Abstract
Objective
DARPP-32 is a frequently amplified and overexpressed gene that promotes several oncogenic functions in gastric cancer. Herein, we investigated the relationship between Helicobacter pylori infection, proinflammatory NF-κB activation and regulation of DARPP-32.
Design
The study used in vivo and in vitro experiments. Luciferase reporter, quantitative real-time PCR, immunoblot, chromatin immunoprecipitation (ChIP), cell viability, H. pylori infection, tissue microarrays and immunohistochemical assays were used.
Results
Our results indicated that H. pylori infection increased the DARPP-32 mRNA and protein levels in gastric cancer cell lines and gastric mucosa of mice. H. pylori infection increased the activity of NF-κB reporter and p-NF-κB (S536) protein level in vitro and in vivo. To investigate the transcriptional regulation of DARPP-32, we cloned a 3019 bp of the DARPP-32 promoter into the luciferase reporter (pGL3-Luc). Both H. pylori infection and tumour necrosis factor-α treatment induced DARPP-32 reporter activity (p<0.01). Using deletion constructs of DARPP-32 promoter and ChIP assay, we demonstrated that the sequence −996 to −1008 bp containing putative NF-κB-binding sites is the most active region. The induction of DARPP-32 expression by H. pylori infection counteracted H. pylori-induced cell death through activation of serine/threonine-specific protein kinase (AKT), as determined by ATP-Glo and clonogenic survival assays. Immunohistochemistry analysis demonstrated a significant positive correlation between NF-κB and DARPP-32 expression levels in gastric cancer tissues (r2=0.43, p<0.01).
Conclusions
Given the high frequency of DARPP-32 overexpression and its prosurvival oncogenic functions, the induction of DARPP-32 expression following H. pylori infection and activation of NF-κB provides a link between infection, inflammation and gastric tumourigenesis.
INTRODUCTION
Although the incidence of gastric cancer is declining,1 it remains the third leading cause of cancer-associated death worldwide.2 We have previously shown that dopamine and cAMP-regulated phosphoprotein, molecular weight 32 000 (DARPP-32) is a novel cancer gene, which is overexpressed in two-thirds of patients with gastric cancer.3,4 DARPP-32 overexpression is detectable in early stages of gastric carcinogenesis cascade, and immunohistochemical analysis suggested that it may participate in transition from atrophic gastritis to intestinal metaplasia and progression to neoplasia.3 Recent studies have shown that DARPP-32 promotes cancer cell survival, drug resistance and invasion.5–9 However, the mechanisms that regulate DARPP-32 expression and promote gastric carcinogenesis remain unclear.
Infection with Helicobacter pylori has been classified by the WHO as a group 1 carcinogen10 and considered a major risk factor for the development of gastric cancer.11,12 Infection with H. pylori induces inflammatory responses in the host, which lead to chronic inflammation and the development of atrophic gastritis that can progress to cancer.13,14 Previous studies suggest that apoptosis and DNA damage are increased in gastric epithelial cells during infection with H. pylori.15,16 Chronic H. pylori infection results in generation of a subpopulation of gastric epithelial cells with high levels of DNA damage that are resistant to apoptosis.15 The accumulation and survival of cells with damaged DNA heightens the risk of development of gastric carcinoma.17 In most cases, eradication of the microorganism leads to resolution of inflammation, which in many instances can result in a beneficial effect in preventing the development of subsequent gastric dysplasia and gastric cancer.18 In the absence of eradication, infection tends to be life long and the immune response is ineffective in clearing the bacteria.19
NF-κB transcription factors comprised the REL-homology domain proteins p50, p52, RelA (also called p65), RelB and cREL.20 In the absence of specific extracellular signals, NF-κB inhibitors such as IκB, p105 and p100 proteins tether to NF-κB in the cytoplasm to prevent NF-κB-mediated gene transcription.21 When cells receive appropriate stimuli such as tumour necrosis factor-α (TNF-α), IκB phosphorylation occurs leading to IκB ubiquitination and proteasomal degradation, and translocation of NF-κB to the nucleus where it binds to its specific promoter elements to activate gene expression.22 NF-κB signalling controls the expression of several genes implicated in inflammation, cell survival and cancer.23 In addition to its role for survival of cancer cells, NF-κB has recently been shown to be activated in cancer stem cells, where it can promote a proinflammatory environment, inhibit apoptosis and stimulate cell proliferation.24
The aim of this study was to investigate whether proinflammatory H. pylori infection and NF-κB activation play a role in regulating DARPP-32 expression and its prosurvival functions in gastric cancer. Our results demonstrate, for the first time, that DARPP-32 is a novel transcription target of NF-κB that is induced in response to H. pylori infection and activation of NF-κB to counteract infection-induced cell death and promote cell survival in gastric carcinogenesis.
MATERIALS AND METHODS
Cell culture and reagents
Human gastric cancer cell lines including AGS and MKN-45 and the immortalised human embryonic kidney epithelial cell line (HEK-293) were cultured in Dulbecco’s modified Eagle’s medium (GIBCO, Carlsbad, California, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen Life Technologies, Carlsbad, California, USA) and 1% penicillin/streptomycin (GIBCO). Recombinant human TNF-α was purchased from PeproTech (Rocky Hill, New Jersey, USA). Bay 11-7082 and 11-7085 were purchased from Sigma–Aldrich (St Louis, Missouri, USA). DARPP-32 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, California, USA). Horseradish peroxidase (HRP)-conjugated mouse and rabbit secondary antibodies, p-P65 (S536), P65, p-serine/threonine-specific protein kinase (AKT) (S473), AKT and β-actin antibodies, were purchased from Cell Signaling Technology (Danvers, Massachusetts, USA).
DARPP-32 expression, shRNA and siRNA
The flag-tagged coding sequence of DARPP-32 was cloned in pcDNA3.1 mammalian expression plasmid (Invitrogen Life Technologies). AGS cells stably expressing DARPP-32 or pcDNA3.1 empty vector were generated as described previously.4,5 Flag-tagged DARPP-32 was cloned into the adenoviral (pACCMV) shuttle vector, and the adenovirus was generated by cotransfecting HEK-293 cells with the shuttle and (pJM17) backbone adenoviral plasmids using the Calcium Phosphate Transfection Kit (Applied Biological Materials, Richmond, British Columbia, USA). Lentivirus particles expressing DARPP-32 shRNA or control shRNA were produced by GeneCopoeia (Rockville, Maryland, USA) and then used to transduce MKN-45 cells. Control siRNA (universal negative control) was purchased from Sigma–Aldrich; DARPP-32 siRNA (sc-35173) was obtained from Santa Cruz Biotechnology.
H. pylori strains
The wild-type H. pylori Cag+ strains 7.13 and J166 and the rodent-adapted Cag+ H. pylori strain PMSS1 were used in this study. Isogeneic 7.13 CagE− (Cag secretion system ATPase) and CagA− (Cag secretion system effector protein) mutants were constructed by insertional mutagenesis using aphA (conferring kanamycin resistance).25 H. pylori bacteria were cultured on trypticase soy agar with 5% sheep blood agar plates (BD Biosciences, Bedford, Massachusetts, USA) for in vitro passage, as previously described.25,26 Isogenic mutants were also cultured on Brucella agar (BD Biosciences) plates containing 20 µg/mL kanamycin (Sigma–Aldrich) to confirm the presence of the kanamycin antibiotic-resistance cassette. H. pylori strains were then cultured in Brucella broth (BB, BD Biosciences) supplemented with 10% FBS (Atlanta Biologicals, Flowery Branch, Georgia, USA) for 16–18 hours at 37°C with 5% CO2. For in vitro studies, the bacteria were co-cultured with gastric epithelial cells at a multiplicity of infection of 50:1.
Infection of mice with H. pylori
All animal studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Vanderbilt University Medical Center’s Institutional Animal Care and Use Committee approved all protocols and all efforts were made to minimise animal suffering. C57BL/6 mice were purchased from Charles River Laboratories for this study and housed in the Vanderbilt University Animal Care Facilities. Mice (10 mice per group) were orogastrically challenged with BB, as an uninfected (UI) control or with the mouse-adapted wild-type H. pylori strain PMSS1 (109 CFU/mouse).27 Mice were euthanised at 7 days and 2–4 months postchallenge, and gastric tissues were harvested for western blot and real-time PCR analyses.
