onset remain cognitively intact (Katzen et al., 2006).
MN’s overall profile supports a case of either idiopathic left side onset PD, albeit at the end of the normative spectrum, or left onset non-PD parkinsonism. A diagnosis with idiopathic PD is supported by his response to PD-related medications, increased dosage of PD-related medications over time, slowed verbal and motor speed and the results of a recent (2015) clinical single-photon emission computed tomography (SPECT) with ioflupane iodine-123 (DaTscan), which showed significant reduction in right basal ganglia uptake and reduced left putamen uptake. The results of this scan match our asymmetry findings (right<left). It should be noted, however, that a DaTscan does not allow for differentiation between parkinsonism syndromes, implying that diagnoses other than idiopathic PD are possible. Support for a diagnosis of left onset non-PD parkinsonism includes minimal apparent disease progression and the extreme asymmetry of striatal gray matter volumes, which is not typical of left onset idiopathic PD.
While MN had stable UPDRS motor symptoms over time, this can partially be explained by an increased dosage of PD-related medication. Further, Goetz and colleagues (2000) reported general stability of parkinsonism symptoms in levodopa-treated individuals with H&Y stage 2 PD over a four year span. Therefore, MN’s symptom stability over five years is not unexpected at his stage of the disease. Additionally, individuals with left-side onset PD have slower progression of motor symptoms than individuals with right side onset PD do (Baumann et al., 2014). This suggests that MN clinically fits a diagnosis of idiopathic PD but with a unique manifestation of subcortical gray matter and cortical thickness asymmetry. His specific accelerated right caudate nucleus atrophy is also likely unique. What remains unclear is the cause of the striatal volume asymmetry and caudate nucleus atrophy.
Some may consider MN a potential for hemiparkinsonism-hemiatrophy (HPHA) diagnosis. This is a rare form of parkinsonism with an early age of onset where the brain and body contralateral to affected motor side exhibit considerable restriction in size (or atrophy) relative to the other (Dziadkiewicz, Białecka, Janik, & Sławek, 2013; Jankovic, 1988; Klawans, 1981). Body asymmetry typically manifests in childhood (hands first then feet and face; Tessitore et al., 2010). From our assessment, MN does not have physical asymmetry outside the brain suggestive of HPHA and had an age of symptom onset not typical for HPHA, making that diagnosis unlikely.
MN was cognitively stable over the four years of our study but his rapidly shrinking right caudate nucleus, if it results in frontal cortex atrophy, places him at an increased risk for future cognitive decline (Bohnen et al., 2015). Longitudinal neuroimaging coupled with cognitive measurement among all individuals with PD could address questions regarding how degradation of specific subcortical gray, white, and cortical regions relates to cognitive change over time (Kehagia et al., 2013) and at which point atrophy becomes clinically meaningful. Thus, our primary study limitation is our inability to longitudinally model brain gray and white matter anatomy for all participants. Larger studies addressing side of PD symptom onset, brain structure asymmetry over time, and longitudinal cognition are needed.
A related limitation is unknown rates of caudate nucleus atrophy over time in our L-PD and non-PD groups. We can partially mitigate this limitation by comparison to other published data. Typical rates of brain atrophy in older adults without PD range from 0.5% volume loss per year to >4%, depending on the brain region (Rusinek et al., 2003; Jiang et al., 2014). Jiang et al. (2014) reported atrophy of the left and right caudate nuclei of ≤0.13% per year in a community-dwelling longitudinal older adult sample (n≥335). The left putamen, in contrast, atrophied an average of 3.97% per year and the right putamen at 2.74% per year. Compared to those data, MN’s left caudate nucleus atrophied at an expected rate but he had accelerated right caudate nucleus atrophy. Specific subcortical structure atrophy rates in PD are not well described but Tessa and colleagues (2014) did not find different rates of atrophy between newly diagnosed individuals with PD and a control group. There is a need for longitudinal in vivo MRI research to present clear rates of atrophy and clinical relevance by structure in PD. Compared to previous research demonstrating greater contralateral subcortical morphometric changes in PD (Sterling et al., 2013; Caligiuri et al., 2016), MN’s baseline brain structure asymmetry and accelerated atrophy of the right caudate nucleus are highly unusual.
