Abstract
Purpose
Assessment of pathological fracture risk is critical to optimize the use of prophylactic orthopedic fixation to prevent pathological fractures. Better prediction of pathological fracture risk is needed. We evaluated if quantitative measures of FDG-avidity can assess femoral pathological fracture risk in patients with metastatic breast cancer (MBC).
Methods
A HIPAA-compliant retrospective case-control study was performed under IRB waiver. Patients with MBC who received an FDG PET/CT from 1/2008–12/2014 and had pathological fracture of the proximal femur within three months of PET/CT were selected as cases. Patients with MBC who had an FDG PET/CT in 2013 were sequentially screened in reverse chronological order to identify patients with proximal femoral metastases on PET/CT but no subsequent pathological fracture to serve as a control group. The pre-specified goal was to have twice the number of controls as cases. Target lesions in the proximal femur, from femoral head to 5 cm below the lesser trochanter, were analyzed on FDG PET/CT for SUVmax, SUVmean, metabolic tumor volume (MTV), and total lesion glycolysis (TLG). Wilcoxon rank-sum test was used to compare continuous variables in cases and controls. A nonparametric receiver operating characteristic (ROC) analysis was performed to assess the ability of quantitative FDG measurements to differentiate between cases and controls.
Results
In 27 cases with pathological fracture and 55 controls without pathological fracture, all four quantitative measures of FDG-avidity were statistically different between cases and controls (p <.001). A TLG of 81 could differentiate between fracture and non-fracture patients with accuracy, sensitivity, and specificity of 0.83, 0.85, and 0.80, respectively.
Conclusion
Quantitative measures of FDG avidity may help identify breast cancer patients at high risk of subsequent pathological fracture of the proximal femur.
INTRODUCTION
Bone is the most common site of distant metastasis from breast cancer, as well as many other malignancies [1–4]. One of the most debilitating sites of osseous metastasis is the proximal femur, which may result in pain, functional limitations, and pathological fractures. Orthopedic fixation of proximal femoral osseous metastases palliates the disease, substantially improving the patient’s quality of life [4, 5]. Prophylactic fixation of proximal femoral lesions, before pathologic fracture, reduces surgical complications, improves quality of life, and even lengthens patient survival [5, 6]. Thus, accurate assessment of pathological fracture risk is critical to optimize the use of prophylactic orthopedic fixation, prevent morbidity, and improve patient outcomes.
The assessment of pathological fracture risk is a difficult and imperfect task. Pathological fracture risk is often estimated using Mirel’s scoring [7], which combines the use of radiographs to grade size, location, and radiopacity of metastases, as well as the patient’s level of pain, to determine a composite score up to 12. Mirel’s scores of 9 or above suggested high fracture risk in initial reports [7], however subsequent evaluations found that the specificity of Mirel’s scoring is only 35–50% [8–10]. Accordingly, better prediction of pathological fracture risk is needed.
18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) allows for evaluation of the level of metabolic activity of osseous metastases [11–14], which is known to be important for prognosis of patients with metastatic breast cancer [13, 14]. The purpose of our study was to determine if quantitative measures of FDG-avidity can assess the risk of femoral pathological fractures in patients with metastatic breast cancer.
MATERIALS AND METHODS
Patients
This retrospective single-institution study was performed in compliance with the Health Insurance Portability and Accountability Act (HIPAA) and was approved by the Institutional Review Board. The requirement to obtain informed consent was waived by the board.
