Abstract
Aims
Severe iron overload is associated with cardiac damage, while iron deficiency has been related to worse outcomes in subjects with heart failure (HF). This study investigated the relationship between ferritin, a marker of iron status, and the incidence of HF in a community-based cohort.
Methods and results
We examined 1,063 participants who were free of heart failure from the Atherosclerosis Risk in Communities (ARIC) study in whom ferritin serum levels were measured at baseline (1987–1989). The participants (mean age 52.7 ± 5.5 years, 62% women), were categorized in low (<30 ng/mL; n=153), normal (30–200 ng/mL in women and 30–300 ng/mL in men; n=663) and high (>200 ng/mL in women and >300 ng/mL in men; n=247) ferritin levels. Multivariable Cox proportional hazards models were used to evaluate the relationship between ferritin and incident HF. After 20.9±4.6 years of follow-up, HF occurred in 144 (13.5%) participants. When compared to participants with normal ferritin levels, participants with low ferritin levels had a higher risk of HF (HR= 2.24, 95% CI= 1.15–4.35; p=0.02) as did those with high ferritin levels (HR=1.81, 95% CI=1.01–3.25; p=0.04), after adjusting for potential confounders. Notably, low ferritin levels remained associated with incident HF even after excluding subjects with anemia (HR= 2.28, 95% CI= 1.11–4.68; p= 0.03).
Conclusion
Derangements in iron metabolism, either low or high ferritin serum levels, were associated with higher risk of incident HF in a general population, even without concurrent anemia. These findings suggest that iron imbalance might play a role in the development of HF.
Keywords: heart failure, ferritin, iron deficiency, iron overload
INTRODUCTION
Disorders of iron metabolism, either deficiency or overload, have been associated with increased cardiovascular morbidity and mortality.1,2,3 Severe iron overload, due to acquired and genetic hemochromatosis, has been consistently associated with cardiomyopathy and higher incidence of heart failure (HF).1 Iron overload cardiomyopathy is found in patients with primary or secondary hemochromatosis and with ferritin levels between 539 and 7,700 ng/mL, which represents a specific population with highly increased body iron stores.4 However, so far, whether mild or moderate increases in iron storages are associated with higher risk of incident HF in the general population has not been addressed.
Iron deficiency has been associated with impaired cardiac function and altered myocardial structure in animal models.5 In humans, up to 73% of patients with HF6,7 were iron deficient in cross-sectional analysis. Moreover, low iron storage has been associated with worse cardiovascular outcome independent of anemia in subjects with heart failure.7 Altogether, these data raise the possibility that iron deficiency might play a role in the physiopathogenesis of cardiac dysfunction. This study evaluated whether iron imbalance, characterized by low or high ferritin serum levels, was associated with an increased incidence of HF in a community-based population.
METHODS
Study population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort study of 15,792 subjects aged between 45 and 64 years old at recruitment between 1987 and 1989. The rationale, design, and procedures have been previously published.8 Participants were recruited in four different centers in United States: Forsyth County, North Carolina; Jackson, Mississippi; the northwest suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Since the recruitment between 1987 and 1989 (Visit 1), four other visits were performed, and the last one (Visit 5) occurred between 2011 and 2013. The study was approved by Institutional review boards from each site, and all participants gave written consent.
For this study, we considered 1,145 participants who had ferritin levels measured in Visit 1 and evaluated the association between ferritin levels and incident HF through the period of follow-up from 1987–1989 through December 31, 2011. This sample was free of diabetes at baseline and was originally enrolled in an case-cohort ARIC sub-study that investigated the relationship between ferritin levels and incident type 2 diabetes ascertained through Visit 4 (1996–1998).9 We excluded patients with prevalent HF (n=61) or unknown HF status at Visit 1 (n=21) leaving 1,063 participants for this analysis.
Definition of incident heart failure
HF incidence was defined as the first occurrence of HF as either hospitalization according to the International Classification of Diseases- 9th Revision (ICD-9), code 428 (428.0 to 428.9) in any position obtained by ARIC Study retrospective surveillance of hospital discharges or a death certificate with death from HF in any position or death certificate with an ICD-9 code of 428 or an ICD-10 code of I50 among any of the listed diagnoses or underlying causes of death.10 Death was ascertained through linkage with the National Death Index.
Ferritin measurement and definition of iron status
Ferritin level was measured using an immunoturbidimetric assay (Roche Diagnosis, Indianapolis, Indiana). Plasma samples were collected at ARIC Visit 1 and frozen at −70°C. The reliability coefficient for measures in 35 subjects with blinded replicate samples collected at Visit 1 was 0.85. The lower limit of detection of ferritin was 3.4 ng/mL. Twenty five subjects had undetectable values that were imputed as 1.7 (half of lower limit detection value) for analyses.
