Abstract
Background
Recent incidence, treatment patterns and outcomes for node negative microscopically invasive breast cancer (MIBC) have not been reported.
Methods
State Health Registry of Iowa data identified women with ductal carcinoma in situ (DCIS), MIBC, and Stage I breast cancer excluding MIBC (Stage 1BC).
Results
From 2000–2013, 1,706, 193 and 4,514 women were diagnosed with DCIS, MIBC and Stage 1BC, respectively. MIBC increased at an annual percentage change of 2.1 (p=0.041). MIBC was more frequently human epidermal-growth-factor-receptor-2 positive than Stage 1BC (39.7% vs 9.6%, p<0.001). Mastectomy was performed more frequently in MIBC than DCIS (40.9% vs 30.6%, p=0.014) or Stage 1BC (40.9% vs 33.8%, p=0.119). Chemotherapy was given to 4.1% of women with MIBC. Survival for women with MIBC was intermediate between DCIS and Stage 1BC.
Conclusions
Management of MIBC is an increasingly frequent clinical scenario. Women with MIBC receive more aggressive local and systemic therapy than women with DCIS.
Keywords: Breast neoplasms, microinvasive tumor, mastectomy, cancer incidence, SEER program
Introduction
Microscopically invasive breast cancer (MIBC) with pathologically negative lymph nodes and without evidence of distant disease represents the smallest of invasive breast tumors. The microscopic foci of invasion, defined by the American Joint Commission on Cancer (AJCC) staging as ≤1mm,1 largely occur in the setting of high-grade ductal carcinoma in situ (DCIS) but can also be seen with other forms of DCIS or lobular carcinoma in situ.2 Previous series have reported that MIBC represents 0.7%–2.4% of breast cancer diagnoses.3 Microinvasion occurs more commonly with larger foci of DCIS4 and in multicentric DCIS tumors.5 The detection of DCIS has increased with mammography,6 making the management of MIBC, reported to occur in 5–10% of DCIS cases,7 an increasingly common clinical scenario.
Previous work reported recurrence risks that were lower for MIBC than larger invasive tumors, closer to pure DCIS.8–10 One series reported 5-year recurrence-free survival of 97.4% for 120 patients with MIBC.11 Recent series have sought to understand treatment and recurrence patterns by receptor status. These have largely shown that human epidermal growth factor receptor 2 (HER2) over-expression, seen in 39–49% of MIBC, exceeds that observed in higher stage invasive tumors.11–13 A series from Memorial Sloan Kettering reported 27% of MIBC tumors with a single focus of microinvasion were HER2-positive compared with 49% of those with two or more sites of microinvasion.13 A recent case report describes a patient with HER2-positive MIBC who developed a rapid fulminant distance recurrence within months of initial diagnosis.14 Previous reports on MIBC treatment and outcomes are largely single institutional and span the pre-trastuzumab era.
Incidence and survival trends for MIBC have not been reported in a recent population-based study sample that discriminates tumors by receptor status, including HER2. In this context, we describe the incidence of MIBC relative to DCIS and higher stage breast cancer in Iowa from 2000–2013. We also present surgical and systemic therapy treatment patterns, along with survival and subsequent breast cancer events for women diagnosed 2010 and later, when HER2 receptor status information became available.
Materials and Methods
Data were provided by the State Health Registry of Iowa, a member of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program since its inception in 1973. The initial sample included microscopically confirmed, DCIS and Stage 1BC women diagnosed in Iowa from 2010–2013 (N=6,529). Those who received neoadjuvant systemic therapy or where the order of surgical treatment and systemic therapy was unknown were excluded (N=96). Those with missing information regarding receipt of chemotherapy (N=1) or type of surgical procedure (N=19) were also excluded. This project was reviewed by our Institutional Review Board and determined not to be human subject research.
Women were categorized by stage at presentation as DCIS, MIBC (T1micN0M0), and Stage 1 excluding T1micN0M0 (hereafter Stage 1BC). Four women with N0(i+) tumors were included in the MIBC sample.
