Abstract
Approximately 10% of all deep vein thromboses occur in the upper extremity, and that number is increasing due to the use of peripherally inserted central catheters. Sequelae of upper extremity deep vein thrombosis (UEDVT) are similar to those for lower extremity deep vein thrombosis (LEDVT) and include postthrombotic syndrome and pulmonary embolism. In addition to systemic anticoagulation, there are multiple interventional treatment options for UEDVT with the potential to reduce the incidence of these sequelae. To date, there have been no randomized trials to define the optimal management strategy for patients presenting with UEDVT, so many conclusions are drawn from smaller, single-center studies or from LEDVT research. In this article, the authors describe the evidence for the currently available treatment options and an approach to a patient with acute UEDVT.
Keywords: upper extremity deep vein thrombosis, Paget-Schroetter syndrome, thrombolysis, pharmacomechanical thrombectomy, interventional radiology
Objectives: Upon completion of this article, the reader will be able to outline the incidence and causes of upper extremity deep vein thrombosis (UEDVT) as well as describe the indications and technique for endovascular management of UEDVT.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Over the past decade, upper extremity deep vein thrombosis (UEDVT) has seen an increasing prevalence and has been recognized as a significant clinical entity with potential complications as severe as those of lower extremity deep vein thrombosis (LEDVT). It is estimated that 10% of acute DVT occurs in the upper extremities, and the incidence of UEDVT is approximately 0.4 to 1 per 10,000 people, and approximately 6 per 10,000 hospitalized patients.1 2
Complications of UEDVT mirror those of LEDVT, including thrombophlebitis, postthrombotic syndrome (PTS), and pulmonary embolism (PE). Rare and potentially life-threatening phlegmasia cerulea dolens has also been reported.3 A retrospective review of a large patient registry found that although the incidence of PE on diagnosis of UEDVT was lower than that for LEDVT, the 3-month outcomes—including overall PE incidence, recurrent DVT, major bleeding, and death—for both groups of patients was similar.4 A more recent study found the incidence of PE to be as high as 15% in intensive care unit (ICU) patients who developed symptomatic UEDVT, significantly higher than the 8% observed incidence of PE related to LEDVT in the same study.5 The reason for the surprising difference is unclear, but may have been related to either a greater propensity to anticoagulate LEDVT patients or a lesser propensity to aggressively diagnose PE in therapeutically anticoagulated patients. There was no significant difference in mortality.5 Estimates on the incidence of PTS following UEDVT vary widely, from 7 to 46%, complicated in part by differing definitions between studies.6 7 8 In a prospective study of 53 patients with symptomatic UEDVT managed with anticoagulation alone, the cumulative incidence of PTS was 27.3% at 2 years.8 The presence of residual thrombosis was associated with the development of PTS, and thrombosis affecting the axillary and subclavian veins showed a trend toward association with PTS, but was not statistically significant.8
To date, there are no prospective randomized controlled trials to determine the optimal treatment course for patients with UEDVT. As a result, much of the evidence driving management of this important entity is derived from single-center and retrospective studies, or from literature related to LEDVT. However, interventional therapies have emerged as a first-line therapy for DVT in many institutional algorithms, as well as some societal guidelines.9 Although multiple options exist for catheter-directed management, none have been demonstrated to be clearly superior. Currently, the exact therapeutic algorithm is frequently determined by operator comfort and institutional preference, and more research is needed to clearly define optimal management.
Classification
Traditionally, UEDVT is classified broadly as either primary or secondary. Management is determined by both the type and acuity of the thrombosis.