Participants were categorized in three different groups according to ferritin serum levels: low-ferritin (<30 ng/mL);11,12 normal (30–200 ng/mL in women and 30–300 ng/mL in men); and high ferritin (>200 ng/mL in women and >300 ng/mL in men).4 In addition, as supplementary analysis, we divided participants in four groups adding a “low-normal” ferritin group, which was defined by ferritin levels between 30 and 100 ng/mL. For another supplementary analysis, we added a fourth ferritin category called “high-normal”, defined by ferritin between 200 and 500 in women and 300 and 500 in men.
Covariates
Other definitions and information about demographic variables (age, gender, race), self-reported smoking and alcohol intake, diabetes, hypertension, body mass index were used as previously described.13 Anemia was defined as hemoglobin levels < 12mg/dL in women and < 13mg/dL in men at Visit 1. Incident anemia was ascertained when anemia appeared during the follow-up. The daily iron intake was estimated by a semi-quantitative food frequency questionnaire. Women were classified as being in postmenopausal status if they had no history of menstruation in the past two years. Data regarding use of nonsteroidal anti-inflammatory drugs (NSAID) were obtained by means of an interviewer-administered questionnaire. Measurements of low density lipoprotein cholesterol, high density lipoprotein cholesterol, glucose, C reactive protein, hemoglobin and albumin were performed as previously reported9. High education level was defined as college, graduate or professional school attendance.
Statistical analyses
Descriptive data are presented as the mean ± standard deviation for normally distributed variables and median (25th percentile, 75th percentile) for non-normally distributed variables. Significant pairwise comparisons were adjusted for multiple testing using Bonferroni correction and are shown only for variables in which a significant global difference was detected using one-way ANOVA or Kruskal-Wallis tests. When appropriate, skewed variables were log transformed for analysis. The association between ferritin and covariates was assessed using liner regression. Categorical data were reported as percent frequencies and compared by chi-squared test. Incident HF was calculated and presented as events per 1000 person-years at risk.
The association between ferritin categories and incidence of heart failure was evaluated using Cox proportional hazard regression models that were weighted to account for the differences in the proportions of diabetes and race in the case-cohort sampling design. Three regression models were created: model 1 included age, gender, and race; model 2 additionally adjusted for: body mass index, heart rate, hypertension, smoking, alcohol drinking, hemoglobin, C reactive protein, glucose, low-density-lipoprotein cholesterol, glomerular filtration rate, prevalent coronary disease; model 3 additionally adjusted for: albumin, menopausal status, education level, incidence of anemia, incidence of diabetes. We included a time-varying covariate to adjust for incident CHD. Cox-regression analyses were performed in the whole sample (n=1,063) and additionally in subjects without anemia (n=925). Kaplan–Meier survival analysis was used to compare the survival free of HF (hospitalization and death) among the three groups. The curvilinear association between ferritin and incident HF was assessed using restricted cubic splines with 4 knots adjusted for covariates included in Model 1. Tests for interaction were performed using the likelihood ratio test for the cross-product interaction term between sex and ferritin categories. A sensitivity analysis was performed using only the non-diabetics participants to investigate whether the results would change after excluding subjects with incident diabetes.
Two-sided p-values <0.05 were considered significant. All the statistical analyses were performed using Stata version 13.1 (Stata Corp., College Station, Texas).
RESULTS
Population characteristics
The mean age of the study participants at baseline was 52.7 ± 5.5 years, 62% were women and 45% were black. The characteristics of the studied participants according to ferritin serum levels are described in Table 1. The median ferritin level was 121 (56–226) ng/mL and among the 1,063 ARIC participants who were included in our analysis, 153 (14%), 663 (62%) and 247 (23%) had low, normal-range and high ferritin levels, respectively. The univariate association between ferritin and the covariates is shown in the Supplementary Material (Table 1S). Subjects with low ferritin were younger, were more likely to be women and had higher heart rate and lower hemoglobin levels than the normal range and high ferritin level groups. Furthermore, low ferritin group had lower iron intake and were less likely to have reached menopausal status in comparison with other groups. Participants with high ferritin were more likely to be black, with higher blood pressure levels than other groups. Also, BMI, LDL cholesterol and glucose were higher in high-ferritin group in comparison with low and normal-range ferritin groups. There were no significant differences in the education level and current smoking or drinking status among the groups as well as the prevalence of hypertension. (Table 1)
Table 1.