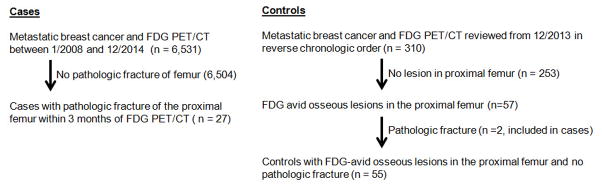
A Standards for Reporting of Diagnostic Accuracy flow chart of the methods used to select cases and controls is provided in Figure 1. The Memorial Sloan Kettering Cancer Center Health Information System (HIS) was searched for patients with metastatic breast cancer, FDG PET/CT from 1/2008 and 12/2014, and pathological fracture of the proximal femur (femoral head to 5 cm below the lesser trochanter) within three months of PET/CT. We used the standard definition of the proximal femur as extending from the femoral head to 5 cm below the lesser trochanter [15]. The presence of pathological fracture was defined as a break in the continuity of bone at the location of an osseous metastasis on x-ray, CT, or MR, or the combination of an incomplete cortical lesion on imaging studies and pain elicited during physical exam during range of motion or manual strength testing against resistance [16, 17] as determined by a fellowship trained orthopedic oncologist with thirty-two years of experience (JHH), blinded to the outcome of FDG PET/CT analyses. These patients were defined as patients with proximal femoral pathological fracture (cases). All cases were examined by the same orthopedic oncologist (JHH). The 6 year screening period was chosen to identify an adequate number of pathologic fracture cases within three months of PET/CT. The occurrence of fracture within three months of PET/CT was chosen to limit alterations in disease status at the time of the PET/CT and the time of fracture. The same HIS was searched for patients with metastatic breast cancer and FDG PET/CT in 2013, which were sequentially screened in reverse chronological order for a list of comparison patients. The imaging and records of these patients were reviewed for (a) the presence of FDG-avid osseous lesions in the proximal femur; and (b) the absence of pathological fracture over the following 12 months. Patients meeting these criteria were defined as patients without pathologic fracture (controls). We set a pre-specified goal of having twice as many controls as cases.
Figure 1.
Standards for Reporting of Diagnostic Accuracy (STARD) flow chart.
Medical records of both cases and controls were reviewed to document breast cancer histology, histologic grade, estrogen receptor (ER)/progesterone receptor (PR)/human epidermal growth factor receptor 2 (HER2) phenotype, and history of radiation therapy to the femoral lesion.
FDG PET/CT scans and review
PET/CT examinations were performed according to our institution’s clinical protocols on GE Discovery STE, 600, 690, and 710 PET/CT systems, including acquisition of images from the mid skull to upper thigh approximately 60 minutes after intravenous administration of 12–15 mCi of FDG. Patients fasted for >6 hours, and finger stick blood glucose levels were <200mg/dl prior to injection. Spiral CT was obtained for attenuation correction at 60mAs, 120–140kVp, with a 5mm slice thickness during free breathing. PET was acquired at 3–5 minutes per bed position using the 3D mode, typically for 6–7 bed positions.
PET/CT scans were reviewed on GE PET VCAR workstations. Reference lesions in the proximal femur (defined as the femoral head to 5 cm below the lesser trochanter) were selected by a dual ABR- and ABNM-boarded radiologist with eight years of PET/CT experience (GAU) blinded to the presence or absence of subsequent pathological fracture. A region of interest (ROI) was drawn around each reference lesion using transaxial, coronal, and sagittal images (Figure 2). The lesion location was defined as (i) Head/Neck, if the lesion was entirely above the superior aspect of the greater trochanter; (ii) Trochanteric, if the lesion was entirely beneath the superior aspect of the greater trochanter; or (iii) Both, if the lesion extended both above and below the superior aspect of the greater trochanter. The lesion location was established based on the FDG PET. Maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) were measured from the ROI using GE PET VCAR software. SUVmax was the single voxel within the ROI with the greatest SUV. MTV was defined as the cubic centimeter (cm3) volume of voxels with SUV > 42% of SUVmax, as described in a prior publication[18]. TLG was defined as the product of MTV and SUVmean of voxels within the MTV[18].
Figure 2.
Measurement of SUVmax, SUVmean, MTV, and TLG in proximal femoral lesions.
CT, FDG PET, and fused FDG PET/CT images in transaxial, coronal, and sagittal sections of an FDG-avid proximal femoral osseous metastasis. A region of interest (ROI) was drawn for each reference lesion (green boxes) and checked for correct positioning in all three axial planes. SUVmax, SUVmean, MTV, and TLG were then obtained using GE PET VCAR software.