A second limitation is our lack of more sophisticated white matter diffusion metrics (e.g., Tanner et al., 2015). Due to concerns about variability in diffusion fiber tracking results between the caudate and frontal lobe (Kuhn et al., 2015), we chose to use fractional anisotropy, which is a well-established but simplistic metric (Jones, Knösche, & Turner, 2013). In spite of the limitations of FA as a metric, particularly within regions with numerous crossing fibers, FA is useful for investigating basic white versus gray matter structural contributions to cognition in PD (e.g., Price et al., 2016). Interpreting what might cause FA changes or what the effects of such changes might be, however, in one individual are difficult.
A third limitation is our lack of off-medication UPDRS motor scores. Off drug metrics may provide more insight into MN’s progressive motor deficits. Due to study limitations and ethical considerations to wanting his best performance during tests (which mimics typical testing environments conducted within neuropsychological clinics), we did not assess brain structure or cognition during off-drug periods.
A fourth limitation is related to the questions that arise from the presence of MN’s small cerebral capillary telangiectasia (CCT) near the right caudate nucleus. CCTs are intracerebral vascular malformations that are generally stable in size over time. They are difficult to resolve without contrasted MRI (which were not acquired for this study) so precise localization and size measurement were not possible. Little is known about the effects of CCTs but >94% of them are asymptomatic (Gross, Puri, Popp, & Du, 2013). Small ones (<1cm) have no known clinical symptoms and large ones (>1cm) result in clinical symptoms in fewer than 30% of cases (Sayama, Osborn, Chin, & Couldwell, 2010).
Many diffuse CCTs can result in ischemia and atrophy (Tang, Jeng, Liu, & Yip, 2003) but it is unknown if MN’s single small CCT contributes to the volume reduction of the caudate nucleus through chronic mild hypoperfusion. It is also unclear if MN’s CCT is related with his small baseline but relatively stable right putamen and globus pallidus volumes or if both are related with an ‘upstream’ vascular issue (e.g., chronic mild hypoperfusion through the right internal carotid artery). As a result of these unanswered questions, we encourage careful volumetric analyses of brain regions in individuals with CCTs, particularly when they occur in the basal ganglia.
In conclusion, we integrated normative-based neuroimaging and normative-based cognition to present a case of an individual diagnosed with idiopathic PD who had conspicuous striatal volume asymmetry and moderate frontal cortex thickness asymmetry with suggestions of mild frontal white matter FA asymmetry. MN’s peculiar asymmetry suggests an interaction between subcortical gray matter volume and cortical thickness, possibly through white matter (i.e., transneuronal degeneration driven primarily from subcortical gray matter) or vascular involvement. This asymmetry and atrophy occurs in the context of limited Parkinson’s disease progression and focal visuospatial working memory decline over time. MN’s visuospatial working memory specifically showed a decline at year 4, which decline could be linked to ongoing atrophy of his right caudate nucleus. Questions remain regarding the cause of MN’s brain atrophy but his case represents a unique manifestation of subcortical gray matter and cortical asymmetry in PD.
Supplementary Material
Figure 4.
Table 1.
Baseline standardized z scores for neuropsychological scores for MN, peers with left onset Parkinson’s disease (n = 15), and peers without Parkinson’s disease (n = 43)
| MN | L-PD (N=15) | Non-PD (N=43) | |
|---|---|---|---|
| Verbal Processing Speed (StroopW) | −1.20 | −0.51±0.64 | −0.11±0.61 |
| −1.60/0.70* | −2.10/0.70γ | ||
| Visual Processing Speed (DigitSym) | 0.00 | −0.09±0.75 | 0.50±0.78 |
| −1.33/1.33 | −1.00/2.00ϕ | ||
| Verbal Working Memory (DS_B) | −0.35 | 0.23±0.87 | 0.81±0.93 |
| −1.65/2.20 | −0.35/3.10 | ||
| Visual Working Memory (SS_B) | 0.67 | 0.35±1.04 | 0.78±1.00 |
| −1.33/2.00 | −2.33/2.67 | ||
| Verbal Memory (LM II) | 1.00 | 0.71±0.92 | 1.16±0.69 |
| −0.67/2.00 | −0.33/3.00 | ||
| Visual Memory (VR II) | 2.00 | 1.04±0.92 | 1.22±1.00 |
| −1.00/2.33 | −0.67/3.00 | ||
| Abstract Reasoning Composite | 1.57 | 0.38±0.83 | 0.78±0.50 |
| −0.81/2.01 | −0.36/1.76 | ||
| Visuospatial Composite | 0.39 | 0.15±0.84 | 0.53±0.56 |
| −2.50/0.92 | −0.64/1.59 | ||
| Language Composite | −0.20 | 0.30±0.59 | 0.57±0.68 |
| −1.07/1.17 | −0.63/2.30 | ||
| Motor Composite | −1.20 | −0.54±1.42 | −0.34±0.73 |
| −2.75/3.05 | −1.80/1.50 |
Note: Mean, standard deviation, and minimum/maximum scores are shown.
