Abstract
IMPORTANCE
Postoperative pulmonary complications (PPCs), a leading cause of poor surgical outcomes, are heterogeneous in their pathophysiology, severity, and reporting accuracy.
OBJECTIVE
To prospectively study clinical and radiological PPCs and respiratory insufficiency therapies in a high-risk surgical population.
DESIGN, SETTING, AND PARTICIPANTS
We performed a multicenter prospective observational study in 7 US academic institutions. American Society of Anesthesiologists physical status 3 patients who presented for noncardiothoracic surgery requiring 2 hours or more of general anesthesia with mechanical ventilation from May to November 2014 were included in the study. We hypothesized that PPCs, even mild, would be associated with early postoperative mortality and use of hospital resources. We analyzed their association with modifiable perioperative variables.
EXPOSURE
Noncardiothoracic surgery.
MAIN OUTCOMES AND MEASURES
Predefined PPCs occurring within the first 7 postoperative days were prospectively identified. We used bivariable and logistic regression analyses to study the association of PPCs with ventilatory and other perioperative variables.
RESULTS
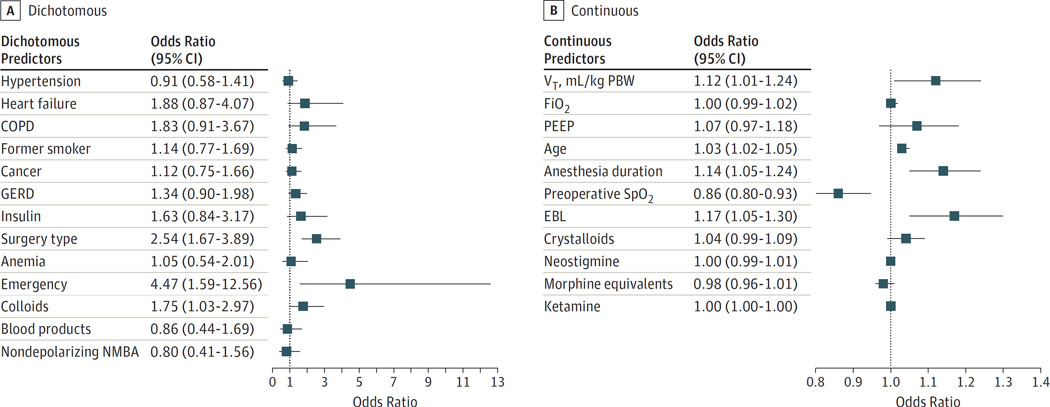
This study included 1202 patients who underwent predominantly abdominal, orthopedic, and neurological procedures. The mean (SD) age of patients was 62.1 (13.8) years, and 636 (52.9%) were men. At least 1 PPC occurred in 401 patients (33.4%), mainly the need for prolonged oxygen therapy by nasal cannula (n = 235; 19.6%) and atelectasis (n = 206; 17.1%). Patients with 1 or more PPCs, even mild, had significantly increased early postoperative mortality, intensive care unit (ICU) admission, and ICU/hospital length of stay. Significant PPC risk factors included nonmodifiable (emergency [yes vs no]: odds ratio [OR], 4.47, 95% CI, 1.59–12.56; surgical site [abdominal/pelvic vs nonabdominal/pelvic]: OR, 2.54, 95% CI, 1.67–3.89; and age [in years]: OR, 1.03, 95% CI, 1.02–1.05) and potentially modifiable (colloid administration [yes vs no]: OR, 1.75, 95% CI, 1.03–2.97; preoperative oxygenation: OR, 0.86, 95% CI, 0.80–0.93; blood loss [in milliliters]: OR, 1.17, 95% CI, 1.05–1.30; anesthesia duration [in minutes]: OR, 1.14, 95% CI, 1.05–1.24; and tidal volume [in milliliters per kilogram of predicted body weight]: OR, 1.12, 95% CI, 1.01–1.24) factors.
CONCLUSIONS AND RELEVANCE
Postoperative pulmonary complications are common in patients with American Society of Anesthesiologists physical status 3, despite current protective ventilation practices. Even mild PPCs are associated with increased early postoperative mortality, ICU admission, and length of stay (ICU and hospital). Mild frequent PPCs (eg, atelectasis and prolonged oxygen therapy need) deserve increased attention and intervention for improving perioperative outcomes.
Postoperative pulmonary complications (PPCs) adversely influence surgical morbidity and mortality,1,2 particularly within the first postoperative week.3–5 Their incidence ranges from 6% to 80%, depending on definitions, severity considered (from atelectasis to acute respiratory distress syndrome [ARDS]), and presence of risk factors.2,6–9 Estimates suggest that more than 1 million PPCs occur annually in the United States, with 46 200 related deaths and 4.8 million additional hospitalization days.1 Previous studies on PPCs1,2,6,10,11 have been mostly retrospective10,11 or based on administrative data,1,6 or they included patients at very different risk levels for PPCs.2,11 Assessing the effect of PPCs on outcomes with administrative data, particularly those presumed mild and often omitted (eg, atelectasis or the supplemental oxygen postoperative requirement), is a challenge that hinders the development of effective specific measures.1
Interventions to reduce PPCs include intraoperative intensive care unit (ICU)–like mechanical ventilation strategies,5,10,12,13 which have been adopted over the past decade by US academic centers.14,15 Yet, the contemporary incidence, clinical impact, and risk factors of individual PPCs after these practice changes are unknown. Detailed knowledge on current mild-to-severe PPCs is needed to identify modifiable risk factors in order to maximize the effectiveness of new interventions. Such an approach matches well with initiatives such as the perioperative surgical home16,17 and enhanced recovery after surgery.18,19
We performed a multicenter prospective observational study in 7 US academic institutions of patients with severe systemic disease (American Society of Anesthesiologists [ASA] physical status class 3) undergoing prolonged noncardiothoracic surgery with general anesthesia and mechanical ventilation. This surgical population subset is particularly susceptible to PPCs2 and would be an ideal target for future interventions based on their volume and severe, but not life-threatening, disease burden. We hypothesized that in this patient cohort, PPCs, even those presumed mild, would be significantly associated with early postoperative mortality and use of hospital resources. To test our hypotheses, we prospectively quantified the incidence of early PPCs in this high-risk patient cohort and assessed the association of mild-to-severe PPCs with these outcomes. In addition, we analyzed the association between the incidence of PPCs and intraoperative ventilation settings and other relevant perioperative variables.
Methods
Study Design
In this study by members of the Perioperative Research Network, institutional review board approval was obtained at each participating institution. Either waiver of consent or an opt-out opportunity were approved as required by individual institutional review boards, based on the observational nature of the study and for feasibility of enrollment immediately before surgery.