Quantitative real-time PCR analysis
Total RNA was isolated from cell lines by using the RNeasy Mini Kit (Qiagen, Valencia, California, USA). Total RNA (1 µg) was reverse transcribed by an iScript cDNA synthesis kit (Bio-Rad, Hercules, California, USA). The quantitative real-time PCR (qRT-PCR) was performed using a Bio-Rad CFX Connect Real-time System (Bio-Rad), with the threshold cycle number determined by Bio-Rad CFX manager software V.3.0. The primers for human DARPP-32 were forward: 5′-CTACACACCACCTTCGCTGA-3′; reverse: 5′-CAGCTCATCCTCCTCCTCTG-3′. The primers for p65 were forward: 5′-TGTTTCATTTGGATCCTTCTTTG-3′; reverse: 5′-GGATGGGCCTTCACATACAT-3′. The primers for IL-8 were forward: 5′-TCCTGATTTCTGCAGCTCTGT-3′; reverse: 5′-AAATTTGGGGTGGAAAGGTT-3. The primers for TNF-α were forward: 5′-GCCAGAGGGCTGATTAGAGA-3′; reverse: 5′-AAATTTGGGGTGGAAAGG TT-3′. The primers for human HPRT1 were forward: 5′-TTGGAAAGGGTGTTTATTCCTCA-3′; reverse: 5′-TCCAGCAGGTCAGCAAAGAA-3′. The primers for mouse DARPP-32 were forward: 5′-AAGGACCGCAAGAAGATTCA-3′; reverse: 5′-GCTCTGAGACCCGGAAAAG-3′. The primers for mouse HPRT were forward: 5′-TATGCCGAGGATTTGGAAAA-3′; reverse: 5′-ACAGAGGGCCACAATGTGAT-3′. The primers for PMSS1 were forward: 5′-CGTCCGGCAATAGCTGCCATAGT-3′; reverse: 5′-GTAGGTCCTGCTACTGAAGCCTTA-3′. Reactions were performed in duplicate, and results of three independent experiments were subjected to statistical analysis. Fold change was calculated using the ΔΔC(t) method.28 HPRT1 was used as a normalisation control.
DARPP-32 promoter and luciferase activity assays
To construct the DARPP-32 promoter, we amplified the promoter region from −96 to −3115 from transcription start site by PCR using hot-start DNA polymerase (New England Biolabs, Ipswich, Massachusetts, USA). A 3-kb fragment containing KpnI and HindIII sites was then ligated into the PCR8 vector by using a TA cloning kit (Invitrogen Life Technologies). The promoter fragment was excised from PCR8-DARPP-32 by digesting it with KpnI and HindIII, and then cloned in the pGL3-basic vector to produce the pGL3-DARPP-32 promoter luciferase reporter. The deletion constructs were made by amplifying the pGL3-DARPP-32 plasmid with hot-start DNA polymerase, using specific primers designed with KpnI and HindIII restriction sites, followed by ligation of the digested fragments.
Subconfluent cells cultured in 12-well plates were transiently cotransfected with luciferase reporter and internal control plasmids, using the DNAfectin Transfection Reagent according to the manufacturer’s protocol (Applied Biological Materials). Forty-eight hours post-transfection, cells were lysed in passive lysis buffer, and luciferase assay was performed using dualluciferase reporter assay kit (Promega, Madison, Wisconsin, USA). Renilla luciferase activity was used as control for the transfection. Each transfection was performed in triplicate.
Western blotting
Western blotting was performed using standard methods. Cells were washed with cold phosphate-buffered saline (PBS) and lysed using radio immunoprecipitation assay (RIPA) buffer (10 mM Tris–HCl (pH 7.2), 150 mM NaCl, 5 mM EDTA, 0.1% sodium dodecyl sulfate (SDS), 1.0% Triton X-100, 1% deoxycholate). Lysates were centrifuged at 13 000 rpm for 5 min at 4°C. Proteins were separated on 12.5% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to Immobilon (polyvinylidene difluoride) transfer membrane (PVDF) membrane (Millipore, Billerica, Massachusetts, USA). Membranes were probed with specific antibodies. Proteins were then visualised by using HRP-conjugated secondary antibodies and Immobilon Western Chemiluminescent HRP Substrate detection reagent (Millipore). β-Actin was used as the loading control. All blots were imaged using Bio-Rad ChemiDoc XRS+ System (Bio-Rad).
Chromatin immunoprecipitation assay
AGS cells were fixed with formaldehyde (1% final concentration, Sigma–Aldrich) and chromatin fragmentation was done by sonication on ice for four cycles (30 s ‘ON’, 30s ‘OFF’ at 40% amplitude) to yield an average length of <500 bp. Chromatin immunoprecipitation (ChIP) assay was performed using Zymo-Spin ChIP Kit (Irvine, California, USA) following the manufacturer’s protocol. Briefly, the supernatants of the fragmented lysates were diluted 10-fold with chromatin dilution buffer. Chromatin solutions were immunoprecipitated with P65 antibody at 4°C overnight. ZymoMag Protein A beads were added to the lysate to isolate the antibody-bound complexes. The elute was reverse cross-linked by heating at 65°C for 30 min. Samples were then treated with proteinase K for 90 min at 65°C to digest the proteins that were immunoprecipitated. To identify the P65-binding sequence, two sets of primers were used for PCR amplification of the DARPP-32 promoter region: primers for P65-binding sites: DARPP-32-CHIP-F: 5′-GAAGCATTCAGACCCTCTGC-3′, DARPP-32-CHIP-R: 5′-TGTGAGCCTCTCTCTCTCCAG-3′; and control primers: DARPP-32-CTRL-F: 5′-TGTCCTCCCACACACAACTC-3′, DARPP-32-CTRL-R: 5′-GAGGCAGGACTCTGGCTTTA-3′.
Cell viability assay
For the quantitative estimation of cell growth and survival, we have used the CellTiter-Glo Cell Viability Assay (Promega). Cells were plated in 96-well microplates at 1000 cells per well. Measurements using BMG FLUOstar OPTIMA Microplate Reader (BMG LABTECH, Cary, North Carolina, USA) were conducted following the manufacturer’s protocol.
Tissue microarray and immunohistochemistry
A total of 159 paraffin-embedded human gastric tissue samples (32 normal, 33 metaplasia, 25 dysplasia and 69 adenocarcinoma) were available for immunohistochemical analysis. All tissue samples were deidentified in accordance with Institutional Review Board-approved protocols. Tissues were stained with H&E and representative regions were selected for inclusion in a tissue array. Control samples from normal epithelial specimens were punched in each sample row. Sections (5 µm) were transferred to polylysine-coated slides (SuperFrostPlus; Menzel-Glaser, Braunschweig, Germany) and incubated at 37°C for 2 hours. DARPP-32 (H62) antibody was obtained from Santa Cruz Biotechnology, and Rabbit p-NF-κB (Ser536) antibody was purchased from Cell Signaling.29 The resulting tumour tissue array was subjected to immunohistochemical analysis for DARPP-32 and NF-κB. The immunoreactivity of the samples tested was assessed by a trained pathologist and scored for intensity (scaled 0–3) and frequency (scaled 0–4). For purposes of statistical analysis, DARPP-32 and NF-κB proteins’ intensity and frequency were transformed into a composite expression score (CES) using the formula CES=4(intensity−1)+frequency. The range of CES was from 0 to 12.3,29
Statistical analyses
Data were expressed as mean±SD of three independent experiments. Statistical significance of the in vitro and in vivo studies was analysed by one-way Analysis of variance (ANOVA), Student’s t-test and Pearson’s method. Differences with p values ≤0.05 are considered significant.