Primary UEDVT encompasses effort thrombosis, also known as Paget-Schroetter syndrome (PSS), venous thoracic outlet syndrome, and idiopathic cases.1 PSS most commonly occurs in otherwise healthy young individuals following vigorous, repetitive arm motions, with a male to female incidence of approximately 2:1.10 Common examples include weightlifting, playing tennis, pitching a baseball, or painting a ceiling. PSS is most commonly associated with concurrent venous thoracic outlet syndrome, an extrinsic compression of the subclavian vein as it traverses the muscular and boney structures of the thoracic outlet, frequently within the costoclavicular space.11 Repetitive microtrauma during activity leads to endovenous inflammation, and ultimately acute activation of the coagulation cascade and thrombosis of the axillary-subclavian vein. Although this is a rare entity, recognition and treatment is critical as significant long-term disability can occur in as many as 48% of patients treated with rest and elevation alone.12
Secondary UEDVT is significantly more common, accounting for approximately 80% of all cases.1 Thrombosis associated with central venous catheters, cancer, surgery, implanted cardiac devices, or transient hypercoagulability such as pregnancy or oral contraceptive use are considered secondary.1 2 13 Instances secondary to inherited thrombophilia are varyingly considered primary or secondary.1 10
To date the most frequent cause of secondary UEDVT, and the most common cause of UEDVT altogether, is catheter-associated thrombosis. This accounts for at least 50% of all cases, and the relative incidence is increasing, likely due to the increased use of peripherally inserted central catheters (PICCs).1 In a prospective study of 332 patients screened for DVT after placement of one of two different PICCs, the overall rate of thrombosis was found to be 72%, and 4% of patients were symptomatic.14 A retrospective cohort study of 76,242 hospitalized patients across 48 Michigan hospitals found that PICC use was associated with a hazard ratio of 10.49 for UEDVT.15 Other studies have found rates of symptomatic PICC-associated DVTs between 3.5 and 12%.16 17 18 Furthermore, in a single-center analysis of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, more than half of all postoperative DVTs were in the upper extremity, and all but one of those were related to a central venous catheter.19 Larger PICC diameter is also a significant risk factor for UEDVT, as well as the ratio of PICC diameter to vessel diameter.14 20 Patients with cancer are at increased risk for PICC-associated DVT and DVT in general.21
Therapeutic Options
Multiple therapeutic options exist, and treatment must be tailored to the individual patient based on the cause and severity of the thrombosis (Fig. 1). There are no randomized controlled trials and few society guidelines exist that specifically address UEDVT management, so, many recommendations are derived from LEDVT literature.9 22 Therapeutic anticoagulation underpins all treatment options, but catheter-directed therapies represent an increasingly important first-line option to resolve symptoms and prevent complications.
Fig. 1.

Management algorithm for patients with acute UEDVT diagnosed on Doppler ultrasound. DOAC, direct oral anticoagulant; LMWH, low-molecular-weight heparin; PICC, peripherally inserted central catheter; UEDVT, upper extremity deep vein thrombosis. *Indications for removal are catheter malfunction, improper positioning, infection, progressive symptoms, and no longer necessary catheters.
Anticoagulation
Unless there is a contraindication, anticoagulation remains the mainstay of treatment for both UEDVT and LEDVT. In fact, current guidelines from the American College of Chest Physicians (ACCP) recommend only anticoagulation, without catheter-directed therapy, as a first-line treatment for secondary UEDVT.22 In addition to the lack of resources and expertise to do CDT at most centers, these guidelines are based on the fact that there are no large prospective randomized trials addressing the benefit of CDT for UEDVT. While no guidelines for specific anticoagulation agents are made for UEDVT, current ACCP guidelines suggest direct oral anticoagulants (rivaroxaban, apixaban, or dabigatran) for LEDVT in noncancer patients and low-molecular-weight heparin (LMWH) over warfarin in cancer patients.22 A standard course of anticoagulation is 3 months for a first provoked LEDVT or at least 6 to 12 months for an unprovoked LEDVT, and this course is typical for UEDVT, although there are no separate guidelines.21 23
The use of prophylactic anticoagulation is not routinely recommended to prevent UEDVT in patients with central catheters.6 22 23 However, a randomized phase III trial of 420 patients found a significant reduction in catheter-related DVT, without a significant increase in adverse events, in cancer patients treated with low-dose LMWH or low-dose warfarin as prophylaxis.24 The differences in the rates of catheter-associated DVT among the control (14.8%), warfarin (6%), and LMWH groups (10%) were statistically significant. There was no significant difference in the bleeding rate between groups.24 This issue is likely to remain controversial until further studies are conducted, and therefore the risk of prophylactic anticoagulation must be assessed on an individual patient basis.