Participants baseline characteristics according to ferritin serum levels (n= 1,063)
| Variables | Low ferritin n=153 |
Normal ferritin n=663 |
High ferritin n=247 |
|---|---|---|---|
| Ferritin (ng/mL) | 14 (6–22) | 109 (67–163) | 358 (301–477) |
| Age-years | 50 ± 5* | 53 ± 6 | 53 ± 5 |
| Male (%) | 23 (15)* | 262 (40) | 117 (47)† |
| Race – black (%) | 65 (43) | 287 (43) | 130 (53)† |
| Heart rate (bpm) | 69 ± 9* | 66 ± 10 | 65 ± 10 |
| Systolic blood pressure (mmHg) | 121 ± 17 | 122 ± 17 | 125 ± 17† |
| Diastolic blood pressure (mmHg) | 75 ± 8 | 75 ± 11 | 77 ± 11† |
| BMI (Kg/m2) | 28 (24.5–33.2) | 27.5 (24.7–31.7) | 29.5 (26.3–32.7) † |
| Hypertension (%) | 48 (31) | 251 (38) | 107 (43) |
| Current smokers (%) | 27 (18) | 152 (23) | 56 (23) |
| Current drinkers (%) | 66 (43) | 338 (51) | 131 (53) |
| Iron intake (mg/day) | 10.1 ± 3.7* | 11.2 ± 5.0 | 10.9 ± 4.3 |
| Hemoglobin (g/dL) | 12.7 (11.8–13.6)* | 13.6 (12.7–14.5) | 13.9 (12.8–15.0) |
| CRP (mg/mL) | 2.1 (0.7–4.6) | 2.0 (0.9–4.4) | 2.3 (1.0–4.9) |
| Albumin (mg/dL) | 3.8 ± 0.2* | 3.9 ± 0.2 | 3.9 ± 0.2† |
| GFR (ml/min/1.73m2) | 110 (101–121)* | 107 (97–117) | 105 (96–117) |
| Glucose (mg/dL) | 99 (93–107) | 101 (95–109) | 104 (97–113)† |
| LDL (mg/dL) | 132 (108–155) | 132 (110–160) | 135 (107–163) |
| Use of NSAID (%) | 14 (9) | 69 (10) | 27 (11) |
| Postmenopausal status (%)‡ | 39/130 (30)* | 307/401 (75) | 123/130 (95) |
| High education level (%) | 61 (40) | 241 (36) | 74 (30) |
BMI: body mass index; CRP: C reactive protein; GFR: glomerular filtration ratio; LDL: low density lipids cholesterol; NSAID: non-steroidal anti-inflammatory drugs.
Proportion among women in each group.
p<0.05 low vs. normal ferritin and
p<0.05 high vs. normal ferritin
Ferritin level and risk of incident heart failure
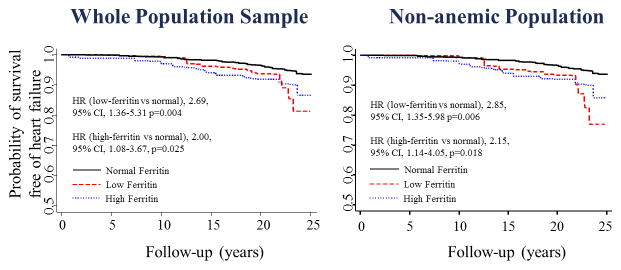
The mean follow-up was 21±4.6 years, and incident HF occurred in 144 participants. Subjects with low ferritin showed the highest rate of HF events in the whole sample [7.7 (5.2–11.4 /1000)] and in non-anemic participants [7.5 (4.7–12.0 /1000)] among the studied groups (Table 2). Participants with high ferritin also had a higher rate of heart failure [6.9 (4.9–9.6 /1000)] than the normal range group of individuals (Figure 1). After adjusting for age, sex, race, body mass index, heart rate, hypertension, smoking, alcohol drinking, hemoglobin, C reactive protein, glucose, low-density-lipoprotein cholesterol, glomerular filtration rate, prevalent coronary disease, albumin, menopausal status, incidence of anemia, incidence of diabetes and education level, low-ferritin levels were associated with a higher risk of HF [HR (95%CI) of 2.24 (1.15–4.35)] when compared to normal-range levels (Table 3). These results were reproduced when only participants without anemia were included in the analysis. We also observed an increased risk of HF between participants with high-ferritin levels [HR (95%CI) of 1.81 (1.01–3.25)] when comparing with those with normal-range levels (Table 3). When adjusting for time-varying incident CHD, the association between low-ferritin levels and incident HF remained significant [HR (95%CI) of 2.06 (1.03–4.11) p=0.040] while the association between high-ferritin and HF was no longer significant [HR (95%CI) of 1.73 (0.96–3.12) p=0.068]. When ferritin was split in four categories, considering individuals with values between 30 and 100 ng/mL in a different group (low-normal ferritin), participants with low and high ferritin remained with a higher risk of HF. Subjects with low-normal ferritin were not significantly associated with incident HF [HR (95%CI) of 1.32, (0.65–2.66) p=0.44] (Table 2S). Participants with normal-high ferritin did not have a significant higher risk of incident HF [HR (95%CI) of 1.78 (0.95–3.35) p=0.072] (Table 3S).