RESULTS
H. pylori induces DARPP-32 expression in gastric epithelial cells in vitro and in vivo
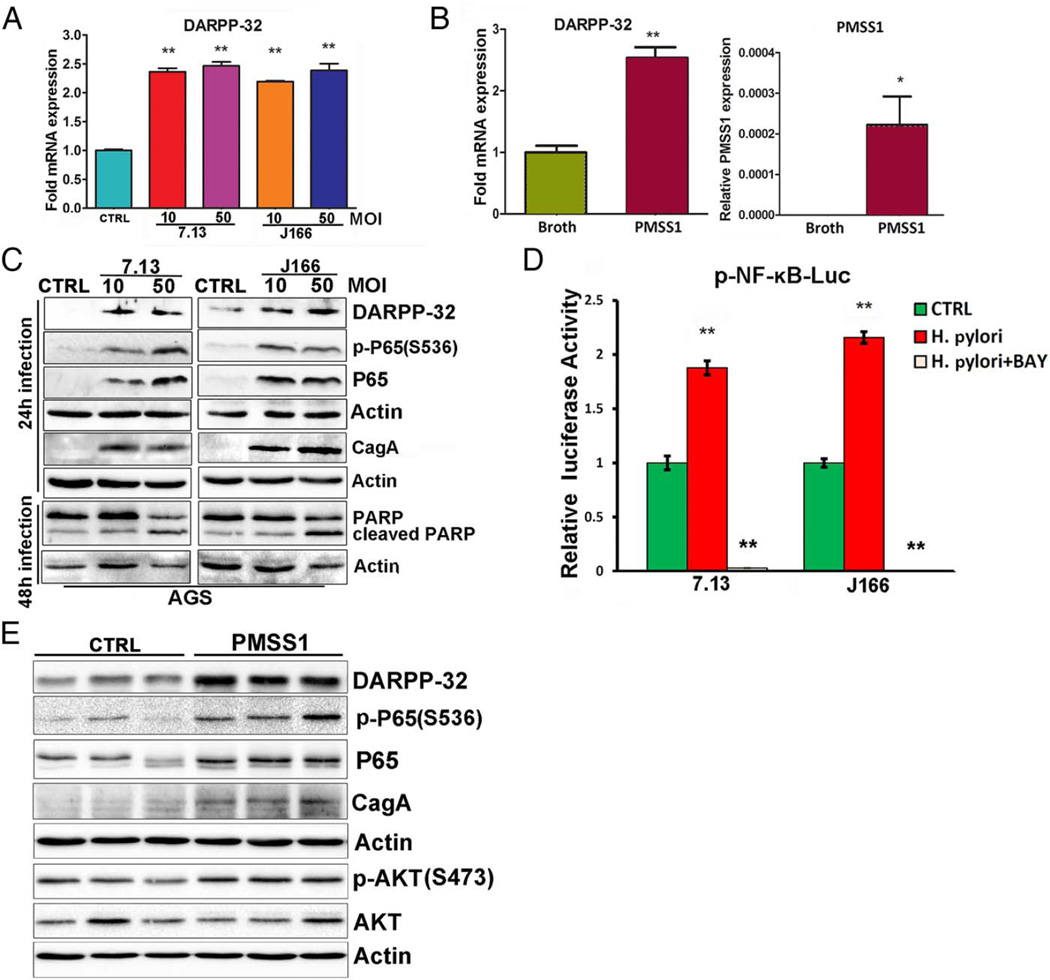
H. pylori infection activates NF-κB through both the canonical and non-canonical pathways.30 Because of the frequent overexpression of DARPP-32 in early stages of gastric tumourigenesis, we investigated whether H. pylori infection could upregulate DARPP-32 expression through activation of NF-κB. Following cultures of AGS cells alone or cocultured with the wild-type Cag+ H. pylori strain 7.13 or J166, the qRT-PCR results showed that H. pylori infection significantly increased DARPP-32 mRNA (figure 1A). To further support our in vitro results, we used an in vivo mouse model of H. pylori infection. C57BL/6 mice were challenged with BB as a negative UI control, or wild-type mouse-adapted Cag+ H. pylori strain PMSS1. The qRT-PCR analysis of gastric tissues showed that infection with H. pylori strain PMSS1 resulted in significantly increased DARPP-32 mRNA expression as compared with UI controls (p<0.01, figure 1B). Western blot analysis data showed that H. pylori infection upregulated DARPP-32, activated cleavage of poly ADP ribose polymerase (PARP) and promoted induction of p-P65 (S536) and P65 protein expression levels, confirming activation of NF-κB (figure 1C). To confirm the proinflammatory effect of H. pylori, we evaluated the mRNA expression of inflammation-mediating cytokines in AGS cells infected with H. pylori. The qRT-PCR data indicated that H. pylori infection significantly induced TNF-α (p<0.05, see online supplementary figure S1A) and IL-8 (p<0.01, see online supplementary figure S1B). To confirm that H. pylori infection leads to activation of NF-κB, we cultured AGS cells alone or cocultured with the wild-type Cag+ H. pylori strain 7.13 or J166, and with or without transfection of the p-NF-κB luciferase reporter plasmid. The luciferase data indicated a significant induction of NF-κB transcription activity (p<0.01) following H. pylori infection; an effect that was blocked by Bay 11-7082, a NF-κB inhibitor (figure 1D). In support of the in vitro findings, the results from the C57BL/6 mice that were challenged with BB as a negative UI control; or wild-type mouse-adapted Cag+ H. pylori strain PMSS1 for 7 days showed that H. pylori infection upregulated DARPP-32 and promoted induction of p-P65 (S536) and p-AKT (S473) protein expression levels (figure 1E).
Figure 1.
Helicobacter pylori infection promotes DARPP-32 expression and activation of NF-κB–P65 in vitro and in vivo. (A) The quantitative real-time (qRT) PCR of DARPP-32 was performed in AGS cells with and without H. pylori infection (7.13 and J166 CagA+ strains). (B) The qRT-PCR analysis of DARPP-32 in gastric tissues collected from mice that were orogastrically challenged with Brucella broth or with CagA+ mouse-adapted H. pylori strain (PMSS1). The expression level of PMSS1 CagA is also shown. (C) Western blot analysis of p-P65 (S536), P65, CagA, cleaved PARP, PARP and DARPP-32 in AGS cells with and without H. pylori infection. (D) Luciferase reporter assay for p-NF-κB-Luc in AGS cells without infection (CTRL), with H. pylori infection alone or in combination with NF-κB inhibition using Bay 11-7082 treatment (BAY). (E) Western blot analysis of p-P65 (S536), P65, CagA, p-AKT (S473), AKT and DARPP-32 using gastric tissues collected from mice that were orogastrically challenged with Brucella broth or with CagA+ mouse-adapted H. pylori strain (PMSS1) for 7 days. MOI, multiplicity of infection.
NF-κB transcriptionally upregulates DARPP-32 expression
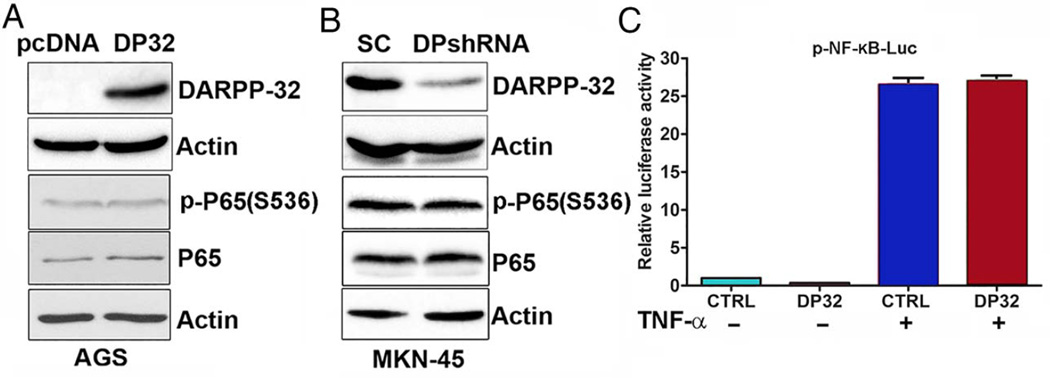
The aforementioned results raised questions whether DARPP-32 regulates NF-κB or NF-κB regulates DARPP-32. To investigate this relationship between DARPP-32 and NF-κB signalling, we first investigated whether DARPP-32 regulates NF-κB-P65 phosphorylation or activity using AGS and MKN-45 cell models. Our data indicated that stable overexpression of DARPP-32 had no significant effects on P65 and p-P65 (S536) protein levels as evaluated by western blot analysis (figure 2A). Furthermore, knocking down endogenous DARPP-32 in MKN-45 cells did not affect P65 and p-P65 (S536) protein expression (figure 2B). Of note, the NF-κB reporter luciferase data showed that overexpression of DARPP-32 in AGS cells had no significant effects on NF-κB transcription activity with or without treatment with TNF-α (figure 2C). These findings clearly indicate that DARPP-32 has no effect on transcription activity of NF-κB.
Figure 2.
DARPP-32 does not regulate NF-κB–P65 expression or activation. (A) Western blot analysis of p-P65 (S536), P65 and DARPP-32 proteins in AGS cells stably expressing DARPP-32 (DP32) or empty vector control (pcDNA). (B) p-P65 (S536), P65 and DARPP-32 protein levels were evaluated by immunoblot analysis in control MKN-45/SC shRNA (SC) and MKN-45/DARPP-32 shRNA stable cells (DPshRNA). (C) Luciferase reporter assay for p-NF-κB-Luc in AGS cells stably expressing DARPP-32 or an empty vector control (CTRL) with or without tumour necrosis factor (TNF)-α treatment.