Statistical methods
The Wilcoxon rank-sum test was used to compare continuous variables (age, SUV, tumor volume, and TLG), and Fisher’s exact test was used to compare categorical variables between patients with pathological fracture and patients without pathological fracture. A non-parametric receiver operating characteristic (ROC) analysis was performed to assess the ability of using each quantitative measure of FDG-avidity (SUVmax, SUVmean, MTV, and TLG) to differentiate fractured patients from others. The area under the curve (AUC) was estimated. Exploratory cutoff points were found by maximizing Youden’s index on the ROC curve for each FDG-avidity along with observed sensitivity, specificity, positive predictive value, and negative predictive value. In the multivariable analyses, first among clinical factors, location and radiation were found to be associated with risk of fracture via stepwise selection. Then for each measure of FDG-avidity, a multivariable logistic regression was used to examine its association with risk of fracture, controlling for location and radiation. All analyses were performed with software packages SAS 9.2 (SAS Institute, Cary, NC) and R version 3.1 (The R Foundation for Statistical Computing).
RESULTS
Characteristics of cases and controls
Twenty-seven patients met criteria for cases with pathological fracture of the proximal femur (Figure 1). The median age of these patients was 62 (range 35–86). All 27 cases had an FDG-avid osseous lesion in the proximal femur which subsequently fractured. Fifty-five patients met criteria for controls without pathological fracture of the proximal femur. The median age of these patients was 58 years (range 32–82). Most patients were ductal in histology (59/82, 72%), with high histologic grade (grade 3 51/82, 62%). Eleven of 82 (13%) lesions had been previously radiated, including seven of twenty-seven (20%) of patients with fracture and four of fifty-five (7%) of patients without fracture. In these eleven patients, radiation therapy was performed between 8 and 26 months prior to fracture. The majority of lesions (50/82, 61%) extended from the head/neck of the femur into the trochanteric region. Seventy-four (90%) were lesions from ER-positive/HER2-negative malignancies, while 4 (5%) were HER2-positive and 4 (5%) were triple-negative. Characteristics of cases and controls are summarized in Table 1.
Table 1.
Patient and imaging characteristics
| No fracture (n=55) | Fracture (n=27) | p Value | |
|---|---|---|---|
| Median (Range) | Median (Range) | ||
|
|
|||
| Age, year | 58 (32, 82) | 62 (35, 86) | 0.355 |
| SUVmax | 4.8 (1.3, 16.5) | 9.9 (1.7, 27) | <.001 |
| SUVmean | 2.8 (0.8, 8.7) | 4.1 (1.5, 13.8) | 0.001 |
| Tumor Volume (cm^3) | 9 (1.4, 69.6) | 52.1 (4, 121) | <.001 |
| Total Lesion Glycolysis | 27.2 (2.66, 605.52) | 184.98 (8.4, 938.4) | <.001 |
| N (%) | N (%) | ||
|
|
|||
| Histology | |||
| Ductal | 40 (73%) | 20 (74%) | 0.999 |
| Lobular | 9 (16%) | 4 (15%) | |
| Both | 6 (11%) | 3 (11%) | |
| Histology Grade | |||
| 2 | 12 (26%) | 7 (30%) | 0.776 |
| 3 | 35 (74%) | 16 (70%) | |
| N/A* | 8 | 4 | |
| Radiation to Lesion | |||
| No | 51 (93%) | 20 (74%) | 0.035 |
| Yes | 4 (7%) | 7 (26%) | |
| Location | |||
| Head/Neck | 4 (7%) | 9 (33%) | 0.006 |
| Troch | 12 (22%) | 7 (26%) | |
| Both | 39 (71%) | 11 (41%) | |
| Lesion Laterality | |||
| Left | 24 (44%) | 9 (33%) | 0.474 |
| Right | 31 (56%) | 18 (67%) | |
| Receptor Status | |||
| ERPR+/HER2− | 52 (95%) | 22 (81%) | 0.020 |
| HER2+ | 3 (5%) | 1 (4%) | |
| TN | 0 (0%) | 4 (15%) | |
Unknown was not included in the test.
Differences in patient characteristics between cases and controls
A greater risk of pathological fracture was correlated with lesions that had been previously radiated (p = .035), those from a triple-negative malignancy (p = .01), and those that involved the head/neck of the femur without extension into the trochanteric region (p = .006, Table 1). No statistically significant differences were noted between the cases and controls for age, histology, or histologic grade.