Population
Eligible study patients were prospectively identified from the daily surgical schedule at all 7 institutions (May-November 2014). Patients were considered eligible and included if they fulfilled the following criteria immediately before surgery: age 18 years or older; ASA class 3 as determined in the preoperative evaluation and confirmed by the institutional principal investigator; any type of noncardiothoracic nonaortic surgery, including elective or emergency procedures; general anesthesia with endotracheal intubation and mechanical ventilation; and expected length of surgical procedure 2 hours or longer. Exclusion criteria included long-term preoperative continuous ventilatory support (continuous positive airway pressure /bilevel positive airway pressure) or oxygen dependency; tracheostomized patients; self-reported pregnancy; or life expectancy equal to or less than 30 days as estimated by the clinician’s notes in the patient’s medical record.
Study Protocol
The following data were collected. Immediately before surgery: age, sex, height, and weight; preexisting comorbidities (see preagreed standardized definitions and other methodological details in the eAppendix in the Supplement); medications; peripheral saturation of oxygen (obtained in the preoperative area with no supplemental oxygen); and hemoglobin concentration in blood (if available within 30 days prior to surgery).
Intraoperative data included the surgical procedure; surgery duration (incision to end of surgery) and anesthesia duration (measure of duration of mechanical ventilation, defined as time from anesthesia induction to extubation and/or out of operating room time if the patient was transferred mechanically ventilated to the next care unit); mechanical ventilation settings; vital signs; neuromuscular blockade monitoring; presence of regional anesthesia; and administered fluids and medications. For ventilation settings and vital signs, the intraoperative median was calculated for each patient. Morphine equivalents of the administered opioids were calculated and adjusted to weight and anesthesia duration.
End Points
The main end point of this study was the incidence of PPCs, occurring within the first 7 postoperative days (PODs). Postoperative pulmonary complications were collected prospectively following preagreed definitions (eAppendix in the Supplement) and included clinical diagnoses (pneumonia,20 bronchospasm, and/or ARDS21), radiological diagnoses (presence of any degree or location of atelectasis,2 pneumothorax,2 and/or pleural effusion2), and therapies for respiratory insufficiency (prolonged [>1 day after end of surgery] supplemental oxygen by nasal cannula [NC], face mask [FM], postoperative noninvasive ventilation [new to the patient or extended in time compared with the patient’s routine use], and/or re-intubation with postoperative mechanical ventilation [POMV]). Radiological diagnoses were reported by attending radiologists independent of the study, with the final consideration as PPC by local study principal investigators. Mechanical ventilation for nonrespiratory reasons (ie, ventilation maintained at end of surgery or initiated after surgery for reasons other than respiratory failure, such as unstable arrhythmia or hemodynamic instability) was not considered as POMV. Additional clinical outcomes collected were mortality within the first 7 PODs and admission and length of stay (LOS) in the ICU/ intermediate care unit and hospital.
Statistical Analysis
The estimated sample size of 1000 patients provided 80% power to detect an odds ratio (OR) of 1.2 or greater as statistically significant for each ventilatory variable of interest and its association with the composite outcome of any of the PPCs, assuming a multiple correlation between the ventilatory variable and the other independent variables in the logistic regression of 0.25 or less, or 90% power to detect an OR of 1.3 or greater, assuming a multiple correlation of 0.50 or less observed earlier.2
We summarized population characteristics, perioperative variables, PPCs, and outcomes. Any missing and outlier data values were individually revised for completion or correction or left as missing data points in our study database. All values represent mean (SD) or number (percentage), unless stated otherwise.
The association between the PPCs (composite and individual PPCs) with demographic data, perioperative data, and clinical outcomes were examined with χ2 or Fisher exact tests for categorical variables and t test or Wilcoxon rank sum test for continuous variables. These PPC composite bivariable associations were then individually adjusted by clustering of patients within sites using simple hierarchical logistic regression with site as a random effect. Relevant covariates to include in the final model were identified as those with P values less than .10, clinical relevance,5 and no significant statistical association with other relevant variables. For the final model, multivariable hierarchical logistic regression was used to model the relationships between the development of PPC and ventilatory settings. The linearity of each continuous predictor with the log odds outcome was checked graphically and, if not present, a variable log transformation was performed (eg, expected blood loss). Pearson correlation coefficients were used to assess collinearity between predictors. To examine the appropriateness of the final model, the intraclass correlation coefficient was calculated. The intraclass correlation coefficient represents the ratio of between-site variance to total variance and ranges from 0 to 1 (larger values representing stronger clustering effects).
All analyses were performed using SAS version 9.4 (SAS Institute). A level significance of .05 was used for all statistical tests previously mentioned.
Results
The 1202 included patients underwent predominantly gastrointestinal, orthopedic, and neurological surgery (descriptive characteristics are shown in Table 1).To reduce the degrees of freedom related to multiple surgery types in the multivariate analysis, procedures were combined into abdominal/pelvic (45.3%) and nonabdominal/pelvic (54.7%) following previous PPC risk assessments.2,22 Albumin was the only colloid fluid used. At least 1 radiological examination was performed after surgery in 381 patients (31.7%). All missing or outlier values of study parameters were identified and corrected. See the eTable in the Supplement for further details.
Table 1.