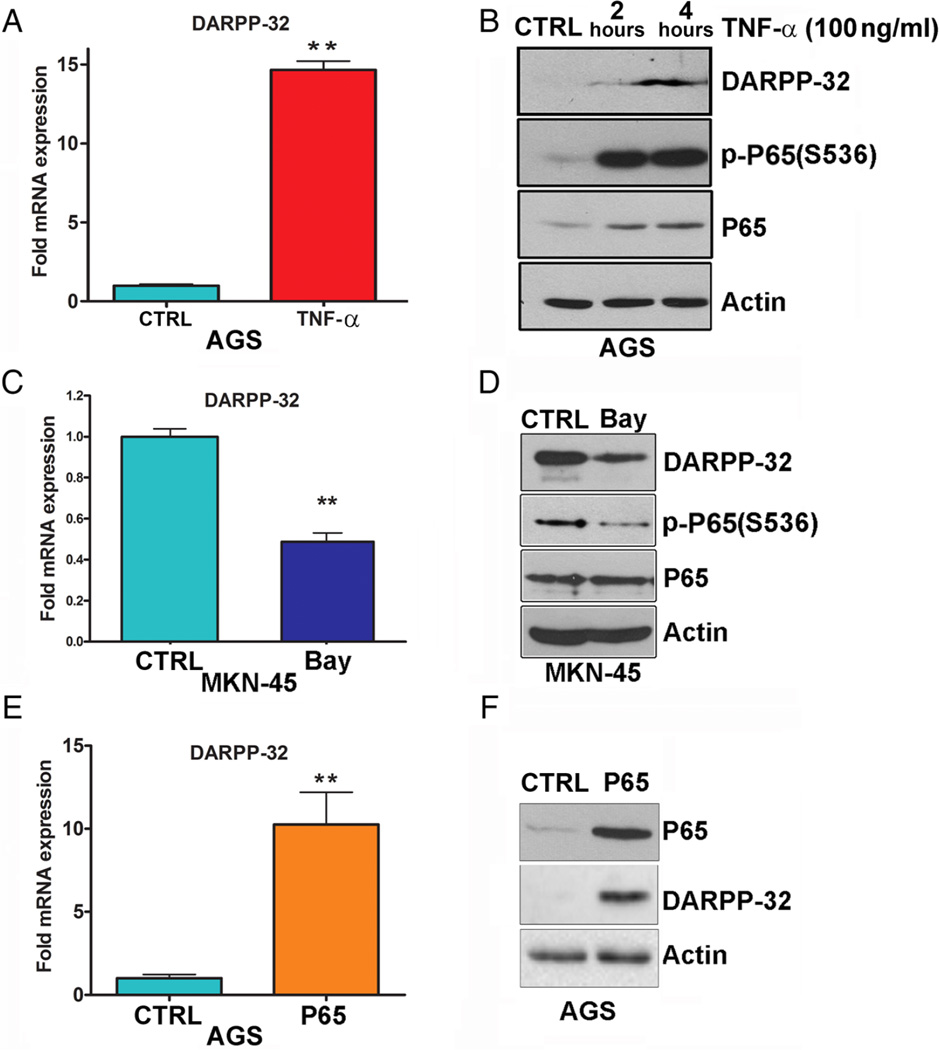
We next investigated whether NF-κB regulates DARPP-32 expression. We treated AGS and MKN-45 gastric cancer cell lines with TNF-α or a NF-κB inhibitor (Bay 11-7082) to activate or inhibit NF-κB, respectively, and evaluated DARPP-32 mRNA and protein expression by qRT-PCR and western blot analysis. The results demonstrated a significant induction of the p-NF-κB reporter luciferase activity following TNF-α treatment in AGS cells (see online supplementary figure S2). We found that TNF-α significantly upregulated DARPP-32 mRNA (figure 3A) and protein (figure 3B) expression in AGS cells. In addition, inhibition of NF-κB with Bay 11-7082 significantly decreased endogenous DARPP-32 mRNA (figure 3C) and protein (figure 3D) expression levels in MKN-45 cells. We further confirmed these findings using transient expression of P65 in AGS cells which led to a 10-fold induction of DARPP-32 mRNA expression (figure 3E) with a notable increase in DARPP-32 protein level (figure 3F). Collectively, these data suggested that NF-κB plays an important role in transcriptional regulation of DARPP-32.
Figure 3.
Activation of NF-κB–P65 upregulates DARPP-32 expression. The quantitative real-time (qRT) PCR (A) and western blot (B) analyses of DARPP-32 was performed in AGS cells following tumour necrosis factor (TNF)-α treatment. (C and D) The qRT-PCR and immunoblot analyses of DARPP-32 were performed in MKN-45 cells after Bay 11-7082 treatment. (E and F) The qRT-PCR and immunoblot analyses were performed to determine DARPP-32 expression in AGS cells transiently transfected with P65 or empty vector.
H. pylori regulates DARPP-32 promoter activity and mRNA expression through NF-κB-P65 binding
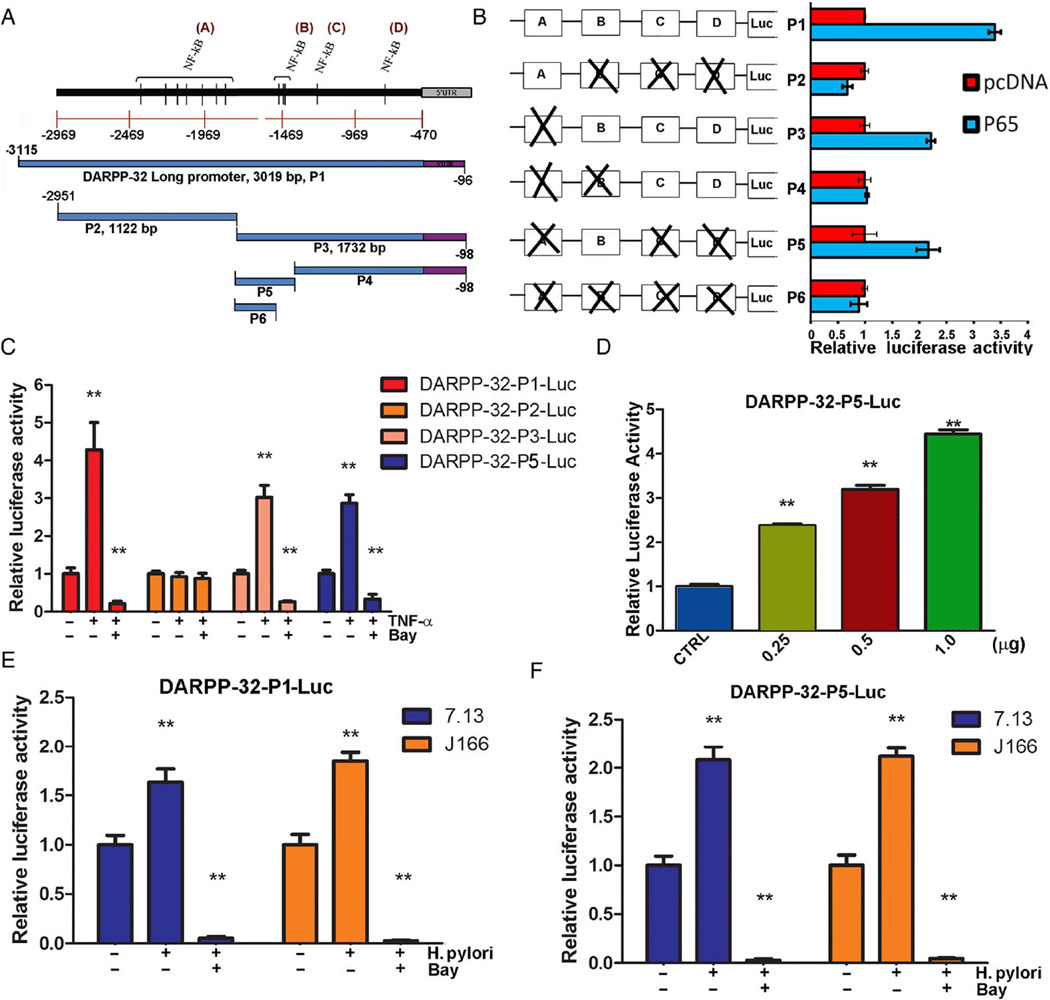
Promoter analysis of DARPP-32, using PROMO tool V.8.3 of TRANSFAC (Beverly, MA, USA), indicated the presence of NF-κB transcription factor putative-binding sites that cluster into four main regions (figure 4A). To characterise the crucial elements for regulation in the DARPP-32 promoter region by NF-κB-P65, we generated six pGL3-based luciferase constructs of DARPP-32 (P1–P6) containing clusters A, B, C or D (figure 4B). A luciferase reporter assay revealed that DARPP-32-P2-Luc, DARPP-32–P4-Luc and DARPP-32-P6-Luc reporter activities were not increased in AGS cells transfected with P65. Conversely, the reporter activities of DARPP-32-P1-Luc, DARPP-32-P3-Luc and DARPP-32-P5-Luc were significantly increased in P65-transfected AGS cells (figure 4B). These results suggest that the regulatory promoter element for regulation of DARPP-32 transcription is located in cluster B.