Central Venous Catheter Removal
In the setting of PICC-associated DVT, removal of the catheter is not always required. When UEDVT is discovered, the position, function, and necessity of the PICC should be assessed. Indications for removal include malfunctioning or improperly positioned catheters, infection, progressive symptoms, and situations where the catheter is no longer necessary.25 ACCP guidelines recommend beginning systemic anticoagulation and keeping the PICC in place, provided that it is necessary, functional, and properly positioned.22
Thrombolytic Agents
Catheter-directed thrombolysis (CDT) has largely replaced systemic therapy in DVT patients where thrombolytic therapy is indicated. Successful thrombus resolution is achieved with lower doses of lytic agent, and the risk of systemic bleeding is decreased.26 A randomized controlled trial of 209 patients in 20 Norwegian hospitals with LEDVT (CaVenT study) found that CDT and anticoagulation together resulted in increased venous patency and an absolute risk reduction of PTS of 14.4% at 24 months when compared with anticoagulation alone.27 However, there was no significant difference in health-related quality of life at the same time point, although patients who developed PTS did report lower quality-of-life scores than those who did not develop PTS.28 No similar studies exist for UEDVT. Newer devices also exist applying ultrasound to CDT. These have the theoretical potential to accelerate clot lysis through heating, direct mechanical effects, and acoustic cavitation.29 However, a trial of 48 patients with acute iliofemoral DVT randomized to either ultrasound-assisted CDT or conventional CDT found no difference in thrombus load reduction, primary venous patency, or PTS severity between the two groups.30
Currently, thrombolytic therapy is appropriate for primary axillo-subclavian UEDVT in patients with severe symptoms, significant thrombus burden, recent thrombosis (symptoms for <14 days), good functional status and life expectancy, and low bleeding risk. CDT is recommended over systemic anticoagulation when the appropriate technology and expertise is available.22 In cases where catheter-directed DVT lysis is performed, a standard course of anticoagulation should be continued after the procedure.22
Mechanical Thrombectomy
Percutaneous mechanical thrombectomy (PMT) can be attempted with or without the concurrent use of thrombolytic agents, termed pharmacomechanical catheter-directed thrombolysis (PCDT) when used in conjunction with thrombolysis. Numerous devices are available for mechanical thrombectomy, but data on their use in UEDVT are limited to retrospective and single-institution studies.31
A retrospective study of 28 patients with either LEDVT or UEDVT managed at one institution using PCDT with the Trellis device found that grade 2 or 3 lysis (≥50% reduction in clot burden) was achieved in all patients (3 patients with grade 2 and 25 patients with grade 3 lysis), and the overall patency (primary plus secondary) was 80% at 12 months.32 Another single-institution experience involving 31 patients found that the use of PCDT reduced treatment duration and length of hospital stay for patients with PSS or iliofemoral DVT.33 Despite its risks and unproven benefit, PCDT has shown promising results in the pediatric population. In small case series, including children younger than 24 months, one center reported successful management of both UEDVT and LEDVT using a multimodality approach involving CDT, PMT, and systemic anticoagulation.34 35 36
A large, phase 3, multicenter randomized trial involving 692 patients comparing the efficacy of PCDT with anticoagulation versus anticoagulation alone for preventing PTS secondary to acute LEDVT (Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis [ATTRACT]) is scheduled for completion in 2016.37 Although this study will focus on LEDVT, the results will likely have significant implications for UEDVT management.