Table 2.
Crude incident rates of heart failure by baseline ferritin serum level (ARIC study)
| Heart failure in the whole population sample
|
Heart failure in non-anemic participants
|
|||||
|---|---|---|---|---|---|---|
| Group | Participants | Number of events | Rates of events per 1000 person-years | Participants | Number of events | Rates of events per 1000 person-years |
| All=1063 | 144 | All=925 | 138 | |||
| Normal range (30–200 ng/mL in women and 30–300 ng/mL in men) | 663 | 84 | 5.9 (4.8–7.4) | 594 | 77 | 6.1 (4.9–7.6) |
| Low ferritin (<30 ng/mL) | 153 | 25 | 7.7 (5.2–11.4) | 111 | 18 | 7.5 (4.7–12.0) |
| High ferritin (>200 ng/mL in women and >300 ng/mL in men) | 247 | 35 | 6.9 (4.9–9.6) | 220 | 32 | 7.1 (5.0–10.0) |
Figure 1.
Ferritin serum levels and incident HF (ARIC study, 1987 to 2011).
Kaplan–Meier curves for patients with low- (red large dashed), normal (black line), and high-ferritin (blue short dashed) serum levels for the outcome of heart failure (hospitalization and death). Adjusted for age, sex, and race, standardized to a population that is 62% male, 55% white, and age 50. On the left, the whole sample was included; on the right, only participants without anemia. Low ferritin: defined as ferritin levels < 30 ng/mL; normal ferritin: 30–200 ng/mL in women and 30–300 ng/mL in men; and high ferritin: >200 ng/mL in women and >300 ng/mL in men. HR (hazard ratio) was from a Cox regression adjusted for age, sex, and race.
Table 3.
Hazard ratio (HR) and 95% confidence interval (95%CI) of heart failure in relation to ferritin serum levels (ARIC study, 1987 to 2011)
| Categories | Heart failure in the whole population sample
|
Heart failure in non-anemic participants
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 n=1063 |
Model 2 n=1034 |
Model 3 n=1034 |
Model 1 n=925 |
Model 2 n=897 |
Model 3 n=897 |
|||||||
|
|
|
|||||||||||
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| Normal range (30–200 ng/mL in women and 30–300 ng/mL in men) | Ref | - | Ref | - | Ref | - | Ref | - | Ref | - | Ref | - |
| Low ferritin (<30 ng/mL) | 2.69 (1.36–5.31) | 0.004 | 2.25 (1.13–4.50) | 0.025 | 2.24 (1.15–4.35) | 0.018 | 2.85 (1.35–5.98) | 0.006 | 2.33 (1.08–4.99) | 0.030 | 2.28 (1.11–4.68) | 0.026 |
| High ferritin (>200 ng/mL in women and >300 ng/mL in men) | 2.00 (1.08–3.67) | 0.025 | 1.86 (1.04–3.35) | 0.037 | 1.81 (1.01–3.25) | 0.046 | 2.15 (1.14–4.05) | 0.018 | 2.09 (1.13–3.85) | 0.018 | 1.97 (1.08–3.59) | 0.027 |
Model 1: adjusted age, sex, and race
Model 2: additionally adjusted for: body mass index, heart rate, hypertension, smoking, alcohol drinking, hemoglobin, C reactive protein, glucose, low-density-lipoprotein cholesterol, glomerular filtration rate, prevalent coronary disease
Model 3: additionally adjusted for: albumin, menopausal status, incidence of anemia, incidence of diabetes and education level.