Figure 4.
NF-κB–P65 regulates DARPP-32 promoter activity. (A) A scheme showing putative NF-κB transcription factor binding sites on DARPP-32 promoter, and the different deletion constructs of the DARPP-32 promoter (P1–P6). (B) Luciferase reporter assay for various DARPP-32-P (1–6)-Luc constructs in AGS cells expressing P65 or empty vector. (C) Luciferase reporter assay for DARPP-32-P1-Luc, DARPP-32-P2-Luc, DARPP-32-P3-Luc and DARPP-32-P5-Luc in AGS cells treated with tumour necrosis factor (TNF)-α alone or in combination with Bay 11-7082. (D) Luciferase reporter assay for DARPP-32-P5-Luc in AGS cells following transient transfection with empty vector or different amounts of P65. (E) Luciferase reporter assay for DARPP-32-P1-Luc in AGS cells infected with Helicobacter pylori alone or in combination with Bay 11-7082 treatment. (F) Luciferase reporter assay for DARPP-32-P5-Luc in AGS cells infected with H. pylori alone or in combination with Bay 11-7082 treatment.
To further validate NF-κB-P65 and DARPP-32 promoter interaction, AGS cells were transfected with DARPP-32-P1-Luc, DARPP-32-P2-Luc, DARPP-32-P3-Luc or DARPP-32-P5-Luc luciferase reporter plasmids, and then treated with TNF-α alone or in combination with Bay 11-7082 (figure 4C). The promoter activity analysis showed that the relative luciferase activity of the DARPP-32-P1-Luc, DARPP-32-P3-Luc and DARPP-32-P5-Luc significantly increased (p<0.01) with TNF-α treatment. Treatment with Bay 11-7082 reversed these effects; in contrast, the relative luciferase activity of the shorter deletion construct (DARPP-32-P2-Luc), containing cluster A, was not increased by TNF-α stimulation (figure 4C). Notably, transient overexpression of P65 led to a dose-dependent increase in luciferase activity of the DARPP-32-P5-Luc construct (p<0.01, figure 4D). To further confirm the role of H. pylori in DARPP-32 transcription regulation, AGS cells were transfected with DARPP-32-P1-Luc or DARPP-32-P5-Luc luciferase reporter plasmid and cocultured with or without H. pylori strains (7.13 or J166) alone or in combination with Bay 11-7082. We found a significant induction of the DARPP-32-P1-Luc and DARPP-32-P5-Luc reporters activity following H. pylori infection; the luciferase activity was completely blocked following inhibition of NF-κB with Bay 11-7082 (p<0.01, figure 4E, F). To further support the role of H. pylori in DARPP-32 transcription regulation, AGS cells were transfected with DARPP-32-P5-Luc luciferase reporter plasmid and cocultured with H. pylori 7.13 strains (wild type, CagA− and CagE− mutant). A threefold induction of the DARPP-32-P5-Luc reporter activity was found following H. pylori 7.13 wild-type infection; the luciferase activity was significantly lower with H. pylori 7.13 mutants’ infection (p<0.01, see online supplementary figure S3).
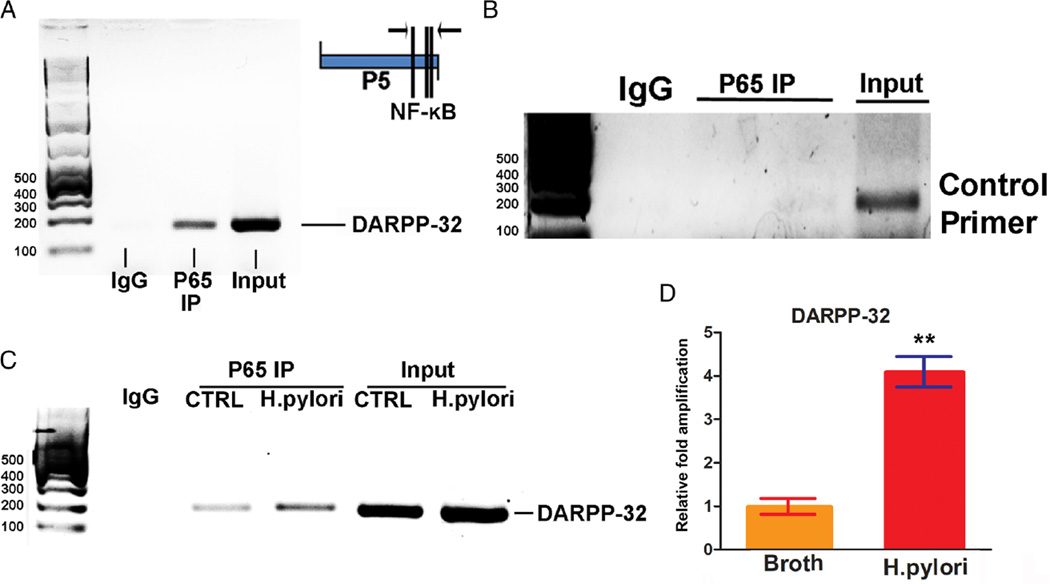
To determine whether NF-κB regulates the expression of DARPP-32 by binding directly to the promoter, we performed ChIP assay by using a specific antibody against P65 to immunoprecipitate formaldehyde-fixed chromatin in AGS cells. We found that P65 directly binds to DARPP-32 promoter and not to negative control (figure 5A, B). Treatment with TNF-α induced a stronger p65 binding to the DARPP-32 promoter, as compared with control (see online supplementary figure S4). To confirm the role of H. pylori in DARPP-32 transcriptional regulation, AGS cells were cocultured with H. pylori strain J166 and subjected to ChIP assay. The data showed that H. pylori infection significantly increased the NF-κB-p65 protein binding to the DARPP-32 promoter, as compared with control group (figure 5C, D).
Figure 5.
Chromatin immunoprecipitation (ChIP) assay confirms the binding of NF-κB–P65 on DARPP-32 promoter. (A) ChIP assay by using a specific antibody against P65 to immunoprecipitate formaldehyde-fixed chromatin in AGS cells, followed by regular PCR with primers designed for NF-κB–P65 binding site of DARPP-32 promoter region (P5, as shown in figure 4B). (B) ChIP assay by using a specific antibody against P65 to immunoprecipitate formaldehyde-fixed chromatin in AGS cells, followed by regular PCR with control primers. (C) ChIP assay in AGS cells infected with Helicobacter pylori, followed by regular PCR with primers designed for NF-κB–P65 binding site of DARPP-32 promoter region (P5). (D) ChIP assay in AGS cells infected with H. pylori, followed by quantitative real-time PCR with primers designed for NF-κB–P65 binding site of DARPP-32 promoter region.