Surgical Decompression
Decompression of the thoracic outlet with resection of the first rib remains integral to most treatment algorithms for PSS. However, there are no randomized data available regarding first-rib resection, and the timing and necessity of surgery remain significant controversies. A recent systematic literature review found that symptom relief at the final recorded follow-up was significantly higher in the surgically managed groups (95%) than in those who did not undergo first-rib resection (63%), and that 40% of patients who initially underwent conservative management subsequently required surgical decompression.38 Other authors have variously supported surgery within the first 14 days, surgery after 3 months of anticoagulation, or a conservative approach that does not necessarily involve first-rib resection.39 40 41 Currently, there are treatment algorithms including surgical decompression during the same hospitalization as thrombolysis, a delay of several weeks after initial thrombolysis prior to surgery, and initial conservative management without surgery.42 Overall consensus remains that first-rib resection is necessary, and that it is safe to perform immediately after thrombolysis, but the controversy is likely to persist given the lack of randomized data. At our institution, we prefer early first-rib resection after thrombolysis.
Superior Vena Cava Filters
Although there is currently no indication by the Food and Drug Administration, superior vena cava (SVC) filter placement for UEDVT has been reported.2 As with inferior vena cava (IVC) filters for LEDVT, the indication for SVC filter placement is a contraindication to anticoagulation coupled with significant clinical concern for developing PE. Given the shorter length of the SVC, this procedure is technically more challenging, and may be more prone to serious complications. Mortality and significant morbidity, including cardiac tamponade, aortic perforation, and recurrent pneumothorax, have been reported in the literature as a result of SVC filter placement, although the overall complication rate remains low.43 There are currently no published guidelines recommending clinical scenarios for SVC filter placement. Given these concerns, without larger, prospective data to support their use, SVC filters should be placed only in rare cases after careful discussion of the risks with the patient, family, and referring physician.
Procedure Technique
Patients are started on therapeutic anticoagulation, using either unfractionated heparin or LMWH prior to the procedure. Access is obtained distal to the thrombus using real-time ultrasound guidance and a micropuncture set, typically via a basilic vein approach. It cannot be emphasized enough that real-time ultrasound guidance be used for vascular access in any patient in whom thrombolysis is being considered. Venography of the affected extremity and central veins is then performed to determine the extent of the thrombus and map the anatomy (Fig. 2a). A 5F sheath is then placed, and a hydrophilic guidewire and 5F catheter are used to cross the thrombus. In situations where the thrombus is challenging to traverse, a chronic total occlusion catheter may be of benefit. A multi-sidehole infusion catheter, such as the Cragg-McNamara (Micro Therapeutics Inc., Irvine, CA) or Unifuse (Angiodynamics, Latham, NY) catheter is then positioned across the thrombus (Fig. 2b). The length of the catheter is selected so that the sideholes cover the entire length of the thrombus. Infusion of the thrombolytic agent (rt-PA, alteplase) is then performed over approximately 24 hours at a rate of 1 mg/hour with concurrent therapeutic or half dose heparin or therapeutic LMWH. The following day, a repeat venogram is performed to evaluate for thrombus resolution (Fig. 2c). At our institution, nearly all cases of thrombolysis are limited to 24 hours, except in unusual circumstances. In addition, we are increasingly using therapeutic LMWH during thrombolysis instead of unfractionated heparin as it not only provides a steady-state level of anticoagulation during rt-PA infusion and mechanical thrombectomy (if needed), but it also eliminates potential dosing errors with heparin infusion and improves nursing efficiency. We do not routinely monitor fibrinogen levels, as there is no prospective evidence that this practice lowers bleeding risk.44 In fact, there is evidence that fibrinogen level can be normal or artificially high due to the accumulation of fragment X, a fibrin degradation product.45 46
Fig. 2.