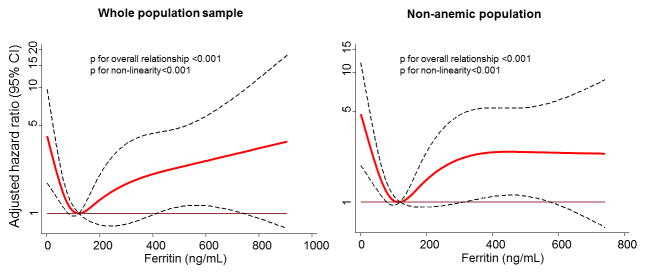
When studying ferritin as a continuous variable, we found a curvilinear association between ferritin and incident HF with a U-shaped pattern in both the whole sample and in the subsample without anemia (Figure 2). Furthermore, there was no significant association between ferritin categories and all-cause mortality in adjusted models. There were no significant interactions between age or gender and ferritin levels on incident HF. We did find a different association between high ferritin and HF in those participants with incident diabetes. Non-diabetic participants with high ferritin had an increased risk of HF in comparison with participants with incident diabetes (p for interaction = 0.045).
Figure 2.
Adjusted hazard ratio of Heart Failure in the whole population sample (left) and in non-anemic participants (right) in function of ferritin – spline regression analysis (ARIC study, 1987 to 2011)
Adjusted for age, sex, and race. The black dashed lines represent the hazard ratios (HR) and the red line the 95%CI. Ferritin=121 ng/mL (median) was considered the reference for the whole population and Ferritin=127 ng/mL (median) for the non-anemic population (HR=1).
In the sensitivity analysis restricted to the 538 participants without incident diabetes, there was an increased risk of HF among low-ferritin participants [HR (95%CI) of 3.08, (1.31–7.25) p=0.010], in comparison with those with normal-range levels in the whole population. Among those without anemia (n=450 participants), low-ferritin was also associated with a higher risk of HF [HR (95%CI) of 3.37, (1.36–8.37) p=0.009] when compared with those with normal-range ferritin. In this subgroup without diabetes cases, we did not observe an increased risk of HF among participants with high-ferritin levels.
DISCUSSION
In a community-based sample of 1,063 patients, iron imbalance, as characterized by either low or high ferritin serum levels, was associated with a higher risk of incident HF, even in non-anemic participants. Overall, these findings suggest that both iron deficiency and iron overload might play a role in the development of HF.
Markers of iron deficiency have been related to an increased incidence of cardiovascular disease, mainly myocardial infarction, CHD and cardiovascular death,2,3 and worse outcomes in patients with heart failure.7,14,15 However, to the best of our knowledge, the association with incident HF had never been tested. In the current study, we found that subjects with ferritin levels below 30 ng/mL had a 2.2-fold greater risk of HF than those with normal-range ferritin. Fourteen percent of participants presented with low-ferritin levels and were mainly women with a lower proportion in post-menopausal status, suggesting that perimenopause bleeding might be a contributing factor for low levels of iron storages among these women. Additionally, a lower ingest of iron in the diet was notice in comparison with those with normal or high ferritin levels. Neither iron intake or menstruation status was independently associated with incident HF, suggesting that these might be causal factors for iron deficiency but not influence in the association between low ferritin and incident HF. Non-steroidal anti-inflammatory drugs are a clinically important cause of gastrointestinal bleeding, iron deficiency, and also are associated with development of HF.16 Although we found a relatively high prevalence of NSAID usage among all individuals, there was no association with iron deficiency. Data regarding gastrointestinal bleeding, another potential cause for low-ferritin, was not available to include in this study. We used a regression model adjusted for demographic parameters, socioeconomic indicators, cardiovascular risk factors and prevalent CHD. It is unlikely that malnutrition status might explain the higher HF incidence rates in subjects with low ferritin. Although albumin was slightly lower in the participants with low-ferritin, the difference in albumin levels was not clinically important; besides, this potential confounder was included in the regression model. Anemia is already known as a factor associated with new-onset HF17 and cardiovascular disease.18 As recently reported, hemoglobin serum levels < 11 g/dL for men and <10 g/dL for women were associated with an increased incidence of HF in eight years of follow-up when compared with normal hemoglobin level concentrations.17 It is possible that anemia plays a role in the mechanism linking iron deficiency and incident HF. Notably, in our study, low ferritin levels were strongly associated with incident HF after excluding anemic subjects at baseline and adjusting for incident anemia, suggesting that iron deficiency is independently associated with incident HF. We also need to take into account the role of erythropoietin in this relationship. A recent study performed by Beverborg and colleagues found that increased levels of serum erythropoietin are associated with new-onset HF in patients with albuminuria in the community.19 In the same study, individuals with high levels of erythropoietin had the lowest ferritin levels and a higher prevalence of anemia than those with low levels of erythropoietin. It is possible that iron deficiency may be associated with high erythropoietin levels leading to HF. Unfortunately, in our study, we do not have this biomarker available to analyze its influence in the association between ferritin and incident HF.