N=14;
N=41;
N=42;
StroopW = Stroop Word; DigitSym = Digit Symbol Coding; DS_B= Digit Span Backward Span; SS_B = WMS III Spatial Span Backward; LM II = Logical Memory II; VR II = Visual Reproductions II; Abstract Reasoning Composite = Matrix Reasoning, Wisconsin Card Sorting Test total errors, D-KEFS Tower total achievement; Visuospatial Composite = Judgment of Line Orientation, Benton Facial Recognition Test; Language Composite = Controlled Oral Word Association Test (FAS), Category Fluency (Animals), Boston Naming Test; Motor Composite = Finger Tapping (left and right).
Table 2.
MN’s reliable change z scores relative to peers with left side onset Parkinson’s disease (N=14) and peers without Parkinson’s disease (N=40): Baseline to year 1
| Related to L-PD Peers (N = 15) | Related to Non-PD Peers (N = 43) | |
|---|---|---|
| Verbal Processing Speed (StroopW) | 0.25 | −0.04 |
| Visual Processing Speed (DigitSym) | −0.21 | −1.20 |
| Verbal Working Memory (DS_B) | 0.86 | 0.46 |
| Visual Working Memory (SS_B) | 0.78 | 0.50 |
| Verbal Memory (LM II) | 0.13 | 0.75 |
| Visual Memory (VR II) | 2.19* | 1.45 |
| Abstract Reasoning Composite | 2.18* | 0.70 |
| Visuospatial Composite | −1.01 | −1.21 |
| Language Composite | −1.45 | −0.90 |
| Motor Composite | 0.35 | −0.69 |
Note: Scores were calculated based on regression reliable change methods (Martin et al., 2002).
Indicates values that are ‘reliably changed’ (only values ≥|1.645|).
StroopW = Stroop Word; DigitSym = Digit Symbol Coding; DS_B= Digit Span Backward Span; SS_B = WMS III Spatial Span Backward; LM II = Logical Memory II; VR II = Visual Reproductions II; Visuospatial Composite = Judgment of Line Orientation, Benton Facial Recognition Test; Language Composite = Controlled Oral Word Association Test (FAS), Category Fluency (Animals), Boston Naming Test; Motor Composite = Finger Tapping (left and right)
Table 3.
Neuropsychological composite and individual test scores for MN across all visits
| Baseline | Year 1 | Year 2 | Year 3 | Year 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Z | %ile | Z | %ile | Z | %ile | Z | %ile | Z | %ile | |
| Processing Speed | ||||||||||
| Stroop W | −1.20 | 12th | −1.10 | 14th | −0.70 | 25th | −1.00 | 16th | −0.90 | 19th |
| DigitSym | 0.00 | 50th | −0.33 | 38th | 0.00 | 50th | 0.00 | 50th | 0.33 | 62nd |
| Working Memory | ||||||||||
| DS_B | −0.35 | 37th | 1.05 | 86th | 1.05 | 86th | 0.50 | 70th | 0.50 | 70th |
| SS_B | 0.67 | 76th | 2.00 | 98th | 1.67 | 95th | * | * | −1.00 | 16th |
| Memory | ||||||||||
| LM II | 1.00 | 84th | 1.67 | 95th | 2.00 | 98th | 2.33 | 99th | 2.00 | 98th |
| VR II | 2.00 | 98th | 3.00 | 99th | 1.67 | 95th | 2.67 | 99th | * | * |
| Abstract Reasoning | 1.57 | 94th | 1.56 | 94th | 1.12 | 87th | * | * | * | * |
| MR | 1.70 | 96th | 2.30 | 99th | 1.70 | 96th | 2.10 | 98th | 1.50 | 94th |
| Tower | 2.00 | 98th | 1.67 | 95th | 0.67 | 75th | 3.00 | 99th | 2.33 | 99th |
| WCST | 1.00 | 84th | 0.70 | 77th | 1.00 | 84th | * | * | * | * |
| Visuospatial | .39 | 66th | −.20 | 42nd | 1.39 | 92nd | .84 | 81st | .31 | 61st |
| JOLO | .19 | 58th | .60 | 73rd | 1.08 | 86th | 1.08 | 86th | 1.08 | 86th |
| FACES | .59 | 73rd | −1.00 | 16th | 1.70 | 96th | .59 | 73rd | −.46 | 32nd |
| Language | −.20 | 42nd | −.33 | 38th | .03 | 52nd | −.10 | 45th | −.07 | 46th |
| FAS | −1.10 | 14th | −1.10 | 14th | −.40 | 34th | −.70 | 25th | −1.40 | 8th |
| Animals | .60 | 73rd | .60 | 73rd | .60 | 73rd | −.50 | 32nd | −.50 | 32nd |
| BNT | −.10 | 45th | −.50 | 32nd | −.10 | 45th | .90 | 82nd | 1.30 | 91st |
| Motor | −1.20 | 12th | −1.20 | 12th | −1.20 | 12th | −.65 | 25th | −1.05 | 14th |
| FT_Dom | −.40 | 34th | −.40 | 34th | −.40 | 34th | .20 | 58th | −.20 | 42nd |
| FT_nonDom | −2.00 | 2nd | −2.00 | 2nd | −2.00 | 2nd | −1.50 | 7th | −1.90 | 3rd |
Missing data due to abbreviated protocol;