Study Group Characteristics and Bivariable Analys is Results for PPC Composite
| Variable | No. (%) | RR (95% CI) | P Value | Site-Adjusted P Value |
||
|---|---|---|---|---|---|---|
| Total Patients (n = 1202) |
≥1 PPC (n = 401) |
No PPC (n = 801) |
||||
| Demographics | ||||||
| Age | 62.1 (13.8) | 65.0 (12.4) | 60.6 (14.2) | NA | <.001 | <.001 |
| Male | 636 (52.9) | 197 (49.1) | 439 (54.8) | NA | .06 | .20 |
| BMI | 30.0 (7.5) | 30.2 (7.9) | 29.8 (7.3) | NA | .41 | .48 |
| Comorbidities | ||||||
| Hypertension | 790 (65.7) | 284 (70.8) | 506 (63.2) | 1.27 (1.06–1.51) | .008 | .04 |
| Heart failure | 68 (5.7) | 31 (7.7) | 37 (4.6) | 1.40 (1.06–1.83) | .03 | .07 |
| Anemia, hemoglobin <10 g/dL | 124 (10.3) | 50 (12.4) | 74 (9.2) | 1.24 (0.98–1.56) | .08 | .10 |
| Chronic obstructive pulmonary disease |
103 (8.6) | 52 (13.0) | 51 (6.4) | 1.59 (1.29–1.97) | <.001 | .007 |
| Asthma | 172 (14.3) | 52 (13.0) | 120 (15.0) | 0.89 (0.70–1.14) | .35 | .24 |
| Obstructive sleep apnea | 234 (19.5) | 88 (21.9) | 146 (18.2) | 1.16 (0.96–1.40) | .12 | .34 |
| Smoking status | ||||||
| Current | 161 (13.4) | 59 (14.7) | 102 (12.8) | 1.11 (0.89–1.39) | .35 | .24 |
| Former | 478 (42.0) | 177 (46.2) | 301 (39.9) | 1.19 (1.01–1.40) | .04 | .05 |
| Cancer | 499 (41.5) | 187 (46.6) | 312 (39.0) | 1.23 (1.05–1.44) | .01 | .02 |
| Gastroesophageal reflux disease | 431 (35.9) | 162 (40.5) | 269 (33.6) | 1.21 (1.03–1.43) | .02 | .09 |
| Diabetes | 301 (25.0) | 110 (27.4) | 191 (23.8) | 1.13 (0.95–1.35) | .18 | .24 |
| Alcohol abuse | 92 (7.7) | 35 (8.7) | 57 (7.1) | 1.15 (0.88–1.52) | .32 | .25 |
| Surgical procedure | ||||||
| Surgery category | ||||||
| Abdominal/pelvic | 545 (45.3) | 240 (59.9) | 305 (30.1) | 1.79 (1.52–2.11) | <.001 | <.001 |
| Nonabdominal/pelvic | 657 (54.7) | 161 (40.1) | 496 (61.9) | |||
| Emergency | 61 (5.1) | 34 (8.5) | 27 (3.4) | 1.73 (1.37–2.20) | <.001 | .04 |
| Any regional block | 266 (22.1) | 112 (27.9) | 154 (19.2) | 1.36 (1.15–1.62) | <.001 | .007a |
| Surgery duration, h | 3.6 (2.0) | 4.2 (2.4) | 3.4 (1.7) | NA | <.001 | <.001a |
| Anesthesia duration, h | 4.7 (2.2) | 5.3 (2.6) | 4.3 (1.9) | NA | <.001 | <.001 |
| Preoperative SpO2, No. (%) | 97.2 (2.1) | 96.8 (2.3) | 97.4 (2.0) | NA | <.001 | <.001 |
| Preoperative hemoglobin, g/dL | 12.4 (2.1) | 12.1 (2.1) | 12.5 (2.1) | NA | .01 | .02a |
| Intraoperative mechanical ventilation | ||||||
| Ventilatory modes | ||||||
| Volume control, VCV | 824 (68.6) | 260 (65.0) | 564 (70.4) | NA | .002b | NA |
| Pressure control, PCV, PCV-VG | 178 (14.8) | 80 (20.0) | 98 (12.2) | NA | NA | .37 |
| Assisted/supported, PSV, SIMV | 48 (4.0) | 11 (2.8) | 37 (4.6) | NA | NA | .13 |
| Unspecified | 151 (12.6) | 49 (12.3) | 102 (12.7) | NA | NA | .68 |
| Median exhaled VT, mL/kg PBW | 8 (1.6) | 8 (1.6) | 8 (1.7) | NA | .53 | .25c |
| Median FiO2, No.(%) | 54.4 (13.9) | 56.1 (14.8) | 53.6 (13.3) | NA | .002 | .15c |
| Median PEEP, cm H2O | 5.1 (1.7) | 5.2 (1.7) | 5 (1.7) | NA | .07 | .01c |
| Respiratory rate, breaths/min | 12 (2.2) | 11.9 (2.3) | 12.1 (2.2) | NA | .12 | .24 |
| Peak inspiratory pressure, cm H2O | 21.3 (5.4) | 21.4 (5.5) | 21.2 (5.4) | NA | .53 | .13 |
| Plateau airway pressure, cm H2O | 20.1 (4.9) | 20.4 (5.0) | 19.9 (4.9) | NA | .25 | .25 |
| SpO2, No. (%) | 98.8 (1.4) | 98.8 (1.4) | 98.8 (1.3) | NA | .78 | .86 |
| ETCO2, mm Hg | 35.1 (3.1) | 35.3 (2.9) | 35.1 (3.2) | NA | .38 | .18 |
| Intraoperative hemodynamic data | ||||||
| Mean blood pressure, mm Hg | 82.6 (10.5) | 82.2 (10.0) | 82.8 (10.7) | NA | .38 | .98 |
| Mean blood pressure <60 mm Hg, min |
4.3 (14.0) | 5.3 (15.7) | 3.7 (13.0) | NA | .005 | .07a |
| Median temperature, °C | 36.1 (0.6) | 36.6 (1.1) | 36.7 (1.3) | NA | .46 | .07a |
| Perioperative fluid management | ||||||
| Crystalloids, mL/kg/h | 6.3 (3.7) | 6.7 (3.8) | 6.1 (3.6) | NA | .02 | .03 |
| Colloids, mL/kg/h | 0.3 (0.8) | 0.5 (1.1) | 0.2 (0.6) | NA | <.001 | <.001 |
| Any blood product | 132 (11.0) | 70 (17.5) | 62 (7.7) | NA | <.001 | .003 |
| Packed red blood cells | 118 (9.8) | 62 (15.5) | 56 (7.0) | NA | <.001 | .004 |
| Fresh frozen plasma | 42 (3.5) | 29 (7.2) | 13 (1.6) | NA | <.001 | .003 |
| Platelet | 19 (1.6) | 11 (2.7) | 8 (1.0) | NA | .02 | .08 |
| Estimated blood loss, mL | 343 (650) | 484 (867) | 273 (493) | NA | <.001 | <.001 |
| Urine output, mL/kg/h | 1.2 (1.5) | 1.2 (1.3) | 1.3 (1.7) | NA | .57 | .09a |
| Intraoperative medications | ||||||
| Analgesia, patients, No. (%) | ||||||
| Fentanyl | 1114 (92.7) | 376 (93.8) | 738 (92.1) | NA | .31 | .50 |
| Sufentanil | 59 (4.9) | 21 (5.2) | 38 (4.7) | NA | .71 | .58 |
| Hydromorphone | 635 (52.8) | 192 (47.9) | 443 (55.3) | NA | .02 | .83 |
| Morphine | 69 (5.7) | 17 (4.2) | 52 (6.5) | NA | .11 | .28 |
| Remifentanil | 210 (17.5) | 56 (14.0) | 154 (19.2) | NA | .02 | .19 |
| Ketamine | 159 (13.2) | 72 (18.0) | 87 (10.9) | NA | <.001 | .03a |
| Dose | ||||||
| Morphine equivalents, µg/kg/h | 2.2 (8.6) | 1.4 (5.0) | 2.6 (9.9) | NA | .02 | .06 |
| Ketamine, mg | 21 (184) | 41 (272) | 12 (118) | NA | .01 | .049 |
| Neuromuscular blockade | ||||||
| Nondepolarizing NMBA | 1038 (86.4) | 357 (89.3) | 681 (85.0) | NA | .04 | .05 |
| NMBA reversal | 883 (73.7) | 300 (75.0) | 583 (73.1) | NA | .47 | .38 |
| Neostigmine, µg/kg | 31.5 (24.1) | 33.9 (24.8) | 30.2 (23.7) | NA | .008 | .001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ETCO2, end-tidal partial pressure of carbon dioxide; FiO2, inspired oxygen fraction; NA, not applicable; NMBA, neuromuscular blocking agent; PBW, predicted body weight; PEEP, positive end-expiratory pressure; PCV, pressure-controlled ventilation; PPC, postoperative pulmonary complication; PSV, pressure-supported ventilation; RR, relative risk; SIMV, synchronized intermittent mandatory ventilation; SpO2, peripheral saturation of oxygen; VCV, volume-control ventilation; VG, volume guaranteed; VT, tidal volume.