Differences in quantitative measurements of FDG-avidity between cases and controls
All four quantitative measures of FDG-avidity demonstrated statistically significant differences between cases with subsequent pathologic fracture and controls without subsequent pathologic fracture (Table 1). SUVmax, MTV, and TLG were all significant with p < .001, while SUVmean was significant with p = .001. For all four measures of FDG-avidity, cases with pathological fracture demonstrated higher values than for controls without pathological fracture. Boxplots for median and interquartile range for each of the four measures of FDG-avidity in cases and controls are visually represented in Figure 3.
Figure 3.
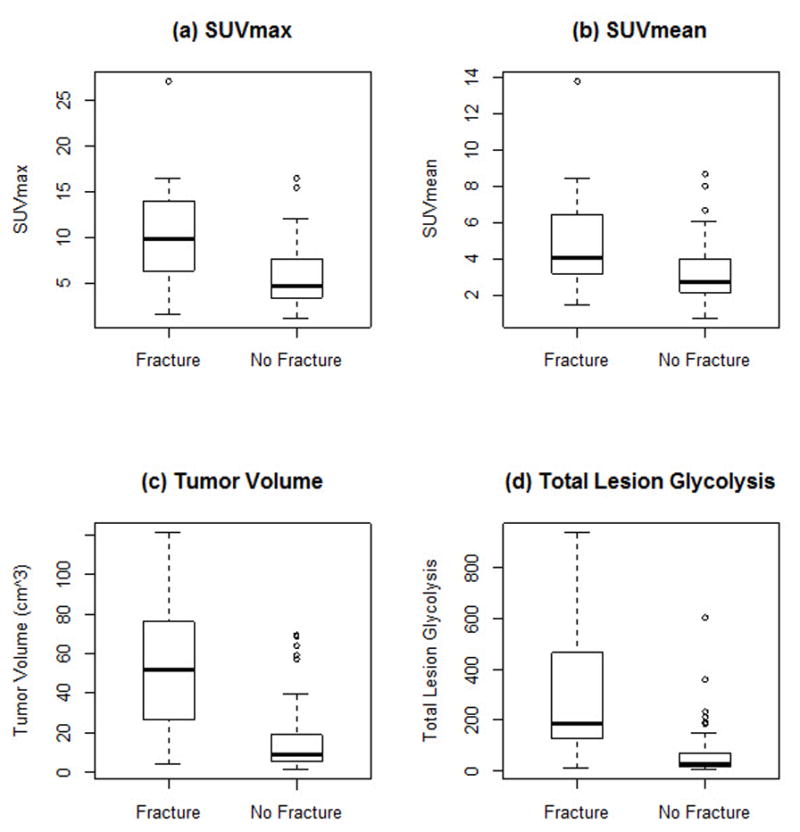
Boxplots of (a) SUVmax (b) SUVmean (c) tumor volume and (d) total lesion glycolysis for cases with pathological fracture and controls without fracture.
Boxplots of quantitative measures of FDG-avidity for cases with pathological fracture and controls without fracture. The thick solid line inside the box indicates the median, the top and the bottom bars are the window of 1.5 times of interquartile range (IQR), and the circle dots are observed extreme values falling outside of the window.
Receiver operating characteristic (ROC) analysis based on measures of FDG-avidity
Cutoff values were determined to maximize the combination of sensitivity and specificity of quantitative measures of FDG avidity to identify patients with subsequent risk of pathological fracture (Table 2). Optimal cutoffs were SUVmax 9.55 g/cm3, SUVmean 3.35 g/cm3, MTV 18.9 cm3, and TLG 81.02 g. Area under the curve (AUC) was greatest for the volumetric measures MTV and TLG. A TLG of 81.02 g could differentiate patients who would suffer subsequent pathological fracture from those who would not with accuracy, sensitivity, and specificity of 0.83, 0.85, and 0.80, respectively. ROC curves for the four measures of FDG avidity to differentiate patients with pathological fracture are shown in Figure 4.
Table 2.