Different pathophysiological mechanism can explain the association between low ferritin levels and incident HF. In conditions of iron deficiency, there is a reduction in concentration and activity of muscular oxidative enzymes and respiratory proteins, causing impairment in cellular energetics.20 Concomitantly, structural alterations such as mitochondrial swelling and irregularities in sarcomere organization could develop.5 Also, iron deprivation affects the cell proliferation cycle, trigging G1/S phase mitotic arrest and apoptosis,21 altering myocardial composition. Independently of presence of anemia, it might increase catecholamines levels,22 contributing to cardiac hypertrophy.23 Altogether, these intracellular and neurohormonal disarrangements might contribute to the development of HF in patients with iron deficiency.
It has been estimated that iron deficiency affects 30% of adults in Western countries, and 39% in Asia.24 In the community, poor iron status affects work productivity, physical performance, mental function and, has been related to an increase in all-cause mortality in an elderly population.25 Here, we didn’t find a significant association between ferritin levels and the risk of death. However the association of low-ferritin level with incident heart failure in a general population is a factor that could impact the public health system. The benefits of iron supplementation in terms of cardiovascular outcomes in the general population are not known. Previous studies demonstrated that the iron-supplemented women have improvement in productivity and a significant decline in fatigue.26 Among iron-deficient patients with chronic heart failure, treatment with iron has shown to improve functional capacity and quality of life.27 Whether iron supplementation might reduce the incidence of HF remains unknown.
Iron overload cardiomyopathy has been described in patients with primary hemochromatosis and also in transfusion-dependent anemias.1 Ferritin serum levels higher than 2500 ng/mL were associated with a higher risk of HF in a previous report.28 In our study, we defined high ferritin when >200 ng/mL for women and >300 ng/mL for men, because we sought to include those subjects considered at a higher risk of developing iron overload cardiomyopathy, although without a significant cardiac siderosis.1 In our community-based study, there was an increased risk of HF in the group with high ferritin, in whom the average ferritin level was 358 ng/mL, suggesting that moderately increased body iron storage might also be harmful to the heart. It is unlikely that only inflammatory conditions might explain the higher HF incidence rates in subjects with mild- and moderately elevated ferritin levels. The association remains consistent after adjusting for CRP and for common cardiovascular diseases as diabetes and hypertension. Other causes of iron overload as hepatic diseases and malignancies might be considered for this discussion, but are unlikely to confound or mediate the relationship between high ferritin and HF. Although the association between iron overload and CHD is still controversy in the literature,3,29 our findings suggest that incident CHD is at least partially involved in the association because no significant relationship between high-ferritin and HF was found after including time-varying covariate for CHD. In addition, concomitant conditions, such as myocardial ischemia, may act in synergy with iron overload to cause damage to the cardiomyocyte, in which iron overload accelerate the catalytic process leading to cardiomyopathy.30 Consequently, the presence of iron in excess might make the myocardium vulnerable to other types of causal agents increasing the incidence of HF in the long-term. These data suggest that patients with mild to moderate elevation in ferritin should be considered with increased risk to develop HF. Whether different approach to reduce ferritin serum level would decrease the incidence of HF is a reason for further studies. Differently, in cases of severe iron overload, the accumulation of iron inside the cell leads to the Fenton reaction and the release of free radicals, causing oxidant-mediated cellular injury31 and development of cardiomyopathy. Only about 2.5% of the general population are homozygous for hemochromatosis,32 which likely explains why few cases of iron overload were found in our study when we defined high-ferritin as > 500 ng/mL, limiting the power to detect an association with HF.