Breast cancers were categorized into three exclusive groups, 1) HER2-positive (both hormone receptor positive and hormone receptor negative), 2) hormone receptor positive and HER2-negative (HR-positive), and 3) triple negative breast cancer (TNBC). Tumors were considered hormone receptor positive if either estrogen receptor (ER) or progesterone receptor (PR) was positive. To be designated TNBC, HER2, PR and ER status must all be known and be negative. Information regarding individual receptor status (whether positive, negative, or unknown) was collected by the registry.
Extent of surgical treatment was categorized as none, lumpectomy, and mastectomy. Receipt of radiation post-lumpectomy was also identified. Patients were categorized by whether they received systemic therapy, or chemotherapy in particular. Information regarding systemic therapy included chemotherapy, hormone therapy, and biological response therapy/immunotherapy.
Multivariate logistic regressions assessed factors associated with mastectomy, versus lumpectomy, for MIBC. Incidence rates were calculated annually for 2000–2013 by stage. Trends were assessed and annual percentage change (APC) was estimated using Joinpoint software, version 4.2.0.2 (NCI). Outcomes were assessed for those with no prior malignancies (N=4,951), and included Kaplan-Meier estimated five-year overall survival and subsequent breast cancers, as reported by SEER. Even though not all subsequent breast events are captured by SEER, given the consistency in coding rules, any differences in the reported rate of subsequent breast cancers across stage may be informative. Analyses were conducted using STATA MP, version 12.0 (StataCorp LP, College Station, TX).
Results
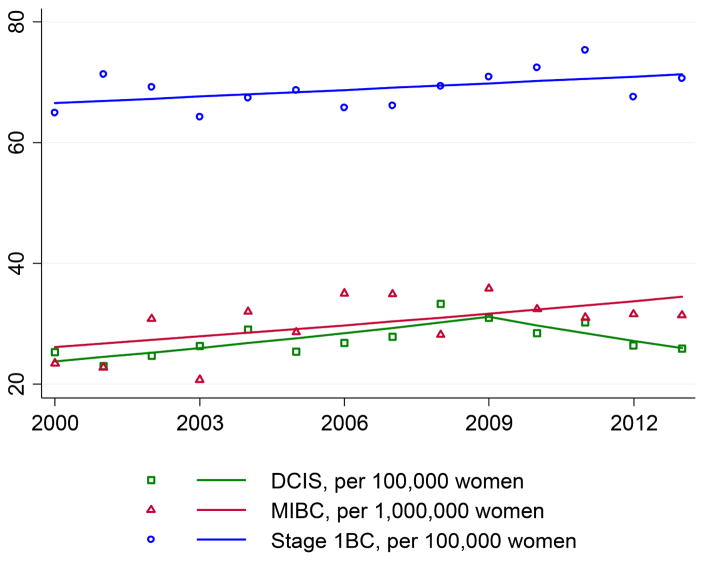
From 2000–2013, mean incidence per 100,000 women for DCIS, MIBC, and Stage 1BC was 27.4, 3.0, and 68.9, respectively. DCIS incidence increased with an APC of 3.0 (p=0.001) from 2000–2009, and decreased thereafter, although insignificantly (APC=−4.4, p=0.183) (Figure 1). In contrast, MIBC increased at an average APC of 2.1 (p=0.041) throughout the period. Incidence of Stage 1BC was stable (APC=0.53, p=0.266).
Figure 1.
Incidence of breast cancers in Iowa by stage, 2000–2013.
The final study sample included 6,413 women diagnosed from 2010–2013. Of these 1,706, 193 and 4,514 had DCIS, MIBC and Stage 1BC respectively (Table 1). Median follow-up was 41 months, with a standard deviation of 15 months. The HER2-positive tumor phenotype was overrepresented in the MIBC sample. Systemic therapy, presumably predominately anti-estrogen therapy, was given at similar rates to women with DCIS and MIBC (36.9% vs. 39.4%, p=0.494). Chemotherapy was delivered to 8 (4.1%) women with MIBC. Women with MIBC underwent mastectomy more often than those with DCIS (40.9% vs. 30.6%, p=0.014) and proportionally, but not significantly, more frequently than those with higher stage invasive disease (40.9% vs 33.8%, p=0.119). Following lumpectomy, women with MIBC were more likely to receive radiation (90.1%) than women with DCIS (80.6%, p=0.007) or Stage 1BC (83.4%, p=0.034).