H. pylori activates the prosurvival AKT pathway through regulation of DARPP-32 expression
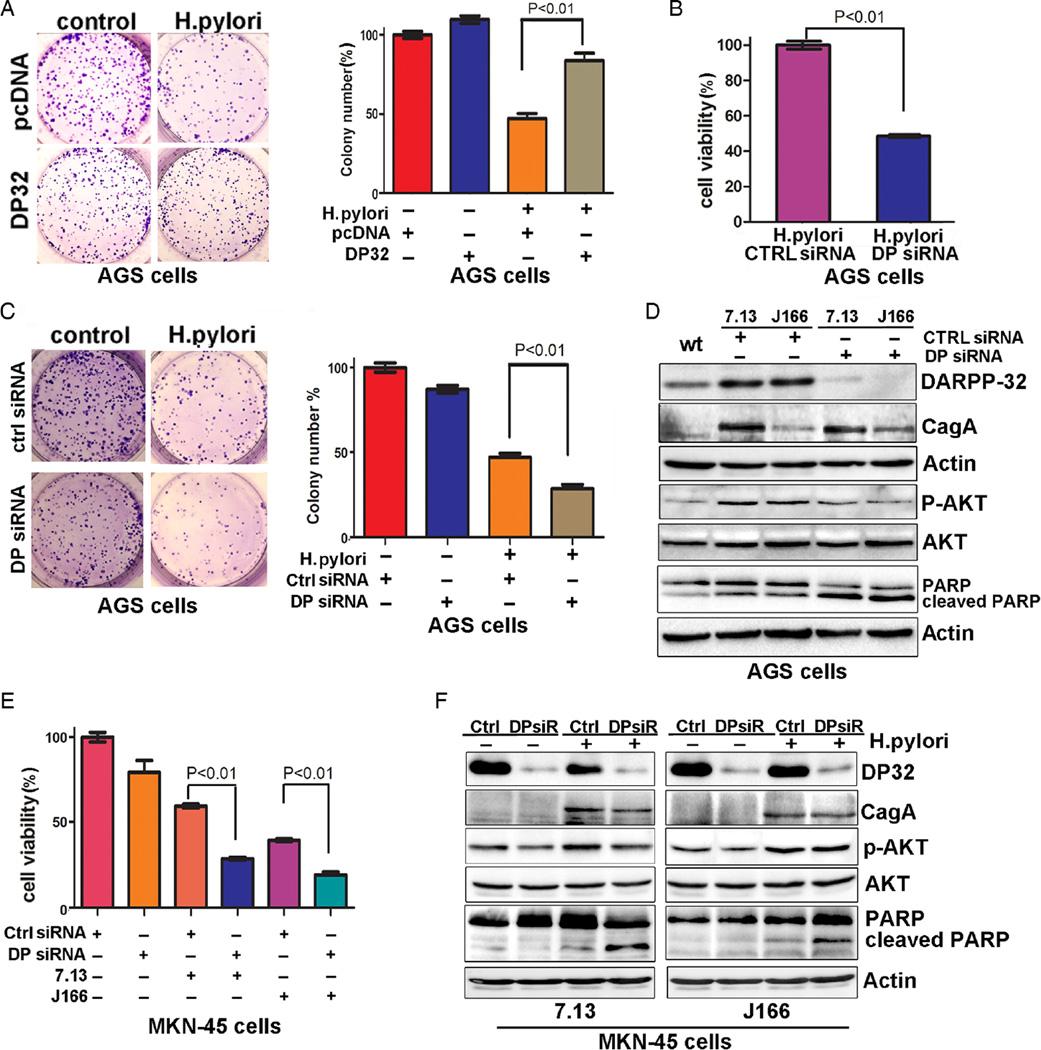
We have previously shown that DARPP-32 mediates the activation of the PI3K/AKT pathway.6,31 As our data indicated that H. pylori infection promotes DARPP-32 overexpression, we investigated whether DARPP-32 induction can promote activation of AKT and cell survival in response to H. pylori infection. Indeed, the clonogenic survival assay results showed significantly increased cell survival of AGS cells stably expressing DARPP-32, as compared with control cells, following infection with H. pylori (p<0.01, figure 6A). Consistent with the cell survival data, knocking down endogenous DARPP-32 by siRNA significantly reduced cell survival following infection with H. pylori in AGS cells (p<0.01, figure 6B). For confirmation, we performed clonogenic survival assay. The results showed that knockdown of DARPP-32 by siRNA significantly decreased cell survival following infection with H. pylori in AGS cells (p<0.01, figure 6C). The western blot data showed that H. pylori infection induced DARPP-32 expression with an increase in p-AKT (S473). On the other hand, knockdown of DARPP-32 expression by siRNA decreased p-AKT (S473) protein and increased the level of cleaved PARP; following H. pylori infection in AGS cells (figure 6D). Furthermore, our results also suggested that genetic knockdown of endogenous DARPP-32 by siRNA significantly reduced cell survival following infection with H. pylori strains (7.13 or J166) in MKN-45 cells (p<0.01, figure 6E). Consistent with these results, the western blot data showed that H. pylori infection following knockdown of endogenous DARPP-32 expression in MKN-45 cells by siRNA activated cleavage of PARP (figure 6F). These findings indicate that H. pylori-mediated cell death is abrogated by the induction of DARPP-32 and activation of the PI3K/AKT pathway in gastric cancer cells.
Figure 6.
Helicobacter pylori activates AKT pathway through regulation of DARPP-32 expression. (A) The clonogenic cell survival assay demonstrates a significant increase in relative colony number in AGS cells stably expressing DARPP-32 (DP32). The data were normalised to uninfected cells; error bars indicate SD. (B) The knockdown of DARPP-32 (DP32) in AGS cells led to a significant reduction in cell viability following H. pylori infection; error bars indicate SD. (C) The clonogenic survival assay shows that the knockdown of DARPP-32 (DP siRNA) in AGS cells led to a significant reduction in number of colonies following H. pylori infection; error bars indicate SD. (D) Western blot analysis of DARPP-32, cleaved PARP, PARP, AKT, p-AKT (S473) and CagA protein following H. pylori infection and transfection with DARPP-32 siRNA (DP siRNA) or control siRNA in AGS cells. (E) The knockdown of DARPP-32 in MKN-45 cells led to a significant reduction in cell survival following H. pylori infection; error bars indicate SD. (F) Western blot analysis of DARPP-32, cleaved PARP, PARP, AKT and p-AKT (S473) proteins following H. pylori infection and transfection with DARPP-32 siRNA (DPsiR) or control siRNA in MKN-45 cells.
Activation of NF-κB and induction of DARPP-32 expression in the human gastric tumourigenesis cascade
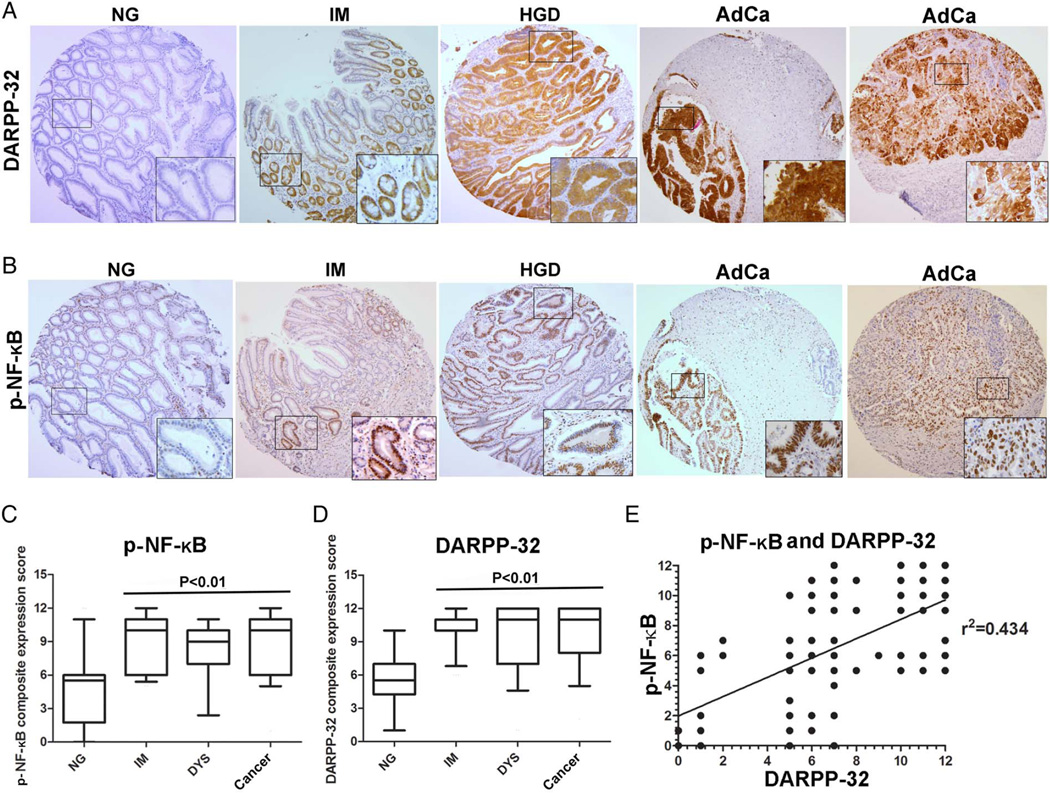
Immunohistochemistry (IHC) staining of p-NF-κB–p65 (S536) and DARPP-32 on human gastric tissue samples revealed weak immunostaining of both proteins in normal gastric mucosa (figure 7A, B, see online supplementary figure S5A, B). Conversely, we observed strong immunostaining of p-NF-κB–p65 and DARPP-32 in intestinal metaplasia, dysplasia and adenocarcinomas. The data showed diffuse cytosolic and nuclear immunostaining of DARPP-32, whereas p-NF-κB-P65 (S536) immunostaining was predominantly nuclear, indicative of NF-κB activation (figure 7A, B, see online supplementary figure S5A, B). To investigate the expression of these two proteins in the multistep progression cascade of gastric cancer, we developed a CES as described in Materials and methods section. We also investigated the potential relationship between p-NF-κB–P65 and DARPP-32 expression. Indeed, the IHC data clearly indicated an increase in the expression of both p-NF-κB–P65 and DARPP-32 (p<0.01) in the early stages of gastric tumourigenesis (figure 7C, D) and a statistically significant positive correlation (p<0.01) between their expression (figure 7E). Taken together, the expression levels of DARPP-32 and NF-κB during gastric tumourigenesis provide an in vivo support for the link between inflammation, NF-κB activation and DARPP-32 expression in human gastric cancer.