Stroop W = Stroop Word Reading; DigitSym = Digit Symbol Coding; Trails A = Trail Making Test Part A; DS_B = Digit Span Backward Span; LNSEQ = Letter Number Sequencing; SS_B = Spatial Span Backward; LM II = Logical Memory II; VR II = Visual Reproductions II; VR Recog. = Visual Reproductions Recognition; MR = WAIS-III Matrix Reasoning; Tower = D-KEFS Tower Test; WCST = Wisconsin Card Sorting Test; JOLO = Judgment of Line Orientation; FACES = Benton Facial Recognition Test; FAS = Controlled Oral Word Association Test; Animals = Category Fluency; BNT = Boston Naming Test; FT-Dom = Finger Tapping Dominant Hand; FT-nonDom = Finger Tapping Non-dominant
Acknowledgments
This work was supported by the National Institute for Neurological Disorders and Stroke (C.C.P; K23NS060660, R01NS082386) and in part by the Center for Movement Disorders and Neurorestoration, as well as the National Institutes of Health/National Center for Advancing Translational Sciences (NIH/NCATS) and Clinical and Translational Science Award to the University of Florida UL1TR000064. We are most grateful for the participants and their time. We are also grateful to the editors and reviewers who dedicated time to help us improve the analyses and paper. Portions of these data were presented at the 39th annual meeting of the International Neuropsychological Society. The authors report no conflicts of interest.
References
- Alexander G, DeLong M, Strick P. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9(1):357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amick MM, Grace J, Chou KL. Body side of motor symptom onset in Parkinson’s disease is associated with memory performance. Journal of the International Neuropsychological Society. 2006;12(5):736–740. doi: 10.1017/S1355617706060875. [DOI] [PubMed] [Google Scholar]
- Baumann CR, Held U, Valko PO, Wienecke M, Waldvogel D. Body side and predominant motor features at the onset of Parkinson's disease are linked to motor and nonmotor progression. Movement Disorders. 2014;29(2):207–213. doi: 10.1002/mds.25650. [DOI] [PubMed] [Google Scholar]
- Bentin S, Silverberg R, Gordon HW. Asymmetrical cognitive deterioration in demented and Parkinson patients. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 1981 doi: 10.1016/s0010-9452(81)80060-3. http://doi.org/10.1016/S0010-9452(81)80060-3. [DOI] [PubMed]
- Benton AL, Sivan AB, Hamsher K, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. 2. New York: Oxford University Press; 1994. [Google Scholar]
- Blonder LX, Gur RE, Gur RC, Saykin AJ, Hurtig HI. Neuropsychological functioning in hemiparkinsonism. Brain and Cognition. 1989;9(2):244–257. doi: 10.1016/0278-2626(89)90034-1. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL, Müller MLTM, Petrou M, Kotagal V, Koeppe RA, … Frey KA. Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson disease and evidence of interaction effects. JAMA Neurology. 2015;72(2):194. doi: 10.1001/jamaneurol.2014.2757. http://doi.org/10.1001/jamaneurol.2014.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor D, Duncan J, Lee ACH, Parr A, Owen AM. Frontal lobe involvement in spatial span: Converging studies of normal and impaired function. Neuropsychologia. 2006;44(2):229–237. doi: 10.1016/j.neuropsychologia.2005.05.010. http://doi.org/10.1016/j.neuropsychologia.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell and Tissue Research. 2004;318(1):121–134. doi: 10.1007/s00441-004-0956-9. http://doi.org/10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Briggs GG, Nebes RD. Patterns of hand preference in a student population. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 1975;11(3):230–238. doi: 10.1016/s0010-9452(75)80005-0. [DOI] [PubMed] [Google Scholar]
- Caligiuri ME, Nisticò R, Arabia G, Morelli M. Alterations of putaminal shape in de novo Parkinson's disease. Movement Disorders. 2016;31(5):676–683. doi: 10.1002/mds.26550. http://doi.org/10.1002/mds.26550. [DOI] [PubMed] [Google Scholar]