Table 1.
Patient and breast cancer characteristics by stage.
| DCIS | MIBC | Stage 1BC | p-valuea | p-valueb | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Sample size | 1,706 | 193 | 4,514 | |||||
| Median age | 62 | 61 | 65 | 0.859 | 0.004 | |||
| Age at diagnosis | ||||||||
| <60 | 743 | 43.6 | 93 | 48.2 | 1,502 | 33.3 | 0.219 | <0.001 |
| ≥60 | 963 | 56.4 | 100 | 51.8 | 3,012 | 66.7 | ||
| Receptor subtypec | ||||||||
| HER2-positive | 52 | 39.7 | 385 | 9.6 | <0.001 | |||
| HR-positive | 64 | 48.9 | 3,324 | 82.5 | ||||
| TNBC | 15 | 11.5 | 319 | 7.9 | ||||
| Systemic therapy | ||||||||
| No | 1,077 | 63.1 | 117 | 60.6 | 1,395 | 30.9 | 0.494 | <0.001 |
| Yes | 629 | 36.9 | 76 | 39.4 | 3,119 | 69.1 | ||
| Chemotherapy | ||||||||
| No | 1,674 | 98.1 | 185 | 95.9 | 3,666 | 81.2 | 0.037 | <0.001 |
| Yes | 32 | 1.9 | 8 | 4.1 | 848 | 18.8 | ||
| Surgical treatment | ||||||||
| Lumpectomy | 1,150 | 67.4 | 111 | 57.5 | 2,917 | 64.6 | 0.014 | 0.119 |
| Mastectomy | 522 | 30.6 | 79 | 40.9 | 1,524 | 33.8 | ||
| None | 34 | 2.0 | 3 | 1.6 | 73 | 1.6 | ||
| Post-lumpectomy radiation | ||||||||
| No | 223 | 19.4 | 10 | 9.0 | 484 | 16.6 | 0.007 | 0.034 |
| Yes | 927 | 80.6 | 101 | 91.0 | 2,433 | 83.4 | ||
Abbreviations: HER2=human epidermal growth factor receptor 2; HR=hormone receptor; TNBC=triple negative breast cancer; DCIS=ductal carcinoma in situ; MIBC=microscopically invasive breast cancer (T1micN0M0); Stage 1BC=stage 1 breast cancer excluding T1micN0M0
Comparing MIBC with DCIS.
Comparing MIBC with Stage 1BC.
Those with missing subtype information not included in analysis. Subtype is not reported for DCIS as HER2 testing is not standard of care. Of the DCIS patients with available data (N=1,561), 85% (N=1,327) were hormone receptor positive.
Women with HER2-positive and TNBC MIBC were younger than their counterparts with HR-positive disease, with median ages of 58, 59 and 63, respectively (p=0.098) (Table 2). Systemic therapy was given to 61 (46.6%) of the 131 women for whom subtype information was available. Of these 61 women, 55 (90.2%) had ER/PR-positive disease (either HER2-positive or HER2-negative). Of the 23 women with HER-positive/HR-negative disease, five (21.7%) received systemic therapy. Of the eight women with MIBC who received chemotherapy, four had HER2-positive tumors and four had HR-positive tumors.
Table 2.