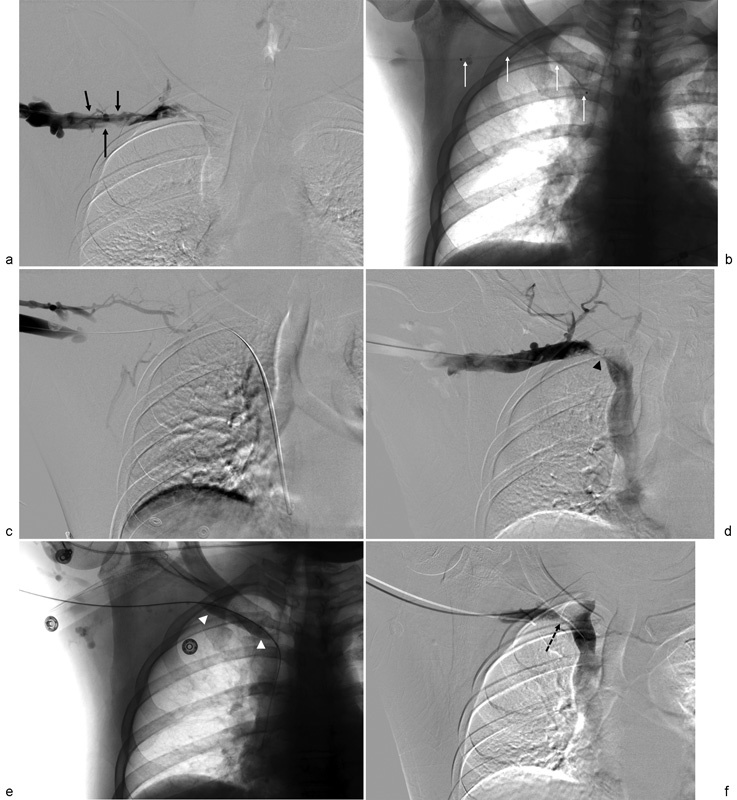
Extensive upper extremity deep vein thrombosis requiring mechanical thrombectomy and balloon angioplasty in a 31-year-old man with axillo-subclavian thrombosis secondary to Paget-Schroetter syndrome. (a) Digital subtraction venogram showing significant thrombus in the right axillary and subclavian veins (solid black arrows) with no appreciable contrast reaching the superior vena cava. Further examination (not shown) demonstrated a patent superior vena cava. (b) Thrombolysis catheter placement spanning the entire extent of the thrombus (white arrows). (c) Venogram obtained following 24 hours of thrombolytic infusion demonstrates persistent axillo-subclavian thrombosis. (d) Mechanical thrombectomy resulted in reduction in thrombus burden, but significant residual stenosis is noted at the site of subclavian vein compression by the first rib (black arrowhead). (e) Prolonged angioplasty was performed at the site of the stenosis (white arrowheads). (f) Residual extrinsic compression remained (dashed black arrow), and the patient was placed on anticoagulation and then underwent first-rib resection 5 days later.
In patients for whom CDT does not completely resolve the thrombus, PMT or PCDT is performed (Fig. 2d). At our institution, the AngioJet device (Boston Scientific, Marlborough, MA) or the Arrow-Trerotola device (Teleflex, Wayne, PA) is used in conjunction with CDT or, in some cases, as the primary treatment modality depending on the age of the thrombus. Angioplasty is performed for significant, flow-limiting stenosis (Fig. 2e), and stenting is not used in acute UEDVT cases, and is contraindicated in the subclavian vein except in unusual situations due to the high risk of stent fracture. Following thrombectomy, patients are monitored overnight prior to discharge. After the procedure, anticoagulation with warfarin, LMWH, or a direct oral anticoagulant is continued for a minimum of 3 to 6 months. In cases of PSS, first-rib resection is typically performed during the same hospitalization (Fig. 2f). Close follow-up in clinic is critical to asses for symptoms of recurrent thrombosis, PTS, or PE. The axillary and subclavian veins are also reassessed with Doppler ultrasonography and/or CT venography on follow-up to monitor patency.
Conclusion
Even as an increasingly recognized clinical entity, UEDVT remains underdiagnosed and inadequately researched. Interventional radiologists are in a unique position to both prevent and treat UEDVT. As central venous catheters are responsible for approximately half of all UEDVT, and larger PICC diameter has been shown to be a risk factor for PICC-associated DVT,14 20 placing the smallest line appropriate for the clinical indication can help reduce UEDVT. Furthermore, interventional therapies appear to be safe and effective first-line therapies for both primary and secondary UEDVT and have promising potential to reduce the incidence of PTS and improve patient's quality of life.