SI conversion factor: to convert hemoglobin to grams per liter, multiply by 10.
Variables that were statistically significant in the univariable analysis but were excluded from the logistic regression analysis because of their clinical and/or statistical correlation with other included covariates.
Compared with VCV as reference mode.
Variables that were included in the logistic model as focus of this study, independently of the P value.
Patients who developed 1 or more PPCs (n = 401; 33.4%) were significantly older and presented with more frequent preoperative diagnoses of hypertension, chronic obstructive pulmonary disease, and cancer (Table 1). No medications were significantly different between patients with and without PPCs. Patients with 1 or more PPCs were significantly more likely to undergo abdominal/pelvic surgery, emergent and/or longer surgical procedures, and a general/regional combined anesthetic approach (compared with exclusively general anesthesia) (Table 1). Preoperative hemoglobin and room-air oxygen saturation were lower in patients who later developed PPCs. During the intraoperative period, patients presenting 1 or more PPCs were more likely to have received volume control ventilation with a slightly higher positive end-expiratory pressure (PEEP), but the median tidal volume (VT) and inspired oxygen fraction (FiO2) were not different from those received by patients with no PPC in the site-adjusted analysis (Table 1). This site-adjusted analysis also showed that patients with 1 or more PPCs had greater blood loss, volume of infused crystalloids or colloids, percentage of transfused blood products, and intra-operative phenylephrine for hemodynamic support. Patients with 1 or more PPCs more often received desflurane or ketamine and lower doses of morphine equivalents (in micrograms per kilogram per hour). The use of neuromuscular blocking agents, neuromuscular blocking agent monitoring, and neuromuscular blocking agent reversal were similar in all patients, but the dose of neostigmine was higher in those with 1 or more PPCs.
A third of the studied patients (n = 401) developed at least 1 PPC (Table 2). The most frequent PPC was the prolonged (>1 day) need for oxygen supplementation by NC, followed by atelectasis and pleural effusion (Table 2). Acute respiratory distress syndrome and pneumothorax were the least frequent complications. While most patients presented a single PPC, 14% of patients experienced 2 or more PPCs (Table 3).
Table 2.
Incidence of PPCs
| Complication | No. (%) |
|---|---|
| Pneumonia | 22 (1.8) |
| Pneumothorax | 4 (0.3) |
| Atelectasis | 206 (17.1) |
| Pleural effusion | 116 (9.7) |
| Bronchospasm | 13 (1.1) |
| ARDS | 2 (0.2) |
| Respiratory dysfunction requiring prolonged (>1 d) PO O2 | |
| By nasal cannula | 235 (19.6) |
| By face mask | 12 (1.0) |
| PO noninvasive ventilation | 46 (3.8) |
| Reintubation + PO mechanical ventilation | 21 (1.7) |
| At least 1 PPC | 401 (33.4) |
Abbreviations: ARDS, acute respiratory distress syndrome; O2, oxygen; PO, postoperative; PPC, postoperative pulmonary complication.
Table 3.
Number of PPCs and Clinical Outcomes
| Variable | All Patients (N = 1202) |
Hospital | ICU | 7-d Mortality (n = 9) |
||
|---|---|---|---|---|---|---|
| Patients, No. (%) (n = 1179) |
LOS, Median (IQR), d |
Patients, No. (%) (n = 270) |
LOS, Median (IQR), d |
|||
| No. of PPCs | ||||||
| 0 | 801 (66.6) | 781 (97.5) | 3 (2–6) | 133 (16.6) | 1 (1–2) | 0 (0) |
| 1 | 231 (19.2) | 228 (98.7) | 5 (4–8) | 57 (24.7) | 2 (1–4) | 0 (0) |
| 2 | 91 (7.6) | 91 (100.0) | 8 (5–14) | 35 (38.5) | 3 (1–6) | 3 (3.3) |
| 3 | 58 (4.8) | 58 (100.0) | 9 (5–15) | 32 (55.2) | 4 (2–7) | 4 (6.9) |
| 4 | 17 (1.4) | 17 (100.0) | 8 (7–18) | 9 (52.9) | 7 (6–15) | 2 (11.8) |
| 5 | 2 (0.2) | 2 (100.0) | 22 (21–22) | 2 (100.0) | 14 (9–18) | 0 (0) |
| 6 | 2 (0.2) | 2 (100.0) | 25 (24–26) | 2 (100.0) | 9 (6–11) | 0 (0) |
| At least 1 PPC | 401 (33.4) | 398 (99.3)a | 6 (4–11)b | 137 (34.2)b | 3 (2–6)b | 9 (2.3)b |
| RR or MDiff (95% CI), (≥1PPCvs0PPCs) |
NA | 1.02 (1.00–1.03) | 3.0 (2.0–5.3) | 2.1 (1.7–2.5) | 2.0 (0.7–3.0) | NDc |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; MDiff, median difference; NA, not available; ND, not defined; PPC, postoperative pulmonary complication; RR, relative risk.
P < .05 in patients with at least 1 PPC vs 0 PPCs.
P < .001 in patients with at least 1 PPC vs 0 PPCs.
RR or MDiff (95% CI) calculated as not defined or not calculated.
Twenty-three patients were discharged from the hospital within 24 hours (Table 3). All patients with more than 1 PPC were admitted to the hospital. Median hospital LOS increased as the number of PPCs per patient increased. Two hundred and seventy patients (22.5%) were admitted to the ICU (Table 3): 214 were admitted immediately after surgery and 56 within the following 7 PODs. The number of PPCs per patient was directly associated with the likelihood of admission to the ICU and median ICU LOS (Table 3).Not only were severe PPCs— such as ARDS, reintubation with POMV, pneumonia, and rescue postoperative noninvasive ventilation—associated with increased ICU admission and LOS, but also atelectasis, pleural effusion, and prolonged oxygen requirement by NC/FM (Table 4).
Table 4.