MIBC: patient and tumor characteristics by receptor subtype.
| HER2-postive | HR-positive | TNBC | p-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Sample size | 52 | 64 | 15 | ||||
| Median age | 58 | 63 | 59 | 0.098 | |||
| Age at diagnosis | |||||||
| <60 | 31 | 59.6 | 28 | 43.8 | 8 | 53.3 | 0.232 |
| ≥60 | 21 | 40.4 | 36 | 56.3 | 7 | 46.7 | |
| Systemic therapy | |||||||
| No | 30 | 57.7 | 26 | 40.6 | * | * | <0.001 |
| Yes | 22 | 42.3 | 38 | 59.4 | * | * | |
| Chemotherapy | |||||||
| No | 48 | 92.3 | 60 | 93.8 | 15 | 100.0 | 0.547 |
| Yes | 4 | 7.7 | 4 | 6.3 | 0 | 0.0 | |
| Surgical treatment a | |||||||
| Lumpectomy | 29 | 55.8 | 40 | 63.5 | 11 | 73.3 | 0.424 |
| Mastectomy | 23 | 44.2 | 23 | 36.5 | 4 | 26.7 | |
Abbreviations: HER2=human epidermal growth factor receptor 2; HR=hormone receptor; TNBC=triple negative breast cancer; MIBC=microscopically invasive breast cancer (T1micN0M0)
For those who received surgical treatment.
Numbers suppressed due to small size.
Women with MIBC who underwent mastectomy were significantly younger than those undergoing lumpectomy with median ages of 58 and 63, respectively (p=0.024). Treatment with any type of systemic therapy was significantly more common for women who received lumpectomy than for women who underwent mastectomy (p<0.001) (Table 3). On multivariate analysis, age <60 years and receipt of systemic therapy were both inversely associated with mastectomy. Women with MIBC of the TNBC subtype tended to be less likely to undergo mastectomy.
Table 3.
Characteristics of MIBC by surgical procedure and multivariate analysis to predict mastectomy.
| Lumpectomy | Mastectomy | OR | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Sample size | 111 | 79 | |||||
| Age at diagnosis | |||||||
| <60 | 44 | 39.6 | 48 | 60.8 | ref | ||
| ≥60 | 67 | 60.4 | 31 | 39.2 | 0.36 | 0.17–0.81 | 0.013 |
| Subtype a | |||||||
| HER2-positive | 29 | 36.3 | 23 | 46.0 | 0.89 | 0.38–2.06 | 0.777 |
| HR-positive | 40 | 50.0 | 23 | 46.0 | ref | ||
| TNBC | 11 | 13.8 | 4 | 8.0 | 0.26 | 0.07–1.03 | 0.055 |
| Systemic therapy | |||||||
| No | 54 | 48.6 | 60 | 75.9 | ref | ||
| Yes | 57 | 51.4 | 19 | 24.1 | 0.20 | 0.08–0.49 | <0.001 |
| Chemotherapy | |||||||
| No | 107 | 96.4 | 76 | 96.2 | ref | ||
| Yes | 4 | 3.6 | 3 | 3.8 | 2.58 | 0.48–13.90 | 0.269 |
Abbreviations: HER2=human epidermal growth factor receptor 2; HR=hormone receptor; TNBC=triple negative breast cancer; MIBC=microscopically invasive breast cancer (T1micN0M0)
Those with missing information not included in this analysis.
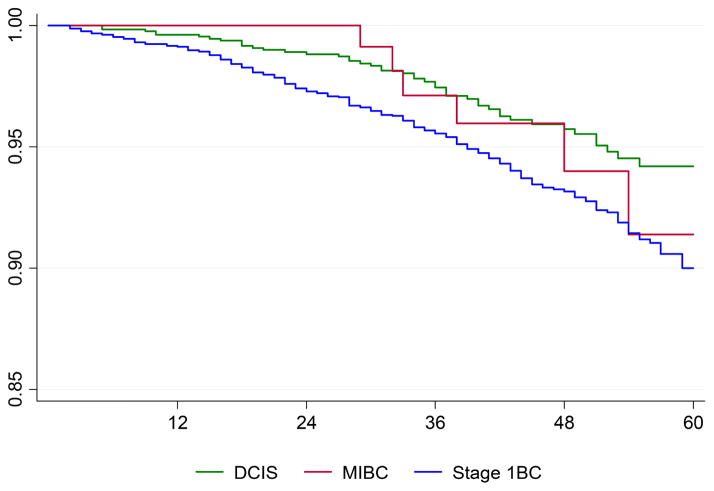
Five-year overall survival for women with no prior malignancy was 94.2% (92.1%–95.8%), 91.4% (80.4%–96.3%), and 90.0% (88.3%–91.5%) for DCIS, MIBC, and Stage 1BC, respectively (Figure 2). The survivor functions differed, per log-rank test, for all three (p<0.001). Under pairwise comparison, the survivor functions did not differ between the MIBC and DCIS breast cancers (p=0.759) or between MIBC and Stage 1BC breast cancers (p=0.343). Mean (median) age at diagnosis for the six women with MIBC who died during the follow-up period was 79 (83). None of these women had a subsequent breast cancer event, local or distant. For the 48 women with DCIS who died during the follow-up period, mean (median) age was 73 (76.5). Three of these women had a subsequent breast cancer, however none were distant recurrence.