- Cook SE, Marsiske M, McCoy KJM. The Use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the Detection of Amnestic Mild Cognitive Impairment. Journal of Geriatric Psychiatry and Neurology. 2009;22(2):103–109. doi: 10.1177/0891988708328214. http://doi.org/10.1177/0891988708328214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CA, Mikos AE, Wood MF, Kirsch-Darrow L, Jacobson CE, Okun MS, … Fernandez HH. Parkinsonism and related disorders. Parkinsonism & Related Disorders. 2009;15(4):315–317. doi: 10.1016/j.parkreldis.2008.07.009. http://doi.org/10.1016/j.parkreldis.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Cubo E, Martín PM, Martin-Gonzalez JA, Rodríguez-Blázquez C, Kulisevsky J ELEP Group Members. Motor laterality asymmetry and nonmotor symptoms in Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society. 2010;25(1):70–75. doi: 10.1002/mds.22896. http://doi.org/10.1002/mds.22896. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system: Technical manual. Psychological Corporation; 2001. [Google Scholar]
- D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Experimental brain research. 2000;133(1):3–11. doi: 10.1007/s002210000395. http://doi.org/10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Direnfeld LK, Albert ML, Volicer L. Parkinson's disease: The possible relationship of laterality to dementia and neurochemical findings. Archives of Neurology. 1984;41(9):935–941. doi: 10.1001/archneur.1984.04050200041016. http://doi.org/10.1001/archneur.1984.04050200041016. [DOI] [PubMed] [Google Scholar]
- Douaud G, Jbabdi S, Behrens TEJ, Menke RA, Gass A, Monsch AU, … Smith S. DTI measures in crossing-fibre areas: Increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer's disease. NeuroImage. 2011;55(3):880–890. doi: 10.1016/j.neuroimage.2010.12.008. http://doi.org/10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziadkiewicz A, Białecka M, Janik P, Sławek J. Hemiparkinsonism-hemiatrophy syndrome – report on two cases and review of the literature. Neurologia I Neurochirurgia Polska. 2013;47(4):387–392. doi: 10.5114/ninp.2013.34557. http://doi.org/10.5114/ninp.2013.34557. [DOI] [PubMed] [Google Scholar]
- Feis DL, Pelzer EA, Timmermann L, Tittgemeyer M. Classification of symptom-side predominance in idiopathic Parkinson’s disease. Npj Parkinson's Disease. 2015;1:15018. doi: 10.1038/npjparkd.2015.18. http://doi.org/10.1038/npjparkd.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. http://doi.org/10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Foster JK, Eskes GA, Stuss DT. The cognitive neuropsychology of attention: A frontal lobe perspective. Cognitive Neuropsychology. 1994;11(2):133–147. http://doi.org/10.1080/02643299408251971. [Google Scholar]
- Foster PS, Yung RC, Drago V, Crucian GP, Heilman KM. Working memory in Parkinson’s disease: The effects of depression and side of onset of motor symptoms. Neuropsychology. 2013;27(3):303–313. doi: 10.1037/a0032265. http://doi.org/10.1037/a0032265. [DOI] [PubMed] [Google Scholar]
- Garg A, Appel-Cresswell S, Popuri K, McKeown MJ, Beg MF. Morphological alterations in the caudate, putamen, pallidum, and thalamus in Parkinson’s disease. Frontiers in Neuroscience. 2015;9:101. doi: 10.3389/fnins.2015.00101. http://doi.org/10.3389/fnins.2015.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2(6):568–573. doi: 10.1038/9224. http://doi.org/10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Geng DY, Li YX, Zee CS. Magnetic resonance imaging-based volumetric analysis of basal ganglia nuclei and substantia nigra in patients with Parkinson’s disease. Neurosurgery. 2006;58(2):256–262. doi: 10.1227/01.NEU.0000194845.19462.7B. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Stebbins GT, Blasucci LM. Differential progression of motor impairment in levodopa-treated Parkinson's disease. Movement Disorders. 2000 doi: 10.1002/1531-8257(200005)15:3<479::AID-MDS1009>3.0.CO;2-P. http://doi.org/10.1002/1531-8257(200005)15:3<479::aid-mds1009>3.0.co;2-p. [DOI] [PubMed]
- Golden CJ, Freshwater SM. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, IL: Stoelting; 2002. [Google Scholar]
- Goldman-Rakic PS. Handbook of Physiology, The Nervous System, Higher Functions of the Brain. Hoboken, NJ, USA: John Wiley & Sons, Inc; 1987. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. http://doi.org/10.1002/cphy.cp010509. [Google Scholar]