Individual PPCs and Clinical Outcomes
| Individual PPCs | All Patients (n = 1202) |
Hospital | ICU | 7-d Mortality (n = 9) |
||
|---|---|---|---|---|---|---|
| Patients, No. (%) (n = 1179) |
LOS, Median (IQR), d |
Patients, No. (%) (n = 270) |
LOS, Median (IQR), d |
|||
| Pneumonia | ||||||
| Yes | 22 (1.8) | 22 (100.0) | 12.0 (7.0–21.0)a | 10 (45.5)b | 6.1 (3.0–9.0)c | 2 (9.1)b |
| No | 1180 (98.2) | 1157 (98.1) | 4.0 (2.0–7.0) | 260 (22.0) | 2.0 (1.0–4.0) | 7 (0.6) |
| RR or MDiff (95% CI) | NA | 1.02 (1.01–1.03) | 8.00 (2.44–15.68) | 2.06 (1.29–3.30) | 4.0 (0.95–7.11) | 15.3 (3.4–69.4) |
| Pneumothorax | ||||||
| Yes | 4 (0.3) | 4 (100.0) | 6.7 (5.0–10.7) | 2 (50.0) | 1.3 (0.5–2.0) | 0 (0) |
| No | 1188 (99.7) | 1175 (98.1) | 4.0 (2.1–7.0) | 268 (22.4) | 2.0 (1.0–4.0) | 9 (0.8) |
| RR or MDiff (95% CI) | NA | 1.02 (1.01–1.03) | 1.0 (1.0–8.81) | 2.24 (0.83–5.99) | −1.50d | NDd |
| Atelectasis | ||||||
| Yes | 206 (17.1) | 206 (100.0)b | 7.4 (5.0–13.0)a | 87 (42.2)a | 3.0 (1.8–6.0)a | 9 (4.4)a |
| No | 996 (82.9) | 973 (97.7) | 4.0 (2.0–6.0) | 183 (18.4) | 2.0 (1.0–3.0) | 0 (0.0) |
| RR or MDiff (95% CI) | NA | 1.02 (1.01–1.03) | 3.29 (0.56–6.81) | 2.30 (1.87–2.83) | 1.0 (0.87–3.25) | NDd |
| Pleural effusion | ||||||
| Yes | 116 (9.7) | 115 (99.1) | 8.4 (5.0–16.0)a | 61 (52.6)a | 3.6 (2.0–7.0)a | 8 (7.0)a |
| No | 1086 (90.3) | 1064 (98.0) | 4.0 (2.0 to 6.3) | 209 (19.2) | 2.0 (1.0–3.0) | 1 (0.1) |
| RR or MDiff (95% CI) | NA | 1.01 (0.99–1.03) | 4.38 (0.52–9.17) | 2.73 (2.21–3.38) | 1.56 (−0.07 to 4.32) | 75.3 (9.5–597.0) |
| Bronchospasm | ||||||
| Yes | 13 (1.1) | 13 (100.0) | 4.0 (2.8–7.0) | 2 (15.4) | 4.5 (2.0–7.0) | 0 (0.0) |
| No | 1189 (98.9) | 1166 (98.1) | 4.0 (2.1–7.0) | 268 (22.5) | 2.0 (1.0–4.0) | 9 (0.8) |
| RR or MDiff (95% CI) | NA | 1.02 (1.01–1.03) | 0 (−1.26 to 33.12) | 0.68 (0.19–2.45) | 0d | NDd |
| ARDS | ||||||
| Yes | 2 (0.2) | 2 (100.0) | 17.0 (7.5–26.4) | 2 (100.0) | 6.9 (6.3–7.5)b | 1 (50.0)b |
| No | 1200 (99.8) | 1177 (98.1) | 4.0 (2.1–7.0) | 268 (22.3) | 2.0 (1.0–4.0) | 8 (0.7) |
| RR or MDiff (95% CI) | NA | 1.02 (1.01–1.03) | 3.50d | 4.48 (4.03–4.98) | 4.25d | 74.8 (15.9–351.6) |
| Respiratory dysfunction requiring prolonged (>1 d) postoperative O2 by NC | ||||||
| Yes | 235 (19.6) | 235 (100.0)b | 6.0 (4.0–9.7)a | 72 (30.6)a | 3.0 (2.0–6.0)a | 1 (0.4) |
| No | 967 (80.4) | 944 (97.6) | 4.0 (2.0–6.0) | 198 (20.5) | 2.0 (1.0–3.0) | 8 (0.8) |
| RR or MDiff (95% CI) | NA | 1.02 (1.01–1.03) | 2.0 (2.0–4.38) | 1.5 (1.19–1.88) | 1.0 (0.48–2.89) | 0.51 (0.06–4.08) |
| Respiratory dysfunction requiring prolonged (>1 d) postoperative O2 by face mask | ||||||
| Yes | 12 (1.0) | 12 (100.0) | 14.5 (5.6–23.0)a | 10 (83.3)a | 6.6 (3.0–11.0)a | 0 (0) |
| No | 1190 (99.0) | 1167 (98.1) | 4.0 (2.0–7.0) | 260 (21.8) | 2.0 (1.0–4.0) | 9 (0.8) |
| RR or MDiff (95% CI) | NA | 1.02 (1.01–1.03) | 7.0 (1.97–18.76) | 3.81 (2.90–5.02) | 4.15 (0.95–9.38) | NDd |
| Postoperative-noninvasive ventilation | ||||||
| Yes | 46 (3.8) | 44 (95.7) | 6.0 (3.0–9.5)b | 16 (34.8)b | 6.1 (2.5–7.5)a | 2 (4.3)b |
| No | 1156 (96.2) | 1135 (98.2) | 4.0 (2.0–7.0) | 254 (22.0) | 2.0 (1.0–3.8) | 7 (0.6) |
| RR or MDiff (95% CI) | NA | 0.97 (0.92–1.04) | 2.0 (−1.52 to 5.74) | 1.58 (1.05–2.39) | 4.0 (1.18–5.82) | 7.2 (1.5–33.5) |
| Reintubation + postoperative mechanical ventilation | ||||||
| Yes | 21 (1.7) | 21 (100.0) | 21.0 (9.0–26.0)a | 19 (90.5)a | 7.0 (5.0–11.6)a | 3 (14.3)a |
| No | 1181 (98.3) | 1158 (98.1) | 4.0 (2.0–7.0) | 251 (21.3) | 2.0 (1.0–3.5) | 6 (0.5) |
| RR or MDiff (95% CI) | NA | 1.02 (1.01–1.03) | 17.0 (5.14–22.40) | 4.26 (3.57–5.08) | 5.0 (4.09–9.37) | 28.0 (7.5–104.6) |
Abbreviations: ARDS, acute respiratory distress syndrome; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; MDiff, median difference; O2, oxygen; NA, not applicable; NC, nasal cannula; ND, not defined; O2, oxygen; PPC, postoperative pulmonary complication; RR, relative risk.
P < .001 yes vs no for individual PPCs.
P < .05
P < .01
RR or MDiff (95% CI) calculated as not defined or not calculated.