Figure 2.
Kaplan-Meier survival by stage.
Subsequent SEER-reported breast cancers occurred in 3.5%, 2.7% and 2.1% of women with DCIS, MIBC and Stage 1BC, respectively (p=0.018). In this early follow-up period, women with DCIS and MIBC who received systemic therapy had fewer subsequent breast cancers than those who did not, 2.8% vs. 3.9% (p=0.218).
Discussion
We report the clinical and pathologic features, treatment, and early outcomes for 193 women who presented with MIBC in Iowa from 2010–2013. This registry-based study sample represents the largest series in the HER2-targeted therapy era and offers insight into current surgical and medical management of these tumors. Incidence trends from 2000–2013 show that women are presenting with MIBC more frequently. This could be due to increased awareness of this entity by pathologists though this T stage has been included in the AJCC staging system for breast cancer since 1997.15 This trend would support that management of MIBC is an increasingly common clinical scenario.
In this sample, women with MIBC, with a median age of 61, were older than in other earlier series.11–13 Notably, median age in Iowa (38.1 years) is slightly, but not markedly higher than for the U.S. (37.2 years).16 As in other reports, age of women with MIBC did not differ significantly from those with DCIS.10 Similar to other series, HER2-positive tumors were more common in DCIS and MIBC, than in higher stage invasive breast cancers.17–19 Reports have been mixed on the prognostic value of HER2 positivity in DCIS.20,21 Less is known about the risk of this marker in MIBC, though a single institution review of 83 patients with MIBC found that HER2-positive status was not associated with local recurrence.12
Overall systemic therapy was delivered with similar frequency to women with DCIS and MIBC, though a greater proportion of women with MIBC received systemic therapy. Chemotherapy was given significantly more frequently to those with MIBC, although the overall sample size is not large. Eight women (4.1% of those with MIBC) were given chemotherapy, half of these having HER2-positive tumors. This systemic therapy trend was noted despite a higher mastectomy rate for MIBC, perhaps suggesting some concern for higher recurrence risk related to MIBC. Similarly, for those undergoing lumpectomy, women with MIBC received radiation therapy with greater frequency than both women with DCIS and Stage 1BC, perhaps again reflecting heightened concern for recurrence. The higher mastectomy rate seen for women with MIBC compared to those with DCIS may be attributable to the microscopic invasion commonly occurring in larger tumors, or that the mastectomies provided larger specimens, increasing the likelihood of upstaging. The lumpectomy rate of 57.5% for MIBC falls within the range reported by others.8,10,11
For women with MIBC, receptor status correlated with systemic therapy, as expected. A higher proportion of HER2-positive MIBC receiving mastectomy, perhaps because these tumors were larger or perceived as higher risk, though receptor status was not associated with type of surgical treatment on multivariate analysis. A single institution report found that HER2-positive status was associated with tumors having >1 foci of microinvasion.13 This same work showed that while number of foci of microinvasion was not associated with the risk of sentinel lymph node metastases, it was associated with systemic therapy decisions.
