Abstract
In this Data in Brief, we have provided data describing the secretion profile of two main immunoregulatory proteins, heme oxygenase-1 (HO-1) and CD200, from bone marrow (BM), Wharton׳s Jelly (WJ) or adipose tissue (AT) mesenchymal stromal cells (MSCs) being cultivated either in the absence or presence of activated T-cells. Whilst HO-1 is a stress-responsive enzyme displaying diverse cytoprotective effects, CD200 is a membrane glycoprotein delivering immunoregulatory signals following interaction with its receptor (CD200R). Using Enzyme-linked immunosorbent assay (ELISA) techniques, these data are presented to show distinct constitutive secretion of both HO-1 and CD200 depending on MSC types. The data presented also demonstrate that the protein levels of HO-1 and CD200 are differentially modulated during co-culture with activated T-cells. All assays were carried out in triplicates and the mean values are reported. The data presented in this article are complementary to our previously published report entitled “The Immunomodulatory Potential of Mesenchymal Stromal Cells: A Story of a Regulatory Network.” [1].
Keywords: Mesenchymal stromal cells, Activated T-cells, Heme oxygenase-1, CD200
Specifications Table
| Subject area | Biology |
|---|---|
| More specific subject area | Mesenchymal stromal cells (MSCs) |
| Type of data | Figures |
| How data was acquired | Enzyme-linked immunosorbent assay (ELISA) |
| Data format | Analyzed |
| Experimental factors | HO-1 and CD200 |
| Experimental features | The protein levels of HO-1 and CD200 secretion by MSCs were assessed using ELISA technique |
| Data source location | Institut Jules Bordet, Brussels, Belgium |
| Data accessibility | Data are provided in the paper |
Value of the data
|
|
1. Data
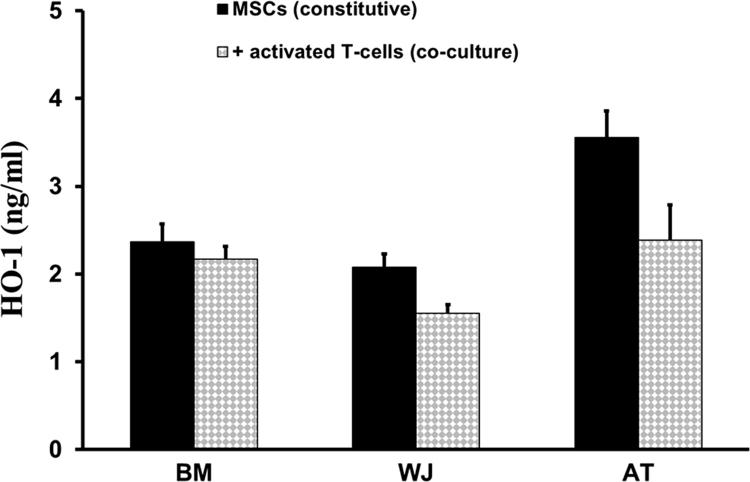
This data article presents the protein level of HO-1 (Fig. 1) and CD200 (Fig. 2) secreted by BM-MSCs, WJ-MSCs and AT-MSCs in the absence (constitutive) or in the presence of activated T-cells (co-culture).
Fig. 1.
HO-1 production by MSCs. BM-MSCs, WJ-MSCs and AT-MSCs were cultivated in the absence (constitutive) or in the presence of activated T-cells (co-culture). Intracellular HO-1 levels from the cell lysates were then measured using an ELISA kit. Reported values correspond to the mean concentrations (ng/ml)±SD.
Fig. 2.
CD200 secretion by MSCs. Following cultivation of BM-MSCs, WJ-MSCs and AT-MSCs in the absence (constitutive) or in the presence of activated T-cells (co-culture), the resulting supernatants were collected and used to assess the levels of CD200 using an ELISA kit. Reported values represent the mean concentrations (pg/ml)±SD.
2. Experimental design, materials and methods
2.1. Isolation and cultivation of MSCs
This study was conducted in accordance with the Declaration of Helsinki (1964) and approved by the local ethics committee of the “Institut Jules Bordet” (Belgium). The samples were obtained from healthy donors who gave informed written consent. Bone-marrow, adipose tissue and Wharton׳s jelly of the umbilical cord were used to isolate MSCs. Cells were cultured in low glucose Dulbecco׳s Modified Eagle׳s Medium (DMEM-LG, Lonza) supplemented with 15% fetal bovine serum (FBS, Sigma-Aldrich, Bornem, Belgium), 2 mM L-glutamine and 50 U/ml penicillin (both from Lonza) and incubated at 37 °C in a 5% CO2 humidified atmosphere to reach 80–90% confluency. Adherent cells were harvested by TrypLE Select (Lonza, Belgium) and expanded by sub culturing at a lower density (1000 cells/cm2).
2.2. Selection of T-cells
Peripheral blood (PB) cells were obtained from healthy donors after informed consent. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (LinfoSep, Biomedics, Madrid, Spain). T-cells were purified by positive selection using MACS system (Miltenyi Biotec GmbH, Bergisch, Germany). Selected T-cell purity (CD3+), determined by flow cytometry, was always above 95%. Mitogenic stimulation by using 5 µg/ml phytohemagglutinin (PHA, Remel Europe, Kent, UK) with 20 U/ml of interleukin 2 (IL2, Biotest AG, Dreieich, Germany) was carried out to induce T-cell activation.
2.3. Co-culture model
Before starting the co-culture, MSCs (25×103) were seeded in flat-bottomed well plates and allowed to adhere overnight. In the next day, activated T-cells (1×105), were added to MSCs for 5 days of co-culture.
2.4. Measurement of HO-1 level
After culture, the respective media were removed and the cells were washed twice with PBS (Phosphate Buffered Saline). Adherent MSCs were then harvested by TrypLE Select (Lonza) and centrifuged. The obtained cell pellets were re-suspended in the HO-1 extraction reagent (Enzo Life Sciences, Belgium), being supplemented with a cocktail of protease inhibitors (Roche Applied Science, Belgium), and then incubated for 30 min on ice. The extracts were centrifuged (20,000 g for 10 min at 4 °C) and the supernatants were carefully collected representing thus the cell lysates. HO-1 levels were then quantified using an ELISA kit (Enzo Life Sciences) following the manufacturer׳s instructions.
2.5. Measurement of CD200 level
After culture, the respective media were recovered and centrifuged. The resulting supernatants were used to quantify the CD200 level using an ELISA kit (Bio-Connect Diagnostics B.V., Nederland) and following the manufacturer׳s instructions.
2.6. Statistical analysis
For each type of MSCs, five different co-cultures were performed. ELISA measurements were carried out in triplicates from and the resulting supernatants. Data are presented as mean±SD (standard deviation) and analysed using Wilcoxon Signed Rank test.
Acknowledgements
We gratefully acknowledge the support received from the "Fonds de la Recherche Scientifique (FNRS), and its Télévie program (Grant no. FC75708).
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.dib.2017.02.036.
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.dib.2017.02.036.
Transparency document. Supplementary material
Supplementary material
.
Appendix A. Supplementary material
Supplementary material
.
Reference
- 1.Najar M., Raicevic G., Crompot E., Fayyad-Kazan H., Bron D., Toungouz M. The immunomodulatory potential of mesenchymal stromal cells: a story of a regulatory network. J. Immunother. 2016;39:45–59. doi: 10.1097/CJI.0000000000000108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material