Nine patients (0.8%) died within the first 7 PODs (early postoperative mortality), all with more than 1 pulmonary complication (Table 3). The presence of 1 or more PPCs of any type was significantly associated with increased early mortality (2.3% in patients with ≥1 PPC vs 0% in patients with no PPCs; P < .001; see relative risk in Table 3). Early mortality rate was the highest in patients with ARDS and reintubation with POMV (50% and 14.3%, respectively, corresponding to relative risks [RR] of 74.8 [95% CI, 15.9–351.6] and 28.0 [95% CI, 7.5–104.6], respectively), but it was also significantly associated with pneumonia, pleural effusion, and atelectasis (observed rates of 9.1%, 7.0%, and 4.4%, respectively, and RRs of 15.3 [95% CI, 3.4–69.4], 75.3 [95% CI, 9.5–597.0], and undefined, respectively). Atelectasis was present in 9 patients and pleural effusion in 8, of the 9 patients with early postoperative death. Pneumothorax, bronchospasm, and requirement of prolonged use of oxygen by NC/FM were not associated with early mortality.
Significant variables from the site-adjusted bivariable analysis were individually evaluated as predictors of PPC development in the hierarchical logistic regression analysis. Variables with significant association with 1 or more PPCs were emergency (OR, 4.47; 95% CI, 1.59–12.56) and abdominal/pelvic surgery (OR, 2.54; 95% CI, 1.67–3.89), colloid use (OR, 1.75; 95% CI, 1.03–2.97), estimated blood loss (OR, 1.17; 95% CI, 1.05–1.30), VT (milliliters per kilogram of predicted body weight) (OR, 1.12; 95% CI, 1.01–1.24), anesthesia duration (OR, 1.14; 95% CI, 1.05–1.24), age (OR, 1.03; 95% CI, 1.02–1.05), and preoperative room-air peripheral saturation of oxygen (OR, 0.86; 95% CI, 0.80–0.93) (Figure). Positive end-expiratory pressure and FiO2 had no significant association with PPC development after adjusting for covariates in the logistic regression analysis. The estimated intraclass correlation coefficient (ratio of be-tween-site variance to total variance) was 0.037, supporting the cluster (hierarchical) logistic regression model.
Figure.
Variables Independently Associated With Postoperative Pulmonary Complications Development by Hierarchical Logistic Regression Analysis
COPD indicates chronic obstructive pulmonary disease; EBL, estimated blood loss; FiO2, inspired oxygen fraction; GERD, gastroesophageal reflux disease; NMBA, neuromuscular blocking agents; PBW, predicted body weight; PEEP, positive end-expiratory pressure; SpO2, peripheral saturation of oxygen; VT, tidal volume.
Discussion
In this prospective multicenter study, we observed that PPCs occur in one-third of noncardiothoracic surgical patients with severe systemic disease (ASA physical status 3) despite current intraoperative protective ventilation practices. The presence of at least 1 PPC, not only severe (eg, ARDS or pneumonia) but also mild (eg, atelectasis or oxygen requirement), was significantly associated with increased early postoperative mortality, ICU admission, and prolonged LOS in the ICU and hospital. In addition, we identified potentially modifiable factors for future targeted interventions, including colloid administration, lower preoperative oxygenation, blood loss, anesthesia duration, and VT setting (even within protective limits).
We selected the ASA physical status 3 surgical population because of its large volume, susceptibility to PPCs,2 and severe but not life-threatening systemic disease burden, which makes them ideal candidates of future interventions and generalizable results. Surgical procedures were classified into abdominal/pelvic and nonabdominal/pelvic following previous PPC risk assessments2,22 reporting the highest risk for PPCs after thoracic procedures (excluded from this study) followed by abdominal surgical procedures, then peripheral procedures (lowest risk). Open and laparoscopic abdominal procedures were combined because the impact on PPCs after varied operations remains unclear.5,23,24
Our results indicate that PPCs affect a substantial number of this patient population. The observed incidence of at least 1 PPC in 33.4% of patients was higher than previous reports,2,6–9 likely due to a combination of different patient characteristics, surgical procedures, and PPC definitions. Our prospective design and the attempt to be comprehensive— including interventions such as oxygen requirement, which are difficult to reliably extract retrospectively—could also contribute to these findings.
Our findings underline that not only severe but also mild PPCs are associated with significantly increased morbidity, mortality, and ICU and hospital LOS. These outcomes varied with the type and number of PPCs. For example, the presence of pneumothorax, bronchospasm, and oxygen requirement by NC/FM were significantly associated with longer hospital LOS despite no association with mortality. The most severe PPCs— ARDS and reintubation with POMV—significantly increased the risk for early mortality risk (RRs, 74.8 and 28.0, respectively) and the ICU LOS but they were rare in ours and previous studies (Table 4).10,11 Importantly, less severe but considerably more frequent PPCs, such as atelectasis or pleural effusion, also increased the risk for mortality (RR calculated as undefined high owing to denominator equal to 0 or 75.3, respectively) and ICU/ hospital LOS (see RRs in Table 4). Pneumonia could be considered a moderate PPC in both frequency and severity, increasing the risk for mortality (RR, 15.3) and the ICU/hospital LOS (Table 4). Therefore, interventions to reduce less-severe PPCs may offer previously unrecognized and substantial opportunities for improvements in postoperative outcomes and use of hospital resources.
We found modifiable and nonmodifiable variables independently associated with PPCs. While some were noted in previous studies, their relative association to outcomes differed, providing a more specific and contemporary measure of their relevance in the studied setting. Nonmodifiable factors (age and abdominal or emergency surgery) were consistent with previous literature2,11,25,26 and emphasize the need for enhanced interventions to specifically address these groups. Modifiable factors—suchaspreoperative oxygenation, procedure duration, and blood loss—offer an opportunity for testing multidisciplinary strategies, such as early identification of high-risk patients and effective prevention strategies. The use of colloids (albumin in milliliters per kilogram per hour) was a modifiable variable independently associated with PPC development. This is consistent with previous literature suggesting an effect of large fluid administration in worsening surgical outcomes,25,27,28 and our observed lower administration of fluids (in milliliters per kilogram per hour) to patients without PPCs. Interestingly, recent studies have shown an increased PPC risk in surgical patients with hypoalbuminemia.2,26 Thus, our results indicate that control of fluid replacement is an important area of intervention for reduction of PPCs. Future studies should address the role of individual fluids and criteria for administration.
Intraoperative VT was another modifiable variable associated with PPC after covariate adjustment. There is an increased interest in perioperative protective mechanical ventilation and a trend to use lower intraoperative VTs despite limited information on the specific optimal settings for different surgical patients.5,10,29 The significant association of VT with PPCs (12% increased risk per milliliters per kilogram of predicted body weight unit of VT; Figure) in the adjusted analysis suggests its role even at the lower range of values used. The effect of PEEP and FiO2 on PPCs was less clear and is controversial in the literature.12,29,30 Our observed narrow range of PEEP and FiO2 may have influenced the lack of a detectable effect. Future specific studies will be required to establish the effect of those variables on surgical outcomes. Of note, our high incidence of PPCs, even in the presence of protective ventilatory strategies,10 implies that interventions to reduce PPCs will need to address factors beyond mechanical ventilation to effectively impact outcome.