As expected, women with MIBC who underwent lumpectomy received systemic therapy significantly more frequently than those who underwent mastectomy. This was likely largely driven by women with HR-positive MIBC who received anti-estrogen therapy to lower both the risk of a recurrence and a second breast cancer. The recently reported benefit of aromatase inhibitors for DCIS in NSABP-35,22 particularly for women <60, could further increase the uptake of these agents for women with MIBC. Systemic therapy offered proportional, but not statistically significant, protection from an early subsequent breast cancer in this sample.
Five-year survival was lower than that reported in other series of MIBC.12,13,19 This is likely attributable to the deceased women having been of advanced age at diagnosis. Further follow-up will more meaningfully elucidate survival trends for this sample. Women with MIBC had an intermediate risk of a subsequent breast cancer in this early follow-up period. This occurred despite a higher mastectomy rate for this stage group.
Limitations to this work are similar to earlier reports on the Iowa SEER registry sample23 and include clinical presentation and treatment choices reported for a single geographic area, one predominately rural and less racially diverse than the national population. The slightly older population could skew treatment decisions. Inherent in this large dataset is the inability to fully capture complete treatment, recurrence and progression trends, and comorbidities for individuals. Some MIBC subtype groups have small numbers. Importantly, by definition MIBC has only small fragments of invasive tumor, often limiting the ability to fully characterize receptor status. The inability to characterize the invasive disease is also a frequent clinical conundrum. The subtypes grouped here are in large categories. Breast cancer is more complex than this, with variation in natural history and treatment response within each subset. Our findings suggest that MIBC remains a relatively rare, but increasing clinical presentation. We report current clinical and pathologic patterns at diagnosis and treatment by receptor status. Follow-up data on subsequent SEER-reported breast cancers offer some early information on risks for patients with MIBC. Most patients with MIBC have an excellent prognosis, but not all. Like other forms of breast cancer, this is a heterogeneous disease. While most do well, some do not, and for these patients stage did not fully capture their risk. Future study from prospective databases and molecular profiling of these tumors will hopefully allow identification of the patients at high risk for recurrence or second breast cancers, for whom more aggressive, risk-adapted therapy may warranted.
Acknowledgments
We would like to acknowledge Dan Olson for his efforts in preparing and providing Iowa Cancer Registry Data.
Funding Support
This research was supported in part by the National Cancer Institute via the State Health Registry of Iowa contract HSN261201000032C and a Cancer Center Support Grant (P30 CA086862).
Footnotes
Conflict of Interest
All authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edge SB. AJCC cancer staging manual. 7. New York: Springer; 2010. American Joint Committee on Cancer. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi S, Vezzosi V. Microinvasive carcinoma of the breast. Pathol Oncol Res. 2008;14(2):105–11. doi: 10.1007/s12253-008-9054-8. [DOI] [PubMed] [Google Scholar]
- 3.Hoda SA, Chiu A, Prasad ML, et al. Are microinvasion and micrometastasis in breast cancer mountains or molehills? Am J Surg. 2000;180(4):305–8. doi: 10.1016/s0002-9610(00)00464-5. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein MJ, Waisman JR, Gamagami P, et al. Intraductal carcinoma of the breast (208 cases). Clinical factors influencing treatment choice. Cancer. 1990;66(1):102–8. doi: 10.1002/1097-0142(19900701)66:1<102::aid-cncr2820660119>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GF, Patchefsky AS, Finklestein SD, et al. Nonpalpable in situ ductal carcinoma of the breast. Predictors of multicentricity and microinvasion and implications for treatment. Arch Surg. 1989;124(1):29–32. doi: 10.1001/archsurg.1989.01410010035007. [DOI] [PubMed] [Google Scholar]