- Gross BA, Puri AS, Popp AJ, Du R. Cerebral capillary telangiectasias: A meta-analysis and review of the literature. Neurosurgical review. 2013;36(2):187–194. doi: 10.1007/s10143-012-0435-9. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, de Gelder B. Neural basis of prosopagnosia: An fMRI study. Human Brain Mapping. 2002;16(3):176–182. doi: 10.1002/hbm.10043. http://doi.org/10.1002/hbm.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. A manual for the Wisconsin Card Sorting Test. Western Psycological Services; 1981. [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism onset, progression, and mortality. Neurology. 1967;17(5):427–427. doi: 10.1212/wnl.17.5.427. http://doi.org/10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- Huber SJ, Freidenberg DL, Shuttleworth EC, Paulson GW, Clapp LE. Neuropsychological similarities in lateralized parkinsonism. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 1989 doi: 10.1016/s0010-9452(89)80059-0. http://doi.org/10.1016/S0010-9452(89)80059-0. [DOI] [PubMed]
- Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: A clinicopathologic study. Neurology. 1992;42(6):1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- Iverson GL. Interpreting change on the WAIS-III/WMS-III in clinical samples. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists. 2001;16(2):183–191. http://doi.org/10.1016/S0887-6177(00)00060-3. [PubMed] [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. http://doi.org/10.1037/0022-006X.59.1.12. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Hemiparkinsonism and hemiatrophy. Neurology. 1988;38(11):1815–1816. doi: 10.1212/wnl.38.11.1815. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jiang J, Sachdev P, Lipnicki DM, Zhang H, Liu T, Zhu W, et al. A longitudinal study of brain atrophy over two years in community-dwelling older individuals. NeuroImage. 2014;86(C):203–211. doi: 10.1016/j.neuroimage.2013.08.022. http://doi.org/10.1016/j.neuroimage.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. NeuroImage. 2013;73(C):239–254. doi: 10.1016/j.neuroimage.2012.06.081. http://doi.org/10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintrab S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Katzen HL, Levin BE, Weiner W. Side and type of motor symptom influence cognition in Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society. 2006;21(11):1947–1953. doi: 10.1002/mds.21105. http://doi.org/10.1002/mds.21105. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Cognitive impairment in Parkinson’s disease: The dual syndrome hypothesis. Neurodegenerative Diseases. 2013;11(2):79–92. doi: 10.1159/000341998. http://doi.org/10.1159/000341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempster PA, Gibb WR, Stern GM, Lees AJ. Asymmetry of substantia nigra neuronal loss in Parkinson's disease and its relevance to the mechanism of levodopa related motor fluctuations. Journal of Neurology, Neurosurgery, and Psychiatry. 1989;52(1):72–76. doi: 10.1136/jnnp.52.1.72. http://doi.org/10.1136/jnnp.52.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. New England Journal of Medicine. 1988;318(14):876–880. doi: 10.1056/NEJM198804073181402. http://doi.org/10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Klawans HL. Hemiparkinsonism as a late complication of hemiatrophy: A new syndrome. Neurology. 1981;31(5):625–628. doi: 10.1212/wnl.31.5.625. [DOI] [PubMed] [Google Scholar]
- Kuhn T, Gullett JM, Nguyen P, Boutzoukas AE, Ford A, Colon-Perez LM, … Bauer RM. Test-retest reliability of high angular resolution diffusion imaging acquisition within medial temporal lobe connections assessed via tract based spatial statistics, probabilistic tractography and a novel graph theory metric. Brain Imaging and Behavior. 2015:1–15. doi: 10.1007/s11682-015-9425-1. http://doi.org/10.1007/s11682-015-9425-1. [DOI] [PMC free article] [PubMed]