ROC analysis on measures of FDG avidity to differentiate fracture and non-fracture.
| AUC (95%CI) | Cutoff Value | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | |
|---|---|---|---|---|---|---|
| SUVmax | 0.771 (0.657, 0.886) | 9.55 | 0.577 (0.369, 0.766) | 0.855 (0.733, 0.935) | 0.652 (0.427, 0.836) | 0.810 (0.686, 0.901) |
| SUVmean | 0.720 (0.598, 0.843) | 3.35 | 0.731 (0.522, 0.884) | 0.673 (0.533, 0.793) | 0.514 (0.344, 0.681) | 0.841 (0.699, 0.934) |
| MTV | 0.839 (0.743, 0.936) | 18.9 | 0.846 (0.651, 0.956) | 0.745 (0.610, 0.853) | 0.611 (0.435, 0.769) | 0.911 (0.788, 0.975) |
| TLG | 0.834 (0.730, 0.937) | 81.02 | 0.846 (0.651, 0.956) | 0.800 (0.670, 0.896) | 0.667 (0.482, 0.820) | 0.917 (0.800, 0.977) |
| No Fracture | Fracture | |
|---|---|---|
| SUVmax < 9.55 | 47 | 11 |
| SUVmax ≥ 9.55 | 8 | 15 |
|
| ||
| SUVmean < 3.35 | 37 | 7 |
| SUVmean ≥ 3.35 | 18 | 19 |
|
| ||
| Tumor Volume < 18.9 cm3 | 41 | 4 |
| Tumor Volume ≥ 18.9 cm3 | 14 | 22 |
|
| ||
| Total Lesion Glycolysis < 81.02 g | 44 | 4 |
| Total Lesion Glycolysis ≥ 81.02 g | 11 | 22 |
The measures of accuracy were estimated assuming patients with values above the cutoff values had fracture.
Figure 4.
ROC curves of using (a) SUVmax (b) SUVmean (c) Tumor volume and (d) total lesion glycolysis to differentiate patients experiencing fracture and patients without fracture.
Multivariate analyses
Four multivariate models were derived, one for each measure of FDG-avidity (SUVmax, SUVmean, MTV, and TLG). After controlling for clinical factors that were statistically significant (tumor location and prior radiation), all four measures of FDG-avidity remained significant for the risk of fracture (Table 3). For SUVmax, an increase in SUVmax of 1 (incremental unit = 1) was associated with an increased odds of fracture of 1.32 (95% confidence interval 1.13 – 1.55). For MTV, an increase in MTV of 5 cm3 was associated with an increased odds of fracture of 1.54 (95% confidence interval 1.25 – 1.91).
Table 3.
Multivariate analyses
| SUVmax Model | SUVmean Model | MTV Model | TLG Model | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P Value | OR (95%CI) | P Value | OR (95%CI) | P Value | OR (95%CI) | P Value | |
| SUVmax (IU=1) | 1.32 (1.13, 1.55) | 0.001 | ||||||
| SUVmean (IU=1) | 1.56 (1.16, 2.09) | 0.003 | ||||||
| MTV (IU =5) | 1.54 (1.25, 1.91) | <0.001 | ||||||
| TLG (IU =10) | 1.13 (1.06, 1.21) | <0.001 | ||||||
| Location | ||||||||
| Head/Neck or Troch | 1 | 0.014 | 1 | 0.017 | 1 | 0.001 | 1 | 0.002 |
| Both | 0.22 (0.07, 0.74) | 0.26 (0.09, 0.79) | 0.03 (0,0.24) | 0.09 (0.02, 0.40) | ||||
| Radiation | ||||||||
| No | 1 | 0.090 | 1 | 0.045 | 1 | 0.777 | 1 | 0.290 |
| Yes | 3.83 (0.81, 18.06) | 4.60 (1.03,20.57) | 1.41 (0.13, 15.17) | 2.77 (0.42, 18.23) | ||||
OR = Odds ratio, IU= Increment unit.
DISCUSSION
Prediction of pathological fractures is important, because prophylactic treatment of proximal femoral lesions before a pathological fracture occurs will reduce surgical complications, enhance quality of life, and lengthen patient survival [5, 6]. In this manuscript, we propose that FDG PET/CT may help identify breast cancer patients at high risk of proximal femoral pathological fracture. We use quantitative measures of FDG avidity to identify subsequent fracture risk. For the most commonly used measure of FDG avidity, SUVmax, sensitivity and specificity of 58% and 85% were achieved, respectively, better than reported values for Mirel’s criteria [8–10]. For the volumetric measurement TLG, which accounts both for size and intensity of FDG-avidity, sensitivity and specificity were 85% and 80%. Quantitative measurements that incorporate lesion size, such as MTV and TLG, account for the possibility that larger lesions may be more likely to fracture. If our results are confirmed, then measures of FDG avidity could be used to help predict risk for pathological fracture and identify patients for referral for prophylactic management of high-risk lesions.