Some limitations in our analyses should be discussed. First, ferritin was the only marker of iron status investigated. Its serum level can also be elevated in inflammatory conditions and in certain liver diseases.33 To minimize this limitation, C-reactive protein, an inflammatory marker, was included as covariate in the model for analysis. Furthermore, serum ferritin is considered the best single test for the diagnosis of iron status, being a highly specific indicator of iron deficiency. The cutoff point of 30 ng/mL was reported to provide better positive predictive values for iron-deficiency anemia (92 to 98 percent) when studied in several populations,11,12 which strengthens our findings associating low ferritin levels with HF. Interestingly, individuals with “low-normal” levels (30 ng/mL - 100 ng/mL) had no increasing in the risk of HF. It is likely that in a general population that is not in the context of chronic disease and inflammation, the “low-normal” ferritin might not represent iron-deficient individuals, as suggested elsewhere.34 Second, ferritin levels were available only at the baseline. It is possible that ferritin values may have varied over time, which could have influenced the estimation of the relationship between its serum levels and HF. In our analysis, we included the incidence of anemia in the follow-up as an approach to attenuate this potential limitation. However, further studies considering repetitive measures of iron status in the exposure variable may be needed to fully address this issue. Third, some can argue that a possible rationale for the relationship between iron deficiency and HF is the development of anemia during follow-up, the low hemoglobin level being the causative factor of heart failure. In our model, we included incident anemia as a covariate to control this confounder, and the effect of incident anemia did not account significantly for the findings. Fourth, although multivariable Cox-regression models were used, residual confounding and also confounding by unmeasured factors cannot be ruled out. Fifth, we did not use reclassification indices to compare the models including ferritin against models without ferritin because these procedures do not allow weighted analysis and we used a sampling weights dataset that were needed to appropriately analyze the data. Finally, this study sample was originally enrolled in an ARIC sub-study that investigated the relationship between ferritin levels and incident type 2 diabetes,9 half of the sample being diabetic patients, with oversampling of African-American in the ARIC cohort. We performed a weighted Cox Regression to account for the differences in the proportions of diabetes and race. Furthermore, a sensitivity analysis was performed including only the non-diabetic participants to avoid the oversampling of incident diabetes.
In conclusion, derangements in iron metabolism, as evidenced by either low or high ferritin serum levels, were associated with a higher risk of incident heart failure when compared with normal ferritin levels in this study population. These findings suggest that iron imbalance may play a role in the incidence of HF.
Supplementary Material
Acknowledgments
FUNDING
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). It is also supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK56918. The authors thank the staff and participants of the ARIC study for their important contributions. This work was also supported NHLBI cooperative agreement NHLBI-HC-11-08 (Dr. Solomon). Dr. Gonçalves was supported by the Portuguese Foundation for Science and Technology Grant HMSP-ICS/007/2012. Dr. Nadruz Junior was supported by the Brazilian National Council for Scientific and Technological Development Grant 249481/2013-8. Dr. Silvestre was supported by the J.P. Lemann Foundation as a Jorge Paulo Lemann Harvard Medical School Cardiovascular Fellow at Brigham and Women’s Hospital.
Footnotes
CONFLICT OF INTEREST: None.
References
- 1.Kremastinos DT, Farmakis D. Iron overload cardiomyopathy in clinical practice. Circulation. 2011;124:2253–2263. doi: 10.1161/CIRCULATIONAHA.111.050773. [DOI] [PubMed] [Google Scholar]
- 2.Hsu H-S, Li C-I, Liu C-S, Lin C-C, Huang K-C, Li T-C, Huang H-Y, Lin W-Y. Iron deficiency is associated with increased risk for cardiovascular disease and all-cause mortality in the elderly living in long-term care facilities. Nutrition. 2013;29:737–743. doi: 10.1016/j.nut.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Das De S, Krishna S, Jethwa A. Iron status and its association with coronary heart disease: systematic review and meta-analysis of prospective studies. Atherosclerosis. 2015;238:296–303. doi: 10.1016/j.atherosclerosis.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Murphy CJ, Oudit GY. Iron-overload cardiomyopathy: pathophysiology, diagnosis, and treatment. J Card Fail. 2010;16:888–900. doi: 10.1016/j.cardfail.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Dong F, Zhang X, Culver B, Chew HG, Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci. 2005;109:277–286. doi: 10.1042/CS20040278. [DOI] [PubMed] [Google Scholar]
- 6.Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, Tsagalou EP, Maroulidis GD, Alexopoulos GP, Kanakakis JE, Anastasiou-Nana MI. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol. 2006;48:2485–2489. doi: 10.1016/j.jacc.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J. 2013;34:816–829. doi: 10.1093/eurheartj/ehs224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 9.Jehn ML, Guallar E, Clark JM, Couper D, Duncan BB, Ballantyne CM, Hoogeveen RC, Harris ZL, Pankow JS. A prospective study of plasma ferritin level and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 2007;165:1047–1054. doi: 10.1093/aje/kwk093. [DOI] [PubMed] [Google Scholar]