- 6.Frykberg ER, Masood S, Copeland EM, 3rd, Bland KI. Ductal carcinoma in situ of the breast. Surg Gynecol Obstet. 1993;177(4):425–40. [PubMed] [Google Scholar]
- 7.Adamovich TL, Simmons RM. Ductal carcinoma in situ with microinvasion. Am J Surg. 2003;186(2):112–6. doi: 10.1016/s0002-9610(03)00166-1. [DOI] [PubMed] [Google Scholar]
- 8.Vieira CC, Mercado CL, Cangiarella JF, et al. Microinvasive ductal carcinoma in situ: clinical presentation, imaging features, pathologic findings, and outcome. Eur J Radiol. 2010;73(1):102–7. doi: 10.1016/j.ejrad.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 9.Wong JH, Kopald KH, Morton DL. The impact of microinvasion on axillary node metastases and survival in patients with intraductal breast cancer. Arch Surg. 1990;125(10):1298–301. doi: 10.1001/archsurg.1990.01410220082011. discussion 301–2. [DOI] [PubMed] [Google Scholar]
- 10.Parikh RR, Haffty BG, Lannin D, Moran MS. Ductal carcinoma in situ with microinvasion: prognostic implications, long-term outcomes, and role of axillary evaluation. Int J Radiat Oncol Biol Phys. 2012;82(1):7–13. doi: 10.1016/j.ijrobp.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Kwon JH, Kim YJ, Lee KW, et al. Triple negativity and young age as prognostic factors in lymph node-negative invasive ductal carcinoma of 1 cm or less. BMC Cancer. 2010;10:557. doi: 10.1186/1471-2407-10-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margalit DN, Sreedhara M, Chen YH, et al. Microinvasive breast cancer: ER, PR, and HER-2/neu status and clinical outcomes after breast-conserving therapy or mastectomy. Ann Surg Oncol. 2013;20(3):811–8. doi: 10.1245/s10434-012-2640-8. [DOI] [PubMed] [Google Scholar]
- 13.Matsen CB, Hirsch A, Eaton A, et al. Extent of microinvasion in ductal carcinoma in situ is not associated with sentinel lymph node metastases. Ann Surg Oncol. 2014;21(10):3330–5. doi: 10.1245/s10434-014-3920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhar CG, Matos E. Human epidermal growth factor receptor 2-positive microinvasive breast carcinoma with a highly aggressive course: a case report. BMC Res Notes. 2014;7:325. doi: 10.1186/1756-0500-7-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming ID American Joint Committee on Cancer., American Cancer Society., American College of Surgeons. AJCC cancer staging manual. 5. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 16.Howden LM, Meyer JA, et al. Age and Sex Composition: 2010. Washington D.C: U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau; 2011. [Google Scholar]
- 17.Yu KD, Wu LM, Liu GY, et al. Different distribution of breast cancer subtypes in breast ductal carcinoma in situ (DCIS), DCIS with microinvasion, and DCIS with invasion component. Ann Surg Oncol. 2011;18(5):1342–8. doi: 10.1245/s10434-010-1407-3. [DOI] [PubMed] [Google Scholar]
- 18.Roses RE, Paulson EC, Sharma A, et al. HER-2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1386–9. doi: 10.1158/1055-9965.EPI-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Zhang W, Lyu S, et al. Clinicopathologic characteristics and molecular subtypes of microinvasive carcinoma of the breast. Tumour Biol. 2015;36(4):2241–8. doi: 10.1007/s13277-014-2652-z. [DOI] [PubMed] [Google Scholar]
- 20.Kerlikowske K, Molinaro AM, Gauthier ML, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst. 2010;102(9):627–37. doi: 10.1093/jnci/djq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stackievicz R, Paran H, Bernheim J, et al. Prognostic significance of HER-2/neu expression in patients with ductal carcinoma in situ. Isr Med Assoc J. 2010;12(5):290–5. [PubMed] [Google Scholar]
- 22.Margolese RG, Cecchini RS, Julian TB, et al. Anastrozole versus tamoxifen in postmenopausal women with ductal carcinoma in situ undergoing lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet. 2016;387(10021):849–56. doi: 10.1016/S0140-6736(15)01168-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder MC, Lynch CF, Abu-Hejleh T, et al. Chemotherapy use and surgical treatment by receptor subtype in node-negative T1a and T1b female breast cancers, Iowa SEER Registry, 2010 to 2012. Clin Breast Cancer. 2015;15(1):e27–34. doi: 10.1016/j.clbc.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]