A case-control design was selected for this study to efficiently examine the risk factor, extent of tumor metabolism, for pathological fracture. Limiting measurements to lesions in the proximal femur of breast cancer patients was prespecified. Metastatic breast cancer patients were selected because they commonly suffer from bone metastases, and the proximal femur was selected because it is the most common site affected by metastases that requires treatment. It is also the focus of the accepted Mirels system to predict fracture risk. Inclusion of multiple sites of fracture or multiple tumor types would have added additional variables, made analysis more difficult, and possibly obscured significant results.
The patient population in this study was similar to the population of patients with breast cancer as a whole, in that the distributions of ductal and lobular histology and histologic grade were representative [19, 20]. Of note, the patient population in this study was more often ER-positive/HER2-negative (74/82; 90%) than the breast cancer population as a whole (70–80%) [21]. The reason for this difference is not clear.
Only FDG-avid osseous lesions of the proximal femur were included in this study. This does not discount the importance of non-FDG-avid osseous metastases, which may be seen in patients with breast cancer, may be more common in certain histologies of breast cancer such as lobular breast cancer [22, 23], and are sources of skeletal events including pathological fracture. The risk of pathological fracture in non-FDG-avid osseous lesions was not assessed in our analysis.
Radiation therapy to proximal femoral lesions was significantly associated with increased risk of pathological fracture (p = .035). This is concordant with prior work suggesting that radiation therapy of osseous lesions, while important for pain control and improvement of function, may increase the risk of pathological fracture [4]. As prior radiation therapy increased risk of pathologic fracture, it was controlled for in multivariate analyses.
Other imaging modalities have also been evaluated for their ability to predict pathological fracture. For example, CT structural rigidity analysis, based on quantitative CT scans of the proximal femur has recently been reported to have improved association with pathological fracture, but this requires special phantoms, calibration, and analysis (21).
Our study had several limitations. The retrospective, single-institution design lends itself to selection biases that are difficult to control. We pre-selected the ranges of patient inclusion and the goal of two controls per case in an effort to minimize these selection biases. In the control group of patients without pathological fracture, proximal femoral lesions were not pathologically confirmed as metastases, although all of the patients had pathologically confirmed metastatic disease and FDG-avid osseous lesions in a patient with known metastatic breast cancer are most consistent with osseous metastases. Our institution uses multiple PET/CT systems, and the use of multiple systems could affect the data.
In conclusion, this retrospective case-control study uses parameters for using quantitative measures of FDG avidity to predict the likelihood of subsequent pathological fracture of the proximal femur in patients with metastatic breast cancer. Measures of FDG avidity may help predict risk for pathological fracture and identify patients for referral for prophylactic management of high-risk lesions. This is a novel application of quantitative FDG PET imaging. Our results need to be substantiated in larger and/or prospective study designs.
Acknowledgments
We acknowledge the support of the Susan G. Komen for the Cure Research Grant KG110441 (GAU) the MSKCC Biostatistics Core (P30 CA008748), the NIH summer student fellowship (AZ), and the Major Family Fellowship in Musculoskeletal Oncology (JHH).