Unfortunately, there have yet to be any randomized trials conducted to optimize the management of UEDVT. As such, the LEDVT research remains the underpinning of UEDVT management. A recent randomized trial (CaVenT) has supported CDT over standard therapy for iliofemoral DVT, and another (ATTRACT) would soon be published regarding PMT for LEDVT.27 37 Randomized trials addressing the efficacy of these techniques for UEDVT will be necessary to improve patient outcomes.
References
- 1.Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869. doi: 10.1056/NEJMcp1008740. [DOI] [PubMed] [Google Scholar]
- 2.Koury J P, Burke C T. Endovascular management of acute upper extremity deep venous thrombosis and the use of superior vena cava filters. Semin Intervent Radiol. 2011;28(1):3–9. doi: 10.1055/s-0031-1272975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg J, Troutman D A, Shubinets V, Dougherty M J, Calligaro K D. Phlegmasia cerulea dolens in the upper extremity: a case report and systematic review and outcomes analysis. Vasc Endovascular Surg. 2016;50(2):98–101. doi: 10.1177/1538574416631645. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz F J, Mismetti P, Poggio R. et al. Clinical outcome of patients with upper-extremity deep vein thrombosis: results from the RIETE Registry. Chest. 2008;133(1):143–148. doi: 10.1378/chest.07-1432. [DOI] [PubMed] [Google Scholar]
- 5.Lamontagne F, McIntyre L, Dodek P. et al. Nonleg venous thrombosis in critically ill adults: a nested prospective cohort study. JAMA Intern Med. 2014;174(5):689–696. doi: 10.1001/jamainternmed.2014.169. [DOI] [PubMed] [Google Scholar]
- 6.Grant J D, Stevens S M, Woller S C. et al. Diagnosis and management of upper extremity deep-vein thrombosis in adults. Thromb Haemost. 2012;108(6):1097–1108. doi: 10.1160/TH12-05-0352. [DOI] [PubMed] [Google Scholar]
- 7.Kahn S R, Galanaud J P, Vedantham S, Ginsberg J S. Guidance for the prevention and treatment of the post-thrombotic syndrome. J Thromb Thrombolysis. 2016;41(1):144–153. doi: 10.1007/s11239-015-1312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prandoni P Bernardi E Marchiori A et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study BMJ 20043297464484–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolaides A Hull R D Fareed J; Cardiovascular Disease Educational and Research Trust; European Venous Forum; North American Thrombosis Forum; International Union of Angiology and Union Internationale du Phlebologie. Thrombolytic therapy Clin Appl Thromb Hemost 2013192198–204. [DOI] [PubMed] [Google Scholar]
- 10.Kurli V, Pryluck D S, Singh C K, Clark T W. Philadelphia, PA: Saunders; 2014. Acute upper extremity deep venous thrombosis; pp. 766–771. [Google Scholar]
- 11.Arnhjort T, Nordberg J, Delle M, Borgis C J, Rosfors S, Lärfars G. The importance of the costoclavicular space in upper limb primary deep vein thrombosis, a study with magnetic resonance imaging (MRI) technique enhanced by a blood pool agent. Eur J Intern Med. 2014;25(6):545–549. doi: 10.1016/j.ejim.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Tilney M L, Griffiths H J, Edwards E A. Natural history of major venous thrombosis of the upper extremity. Arch Surg. 1970;101(6):792–796. doi: 10.1001/archsurg.1970.01340300148026. [DOI] [PubMed] [Google Scholar]