Figure 7.
Immunohistochemistry for DARPP-32 and p-NF-κB in human gastric tissues. (A and B) Immunohistochemical staining for DARPP-32 and NF-κB in serial tissue sections from human gastric mucosa with normal histology (NG), intestinal metaplasia (IM), high-grade dysplasia (HGD) and adenocarcinoma (AdCa). Original magnification, 20×. A progressive increase in DARPP-32 and p-NF-κB–p65 (S536) coexpression was observed from normal mucosa to adenocarcinoma. The graphs summarise the immunohistochemical staining results on gastric tissue microarrays (C and D). (E) A statistically significant positive correlation between the p-NF-κB (S536) and DARPP-32 composite expression score (CES) was detected (r2=0.434, p<0.01).
DISCUSSION
DARPP-32, which is frequently overexpressed in upper GI cancers,3,5,32 has been shown to exhibit oncogenic properties such as cancer cell survival and invasion.5–7 Although DARPP-32 overexpression has been associated with gastric tumour progression cascade,3 the underlying molecular mechanisms are not fully understood. H. pylori, the causative agent in approximately two-thirds of gastric cancers,33 has been classified as a Group I carcinogen by the International Agency for Research on Cancer since 1994.10 Numerous studies have been conducted to elicit the link between H. pylori infection and gastric cancer.33,34 The objective of this study was to investigate whether H. pylori infection-induced inflammation could upregulate DARPP-32 expression, which promotes cell survival, thereby facilitating gastric tumourigenesis. Indeed, we found that H. pylori infection induces the endogenous DARPP-32 expression in in vitro gastric cancer cell models and an in vivo mouse model; this strongly suggests that DARPP-32 plays an important role in H. pylori-mediated gastric tumourigenesis. Due to its essential role in inflammation and immunity, NF-κB activation and modulation by H. pylori have been topics of great interest to many investigators. NF-κB can be activated by numerous proinflammatory stimuli, ranging from toll-like receptors (TLR) activation by pathogen products to cytokines released by other cells, through the canonical and the non-canonical pathways.21,23 In fact, our data demonstrated that H. pylori infection induced activation of NF-κB, which in turn, transcriptionally upregulated DARPP-32 expression in in vitro and in vivo models.
Based on our novel findings, we propose that the proinflammatory transcription factor NF-κB upregulates DARPP-32 expression, which promotes gastric tumourigenesis. This hypothesis is supported by several lines of evidence: (1) both DARPP-32 overexpression and NF-κB activation occur as early as intestinal metaplasia and persist in the subsequent steps of the gastric tumourigenesis progression cascade; (2) we found that TNF-α-mediated activation of NF-κB or transient overexpression of NF-κB-P65 is associated with an increase in DARPP-32 protein and mRNA levels; (3) we demonstrated that the endogenous DARPP-32 protein and mRNA levels are upregulated by H. pylori infection in in vitro and in vivo models.
Our data demonstrated the role of NF-κB in the transcriptional regulation of DARPP-32 and identified the critical NF-κB-binding site within DARPP-32 promoter. Of note, the majority of human gastric cancers are associated with chronic infection with H. pylori and inflammation.35–37 The transcription factor NF-κB is a master regulator of immune and inflammatory responses and regulates many cellular processes important in carcinogenesis, including transformation, proliferation, angiogenesis and metastasis.38,39 Activation of NF-κB is a crucial mediator of inflammation-induced tumour growth and progression, as well as an important modulator of tumour surveillance and rejection.40,41 H. pylori infection of gastric epithelial cells has been shown to modulate an intricate network of signalling pathways that balance cell proliferation, survival and apoptosis; this ultimately may lead to gastric adenocarcinoma (reviewed by refs. 34, 35). In vitro studies have demonstrated that H. pylori can induce apoptosis in gastric epithelial cells.42,43 H. pylori triggers apoptosis via interaction with death receptors in the plasma membrane of cancer cells, leading to cleavage of procaspase-8, release of EGFR - estimated glomerular filtra (AIF) from mitochondria and activation of subsequent downstream events leading to the destruction of nuclear lamins.44 We have previously determined the mechanisms by which DARPP-32 activates the prosurvival PI3K–AKT pathway6 and promotes the CXCR4-dependent cancer cell invasion45 in gastric cancer. H. pylori upregulates the expression of EGF-related growth factors, which activate EGFR and other tyrosine kinase receptors, and then stimulate proliferation and survival through PI3K–AKT and mitogen-activated protein kinases (MAPK) pathways.46 Notably, we have previously shown that DARPP-32 plays an important role in stabilisation of EGFR and activation of AKT in gastric cancer cells.6 Taken together, the fact that DARPP-32 expression is induced by H. pylori-activated NF-κB strongly suggests that DARPP-32 oncogenic functions may contribute to H. pylori-mediated gastric tumourigenesis. These results also establish NF-κB as a key link between H. pylori infection and DARPP-32 overexpression in gastric cancer.
In summary, our findings demonstrate, for the first time, the role of inflammation and H. pylori-mediated activation of NF-κB in DARPP-32 transcriptional regulation in cancer. Importantly, these findings suggest that induction of DARPP-32 is a key step in providing survival properties and abrogating H. pylori-mediated cell death in gastric cells. This novel H. pylori–NF-κB–DARPP-32 axis provides a new paradigm in gastric carcinogenesis.
Supplementary Material
Significance of this study.
What is already known on this subject?
-
◄
Helicobacter pylori infection is associated with increased inflammation.
-
◄
DARPP-32 overexpression was frequently observed in early and late stages of gastric carcinogenesis.
-
◄
Amplification and overexpression of DARPP-32 in gastric cancer promote activation of several oncogenic signalling pathways and resistance to anticancer drugs.
What are the new findings?
-
◄
This study demonstrates that NF-κB-p65 binds to and activates DARPP-32 promoter and transcription.
-
◄
H. pylori-mediated activation of NF-κB transcriptionally regulates DARPP-32 expression to counteract cell death and promote cell survival.
-
◄
Given the DARPP-32 prosurvival oncogenic functions, upregulation of DARPP-32 could be a key component of H. pylori-mediated gastric tumourigenesis.
-
◄
This study strongly suggests that inflammation-regulated DARPP-32 constitutes a key component of H. pylori-mediated gastric tumourigenesis.
How might it impact on clinical practice in the foreseeable future?
-
◄
The study provides a novel understanding of mechanisms by which H. pylori infection
-
◄
promotes cell survival and the development of gastric cancer.
-
◄
H. pylori–NF-κB–DARPP-32 axis provides a new paradigm in gastric carcinogenesis that explains the link between infection, inflammation and gastric carcinogenesis.
-
◄
Development of potential inhibitors against DARPP-32 might be of clinical value in gastric cancer.
Acknowledgments
Funding This study was supported by grants from the National Cancer Institute (R01CA93999), Department of Veterans Affairs, Vanderbilt SPORE in Gastrointestinal Cancer (P50 CA95103), Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404).
Footnotes
Contributors SZ: design of in vitro and in vivo experiments and acquisition of data, analysis and interpretation of data and drafting of the manuscript. MS: assisted in in vivo experiments and interpretation of data. ZC: assisted in in vivo experiments and interpretation of data. DFP: analysis of immunohistochemical data. JR-G: culture of bacteria and coordinated H. pylori infection experiments. USK: culture of wild-type and mutant H. pylori and planning infection experiments. AB: analysis and interpretation of data, experimental troubleshooting and drafting of the manuscript. MKW: histopathology analysis of mouse and human tissues. RP: provided resources for H. pylori culture and planning experiments. WE-R: study concept and design, study supervision, experimental troubleshooting, analysis and interpretation of data, drafting of the manuscript and critical revision of the manuscript.