- Lee HM, Kwon KY, Kim MJ, Jang JW, Suh SI, Koh SB, Kim JH. Subcortical grey matter changes in untreated, early stage Parkinson's disease without dementia. Parkinsonism & Related Disorders. 2014;20(6):622–626. doi: 10.1016/j.parkreldis.2014.03.009. http://doi.org/10.1016/j.parkreldis.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van De Moortele PFO, Francois C, Thivard L, Poupon C, … Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of Neurology. 2004;55(4):522–529. doi: 10.1002/ana.20030. http://doi.org/10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Lewis MM, Smith AB, Styner M, Gu H, Poole R, Zhu H, … Huang X. Asymmetrical lateral ventricular enlargement in Parkinson’s disease. European Journal of Neurology: The Official Journal of the European Federation of Neurological Societies. 2009;16(4):475–481. doi: 10.1111/j.1468-1331.2008.02430.x. http://doi.org/10.1111/j.1468-1331.2008.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Sawrie S, Gilliam F, Mackey M, Faught E, Knowlton R, Kuzniekcy R. Determining reliable cognitive change after epilepsy surgery: Development of reliable change indices and standardized regression-based change norms for the WMS-III and WAIS-III. Epilepsia. 2002;43(12):1551–1558. doi: 10.1046/j.1528-1157.2002.23602.x. http://doi.org/10.1046/j.1528-1157.2002.23602.x. [DOI] [PubMed] [Google Scholar]
- Matteau E, Dupré N, Langlois M, Jean L, Thivierge S, Provencher P, Simard M. Mattis Dementia Rating Scale 2: Screening for MCI and dementia. American Journal of Alzheimer's Disease and Other Dementias. 2011;26(5):389–398. doi: 10.1177/1533317511412046. http://doi.org/10.1177/1533317511412046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeny AJ, Naugle RI, Chelune GJ, Lüders H. “T Scores for Change”: An illustration of a regression approach to depicting change in clinical neuropsychology. The Clinical Neuropsychologist. 1993;7(3):300–312. http://doi.org/10.1080/13854049308401901. [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Research Reviews. 2000a;31(2–3):236–250. doi: 10.1016/s0165-0173(99)00040-5. http://doi.org/10.1016/S0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia output and cognition: Evidence from anatomical, behavioral, and clinical studies. Brain and Cognition. 2000b;42(2):183–200. doi: 10.1006/brcg.1999.1099. http://doi.org/10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Nemmi F, Sabatini U, Rascol O, Péran P. Parkinson's disease and local atrophy in subcortical nuclei: Insight from shape analysis. Neurobiology of Aging. 2015;36(1):424–433. doi: 10.1016/j.neurobiolaging.2014.07.010. http://doi.org/10.1016/j.neurobiolaging.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and behaviour. Current Opinion in Neurobiology. 1994;4(2):207–211. doi: 10.1016/0959-4388(94)90074-4. http://doi.org/10.1016/0959-4388(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Price CC, Favilla C, Tanner JJ, Towler S, Jacobson CE, Hass CJ, … Okun Michael S. Lateral ventricle volume is poor predictor of post unilateral DBS motor change for Parkinson's disease. Parkinsonism & Related Disorders. 2011;17(5):343–347. doi: 10.1016/j.parkreldis.2011.01.018. http://doi.org/10.1016/j.parkreldis.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CC, Tanner J, Nguyen PT, Schwab NA, Mitchell S, Slonena E, et al. Gray and White Matter Contributions to Cognitive Frontostriatal Deficits in Non-Demented Parkinson's Disease. PLoS One. 2016;11(1):e0147332–22. doi: 10.1371/journal.pone.0147332. http://doi.org/10.1371/journal.pone.0147332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Manual for administration of neuropsychological test batteries for adults and children. Neuropsychology Laboratory, Indiana University Medical Center; 1979. [Google Scholar]
- Riklan M, Stellar S, Reynolds C. The relationship of memory and cognition in Parkinson's disease to lateralisation of motor symptoms. Journal of Neurology, Neurosurgery, and Psychiatry. 1990;53(4):359–360. doi: 10.1136/jnnp.53.4.359-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, … Fox PT. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. NeuroImage. 2012;60(1):117–129. doi: 10.1016/j.neuroimage.2011.12.010. http://doi.org/10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinek H, De Santi S, Frid D, Tsui WH, Tarshish CY, Convit A, de Leon MJ. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229(3):691–696. doi: 10.1148/radiol.2293021299. http://doi.org/10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- Sayama CM, Osborn AG, Chin SS, Couldwell WT. Capillary telangiectasias: Clinical, radiographic, and histopathological features: Clinical article. Journal of neurosurgery. 