Our collaborative effort successfully characterized the epidemiology of individual PPCs in a contemporary multicenter high-risk cohort. This study was developed in a detailoriented manner with preagreement of terms and data to be collected. Our investigation exploited information extraction from electronic medical record systems. The prospective enrollment, data collection, and validation add reliability to clinical outcomes that are often more challenging to measure than mortality. This design differentiates the present investigation from prior studies that have relied exclusively on administrative databases.11,25,31 This pioneering collaboration, combining the resources of electronic medical records with prospective patient assessment, may prove an efficient approach for benchmarking in perioperative care and is the foundation of future interventions to improve perioperative care and outcomes.
Limitations
Our study presents several limitations primarily related to its observational nature, preselection of specific PPC definitions, and possible multiple testing. The need for oxygen therapy was determined by local practices and not by the implementation of a common protocol. This has likely introduced heterogeneity with potential avoidable oxygen therapy but also untreated hypoxemia being present in our sample. Indeed, hypoxemia32 has been found to be a common, persistent, and often underdiagnosed postoperative complication. The association of this variable with the reported outcomes, even in the presence of that heterogeneity, emphasizes its importance. Radiological examinations were not obtained in all patients and therefore may induce a selection bias. We addressed the residual bias and confounding effects with adjusted analyses. Our findings reflect statistical associations and do not imply cause-effect relationships. Finally, our patient population limited to ASA physical status 3 and 7-day postoperative follow-up after noncardiothoracic nonaortic surgery may limit the generalizability of our results to other populations.
Conclusions
Postoperative pulmonary complications occurred in one-third of all patients with severe systemic disease (ASA physical status 3) undergoing noncardiothoracic surgery of 2 hours or more in 7 major US academic centers. The development of at least 1 PPC, even those presumed mild, was associated with significantly increased early postoperative mortality, ICU admission, and prolonged LOS in the ICU and hospital. A high incidence of PPCs was observed even in the presence of ventilation settings that were consistent with protective ventilatory strategies. Postoperative pulmonary complications frequently considered mild (eg, atelectasis and prolonged oxygen therapy requirement) deserve increased attention. Efforts to reduce these mild PPCs may contribute to improved perioperative outcomes and shorter hospital stays. These multidisciplinary interventions should include the management of fluid administration, preoperative oxygenation, blood loss, anesthesia duration, and intraoperative VT.
Key Points.
Question
Are postoperative pulmonary complications (PPCs), even mild ones, associated with early postoperative mortality and use of hospital resources?
Findings
In this multicenter study in 1202 American Society of Anesthesiologists physical status 3 patients undergoing noncardiothoracic surgery requiring 2 hours or more of general anesthesia with mechanical ventilation, at least 1 PPC occurred in 401 patients (mainly the need for prolonged oxygen therapy by nasal cannula and atelectasis). Patients with 1 PPC or more, even mild, had significantly increased early postoperative mortality, intensive care unit admission, and intensive care unit/hospital length of stay.
Meaning
Mild frequent PPCs (eg, atelectasis and prolonged oxygen therapy need) deserve increased attention and intervention for improving perioperative outcomes.
Acknowledgments
Funding/Support: This work was partially supported by grants R34HL123438 (Drs Vidal Melo, Fernandez-Bustamante, and Sprung)and K23HL112855 (Dr Kor) from the National Institutes of Health, and by the 2012 Foundation for Anesthesia Education and Research Mentored Research Training Grant–Clinical/Translational (Dr Fernandez-Bustamante).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank the founding members of the Perioperative Research Network who did not contribute patient data for this current study for their contribution to study protocol development and feedback on data interpretation and the manuscript: James Blum, MD (Emory University), Suzanne Karan, MD (University of Rochester Medical Center), Pedro Tanaka, MD, PhD (Stanford University), and Loreta Grecu, MD (Yale School of Medicine). We also thank the following personnel for their assistance with administrative compliance and/or data collection: Hannah Lee, BS (Beth Israel Deaconess Medical Center); Derek P. Guanaga, BS (Brigham and Women’s Hospital); Julio C. M. Brandao, MD (Massachusetts General Hospital); Hemang Yadav, MBBS (Mayo Clinic); Colleen Dingmann, RN, PhD, and Ken Bullard, BS (University of Colorado School of Medicine); and Christina Matadial, MD (University of Miami). They did not receive compensation.
Footnotes
Author Contributions: Dr Fernandez-Bustamante takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Fernandez-Bustamante, Frendl, Sprung, Kor, Subramaniam, Martinez Ruiz, Lee, Henderson, Vidal Melo.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Fernandez-Bustamante, Frendl, Sprung, Kor, Subramaniam, Lee, Moss, Colwell, Giquel, Vidal Melo.
Critical revision of the manuscript for important intellectual content: Fernandez-Bustamante, Frendl, Kor, Subramaniam, Martinez Ruiz, Lee, Henderson, Mehdiratta, Bartels, Kolodzie, Vidal Melo.
Statistical analysis: Fernandez-Bustamante, Henderson, Moss, Vidal Melo.
Administrative, technical, or material support: Fernandez-Bustamante, Frendl, Subramaniam, Martinez Ruiz, Lee, Mehdiratta, Bartels, Giquel, Vidal Melo.
Study supervision: Fernandez-Bustamante, Frendl, Sprung, Kor, Subramaniam, Martinez Ruiz, Lee, Vidal Melo.
Conflict of Interest Disclosures: None reported.
Previous Presentation: This work was presented in abstract format at the Anesthesiology 2015 Annual Meeting; October 24, 2015; San Diego, California.