- 13.Wadhawan A, Laage Gaupp F M, Sista A K. Automatic implantable cardiac defibrillator implantation may precipitate effort-induced thrombosis in young athletes: a case report and literature review. Clin Imaging. 2014;38(4):510–514. doi: 10.1016/j.clinimag.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Itkin M, Mondshein J I, Stavropoulos S W, Shlansky-Goldberg R D, Soulen M C, Trerotola S O. Peripherally inserted central catheter thrombosis--reverse tapered versus nontapered catheters: a randomized controlled study. J Vasc Interv Radiol. 2014;25(1):85–910. doi: 10.1016/j.jvir.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Greene M T, Flanders S A, Woller S C, Bernstein S J, Chopra V. The association between PICC use and venous thromboembolism in upper and lower extremities. Am J Med. 2015;128(9):986–930. doi: 10.1016/j.amjmed.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847–854. doi: 10.1111/jth.12549. [DOI] [PubMed] [Google Scholar]
- 17.Chopra V, Fallouh N, McGuirk H. et al. Patterns, risk factors and treatment associated with PICC-DVT in hospitalized adults: a nested case-control study. Thromb Res. 2015;135(5):829–834. doi: 10.1016/j.thromres.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Bertoglio S, Faccini B, Lalli L, Cafiero F, Bruzzi P. Peripherally inserted central catheters (PICCs) in cancer patients under chemotherapy: a prospective study on the incidence of complications and overall failures. J Surg Oncol. 2016;113(6):708–714. doi: 10.1002/jso.24220. [DOI] [PubMed] [Google Scholar]
- 19.Mino J S, Gutnick J R, Monteiro R, Anzlovar N, Siperstein A E. Line-associated thrombosis as the major cause of hospital-acquired deep vein thromboses: an analysis from National Surgical Quality Improvement Program data and a call to reassess prophylaxis strategies. Am J Surg. 2014;208(1):45–49. doi: 10.1016/j.amjsurg.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 20.Trerotola S O, Stavropoulos S W, Mondschein J I. et al. Triple-lumen peripherally inserted central catheter in patients in the critical care unit: prospective evaluation. Radiology. 2010;256(1):312–320. doi: 10.1148/radiol.10091860. [DOI] [PubMed] [Google Scholar]
- 21.Delluc A, Le Gal G, Scarvelis D, Carrier M. Outcome of central venous catheter associated upper extremity deep vein thrombosis in cancer patients. Thromb Res. 2015;135(2):298–302. doi: 10.1016/j.thromres.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Kearon C, Akl E A, Ornelas J. et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 23.Lenchus J D Biehl M Cabrera J de Moraes A G Dezfulian C In-hospital management and follow-up treatment of venous thromboembolism: focus on new and emerging treatments J Intensive Care Med 2016;pii:0885066616648265 10.1177/0885066616648265 [DOI] [PubMed] [Google Scholar]
- 24.Lavau-Denes S, Lacroix P, Maubon A. et al. Prophylaxis of catheter-related deep vein thrombosis in cancer patients with low-dose warfarin, low molecular weight heparin, or control: a randomized, controlled, phase III study. Cancer Chemother Pharmacol. 2013;72(1):65–73. doi: 10.1007/s00280-013-2169-y. [DOI] [PubMed] [Google Scholar]
- 25.Fallouh N, McGuirk H M, Flanders S A, Chopra V. Peripherally inserted central catheter-associated deep vein thrombosis: a narrative review. Am J Med. 2015;128(7):722–738. doi: 10.1016/j.amjmed.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Mizuno A, Anzai H, Utsunomiya M. et al. Real clinical practice of catheter therapy for deep venous thrombosis: periprocedural and 6-month outcomes from the EDO registry. Cardiovasc Interv Ther. 2015;30(3):251–259. doi: 10.1007/s12928-014-0314-0. [DOI] [PubMed] [Google Scholar]
- 27.Enden T Haig Y Kløw N E et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial Lancet 2012379981031–38. [DOI] [PubMed] [Google Scholar]
- 28.Enden T, Wik H S, Kvam A K, Haig Y, Kløw N E, Sandset P M. Health-related quality of life after catheter-directed thrombolysis for deep vein thrombosis: secondary outcomes of the randomised, non-blinded, parallel-group CaVenT study. BMJ Open. 2013;3(8):e002984. doi: 10.1136/bmjopen-2013-002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bader K B, Bouchoux G, Holland C K. Sonothrombolysis. Adv Exp Med Biol. 2016;880:339–362. doi: 10.1007/978-3-319-22536-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engelberger R P, Spirk D, Willenberg T. et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv. 2015;8(1):e002027. doi: 10.1161/CIRCINTERVENTIONS.114.002027. [DOI] [PubMed] [Google Scholar]