Competing interests None declared.
REFERENCES
- 1.Bertuccio P, Chatenoud L, Levi F, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125:666–673. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Mukherjee K, Peng D, Brifkani Z, et al. Dopamine and cAMP regulated phosphoprotein MW 32 kDa is overexpressed in early stages of gastric tumorigenesis. Surgery. 2010;148:354–363. doi: 10.1016/j.surg.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Rifai W, Smith MF, Jr, Li G, et al. Gastric cancers overexpress DARPP-32 and a novel isoform, t-DARPP. Cancer Res. 2002;62:4061–4064. [PubMed] [Google Scholar]
- 5.Belkhiri A, Zaika A, Pidkovka N, et al. Darpp-32: a novel antiapoptotic gene in upper gastrointestinal carcinomas. Cancer Res. 2005;65:6583–6592. doi: 10.1158/0008-5472.CAN-05-1433. [DOI] [PubMed] [Google Scholar]
- 6.Zhu S, Belkhiri A, El-Rifai W. DARPP-32 Increases Interactions Between Epidermal Growth Factor Receptor and ERBB3 to Promote Tumor Resistance to Gefitinib. Gastroenterology. 2011;141:1738.e2–1748.e2. doi: 10.1053/j.gastro.2011.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belkhiri A, Zhu S, Chen Z, et al. Resistance to TRAIL is mediated by DARPP-32 in gastric cancer. Clin Cancer Res. 2012;18:3889–3900. doi: 10.1158/1078-0432.CCR-11-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vangamudi B, Peng DF, Cai Q, et al. t-DARPP regulates phosphatidylinositol-3-kinase-dependent cell growth in breast cancer. Mol Cancer. 2010;9:240. doi: 10.1186/1476-4598-9-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamel S, Bouchard A, Ferrario C, et al. Both t-Darpp and DARPP-32 can cause resistance to trastuzumab in breast cancer cells and are frequently expressed in primary breast cancers. Breast Cancer Res Treat. 2010;120:47–57. doi: 10.1007/s10549-009-0364-7. [DOI] [PubMed] [Google Scholar]
- 10.IARC. Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 11.McLean MH, El-Omar EM. Genetics of inflammation in the gastrointestinal tract and how it can cause cancer. Recent Results Cancer Res. 2011;185:173–183. doi: 10.1007/978-3-642-03503-6_11. [DOI] [PubMed] [Google Scholar]
- 12.Fox JG, Wang TC. Helicobacter pylori infection: pathogenesis. Curr Opin Gastroenterol. 2002;18:15–25. doi: 10.1097/00001574-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Jackson L, Britton J, Lewis SA, et al. A population-based epidemiologic study of Helicobacter pylori infection and its association with systemic inflammation. Helicobacter. 2009;14:108–113. doi: 10.1111/j.1523-5378.2009.00711.x. [DOI] [PubMed] [Google Scholar]
- 14.Gerhard M, Rad R, Prinz C, et al. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2002;7(Suppl 1):17–23. doi: 10.1046/j.1523-5378.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi R, Asim M, Romero-Gallo J, et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology. 2011;141:1696–1708. e1–e2. doi: 10.1053/j.gastro.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyata H, Yamasaki M, Kurokawa Y, et al. Survival factors in patients with recurrence after curative resection of esophageal squamous cell carcinomas. Ann Surg Oncol. 2011;18:3353–3361. doi: 10.1245/s10434-011-1747-7. [DOI] [PubMed] [Google Scholar]
- 17.Xia HH, Talley NJ. Apoptosis in gastric epithelium induced by Helicobacter pylori infection: implications in gastric carcinogenesis. Am J Gastroenterol. 2001;96:16–26. doi: 10.1111/j.1572-0241.2001.03447.x. [DOI] [PubMed] [Google Scholar]
- 18.Chon I, Choi C, Shin CM, et al. Effect of Helicobacter pylori eradication on subsequent dysplasia development after endoscopic resection of gastric dysplasia. Korean J Gastroenterol. 2013;61:307–312. doi: 10.4166/kjg.2013.61.6.307. [DOI] [PubMed] [Google Scholar]
- 19.Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133:288–308. doi: 10.1053/j.gastro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Caamaño J, Hunter CA. NF-κB family of transcription factors: central regulators of innate and adaptive immune functions. Clin Microbiol Rev. 2002;15:414–29. doi: 10.1128/CMR.15.3.414-429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilmore TD. Introduction to NF-κB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 22.Solt LA, May MJ. The IκB kinase complex: master regulator of NF-κB signaling. Immunol Res. 2008;42:3–18. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 24.Alvero AB, Chen R, Fu HH, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien DP, Romero-Gallo J, Schneider BG, et al. Regulation of the Helicobacter pylori cellular receptor decay-accelerating factor. J Biol Chem. 2008;283:23922–23930. doi: 10.1074/jbc.M801144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franco AT, Johnston E, Krishna U, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–387. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noto JM, Khizanishvili T, Chaturvedi R, et al. Helicobacter pylori promotes the expression of Krüppel-like factor 5, a mediator of carcinogenesis, in vitro and in vivo. PLoS ONE. 2013;8:e54344. doi: 10.1371/journal.pone.0054344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soutto M, Belkhiri A, Piazuelo MB, et al. Loss of TFF1 is associated with activation of NF-κB-mediated inflammation and gastric neoplasia in mice and humans. J Clin Invest. 2011;121:1753–1767. doi: 10.1172/JCI43922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamb A, Chen LF. The many roads traveled by Helicobacter pylori to NFκB activation. Gut Microbes. 2010;1:109–113. doi: 10.4161/gmic.1.2.11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong J, Katsha A, Lu P, et al. Regulation of ERBB2 receptor by t-DARPP mediates trastuzumab resistance in human esophageal adenocarcinoma. Cancer Res. 2012;72:4504–4514. doi: 10.1158/0008-5472.CAN-12-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckler A, Moskaluk CA, Zaika A, et al. Overexpression of the 32-kilodalton dopamine and cyclic adenosine 3′,5′-monophosphate-regulated phosphoprotein in common adenocarcinomas. Cancer. 2003;98:1547–1551. doi: 10.1002/cncr.11654. [DOI] [PubMed] [Google Scholar]
- 33.Peek RM., Jr Orchestration of aberrant epithelial signaling by Helicobacter pylori CagA. Sci STKE. 2005;2005:pe14. doi: 10.1126/stke.2772005pe14. [DOI] [PubMed] [Google Scholar]
- 34.Amieva M, Peek RM., Jr Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wroblewski LE, Peek RM., Jr Helicobacter pylori in gastric carcinogenesis: mechanisms. Gastroenterol Clin North Am. 2013;42:285–298. doi: 10.1016/j.gtc.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peek RM, Jr, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 38.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 39.Sun B, Karin M. The therapeutic value of targeting inflammation in gastrointestinal cancers. Trends Pharmacol Sci. 2014;35:349–357. doi: 10.1016/j.tips.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, et al. Roles of IKK kinases and protein kinase CK2 in activation of nuclear factor-κB in breast cancer. Cancer Res. 2001;61:3810–3818. [PubMed] [Google Scholar]
- 42.Jones NL, Sherman PM. Helicobacter pylori-epithelial cell interactions: from adhesion to apoptosis. Can J Gastroenterol. 1999;13:563–566. doi: 10.1155/1999/848346. [DOI] [PubMed] [Google Scholar]
- 43.Cover TL, Krishna US, Israel DA, et al. Induction of gastric epithelial cell apoptosis by Helicobacter pylori vacuolating cytotoxin. Cancer Res. 2003;63:951–957. [PubMed] [Google Scholar]
- 44.Ashktorab H, Dashwood RH, Dashwood MM, et al. H. pylori-induced apoptosis in human gastric cancer cells mediated via the release of apoptosis-inducing factor from mitochondria. Helicobacter. 2008;13:506–517. doi: 10.1111/j.1523-5378.2008.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu S, Hong J, Tripathi MK, et al. Regulation of CXCR4-mediated invasion by DARPP-32 in gastric cancer cells. Mol Cancer Res. 2013;11:86–94. doi: 10.1158/1541-7786.MCR-12-0243-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaturvedi R, Asim M, Piazuelo MB, et al. Activation of EGFR and ERBB2 by Helicobacter pylori results in survival of gastric epithelial cells with DNA damage. Gastroenterology. 2014;146:1739–1751. e14. doi: 10.1053/j.gastro.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.