REFERENCES
- 1.Shander A, Fleisher LA, Barie PS, Bigatello LM, Sladen RN, Watson CB. Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med. 2011;39(9):2163–2172. doi: 10.1097/CCM.0b013e31821f0522. [DOI] [PubMed] [Google Scholar]
- 2.Canet J, Gallart L, Gomar C, et al. ARISCAT Group. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–1350. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 3.Brooks-Brunn JA. Predictors of postoperative pulmonary complications following abdominal surgery. Chest. 1997;111(3):564–571. doi: 10.1378/chest.111.3.564. [DOI] [PubMed] [Google Scholar]
- 4.Brooks-Brunn JA. Postoperative atelectasis and pneumonia: risk factors. Am J Crit Care. 1995;4(5):340–349. [PubMed] [Google Scholar]
- 5.Futier E, Constantin JM, Paugam-Burtz C, et al. IMPROVE Study Group. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369(5):428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 6.Arozullah AM, Daley J, Henderson WG, Khuri SF The National Veterans Administration Surgical Quality Improvement Program. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. Ann Surg. 2000;232(2):242–253. doi: 10.1097/00000658-200008000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreyra GP, Baussano I, Squadrone V, et al. Continuous positive airway pressure for treatment of respiratory complications after abdominal surgery: a systematic review and meta-analysis. Ann Surg. 2008;247(4):617–626. doi: 10.1097/SLA.0b013e3181675829. [DOI] [PubMed] [Google Scholar]
- 8.Fagevik Olsén M, Hahn I, Nordgren S, Lönroth H, Lundholm K. Randomized controlled trial of prophylactic chest physiotherapy in major abdominal surgery. Br J Surg. 1997;84(11):1535–1538. doi: 10.1111/j.1365-2168.1997.02828.x. [DOI] [PubMed] [Google Scholar]
- 9.Celli BR, Rodriguez KS, Snider GL. A controlled trial of intermittent positive pressure breathing, incentive spirometry, and deep breathing exercises in preventing pulmonary complications after abdominal surgery. Am Rev Respir Dis. 1984;130(1):12–15. doi: 10.1164/arrd.1984.130.1.12. [DOI] [PubMed] [Google Scholar]
- 10.Ladha K, Vidal Melo MF, McLean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ. 2015;351:h3646. doi: 10.1136/bmj.h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brueckmann B, Villa-Uribe JL, Bateman BT, et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology. 2013;118(6):1276–1285. doi: 10.1097/ALN.0b013e318293065c. [DOI] [PubMed] [Google Scholar]
- 12.Serpa Neto A, Hemmes SN, Barbas CS, et al. PROVE Network Investigators. Protective versus conventional ventilation for surgery: a systematic review and individual patient data meta-analysis. Anesthesiology. 2015;123(1):66–78. doi: 10.1097/ALN.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 13.Levin MA, McCormick PJ, Lin HM, Hosseinian L, Fischer GW. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br J Anaesth. 2014;113(1):97–108. doi: 10.1093/bja/aeu054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanderer JP, Ehrenfeld JM, Epstein RH, et al. Temporal trends and current practice patterns for intraoperative ventilation at US academic medical centers: a retrospective study. BMC Anesthesiol. 2015;15:40. doi: 10.1186/s12871-015-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bender SP, Paganelli WC, Gerety LP, et al. Intraoperative lung-protective ventilation trends and practice patterns: a report from the Multicenter Perioperative Outcomes Group. Anesth Analg. 2015;121(5):1231–1239. doi: 10.1213/ANE.0000000000000940. [DOI] [PubMed] [Google Scholar]
- 16.Vetter TR, Ivankova NV, Goeddel LA, McGwin G, Jr, Pittet JF UAB Perioperative Surgical Home Group. An analysis of methodologies that can be used to validate if a perioperative surgical home improves the patient-centeredness, evidence-based practice, quality, safety, and value of patient care. Anesthesiology. 2013;119(6):1261–1274. doi: 10.1097/ALN.0b013e3182a8e9e6. [DOI] [PubMed] [Google Scholar]
- 17.Anderson AD, McNaught CE, MacFie J, Tring I, Barker P, Mitchell CJ. Randomized clinical trial of multimodal optimization and standard perioperative surgical care. Br J Surg. 2003;90(12):1497–1504. doi: 10.1002/bjs.4371. [DOI] [PubMed] [Google Scholar]
- 18.Hughes MJ, Ventham NT, McNally S, Harrison E, Wigmore S. Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta-analysis. JAMA Surg. 2014;149(12):1224–1230. doi: 10.1001/jamasurg.2014.210. [DOI] [PubMed] [Google Scholar]
- 19.Thiele RH, Rea KM, Turrentine FE, et al. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg. 2015;220(4):430–443. doi: 10.1016/j.jamcollsurg.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 20.Cassidy MR, Rosenkranz P, McCabe K, Rosen JE, McAneny D. ICOUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg. 2013;148(8):740–745. doi: 10.1001/jamasurg.2013.358. [DOI] [PubMed] [Google Scholar]
- 21.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 22.Mazo V, Sabaté S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121(2):219–231. doi: 10.1097/ALN.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence VA, Cornell JE, Smetana GW American College of Physicians. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):596–608. doi: 10.7326/0003-4819-144-8-200604180-00011. [DOI] [PubMed] [Google Scholar]
- 24.Fuks D, Cauchy F, Ftériche S, et al. Laparoscopy decreases pulmonary complications in patients undergoing major liver resection: a propensity score analysis. Ann Surg. 2016;263(2):353–361. doi: 10.1097/SLA.0000000000001140. [DOI] [PubMed] [Google Scholar]
- 25.Blum JM, Stentz MJ, Dechert R, et al. Preoperative and intraoperative predictors of postoperative acute respiratory distress syndrome in a general surgical population. Anesthesiology. 2013;118(1):19–29. doi: 10.1097/ALN.0b013e3182794975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kor DJ, Lingineni RK, Gajic O, et al. Predicting risk of postoperative lung injury in high-risk surgical patients: a multicenter cohort study. Anesthesiology. 2014;120(5):1168–1181. doi: 10.1097/ALN.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tandon S, Batchelor A, Bullock R, et al. Peri-operative risk factors for acute lung injury after elective oesophagectomy. Br J Anaesth. 2001;86(5):633–638. doi: 10.1093/bja/86.5.633. [DOI] [PubMed] [Google Scholar]
- 28.Canet J, Gallart L. Postoperative respiratory failure: pathogenesis, prediction, and prevention. Curr Opin Crit Care. 2014;20(1):56–62. doi: 10.1097/MCC.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 29.Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ Prove Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet. 2014;384(9942):495–503. doi: 10.1016/S0140-6736(14)60416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbosa FT, Castro AA, de Sousa-Rodrigues CF. Positive end-expiratory pressure (PEEP) during anaesthesia for prevention of mortality and postoperative pulmonary complications. Cochrane Database Syst Rev. 2014;6(6):CD007922. doi: 10.1002/14651858.CD007922.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramachandran SK, Nafiu OO, Ghaferi A, Tremper KK, Shanks A, Kheterpal S. Independent predictors and outcomes of unanticipated early postoperative tracheal intubation after nonemergent, noncardiac surgery. Anesthesiology. 2011;115(1):44–53. doi: 10.1097/ALN.0b013e31821cf6de. [DOI] [PubMed] [Google Scholar]
- 32.Sun Z, Sessler DI, Dalton JE, et al. Postoperative hypoxemiaiscommon and persistent: a prospective blinded observational study. Anesth Analg. 2015;121(3):709–715. doi: 10.1213/ANE.0000000000000836. [DOI] [PMC free article] [PubMed] [Google Scholar]