Footnotes
Disclosures: No conflicts of interest
References
- 1.Poon M, Zeng L, Zhang L, et al. Incidence of skeletal-related events over time from solid tumour bone metastases reported in randomised trials using bone-modifying agents. Clin Oncol (R Coll Radiol) 2013;25:435–444. doi: 10.1016/j.clon.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Wood TJ, Racano A, Yeung H, et al. Surgical management of bone metastases: quality of evidence and systematic review. Ann Surg Oncol. 2014;21:4081–4089. doi: 10.1245/s10434-014-4002-1. [DOI] [PubMed] [Google Scholar]
- 3.Costa L, Badia X, Chow E, Lipton A, Wardley A. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support Care Cancer. 2008;16:879–889. doi: 10.1007/s00520-008-0418-0. [DOI] [PubMed] [Google Scholar]
- 4.Rordorf T, Hassan AA, Azim H, et al. Bone health in breast cancer patients: a comprehensive statement by CECOG/SAKK Intergroup. Breast. 2014;23:511–525. doi: 10.1016/j.breast.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Ward WG, Holsenbeck S, Dorey FJ, Spang J, Howe D. Metastatic disease of the femur: surgical treatment. Clin Orthop Relat Res. 2003:S230–244. doi: 10.1097/01.blo.0000093849.72468.82. [DOI] [PubMed] [Google Scholar]
- 6.Ratasvuori M, Wedin R, Keller J, et al. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg Oncol. 2013;22:132–138. doi: 10.1016/j.suronc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989:256–264. [PubMed] [Google Scholar]
- 8.Van der Linden YM, Dijkstra PD, Kroon HM, et al. Comparative analysis of risk factors for pathological fracture with femoral metastases. J Bone Joint Surg Br. 2004;86:566–573. [PubMed] [Google Scholar]
- 9.Damron TA, Morgan H, Prakash D, et al. Critical evaluation of Mirels’ rating system for impending pathologic fractures. Clin Orthop Relat Res. 2003:S201–207. doi: 10.1097/01.blo.0000093842.72468.73. [DOI] [PubMed] [Google Scholar]
- 10.Damron TA, Nazarian A, Entezari V, et al. CT-based Structural Rigidity Analysis Is More Accurate Than Mirels Scoring for Fracture Prediction in Metastatic Femoral Lesions. Clin Orthop Relat Res. 2016;474:643–651. doi: 10.1007/s11999-015-4453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du Y, Cullum I, Illidge TM, Ell PJ. Fusion of metabolic function and morphology: sequential [18F]fluorodeoxyglucose positron-emission tomography/computed tomography studies yield new insights into the natural history of bone metastases in breast cancer. J Clin Oncol. 2007;25:3440–3447. doi: 10.1200/JCO.2007.11.2854. [DOI] [PubMed] [Google Scholar]
- 12.Morris PG, Lynch C, Feeney JN, et al. Integrated positron emission tomography/computed tomography may render bone scintigraphy unnecessary to investigate suspected metastatic breast cancer. J Clin Oncol. 2010;28:3154–3159. doi: 10.1200/JCO.2009.27.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris PG, Ulaner GA, Eaton A, et al. Standardized uptake value by positron emission tomography/computed tomography as a prognostic variable in metastatic breast cancer. Cancer. 2012;118:5454–5462. doi: 10.1002/cncr.27579. [DOI] [PubMed] [Google Scholar]
- 14.Ulaner GA, Eaton A, Morris PG, et al. Prognostic value of quantitative fluorodeoxyglucose measurements in newly diagnosed metastatic breast cancer. Cancer Med. 2013;2:725–733. doi: 10.1002/cam4.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vander Schilden J, Bolhofner B, Sanders R, Wiss D, Spiegel P. Surgery of the Musculoskeletal System. 2. New York: 1990. p. 2641. [Google Scholar]
- 16.Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 17.Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 18.Larson SM, Erdi Y, Akhurst T, et al. Tumor Treatment Response Based on Visual and Quantitative Changes in Global Tumor Glycolysis Using PET-FDG Imaging. The Visual Response Score and the Change in Total Lesion Glycolysis. Clin Positron Imaging. 1999;2:159–171. doi: 10.1016/s1095-0397(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 19.Li CI, Anderson BO, Daling JR, Moe RE. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421–1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 20.Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046–1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26–35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Dashevsky BZ, Goldman DA, Parsons M, et al. Appearance of untreated bone metastases from breast cancer on FDG PET/CT: importance of histologic subtype. Eur J Nucl Med Mol Imaging. 2015 doi: 10.1007/s00259-015-3080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan MP, Goldman DA, Dashevsky B, et al. Comparison of 18F-FDG PET/CT for Systemic Staging of Newly Diagnosed Invasive Lobular Carcinoma Versus Invasive Ductal Carcinoma. J Nucl Med. 2015;56:1674–1680. doi: 10.2967/jnumed.115.161455. [DOI] [PMC free article] [PubMed] [Google Scholar]