2010;113(4):709–714. doi: 10.3171/2009.9.JNS09282. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Amick MM, Cronin-Golomb A. Role of a lateralized parietal-basal ganglia circuit in hierarchical pattern perception: Evidence from Parkinson’s disease. Behavioral Neuroscience. 2009;123(1):125–136. doi: 10.1037/a0013734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, … Fitney DE. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spicer KB, Roberts RJ, LeWitt PA. Neuropsychological performance in lateralized parkinsonism. Archives of Neurology. 1988;45(4):429–432. doi: 10.1001/archneur.1988.00520280079019. [DOI] [PubMed] [Google Scholar]
- Sterling NW, Du G, Lewis MM, Dimaio C, Kong L, Eslinger PJ, … Huang X. Striatal shape in Parkinson's disease. Neurobiology of Aging. 2013;34(11):2510–2516. doi: 10.1016/j.neurobiolaging.2013.05.017. http://doi.org/10.1016/j.neurobiolaging.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York: Oxford University Press; 2006. [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: A conceptual view. Psychological Research. 2000;63(3–4):289–298. doi: 10.1007/s004269900007. http://doi.org/10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Tang SC, Jeng JS, Liu HM, Yip PK. Diffuse capillary telangiectasia of the brain manifested as a slowly progressive course. Cerebrovascular Diseases. 2003;15(1–2):140–142. doi: 10.1159/000067136. [DOI] [PubMed] [Google Scholar]
- Tanner JJ, Mareci TH, Okun MS, Bowers D, Libon DJ, Price CC. Temporal lobe and frontal-subcortical dissociations in non-demented Parkinson’s disease with verbal memory impairment. PLoS One. 2015;10(7):e0133792. doi: 10.1371/journal.pone.0133792. http://doi.org/10.1371/journal.pone.0133792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessa C, Lucetti C, Giannelli M, Diciotti S, Poletti M, Danti S, … Toschi N. Progression of brain atrophy in the early stages of Parkinson's disease: A longitudinal tensor-based morphometry study in de novo patients without cognitive impairment. Human Brain Mapping. 2014;35(8):3932–3944. doi: 10.1002/hbm.22449. http://doi.org/10.1002/hbm.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Russo A, Cirillo M, Giordano A, Marcuccio L, Tedeschi G. Hemiparkinsonism and hemiatrophy syndrome: A rare observation. Clinical Neurology and Neurosurgery. 2010;112(6):524–526. doi: 10.1016/j.clineuro.2010.03.016. http://doi.org/10.1016/j.clineuro.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang R, Telang F, Wang GJ, Chang L, … Fowler JS. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One. 2009;4(6):e6102. doi: 10.1371/journal.pone.0006102. http://doi.org/10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Levin BE, Weiner WJ. Side of onset of motor symptoms influences cognition in Parkinson's disease. Annals of Neurology. 1993;34(4):579–584. doi: 10.1002/ana.410340412. http://doi.org/10.1002/ana.410340412. [DOI] [PubMed] [Google Scholar]
- Verreyt N, Nys GMS, Santens P, Vingerhoets G. Cognitive differences between patients with left-sided and right-sided Parkinson’s disease: A review. Neuropsychology Review. 2011;21(4):405–424. doi: 10.1007/s11065-011-9182-x. http://doi.org/10.1007/s11065-011-9182-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) San Antonio, TX: Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. San Antonio, TX: The Psychological Corporation; 1997b. (WMS-III) [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376(6541):572–575. doi: 10.1038/376572a0. http://doi.org/10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. http://doi.org/10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. Journal of Neuroscience. 1997;17(21):8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ, Gordon MF, Feigin A, Eidelberg D. An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson's disease. Journal of Clinical and Experimental Neuropsychology. 2006;28(7):1127–1144. doi: 10.1080/13803390500246910. http://doi.org/10.1080/13803390500246910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.