- 10.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 11.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 12.Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol. 2005;18:319–332. doi: 10.1016/j.beha.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Folsom AR, Yamagishi K, Hozawa A, Chambless LE Atherosclerosis Risk in Communities Study Investigators. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okonko DO, Mandal AKJ, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58:1241–1251. doi: 10.1016/j.jacc.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 15.Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575–582.e3. doi: 10.1016/j.ahj.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Scott PA, Kingsley GH, Scott DL. Non-steroidal anti-inflammatory drugs and cardiac failure: meta-analyses of observational studies and randomised controlled trials. Eur J Heart Fail. 2008;10:1102–1107. doi: 10.1016/j.ejheart.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Klip IT, Postmus D, Voors AA, Brouwers FP, Gansevoort RT, Bakker SJ, Hillege HL, de Boer RA, van der Harst P, van Gilst WH, van Veldhuisen DJ, van der Meer P. Hemoglobin levels and new-onset heart failure in the community. Am Heart J. 2015;169:94–101. e2. doi: 10.1016/j.ahj.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Sarnak MJ, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, Levey AS. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol. 2002;40:27–33. doi: 10.1016/s0735-1097(02)01938-1. [DOI] [PubMed] [Google Scholar]
- 19.Beverborg NG, van der Wal HH, Klip IT, Voors AA, de Boer RA, van Gilst WH, van Veldhuisen DJ, Gansevoort RT, Hillege HL, van der Harst P, Bakker SJL, van der Meer P. High serum erythropoietin levels are related to heart failure development in subjects from the general population with albuminuria: data from PREVEND. Eur J Heart Fail. 2016;18:814–821. doi: 10.1002/ejhf.484. [DOI] [PubMed] [Google Scholar]
- 20.Brownlie T, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79:437–443. doi: 10.1093/ajcn/79.3.437. [DOI] [PubMed] [Google Scholar]
- 21.Lederman HM, Cohen A, Lee JW, Freedman MH, Gelfand EW. Deferoxamine: a reversible S-phase inhibitor of human lymphocyte proliferation. Blood. 1984;64:748–753. [PubMed] [Google Scholar]
- 22.Dillmann E, Johnson DG, Martin J, Mackler B, Finch C. Catecholamine elevation in iron deficiency. Am J Physiol. 1979;237:R297–R300. doi: 10.1152/ajpregu.1979.237.5.R297. [DOI] [PubMed] [Google Scholar]
- 23.Rossi MA, Carillo SV. Pathogenesis of cardiac hypertrophy in iron deficiency anaemia: the role of noradrenaline. Br J Exp Pathol. 1982;63:269–277. [PMC free article] [PubMed] [Google Scholar]
- 24.Yeo TJ, Yeo PSD, Ching-Chiew Wong R, Ong HY, Leong KTG, Jaufeerally F, Sim D, Santhanakrishnan R, Lim SL, Chan MYM, Chai P, Low AF, Ling LH, Ng TP, Richards AM, Lam CSP. Iron deficiency in a multi-ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis. Eur J Heart Fail. 2014;16:1125–1132. doi: 10.1002/ejhf.161. [DOI] [PubMed] [Google Scholar]
- 25.Corti MC, Guralnik JM, Salive ME, Ferrucci L, Pahor M, Wallace RB, Hennekens CH. Serum iron level, coronary artery disease, and all-cause mortality in older men and women. Am J Cardiol. 1997;79:120–127. doi: 10.1016/s0002-9149(96)00697-2. [DOI] [PubMed] [Google Scholar]
- 26.McClung JP, Murray-Kolb LE. Iron nutrition and premenopausal women: effects of poor iron status on physical and neuropsychological performance. Annu Rev Nutr. 2013;33:271–288. doi: 10.1146/annurev-nutr-071812-161205. [DOI] [PubMed] [Google Scholar]
- 27.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan B-A, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 28.Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, Wu D, Taylor J, Westwood MA, Anderson LJ, Pennell DJ. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120:1961–1968. doi: 10.1161/CIRCULATIONAHA.109.874487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danesh J, Appleby P. Coronary heart disease and iron status: meta-analyses of prospective studies. Circulation. 1999;99:852–4. doi: 10.1161/01.cir.99.7.852. [DOI] [PubMed] [Google Scholar]
- 30.Gujja P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy: better understanding of an increasing disorder. J Am Coll Cardiol. 2010;56:1001–1012. doi: 10.1016/j.jacc.2010.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366:348–359. doi: 10.1056/NEJMra1004967. [DOI] [PubMed] [Google Scholar]
- 32.Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, Dawkins FW, Acton RT, Harris EL, Gordeuk VR, Leiendecker-Foster C, Speechley M, Snively BM, Holup JL, Thomson E, Sholinsky P Hemochromatosis and Iron Overload Screening (HEIRS) Study Research Investigators. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352:1769–1778. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 33.Qaseem A, Aronson M, Fitterman N, Snow V, Weiss KB, Owens DK Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening for hereditary hemochromatosis: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;143:517–521. doi: 10.7326/0003-4819-143-7-200510040-00010. [DOI] [PubMed] [Google Scholar]
- 34.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.