- 31.Zangan S, Funaki B. Philadelphia, PA: Saunders; 2014. Thrombectomy devices; pp. 766–771. [Google Scholar]
- 32.Chaudhry M A, Pappy R, Hennebry T A. Use of the trellis device in the management of deep vein thrombosis: a retrospective single-center experience. J Invasive Cardiol. 2013;25(6):296–299. [PubMed] [Google Scholar]
- 33.Spivack A, Troutman D, Dougherty M, Calligaro K. Changing strategies to treat venous thrombotic occlusions of the upper and lower extremities secondary to compressive phenomena. Vasc Endovascular Surg. 2013;47(4):274–277. doi: 10.1177/1538574413481857. [DOI] [PubMed] [Google Scholar]
- 34.Dandoy C E, Kukreja K U, Gruppo R A, Patel M N, Tarango C. Outcomes in children with deep vein thrombosis managed with percutaneous endovascular thrombolysis. Pediatr Radiol. 2015;45(5):719–726. doi: 10.1007/s00247-014-3209-4. [DOI] [PubMed] [Google Scholar]
- 35.Kukreja K U, Lungren M P, Patel M N. et al. Endovascular venous thrombolysis in children younger than 24 months. J Vasc Interv Radiol. 2014;25(8):1158–1164. doi: 10.1016/j.jvir.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Lungren M P, Ward T J, Patel M N, Racadio J M, Kukreja K. Endovascular thrombolysis to salvage central venous access in children with catheter-associated upper extremity deep vein thrombosis: technique and initial results. J Thromb Thrombolysis. 2015;40(3):274–279. doi: 10.1007/s11239-015-1209-3. [DOI] [PubMed] [Google Scholar]
- 37.Vedantham S, Goldhaber S Z, Kahn S R. et al. Rationale and design of the ATTRACT Study: a multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. Am Heart J. 2013;165(4):523–530000. doi: 10.1016/j.ahj.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lugo J, Tanious A, Armstrong P. et al. Acute Paget-Schroetter syndrome: does the first rib routinely need to be removed after thrombolysis? Ann Vasc Surg. 2015;29(6):1073–1077. doi: 10.1016/j.avsg.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Johansen K H, Illig K A. New York: Springer; 2013. Conservative (non-operative) treatment of VTOS; pp. 395–400. [Google Scholar]
- 40.Johansen K H. New York: Springer; 2013. Controversies in VTOS: is costoclavicular junction decompression always needed in VTOS; pp. 513–515. [Google Scholar]
- 41.Lee J T. New York: Springer; 2013. Controversies in VTOS: timing of first rib resection after thrombolysis; pp. 517–520. [Google Scholar]
- 42.van den Houten M M van Grinsven R Pouwels S Yo L S van Sambeek M R Teijink J A Treatment of upper-extremity outflow thrombosis Phlebology 201631(1, Suppl):28–33. [DOI] [PubMed] [Google Scholar]
- 43.Owens C A, Bui J T, Knuttinen M G, Gaba R C, Carrillo T C. Pulmonary embolism from upper extremity deep vein thrombosis and the role of superior vena cava filters: a review of the literature. J Vasc Interv Radiol. 2010;21(6):779–787. doi: 10.1016/j.jvir.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Morrison H L. Catheter-directed thrombolysis for acute limb ischemia. Semin Intervent Radiol. 2006;23(3):258–269. doi: 10.1055/s-2006-948765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisenberg P R, Sobel B E, Jaffe A S. Characterization in vivo of the fibrin specificity of activators of the fibrinolytic system. Circulation. 1988;78(3):592–597. doi: 10.1161/01.cir.78.3.592. [DOI] [PubMed] [Google Scholar]
- 46.Sakharov D V, Rijken D C. Superficial accumulation of plasminogen during plasma clot lysis. Circulation. 1995;92(7):1883–1890. doi: 10.1161/01.cir.92.7.1883. [DOI] [PubMed] [Google Scholar]


